Abstract
Cognitive impairment in Alzheimer’s disease (AD) is strongly associated with both extensive deposition of amyloid β peptides and oxidative stress, but the exact role of these indices in the development of dementia is not clear. This study was designed to determine the relationship between cognitive impairment, activation of the free radical producing enzyme NADPH oxidase (NOX), and progressive changes in Aβ deposition and solubility in humanized APP × PS1 knock-in mice of increasing age. Data show that cognitive performance and expression of key synaptic proteins was progressively decreased in aging APP × PS1 mice. Likewise, NOX activity and expression of the specific NOX subunit NOX4 was significantly increased in APP × PS1 mice in an age-dependent manner, and NOX activity and cognitive impairment shared a significant linear relationship. Data further show that age-dependent increases in Aβ1-42 had a significant linear relationship with both NOX activity and cognitive performance in APP × PS1 knock-in mice. Collectively, these data show that NOX expression and activity are significantly upregulated with age in this humanized model of Aβ pathogenesis, and suggest that NOX-associated redox pathways are intimately linked to both the loss of cognitive function and the deposition of Aβ1-42.
Keywords: Aging, Dementia, Oxidative stress, Redox signaling pathways, Synapse loss
INTRODUCTION
Alzheimer’s disease (AD) is characterized by a progressive and irreversible loss of cognitive function and is the most common dementing disorder of the elderly (Jalbert et al., 2008). As existing treatments unfortunately have only limited efficacy in slowing clinical decline, there has been concerted effort shared by basic, clinical, and epidemiological researchers to map the etiology of this disease. An important and primary pathological feature of AD is the progressive accumulation of amyloid β-protein (Aβ) into neuritic plaques (Jellinger, 2002), (Selkoe, 2001), (Vinters and Farag, 2003), (Walsh and Selkoe, 2004), and the progression of cognitive decline is associated with accumulation of Aβ peptide, particularly Aβ1-42, and alterations in Aβ solubility (Johnson, 2000), (Murphy et al., 2007). AD brains are also typified by ample pathological evidence of oxidative stress in affected regions, including the cerebral cortex and hippocampal formation (Markesbery and Lovell, 1998), (Aksenov et al., 2001), (Wang et al., 2006). Markers of oxidative stress are elevated in mild cognitive impairment (MCI), which may be the earliest stage of clinical dementia (Keller et al., 2005), (Williams et al., 2006), (Butterfield et al., 2006), (Mosconi et al., 2008). While collectively these data are consistent with the hypothesis that free radical production might drive the initiation of cognitive dysfunction, the nature of the relationship between free radical production and the pathogenesis of Aβ is still not fully understood.
Free radicals are produced in mammalian cells as secondary by-products by many different systems, including mitochondrial electron transport, xanthine oxidase, cyclooxgenases, and monoamine oxidases (reviewed in (Droge, 2002), (Reddy, 2006)). However, the enzyme NADPH oxidase (NOX) is noteworthy as it is dedicated to the specific and deliberate production of free radicals. Although NOX was first described in phagocytic cells such as microglia, it is now well established that NOX subunits are also expressed in neurons and astrocytes (Noh and Koh, 2000), (Kim et al., 2005). Indeed, experimental evidence points to a role for NOX in neuronal physiology, particularly in functions relating to hippocampal electrophysiology (Kishida et al., 2005), (Tejada-Simon et al., 2005). These data suggest that NOX is very well situated to participate in perturbations to cognitive function, and indeed, many reports have proposed that NOX may be involved in AD pathogenesis (reviewed in (Wilkinson and Landreth, 2006), (Block, 2008)). For example, early studies revealed the ability of Aβ to activate NOX and increase superoxide production (Meda et al., 1995), (Bruce et al., 1996), (Thomas et al., 1996), (Bianca et al., 1999), (Jana and Pahan, 2004), (Niikura et al., 2004), suggesting that NOX may be involved in Aβ-induced neuronal injury. Furthermore, published reports show increased NOX activity in AD brains (Shimohama et al., 2000) and (Ansari and Scheff, 2011), and also in MCI brains (Bruce-Keller et al., 2010) . Finally, genetic deletion of specific NOX subunits was recently shown to attenuate neurovascular dysfunction and cognitive decline in transgenic mice overexpressing the Swedish mutation of the human amyloid precursor protein (Park et al., 2008).
As there is ample support indicating that NOX could participate in AD, this study was undertaken to delineate the profile of NOX enzymatic activity and subunit expression in humanized APP × PS1 knock-in mice, which are a second generation, physiologically appropriate mouse model of Aβ pathogenesis. The use of knock-in technology, rather than a transgenic overexpression system, preserves the physiologically appropriate regulation of APP expression by endogenous promoters. Thus, data related to Aβ levels in these particular mice are not confounded by an artificial promoter system in unknown loci that drives supraphysiologic levels of APP expression. Indeed, previous reports from our group show that this model of Aβ pathogenesis is associated with progressive changes in Aβ solubility and deposition that mirror key changes in human AD (Flood et al., 2002), (Murphy et al., 2007). Furthermore, this model recapitulates the development of both diffuse and neuritic plaques (Murphy et al., 2007), which is key to modeling AD pathology (Murphy et al., 2007). Finally, published reports document progressive and age-related increases in markers of oxidative stress in these mice (Abdul et al., 2008), (Huang et al., 2010). Thus, these studies were designed to specifically evaluate the exact relationship between Aβ, NOX activation, and cognitive decline. To this end, APP × PS1 and wild type (WT) mice were examined for cognitive impairment, and cognitive function was analyzed in relation to NOX activation and to parameters of Aβ deposition and solubility. Studies were conducted in young (4-6 month-old) mice, in which neuritic plaques are not detected; and old (16-19 month-old) mice, in which neuritic plaques are present predominantly in the frontal and parietal cortex and (to a lesser degree) in the hippocampus (Murphy et al., 2007).
MATERIALS AND METHODS
APP × PS1 knock-in mice
The Institutional Animal Care and Use Committee at the Pennington Center approved all experimental protocols, which were compliant with NIH guidelines on the use of experimental animals. All mice were housed in standard caging with 12:12 light: dark cycle, and had ad libitum access to food and water throughout the study. Young (age 4-6 months) and aged (16-19 months) mice were evaluated in this study, and data were compiled from 2 complete but separate cohorts of mice, for a total of 12-20 animals in each group.
This study employed APPNLh/NLh × PS1P264L/P246L mutant mice obtained from Cephalon, Inc. This second generation, knock-in model of Aβ pathology is based on Cre-lox knock-in technology, and expression of both genes is driven by their endogenous promoters and genes are not overexpressed (Anantharaman et al., 2006), (Reaume et al., 1996), (Siman et al., 2000), (Flood et al., 2002), (Chang et al., 2006). APP × PS1 mice are maintained on a CD-1/129 background, and wild type (WT) mice obtained from heterozygous APP × PS1 pairings were used as controls. The present study used only male mice.
Behavioral analysis of cognitive performance: Stone T-maze
Procedural memory was tested behaviorally in mice using the segmented Stone T-maze as described in previous reports (Pistell et al., 2010),(Pistell and Ingram, 2010), (Pistell et al., 2010). Briefly, performance in this maze requires the mouse to learn the correct sequence of 13 consecutive left and right turns to reach the goal box and successfully escape the maze. Mice are motivated to escape because they are required to wade (not swim) through the maze, as the apparatus is maintained in a tray of water (21-23°C) filled to a level (1.5 cm) that allows the mice to keep their head out of the water while maintaining contact with the floor, but the height of the maze prevents rearing. The day before acquisition testing, all animals were pre-trained to escape the water using a straight runway constructed of acrylic with opaque sides. For acquisition testing, mice were given 15 sequential trials in the T-maze in a single day. Mice were tested in groups of 8-10, and were run through such that the first trial was completed by all mice before proceeding to the second trial, insuring that each mouse had sufficient rest between trials to prevent any potential effects of fatigue. For the purpose of data analysis and presentation, maze acquisition data was collapsed and averaged into 5 separate “blocks” of 3 trials each. To measure memory retention, mice were given an additional 5 sequential trials in the maze 7 days after acquisition training. All trials were recorded using video tracking software (Viewpoint Lifesciences, Inc), and the numbers of errors committed were recorded and used as the primary measure of learning because it is unbiased by potential confounds resulting from differences in motor function.
Measures of synaptic marker and NOX subunit expression
Brain samples taken from the frontal cortex were homogenized in a Tris-buffered saline (pH 7.4) lysis buffer containing 0.1% Triton X-100, 5 mM EDTA, and protease inhibitor cocktail (Sigma-Aldrich). Samples were then denatured in SDS, and equivalent amounts of protein were electrophoretically separated in polyacrylamide gels and blotted onto nitrocellulose. Blots were processed using the following primary antisera: anti-p47phox (1:500, Millipore); anti-NOX2 (1:500, Santa Cruz Biotenchology, Inc.), anti-NOX4 (1:1000, Novus Biologicals LLC), anti-synapsin 1 (1:2000, Abcam) anti-phospho(S553)-synapsin 1 (1:10000), anti-synapse associated protein 97(1:3000, Thermo Fisher Scientific), and anti-tubulin (1:100, Wako Chemicals USA, Inc.). After incubation with primary antibodies, blots were washed and exposed to horseradish peroxidase-conjugated secondary antibodies, and visualized using a chemiluminescence system (Amersham Biosciences). Blot images were scanned and densitometrically analyzed for quantification. To ensure accurate quantification across multiple blots, samples from APP × PS1 and WT mice of a given age were included in each blot. Data were calculated as a ratio of expression over tubulin expression, which was included as an internal loading control. Protein expression in APP × PS1 mice was then calculated and presented as percent expression in WT mice of the same age so that multiple blots could be accurately reconciled.
Measures of NOX activity
Frozen samples taken from the frontal cortex of mice were homogenized in protease inhibitor-containing buffer at 4°C, and then subjected to differential centrifugation to isolate membranes. Membrane samples (exactly 35 μg total protein) were incubated with 5 μM lucigenin and 100 μM NADPH, and NOX activity was measured immediately by documenting the light produced by each sample at 37°C. Light emission was recorded from each sample in 10 second intervals for exactly 3 minutes. In agreement with published data on this assay (Li et al., 1998), (Janiszewski et al., 2002), initial optimization of this assay revealed that NADPH-driven luminescence was nearly completely prevented (>95% inhibition) by the addition of either diphenyleneiodonium (DPI), which selectively inhibits flavonoid-containing enzymes by irreversibly binding flavin (O’Donnell et al., 1993), or by superoxide dismutase, a catalytic scavenger of superoxide radicals (Fridovich, 1975). Thus, the specific role of NOX in the measured luminescence was determined by subtracting the background level of luminescence for each sample (generated by the inclusion of 1 μM DPI within the sample), and NOX activity is presented as average luminescent counts per minute (CPM) per microgram protein.
Analysis of Aβ solubility
Aβ solubility was measured using a well characterized three-step extraction procedure that uses sequentially more denaturing conditions to serially extract Aβ that is progressively more insoluble, and is followed by measurement of the amount of Aβ using a two-site (sandwich) ELISA. Details of this procedure, and the antibodies used, have been published (Beckett et al., 2010), (McGowan et al., 2005), (Murphy et al., 2007), but briefly, tissue was polytron homogenized in ice-cold PBS with a complete protease inhibitor cocktail (Amresco). The supernatant was collected following centrifugation at 20,000xg for 30 min at 4 °C. The pellet was re-extracted by brief sonication (10 × 0.5 s microtip pulses at 100 W; Fisher Sonic Dismembrator, Model 500) in 2% SDS with complete protease inhibitor cocktail, centrifuged, and the supernatant again collected. The remaining pellet was finally extracted by sonication (as above) in 70% formic acid (FA), and centrifuged at 20,000xg for 1 h at 4 °C. Sample extracts were stored frozen at − 80 °C until time of ELISA assay.
The sandwich ELISA was conducted using antibodies Ab42.5 (human sequence Aβ1–16) for Aβ1-40 capture, and biotinylated 13.1.1 (end specific for Aβ1-40) for detection. Measurement of Aβ1-42 was done with antibodies 2.1.3 (end specific for Aβ1-42) for capture and biotinylated 4G8 (human sequence Aβ17-24, Covance) for detection. All standards and samples were run at least in duplicate. Plates (Immulon 4HBX) were coated with 0.5 μg/well of antibody in PBS, and blocked with Synblock (Serotec) overnight. This is a well characterized system, with a lower limit of detection of approximately 5 pM and a linear range of about two orders of magnitude (20–2000 pM). Following development with TMB reagent, plates were stopped with 6% o-phosphoric acid and read at 450 nm using a BioTek multiwell plate reader. Total levels of Aβ1-40 and Aβ1-42 were determined by calculating the sum of levels in each fraction (PBS, SDS, and formic acid).
Statistical analyses
All data are shown as mean ± standard error of measurement. Stone maze performance was analyzed with 2-way repeated measures analyses of variance (ANOVA) to determine main effects of trail block and treatment, followed by Bonferroni post-hoc comparisons to determine differences between APP × PS1 and WT groups. NOX activity was also analyzed with 2-way ANOVA to determine main effects of genotype and age, followed by Bonferroni post-hoc comparisons to determine differences between APP × PS1 and WT mice of each age. Data generated from Western blot were analyzed by 2-tailed, unpaired t-tests to determine specific differences between genotypes within individual age groups. Linear regression analyses were used to determine statistical relationships of NOX activity and Aβ levels with cognitive decline. Statistical significance for all analyses was accepted at p < 0.05, and *, **, and *** represent p < 0.05, p < 0.01, and p < 0.001, respectively.
RESULTS
Cognitive function in young and aged APP × PS1 and WT mice
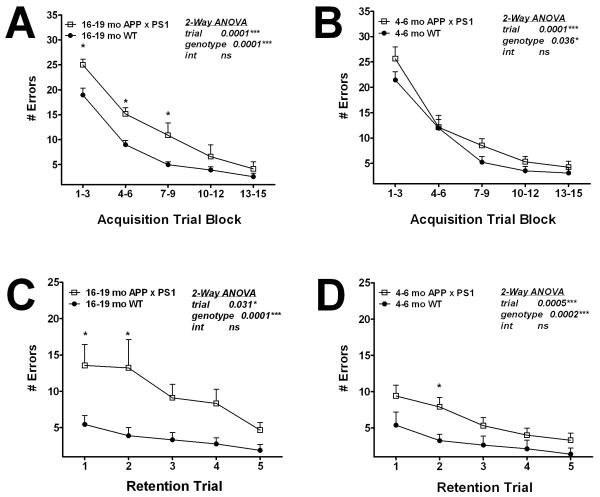
Previous behavioral investigations of cognitive function in APP × PS1 knock-in mice employed the Morris water maze and the 6-arm water maze tasks of spatial memory (Chang et al., 2006). This study did not reveal a working memory deficit in APP × PS1 mice, although APP × PS1 mice did show impaired memory “flexibility”, i.e., the ability to learn the location of a new platform each day without perseverating on the previous location (Chang et al., 2006). However, it is possible that deficits in working memory may have been masked in the water-based assays, as it is generally appreciated that mice perform uniformly poorly in learning tasks that require swimming (Whishaw and Tomie, 1996). Thus, we sought to assess cognitive function in APP × PS1 mice using the Stone T-maze maze, which is a task of procedural learning and memory that is very well suited for use in mice as it does not require the animals to swim, and is not confounded by changes in motor impairment, feeding behavior, or nociception (Pistell and Ingram, 2010), (Pistell et al., 2010), (Pistell et al., 2010). This task, which is described in detail in Methods, was developed following the success of this maze in assessing age-related cognitive deficits in rats (Ingram, 1988), (Spangler et al., 1989), (Markowska et al., 1989). In addition to documenting age-related cognitive decline, this task has been successfully used in both rats and mice to document the detrimental effects of cholinergic antagonists (Spangler et al., 1989), (Bratt et al., 1994) , cerebral ischemia (Caldwell et al., 1997), and diabetes/obesity (Pistell et al., 2010), (Morrison et al., 2010), (Stranahan et al., 2008) on cognitive function. Furthermore, studies in rats in which both Stone maze and Morris water maze tasks were employed demonstrated that errors made during Stone maze training mirrored latency data obtained using the Morris water maze (Lee et al., 2006), (Markowska et al., 1989). Young (age 4-6 months) and aged (16-19 months) mice were thus evaluated for both acquisition and retention in the Stone maze as described in Methods, and data show that aged APP × PS1 knock-in mice were significantly impaired in maze acquisition (Fig 1A). Specifically, ANOVA on errors committed by 16-19 month-old mice revealed significant main effects of trial block (F(4, 80) = 54.2, p < 0.0001) and genotype (F(1,80) = 24.8, p < 0.0001). Post-hoc analyses revealed that APP × PS1 mice committed significantly more errors in the trial blocks 1-3, 4-6, and 7-9 as compared to WT mice (Fig. 1A). Maze acquisition was also impaired in young APP × PS1 knock-in mice, but to a lesser degree (Fig. 1B). Specifically, ANOVA on errors committed by 4-6 month-old mice revealed significant main effects of trial block (F(4, 80) = 54.8, p < 0.0001) and genotype (F(1,80) = 4.6, p < 0.05), but post-hoc analyses did not indicate significant differences in performance in individual trial blocks (Fig. 1B).
Figure 1. Age-related impairment in cognitive ability in APP × PS1 mice.
Male APP × PS1 and WT mice were tested behaviorally in the Stone T-maze as described in Methods. (A) Procedural memory was evaluated in aged (16-19 month-old) APP × PS1 and WT mice by quantifying the number of errors committed over 15 trials of maze acquisition training. Data are means ± S.E.M. of average errors accrued over 3-trial blocks and were generated from 9-12 mice per group. Data were analyzed by 2-way ANOVA, and * indicates significant (p<0.05) increases in errors made by APP × PS1 mice. (B) Procedural memory in young (4-6 month-old) mice was measured by quantifying the number of errors committed over 15 trials of maze acquisition training. Data are means ± S.E.M. of average errors accrued over 3-trial blocks and were generated from 9-12 mice per group, and were analyzed by 2-way ANOVA. (C) Memory retention was tested in aged (16-19 month-old) APP × PS1 and WT mice by quantifying the number of errors committed over 5 additional trials in the maze 7 days following acquisition training. Data are means ± S.E.M. of average errors accrued over 5 individual trails, and were analyzed by 2-way ANOVA. * indicates significant (p<0.05) increases in errors made by APP × PS1 mice. (D) Memory retention in young (4-6 month-old) APP × PS1 and WT mice was measured by 5 additional trials in the maze 7 days following acquisition training. Data are means ± S.E.M. of average errors accrued over 5 individual trails, and were analyzed by 2-way ANOVA. * indicates significant (p<0.05) increases in errors made by APP × PS1 mice.
Maze retention was evaluated 7 days following acquisition as described in Methods, and data show a severe impairment in memory retention in aged APP × PS1 knock-in mice (Fig 1C). 2-way ANOVA on retention errors committed by 16-19 month-old mice revealed a significant main effect of trial number (F(4, 80) = 3.4, p < 0.05) and genotype (F(1,80) = 27.3, p < 0.0001). Post-hoc analyses revealed that aged APP × PS1 mice committed significantly more errors in the first 2 retention trials as compared to WT mice (Fig. 1C). Retention was also impaired in young APP × PS1 knock-in mice, (Fig. 1D). ANOVA on errors committed by 4-6 month-old mice revealed significant main effects of trial number (F(4, 80) = 5.5, p < 0.001) and genotype (F(1,80) = 15.4, p < 0.001), and post-hoc analyses revealed a significant increase in errors committed by APP × PS1 mice in the second retention trial (Fig. 1D).
Synaptic marker expression in young and aged APP × PS1 and WT mice
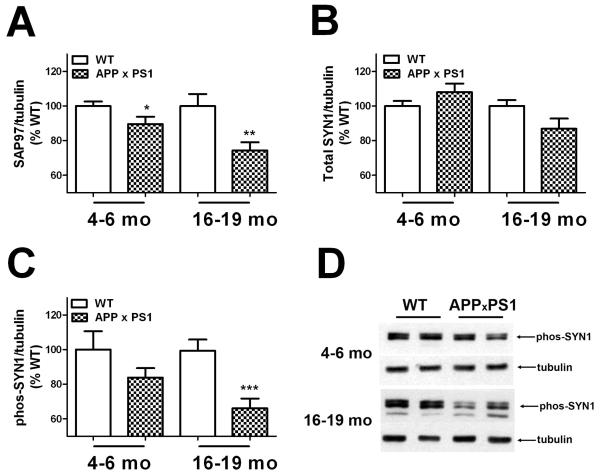
Experiments were next designed to determine if the observed decreases in memory function were associated with altered expression of key synaptic markers. To this end, expression of the post-synaptic protein synapse associated protein 97 (SAP97) and total and phosphorylated forms of the pre-synaptic protein synapsin 1 (SYN1) were quantified in tissue homogenates prepared from the frontal cortex by Western blot as described in Methods. These specific markers were chosen as studies have shown that these proteins reflect most faithfully the number of synapses as determined by EM-based synapse counts (S.W. Scheff, personal communication). Each age (4-6 and 16-19 months) was measured separately by Western blot, and individual samples were normalized first to tubulin and then to WT to reconcile multiple blots, as described in Methods. Thus, for each age, protein expression values from APP ×PS1 mice were compared to values from WT mice by t-test. Quantification of SAP97 expression revealed that levels were significantly decreased in APP × PS1 mice (Fig. 2A). Specifically, 2-tailed, unpaired t-tests indicated significant decreases in SAP97 expression in both 4-6 month old (t(38) = 2.07, p = 0.045) and 16-19 month-old APP × PS1 mice (t(36) = 3.08, p = 0.0039) as compared to WT mice of the same age (Fig. 2A). Conversely, total SYN1 expression in frontal cortex was not altered in APP × PS1 mice as compared to WT mice of either age (Fig. 2B).
Figure 2. Age-related decreases in synaptic marker expression in APP × PS1 mice.
(A) Expression of the post-synaptic protein synapse associated protein 97 (SAP97) was evaluated in tissue homogenates prepared from the frontal cortex of young (4-6 month–old) and aged (16-19 month–old) APP × PS1 and WT mice by Western blot as described in Methods. For consistent quantification across multiple blots, individual samples were normalized first to tubulin and then to levels in WT mice, as described in Methods. Data are mean ± SEM SAP97 expression in 19-20 mice/group, and were analyzed by 2-tailed, unpaired t-tests. * and ** indicate significant (p < 0.05 and 0.01, respectively) decreases in SAP97 expression detected in young and aged APP × PS1 mice, respectively. (B) Expression of the pre-synaptic protein synapsin 1 (SYN1) was evaluated in tissue homogenates prepared from the frontal cortex of young (4-6 month–old) and aged (16-19 month–old) APP × PS1 and WT mice as described in Methods. Data are mean ± SEM SYN1 expression in 19-20 mice/group. (C) Expression of phosphorylated synapsin 1 (phos-SYN1) was evaluated in tissue homogenates prepared from the frontal cortex of young (4-6 month–old) and aged (16-19 month–old) APP × PS1 and WT mice as described in Methods. Data are mean ± SEM phos-SYN1 expression in 19-20 mice/group, and were analyzed by 2-tailed, unpaired t-tests. *** indicates significant (p < 0.001) decreases in phos-SYN1 expression in aged APP × PS1 mice as compared to aged WT mice. (D) Representative images depict the pattern of phos-SYN1 expression in young and aged APP × PS1 and WT mice.
However, levels of phosphorylated SYN1 were affected in APP × PS1 mice (Fig. 2C and 2D). Specifically, 2-tailed, unpaired t-tests revealed that while the trend towards lower levels in 4-6 month-old APP × PS1 mice did not reach statistical significance,16-19 month-old mice had significantly reduced phosphorylated SYN1 expression (t(36) = 3.89, p = 0.0004) in the cortex as compared to WT mice (Fig. 2C).
NOX activity in young and aged APP × PS1 and WT mice
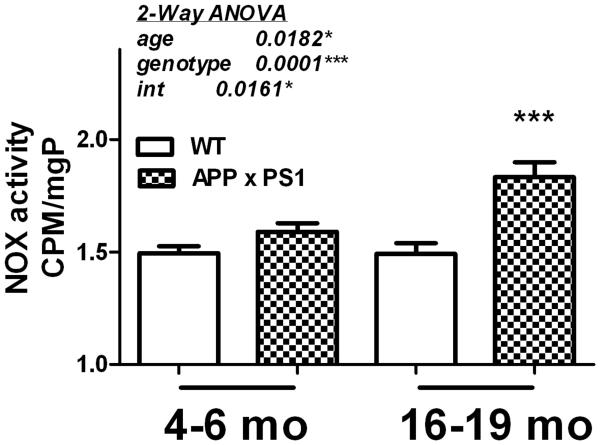
The pro-oxidant and pro-inflammatory enzyme NOX has been proposed as an important potential mediator of AD pathology (Wilkinson and Landreth, 2006), (Block, 2008). While reports from our group and others have shown increased NOX activity in AD and MCI brains (Shimohama et al., 2000), (Bruce-Keller et al., 2010), (Ansari and Scheff, 2011), and in transgenic animal models (Jana and Pahan, 2004), (Niikura et al., 2004), (Park et al., 2008), the profile of NOX enzymatic activity in this humanized, knock-in model of AD has not been established. Thus, NOX activity was determined in frontal cortex of both young and aged WT and APP × PS1 mice as described in Methods. Data show that NOX activity is increased in APP × PS1 mice, particularly in 16-19 month-old mice (Fig. 3). Specifically, 2-way ANOVA of NOX activity in young and old APP × PS1 and WT mice revealed significant main effects of both age (F(1,72) = 5.84, p = 0.018) and genotype (F(1,72) = 18.2, p < 0.0001) on NOX activity, and a significant interaction of age x genotype (F(1,72) = 6.08, p = 0.016). Bonferroni post-hoc analyses revealed that NOX activity in 16-19 month-old APP × PS1 mice was significantly elevated compared to activity in WT mice of the same age (Fig. 3), but no differences were noted in 4-6 month-old mice.
Figure 3. Age-related increases in NOX activity in APP × PS1 mice.
NOX activity was measured in tissue homogenates prepared from the frontal cortex of young (4-6 month–old) and aged (16-19 month–old) APP × PS1 and WT mice using lucigenin as described in Methods. Data were calculated as counts per minute (CPM) per microgram total protein, and are presented as mean ± SEM. 2-way ANOVA indicated an overall significant main effect of age and genotype on NOX activity, and also a significant interaction of age and genotype on NOX activity (text insert). *** indicates significant (p < 0.001) increases in NOX activity in aged APP × PS1 mice as compared to aged WT mice.
NOX subunit expression in young and aged APP × PS1 and WT mice
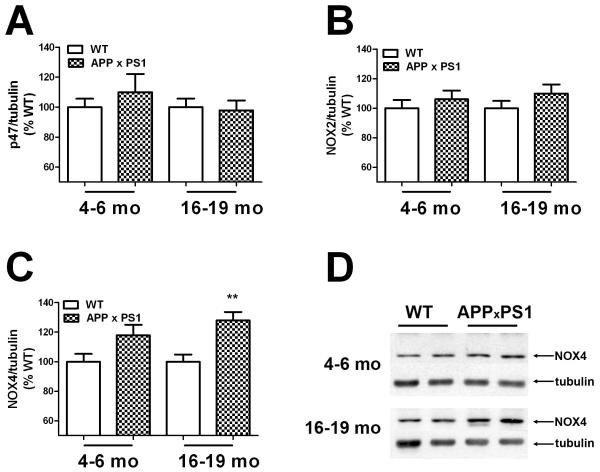
Experiments next determined if the observed increases in NOX activity were associated with enhanced expression of key NOX subunits. To this end, the expression profile of the regulatory subunit p47phox, the catalytic subunit NOX2 (gp91phox), and the NOX2 homolog NOX4 in the frontal cortex of young and aged WT and APP × PS1 mice was measured by Western blot as described in Methods. Evaluation of p47phox expression in frontal cortex did not indicate changes in APP × PS1 mice as compared to WT mice of either age (Fig. 4A). Likewise, expression of NOX2 was not significantly altered in APP × PS1 mice of either age (Fig. 4B). Conversely, levels of NOX4 were significantly increased in APP × PS1 mice (Fig. 4C and 4D). Specifically, 2-tailed, unpaired t-tests revealed significant increases in NOX4 expression in 16-19 month-old APP × PS1 mice (t(20) = 3.76, p = 0.0012) as compared to WT mice, but not in 4-6 month-old mice (Fig. 4C). Levels of the alternate NOX2 homologs NOX1 and NOX3 were also evaluated in young and old WT and APP × PS1 mice, but the expression of these proteins in the brain was beneath the level of detection by Western blot (data not shown).
Figure 4. NOX subunit expression in young and aged WT and APP × PS1 mice.
(A) Expression of the regulatory subunit p47phox (p47) was evaluated in tissue homogenates prepared from the frontal cortex of young (4-6 month–old) and aged (16-19 month–old) APP × PS1 and WT mice by Western blot as described in Methods. For consistent quantification across multiple blots, individual samples were normalized first to tubulin and then to levels in WT mice, as described in Methods. Data are mean ± SEM p47expression in 19-20 mice/group, and were analyzed by 2-tailed, unpaired t-tests. (B) Expression of the catalytic subunit NOX2 was evaluated in tissue homogenates prepared from the frontal cortex of young (4-6 month–old) and aged (16-19 month–old) APP × PS1 and WT mice as described in Methods. Data are mean ± SEM NOX2 expression in 19-20 mice/group. (C) Expression of NOX2 homolog NOX4 was evaluated in tissue homogenates prepared from the frontal cortex of young (4-6 month–old) and aged (16-19 month–old) APP × PS1 and WT mice as described in Methods. Data are mean ± SEM NOX4 expression in 19-20 mice/group, and were analyzed by 2-tailed, unpaired t-tests. ** indicates significant (p < 0.01) increases in NOX4 expression in aged APP × PS1 mice as compared to aged WT mice. (D) Representative images depict the pattern of NOX4 expression in young and aged APP × PS1 and WT mice.
Relationship of NOX activity and behavioral performance in young and aged APP × PS1 and WT mice
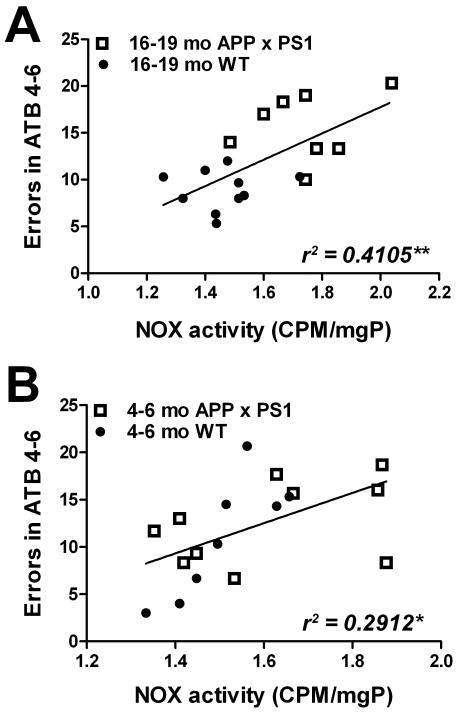
Regression analyses were next undertaken to determine the statistical relationship between NOX activity and cognitive function. Specifically, numerical values for NOX activity were correlated against errors accrued by the same individual mice in acquisition trials 4-6 in young and aged APP × PS1 and WT mice. Data analyses show a strong and statistically significant association between overall NOX activity in the frontal cortex and acquisition errors in 16-19 month-old mice (r2 = 0.4105, p = 0.0042; Fig. 5A). The relationship between NOX activity in the frontal cortex and acquisition errors in 4-6 month-old mice was also significant (r2 = 0.2912, p = 0.0208; Fig. 5B). NOX activity also showed a significant correlation with errors in retention trials 1-3 in both aged and young mice (data not shown).
Figure 5. Linear relationship between maze performance and NOX activity in young and aged WT and APP × PS1 mice.
(A) Scatter plots show the relationship between errors in acquisition trail block 4-6 (errors in ATB 4-6) and NOX activity in aged (16-19 month-old) APP × PS1 (open squares) and WT (closed circles) mice. Each point represents an individual subject, and the line depicts the degree of linear regression. (B) Scatter plots show the relationship between errors in acquisition trail block 4-6 (errors in ATB 4-6) and NOX activity in young (4-6 month-old) APP × PS1 (open squares) and WT (closed circles) mice. Each point represents an individual subject, and the line depicts the degree of linear regression.
Progressive changes in Aβ levels and solubility in APP × PS1 mice
Previous reports from our group show that this humanized model of Aβ pathogenesis recapitulates the development of both diffuse and neuritic plaques, and is furthermore associated with progressive changes in Aβ solubility and deposition that mirror the progressive solubility changes observed in human AD (Flood et al., 2002), (Murphy et al., 2007). To determine how the cognitive abnormalities noted in these mice related to physiologic changes in Aβ indices, alterations in Aβ levels and solubility were evaluated in young and aged APP × PS1 mice. Total levels of Aβ1-40 and Aβ1-42 were evaluated as described in Methods, and showed dramatic increases in 16-19 month-old mice as compared to 4-6 month-old mice (Table 1). Furthermore, Aβ1-40 was increased within the PBS, SDS, and formic acid pools in 16-19 month-old mice relative to young mice (Table 1). Indeed, formic acid extractable Aβ1-40 could not be detected in young mice, but was abundant in the aged group (Table 1). Likewise, neither SDS extractable Aβ1-42 nor formic acid extractable Aβ1-42 could be detected in young mice, but Aβ1-42 partition into these increasingly insoluble fractions was apparent in the aged group (Table 1). Levels of Aβ1-40 and Aβ1-42 in WT mice of the same genetic background were not elevated significantly above background levels (data not shown).
Table 1. Summary of Aβ levels and progressive changes in solubility in young (age 4-6 months) and aged (16-19 months) APP X PS1 mice.
To quantify levels of Aβ1-40 and Aβ1-42 in increasingly insoluble fractions, Aβ proteins were examined by serial extraction from phosphate buffered saline (PBS), sodium dodecyl sulfate (SDS), and formic acid (FA) soluble fractions, followed by ELISA as described in Methods. Total levels of Aβ1-40 and Aβ1-42 were determined by summing levels detected in all fractions. Data are expressed as mean pmol/g tissue ± SEM with 10-12 mice per group. ND = not detected.
| Aβ 1-40 | PBS | SDS | FA | Total |
|---|---|---|---|---|
| 4-6 months | 86.7 ± 6.1 | 1,623.1 ± 74.6 | ND | 1,709.8 ± 73.0 |
| 16-19 months | 153.5 ± 10.5 | 39,518.7 ± 5108.9 | 11,022.2 ± 2418.7 | 50,694.5 ± 4475.7 |
| Aβ 1-42 | PBS | SDS | FA | Total |
| 4-6 months | 22.7 ± 10.4 | ND | ND | 22.7 ± 10.4 |
| 16-19 months | 16.7 ± 7.5 | 26,571.4 ± 4921.8 | 4,653.3 ± 1729.1 | 31,241.4 ± 4450.1 |
Relationship of Aβ to NOX activity and behavioral performance in APP × PS1 mice
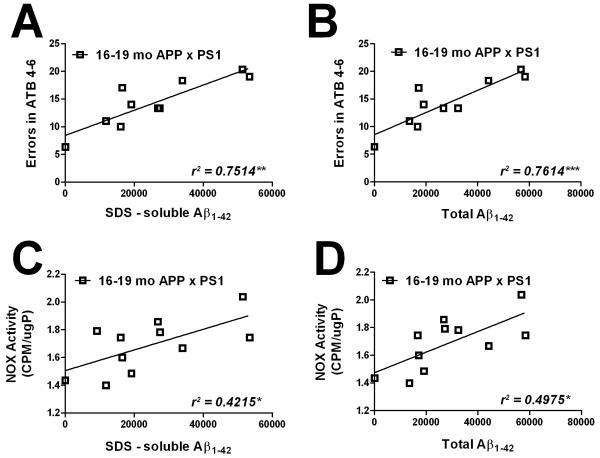
To determine how these Aβ indices relate to the cognitive and physiologic abnormalities noted in these mice, regression analyses were next undertaken to determine the statistical relationship between Aβ levels in specific pools to NOX activity and cognitive function. Specifically, numerical values for total Aβ1-40 and Aβ1-42, and for Aβ1-40 and Aβ1-42 in PBS, SDS, and formic acid extractable fractions were correlated against NOX activity in the frontal cortex and against acquisition errors in the same individual young and aged APP × PS1 mice. Data analyses show that for 4-6 month-old APP × PS1 mice, no correlation could be found for any pool of Aβ1-40 or Aβ1-42 (total, PBS, or SDS soluble) to either acquisition errors or NOX activity (data not shown). Likewise, for 16-19 month-old APP × PS1 mice, no correlation could be found for total Aβ1-40, or for PBS-, SDS-, or formic acid soluble Aβ1-40 with overall NOX activity or acquisition errors (data not shown). However, there was significant correlation between Aβ1-42 and acquisition errors in 16-19 month-old APP × PS1 mice (Fig. 6). Specifically, acquisition errors in 16-19 month-old APP × PS1 mice correlated significantly with both SDS-soluble Aβ1-42 (r2 = 0.7514, p = 0.0012; Fig. 6A) and total Aβ1-42 (r2 = 0.7614, p = 0.001; Fig. 6B). There was not a significant linear relationship between PBS-soluble Aβ1-42 and acquisition errors (r2 = 0.0088, p = 0.7971; not shown), but levels of formic acid-soluble Aβ1-42 did show a modest but significant correlation with acquisition errors in16-19 month-old APP × PS1 mice (r2 = 0.4018, p = 0.049; not shown). The statistical relationship between Aβ1-42 and NOX activity in the frontal cortex of 16-19 month-old APP × PS1 mice was also significant, although not as strong as for acquisition errors (Fig. 6). NOX activity correlated significantly with SDS-soluble Aβ1-42 (r2 = 0.4215, p = 0.0306; Fig. 6C) and total Aβ1-42 (r2 = 0.4975, p = 0.0153; Fig. 6D). However, there was no correlation between PBS-soluble Aβ1-42 and NOX activity (r2 = 0.0409, p = 0.5506; not shown), nor between levels of formic acid-soluble Aβ1-42 and NOX activity (r2 = 0.1249, p = 0.2865; not shown) in 16-19 month-old APP × PS1 mice. These data are generally comparable to the degree of correlation found in published reports employing similar linear regression techniques in conjunction with Aβ analyses. For example, the correlation demonstrated between fronto-cortical fibrillar Aβ accumulation and dementia as measured by MMSE in humans is r = .72 / r2 = .518 (Sarsoza et al., 2009). Additionally, the reported correlation coefficients between the number of synaptophysin-immunoreactive terminals and age in wild-type human amyloid protein precursor transgenic mice (r = 0.53 / r2 = .2809; Mucke et al., 2000) and between memory deficit and neurogenesis in 3xTgAD mice (r = .80 / r2 = .64; Wang et al., 2010) are also comparable to data in this manuscript.
Figure 6. Linear relationship between Aβ1-42 and both maze performance and NOX activity in aged APP × PS1 mice.
(A) Scatter plots show the relationship between errors in acquisition trail block 4-6 (errors in ATB 4-6) and SDS-soluble Aβ1-42 levels in 16-19 month-old APP × PS1 mice. Each point represents an individual subject, and the line depicts the degree of linear regression. (B) Scatter plots show the relationship between errors in acquisition trial block 4-6 (errors in ATB 4-6) and total Aβ1-42 levels in 16-19 month-old APP × PS1 mice. Each point represents an individual subject, and the line depicts the degree of linear regression. (C) Scatter plots show the relationship between NOX activity and SDS-soluble Aβ1-42 levels in 16-19 month-old APP × PS1 mice. Each point represents an individual subject, and the line depicts the degree of linear regression. (D) Scatter plots show the relationship between NOX activity and total Aβ1-42 levels in 16-19 month-old APP × PS1 mice. Each point represents an individual subject, and the line depicts the degree of linear regression.
DISCUSSION
This study uses a second generation mouse model of AD to evaluate the role of NOX in amyloid pathogenesis. Specifically, using APP × PS1 knock-in mice, experiments were designed to document changes in cognitive function and NOX activity, and to further evaluate these indices in relation to changes in Aβ. Data show that NOX activity is significantly increased in aged APP × PS1 mice, and furthermore shares a significant linear relationship with deficits in cognitive function. Data also show that while p47phox and NOX2 (gp91phox) expression are not altered in APP × PS1 mice, expression of the NOX2 homolog NOX4 is significantly increased in aged APP × PS1 mice. Finally, data further show that both NOX activity and cognitive function show a significant linear relationship specifically with levels of Aβ1-42, but not Aβ1-40. Overall, these data are in general agreement with published studies suggesting a role for NOX in the pathogenesis of AD (Ansari and Scheff, 2011), (Park et al., 2008), (Bruce-Keller et al., 2010), but extend these studies by demonstrating the intimate link between NOX activity and both disruptions in cognitive function and the deposition of specific forms of Aβ. The knock-in mice are powerful tool with which to study Aβ pathology, and indeed, our previous reports show that this model of Aβ pathogenesis is associated several key phenomena that are also observed in AD brain, including progressive changes in Aβ solubility and the development of both diffuse and neuritic plaques. Furthermore, data obtained from these mice are not hindered by confounds caused by an artificial promoter system, ectopic expression, or supraphysiologic levels of APP expression. Thus, the combination of this unique animal model of Aβ pathology with stringent behavioral and biochemical analyses has revealed a potentially critically important role for NOX activation in Aβ pathogenesis. While cause and effect relationships remain to be fully resolved, these data strongly suggest that NOX could be a key factor linking SDS-soluble Aβ1-42 to disruptions in synaptic/cognitive function.
While the etiology of AD-related cognitive decline is still not understood, advanced age is the strongest risk factor for AD and the free radical theory of aging (Harman, 1956) may have particular relevance to the development of AD. This oxidative stress-based theory describes a redox imbalance, whereby the production of free radicals overtakes endogenous anti-oxidant capacity, leading to oxidative damage to critical cellular elements (Harman, 1993). Indeed, oxidative stress has been repeatedly implicated in both brain aging and AD (Morrison et al., 2010), (Liu et al., 2003), (Joseph et al., 1998), (Butterfield et al., 2001), (Markesbery and Lovell, 1998), (Stadtman and Levine, 2003), but the primary source of AD-related oxidative stress has not been identified. There is strong evidence supporting mitochondrial dysfunction in AD, and the “mitochondrial cascade hypothesis” posits that age-related increases in basal mitochondrial ROS production lead to neuronal dysfunction and pathological hallmarks of AD (Swerdlow and Khan, 2004). Data in this manuscript suggest that increased NOX might participate with mitochondria in AD-associated oxidative stress and cognitive decline, which is in keeping with many reports implicating NOX in AD (Wilkinson and Landreth, 2006), (Park et al., 2008), (Park et al., 2005). It is also important to note that reactive oxygen species (ROS) are important signaling molecules involved in synaptic plasticity and memory formation, and that NOX in particular is a key regulator of ROS generation in synaptic plasticity and memory formation (reviewed in (Kishida and Klann, 2007)). Thus, aberrant NOX activation could significantly perturb cognitive function even in the absence of overt oxidative stress. In support of this scenario, experimental data indicate a delicate balance of ROS required for signaling, with either too little or too much ROS resulting in impairments in long term potentiation (LTP) and memory (Knapp and Klann, 2002). In further reference to aging, data suggest the presence of an age-related shift in the role of ROS signaling in memory formation (Knapp and Klann, 2002), (Kamsler and Segal, 2004), (Hu et al., 2006), as overexpression of antioxidant enzymes impairs LTP in young mice, but preserves LTP in aged mice (Hu et al., 2006), (Kamsler and Segal, 2004). Likewise, long-term application of antioxidant enzyme mimetics can reverse hippocampus-dependent learning deficits in aged mice (Liu et al., 2003). While the exact biochemical means whereby NOX modulates physiologic signaling are still under investigation, current theories focus on reversible thiol oxidation of cysteine residues, a chemical alteration often utilized by ROS to impact signaling without causing cytotoxicity (Parasassi et al., 2010), and protein phosphatases are well known to be inhibited by reversible cysteine oxidation (Denu and Tanner, 1998). Finally, it should be noted that the detrimental actions of NOX appear to be most strongly associated with age-related chronic disease processes, including Parkinson’s disease and atherosclerosis in addition to AD (reviewed in (Lambeth, 2007)). Indeed, a model of “antagonistic pleiotropy” has been proposed to explain the potentially dual role of NOX with age, in which the physiologic production of ROS garners an advantage in early life but sustained or aberrant activation of NOX culminates in harmful effects with age later in life (Lambeth, 2007), supporting the potential detrimental role of NOX in the pathogenesis of AD.
NOX, which is uniquely dedicated to the deliberate and specific production of reactive oxygen species, consists of membrane (NOX2, also called gp91phox, and p22phox) and cytosolic (p47phox, p67phox, and p40phox) components (Babior, 1991), (DeLeo and Quinn, 1996). The membrane-integrated protein NOX2 is the catalytic core of the enzyme responsible for the electron transfer from NADPH to molecular oxygen for superoxide production. Interestingly, recent expansion of the genome database has led to identification of several novel NOX2 homologues which constitute the NOX family of oxidases. These proteins are distinct from the phagocyte NOX2 and have functions other than host defense (Lambeth, 2004), (Geiszt and Leto, 2004). The human genome contains 5 NOX members: NOX1 through NOX5, with NOX2 as gp91phox. Some of these novel oxidases have a fairly limited tissue expression (Krause, 2004), but both NOX1 and NOX4 are thought to be expressed in the CNS in addition to NOX2 (Lambeth, 2004), (Krause, 2004), (Sorce and Krause, 2009). Indeed, data in this report suggest that increased expression of NOX4 could contribute to the observed increases in NOX activity in aged APP × PS1 mice. While NOX activity is regulated post-translationally, and thus increased subunit expression is not necessary per se for increased enzyme activity, studies have shown that increased expression of either NOX2 or NOX4 is sufficient to increase NOX activity in transgenic mice (Anilkumar et al., 2008). Furthermore, the increased expression of NOX4 in human rennin/angiotensinogen chimeric transgenic mice is associated with both increased NOX activity and cognitive decline (Inaba et al., 2009). Thus, NOX4 may be a key participant in the increased NOX activity that has been repeatedly noted in the progression of AD (Ansari and Scheff, 2011), (Bruce-Keller et al., 2010). Indeed, the contribution of NOX4 to amyloid pathology may partially explain why administration of the widely used NOX-inhibitor apocynin, which has been shown to be protective in models of stroke and ALS (Harraz et al., 2008), (Tang et al., 2008), (Wang et al., 2006), was recently shown to be ineffective in preventing cognitive deficits, amyloid deposition, microgliosis and hyperphosphorylated tau in the Tg19959 mouse model (Dumont et al., 2011). Apocynin is thought to inhibit NOX by preventing the assembly of NOX subunits, particularly the translocation of p47phox (Stolk et al., 1994), which may not be a prerequisite for activation of NOX4, as evidence suggests that NOX4 is constitutively active and does not require the cytosolic subunits (Ray and Shah, 2005) (Martyn et al., 2006).
It is widely thought that AD begins as a malfunction of synapses, eventually leading to cognitive impairment and dementia. Indeed, extensive work on AD indicates that memory failure in early AD patients results from synaptic disruption without frank neuronal loss (reviewed in (Selkoe, 2002)), and this perturbation is attributed to the toxicity of the 42-aa variant of the amyloid β protein (Aβ1-42). Furthermore, work in multiple AD models (Rowan et al., 2003) has shown that Aβ1-42 in particular impairs synaptic plasticity in brain regions that are recognized early targets for AD. Thus, while the specific role that amyloid beta peptides (Aβ) play in causing AD is subject to controversy, it is clear that Aβ-containing senile plaques are associated with degenerating neurons, and that Aβ peptides are potently bioactive both in vitro and in vivo, and collective evidence suggests that AD may be mediated at least in part via the overproduction of Aβ (reviewed in (Selkoe and Schenk, 2003)). Thus, a significant finding in the present study is the significant linear association between both NOX activity and cognitive performance in individual mice with levels of Aβ1-42. As published data convincingly demonstrate that Aβ can increase NOX activity in neurons and microglia (Meda et al., 1995), (Bruce et al., 1996), (Thomas et al., 1996), (Bianca et al., 1999), (Niikura et al., 2004), (Jana and Pahan, 2004), (Shelat et al., 2008), these data suggest that Aβ1-42, particularly within increasingly insoluble SDS fractions, may disrupt synaptic and cognitive function via the induction of NOX.
Highlights.
Cognitive performance and expression of key synaptic proteins is progressively decreased with age in APP × PS1 mice
NOX activity and expression of the NOX4 is progressively increased with age in APP × PS1 mice
Cognitive function shows a significant linear correlation with NOX activity and Aβ1-42
ACKNOWLEDGEMENTS
The authors are grateful to Dr. Barry Robert and Cynthia Kloster for exemplary veterinary assistance. Additional gratitude goes to Teresa Noel for expert training and leadership in animal husbandry and colony management. This work was supported by grants from the NIH (NS46267 and AG05119), and also used PBRC Core facilities (Animal Phenotyping) that are funded by the NIH (P20-RR021945 and P30-DK072476).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdul HM, Sultana R, Clair DK, Markesbery WR, Butterfield DA. Oxidative damage in brain from human mutant APP/PS-1 double knock-in mice as a function of age. Free Radic. Biol. Med. 2008;45:1420–5. doi: 10.1016/j.freeradbiomed.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR. Protein oxidation in the brain in Alzheimer’s disease. Neuroscience. 2001;103:373–83. doi: 10.1016/s0306-4522(00)00580-7. [DOI] [PubMed] [Google Scholar]
- Anantharaman M, Tangpong J, Keller JN, Murphy MP, Markesbery WR, Kiningham KK, Clair DKS. {beta}-amyloid mediated nitration of manganese superoxide dismutase: implication for oxidative stress in a APPNLh/NLh X PS-1P264L/P264L double knock-in mouse model of Alzheimer’s disease. Am. J. Pathol. 2006;168:1608–1618. doi: 10.2353/ajpath.2006.051223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anilkumar N, Weber R, Zhang M, Brewer A, Shah AM. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler. Thromb. Vasc. Biol. 2008;28:1347–54. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Scheff SW. NADPH-oxidase activation and cognition in Alzheimer’s disease progression. Free Radic. Biol. Med. 2011 doi: 10.1016/j.freeradbiomed.2011.03.025. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM. The respiratory burst oxidase and the molecular basis of chronic granulomatous disease. Am. J. Hematol. 1991;37:263–6. doi: 10.1002/ajh.2830370410. [DOI] [PubMed] [Google Scholar]
- Beckett TL, Niedowicz DM, Studzinski CM, Weidner AM, Webb RL, Holler CJ, Ahmed RR, LeVine H. r., Murphy MP. Effects of nonsteroidal anti-inflammatory drugs on amyloid-beta pathology in mouse skeletal muscle. Neurobiol. Dis. 2010;39:449–56. doi: 10.1016/j.nbd.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianca VD, Dusi S, Bianchini E, Dal Pra I, Rossi F. beta-amyloid activates the O-2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer’s disease. J. Biol. Chem. 1999;274:15493–9. doi: 10.1074/jbc.274.22.15493. [DOI] [PubMed] [Google Scholar]
- Block ML. NADPH oxidase as a therapeutic target in Alzheimer’s disease. BMC Neurosci. 2008;9(Suppl 2):S8. doi: 10.1186/1471-2202-9-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt AM, Kelly ME, Domeney AM, Naylor RJ, Costall B. Ondansetron fails to attenuate a scopolamine-induced deficit in a Stone maze task. Neuroreport. 1994;5:1921–4. doi: 10.1097/00001756-199410000-00020. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Gupta S, Parrino TE, Knight AG, Ebenezer PJ, Weidner AM, LeVine H. r., Keller JN, Markesbery WR. NOX activity is increased in mild cognitive impairment. Antioxid. Redox Signal. 2010;12:1371–82. doi: 10.1089/ars.2009.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AJ, Malfroy B, Baudry M. b-amyloid toxicity in organotypic hippocampal cultures: protection by EUK-8, a synthetic catalytic free radical scavenger. Proc. Natl. Acad. Sci. USA. 1996;93:2312–2316. doi: 10.1073/pnas.93.6.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol. Med. 2001;7:548–554. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Poon HF, Clair D, Keller JN, Pierce WM, Klein JB, Markesbery WR. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer’s disease. Neurobiol. Dis. 2006;22:223–32. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Caldwell MA, Reymann JM, Allain H, Leonard BE, Bentué-Ferrer D. Lisuride prevents learning and memory impairment and attenuates the increase in extracellular dopamine induced by transient global cerebral ischemia in rats. Brain Res. 1997;771:305–18. doi: 10.1016/s0006-8993(97)00817-2. [DOI] [PubMed] [Google Scholar]
- Chang EH, Savage MJ, Flood DG, Thomas JM, Levy RB, Mahadomrongkul V, Shirao T, Aoki C, Huerta PT. AMPA receptor downscaling at the onset of Alzheimer’s disease pathology in double knockin mice. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3410–3415. doi: 10.1073/pnas.0507313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo FR, Quinn MT. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J. Leukoc. Biol. 1996;60:677–691. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Dumont M, Stack C, Elipenhali C, Calingasan NY, Wille E, Beal MF. Apocynin administration does not improve behavioral and neuropathological deficits in a transgenic mouse model of Alzheimer’s disease. Neurosci. Lett. 2011;492:150–4. doi: 10.1016/j.neulet.2011.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood DG, Reaume AG, Dorfman KS, Lin YG, Lang DM, Trusko SP, Savage MJ, Annaert WG, De Strooper B, Siman R, Scott RW. FAD mutant PS-1 gene-targeted mice: increased A beta 42 and A beta deposition without APP overproduction. Neurobiol. Aging. 2002;23:335–48. doi: 10.1016/s0197-4580(01)00330-x. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide Dismutases. Ann. rev. Biochem. 1975;44:17–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Geiszt M, Leto TL. The Nox family of NAD(P)H oxidases: host defense and beyond. J. Biol. Chem. 2004;279:51715–51718. doi: 10.1074/jbc.R400024200. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harman D. Free radical involvement in aging. Pathophysiology and therapeutic implications. Drugs Aging. 1993;3:60–80. doi: 10.2165/00002512-199303010-00006. [DOI] [PubMed] [Google Scholar]
- Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Schoneich C, Engelhardt JF. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J. Clin. Invest. 2008;118:659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Serrano F, Oury TD, Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J. Neurosci. 2006;26:3933–3941. doi: 10.1523/JNEUROSCI.5566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Aluise CD, Joshi G, Sultana R, Clair DK, Markesbery WR, Butterfield DA. Potential in vivo amelioration by N-acetyl-L-cysteine of oxidative stress in brain in human double mutant APP/PS-1 knock-in mice: toward therapeutic modulation of mild cognitive impairment. J. Neurosci. Res. 2010;88:2618–29. doi: 10.1002/jnr.22422. [DOI] [PubMed] [Google Scholar]
- Inaba S, Iwai M, Furuno M, Tomono Y, Kanno H, Senba I, Okayama H, Mogi M, Higaki J, Horiuchi M. Continuous activation of renin-angiotensin system impairs cognitive function in renin/angiotensinogen transgenic mice. Hypertension. 2009;53:356–62. doi: 10.1161/HYPERTENSIONAHA.108.123612. [DOI] [PubMed] [Google Scholar]
- Ingram DK. Complex maze learning in rodents as a model of age-related memory impairment Neurobiol. Aging. 1988;9:475–85. doi: 10.1016/s0197-4580(88)80101-5. [DOI] [PubMed] [Google Scholar]
- Jalbert JJ, Daiello LA, Lapane KL. Dementia of the Alzheimer type. Epidemiol. Rev. 2008;30:15–34. doi: 10.1093/epirev/mxn008. [DOI] [PubMed] [Google Scholar]
- Jana A, Pahan K. Fibrillar amyloid-beta peptides kill human primary neurons via NADPH oxidase-mediated activation of neutral sphingomyelinase. Implications for Alzheimer’s disease. J. Biol. Chem. 2004;279:51451–9. doi: 10.1074/jbc.M404635200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiszewski M, Souza HP, Liu X, Pedro MA, Zweier JL, Laurindo FR. Overestimation of NADH-driven vascular oxidase activity due to lucigenin artifacts. Free Radic. Biol. Med. 2002;32:446–53. doi: 10.1016/s0891-5849(01)00828-0. [DOI] [PubMed] [Google Scholar]
- Jellinger K. Alzheimer’s disease and cerebrovascular pathology: an update. J. Neural Transm. 2002;109:813–836. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- Johnson WG. Late-onset neurodegenerative diseases-The role of protein insolubility. J. Anat. 2000;196:609–616. doi: 10.1046/j.1469-7580.2000.19640609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JA, Denisova N, Fisher D, Bickford P, Prior R, Cao G. Age-related neurodegeneration and oxidative stress: putative nutritional intervention. Neurol. Clin. 1998;16:747–55. doi: 10.1016/s0733-8619(05)70092-x. [DOI] [PubMed] [Google Scholar]
- Kamsler A, Segal M. Hydrogen peroxide as a diffusible signal molecule in synaptic plasticity. Mol. Neurobiol. 2004;29:167–178. doi: 10.1385/MN:29:2:167. [DOI] [PubMed] [Google Scholar]
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–6. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Shin KS, Chung YB, Jung KW, Cha CI, Shin DH. Immunohistochemical study of p47Phox and gp91Phox distributions in rat brain. Brain Res. 2005;1040:178–86. doi: 10.1016/j.brainres.2005.01.066. [DOI] [PubMed] [Google Scholar]
- Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid. Redox Signal. 2007;9:233–44. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida KT, Pao M, Holland SM, Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp LT, Klann E. Role of reactive oxygen species in hippocampal long-term potentiation: contributory or inhibitory? J. Neurosci. Res. 2002;70:1–7. doi: 10.1002/jnr.10371. [DOI] [PubMed] [Google Scholar]
- Krause KH. Tissue distribution and putative phusiological function of NOX family NADPH oxidases. Jpn. J. Infect. Dis. 2004;57:S28–S29. [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic. Biol. Med. 2007;43:332–47. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GD, Longo DL, Wang Y, Rifkind JM, Abdul-Raman L, Mamczarz JA, Duffy KB, Spangler EL, Taub DD, Mattson MP, Ingram DK. Transient improvement in cognitive function and synaptic plasticity in rats following cancer chemotherapy. Clin. Cancer Res. 2006;12:198–205. doi: 10.1158/1078-0432.CCR-05-1286. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhu H, Kuppusamy P, Roubaud V, Zweier JL, Trush MA. Validation of lucigenin (bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J. Biol. Chem. 1998;273:2015–23. doi: 10.1074/jbc.273.4.2015. [DOI] [PubMed] [Google Scholar]
- Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, Baudry M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery WR, Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol. Aging. 1998;19:33–36. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Stone WS, Ingram DK, Reynolds J, Gold PE, Conti LH, Pontecorvo MJ, Wenk GL, Olton DS. Individual differences in aging: behavioral and neurobiological correlates. Neurobiol. Aging. 1989;10:31–43. doi: 10.1016/s0197-4580(89)80008-9. [DOI] [PubMed] [Google Scholar]
- Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, Delucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr., Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by b-amyloid protein and interferon-gamma. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce-Keller AJ, Keller JN. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J. Neurochem. 2010;114:1581–9. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann N Y Acad Sci. 2008;1147:180–95. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 2000;20:4050–8. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, Beckett TL, Ding Q, Patel E, Markesbery WR, Clair DK, LeVine H. r., Keller JN. Abeta solubility and deposition during AD progression and in APPxPS-1 knock-in mice. Neurobiol. Dis. 2007;27:301–11. doi: 10.1016/j.nbd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Niikura T, Yamada M, Chiba T, Aiso S, Matsuoka M, Nishimoto I. Characterization of V642I-AbetaPP-induced cytotoxicity in primary neurons. J. Neurosci. Res. 2004;77:54–62. doi: 10.1002/jnr.20139. [DOI] [PubMed] [Google Scholar]
- Noh KM, Koh JY. Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J. Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-23-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell BV, Tew DG, Jones OT, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem. J. 1993;290:41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi T, Brunelli R, Costa G, De Spirito M, Krasnowska E, Lundeberg T, Pittaluga E, Ursini F. Thiol redox transitions in cell signaling: a lesson from N-acetylcysteine. ScientificWorldJournal. 2010;10:1192–202. doi: 10.1100/tsw.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, Iadecola C. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J. Neurosci. 2005;25:1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1347–52. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell PJ, Gupta S, Knight AG, Domingue M, Uranga RM, Ingram DK, Kheterpal I, Ruiz C, Keller JN, Bruce-Keller AJ. Metabolic and neurologic consequences of chronic lopinavir/ritonavir administration to C57BL/6 mice. Antiviral Res. 2010;88:334–42. doi: 10.1016/j.antiviral.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell PJ, Ingram DK. Development of a water-escape motivated version of the Stone T-maze for mice. Neuroscience. 2010;166:61–72. doi: 10.1016/j.neuroscience.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R, Shah AM. NADPH oxidase and endothelial cell function. Clin. Sci. (Lond) 2005;109:217–26. doi: 10.1042/CS20050067. [DOI] [PubMed] [Google Scholar]
- Reaume AG, Howland DS, Trusko SP, Savage MJ, Lang DM, Greenberg BD, Siman R, Scott RW. Enhanced amyloidogenic processing of the beta-amyloid precursor protein in gene-targeted mice bearing the Swedish familial Alzheimer’s disease mutations and a “humanized” Abeta sequence. J. Biol. Chem. 1996;271:23380–23388. doi: 10.1074/jbc.271.38.23380. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer’s disease. J. Neurochem. 2006;96:1–13. doi: 10.1111/j.1471-4159.2005.03530.x. [DOI] [PubMed] [Google Scholar]
- Rowan MJ, Klyubin I, Cullen WK, Anwyl R. Synaptic plasticity in animal models of early Alzheimer’s disease. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2003;358:821–8. doi: 10.1098/rstb.2002.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsoza F, Saing T, Kayed R, Dahlin R, Dick M, Broadwater-Hollifield C, Mobley S, Lott I, Doran E, Gillen D, Anderson-Bergman C, Cribbs DH, Glabe C, Head E. A fibril-specific, conformation-dependent antibody recognizes a subset of Abeta plaques in Alzheimer disease, Down syndrome and Tg2576 transgenic mouse brain. Acta Neuropathol. 2009;118:505–17. doi: 10.1007/s00401-009-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 2003;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, Sun AY, Simonyi A, Sun GY. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J. Neurochem. 2008;106:45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Tanino H, Kawakami N, Okamura N, Kodama H, Yamaguchi T, Hayakawa T, Nunomura A, Chiba S, Perry G, Smith MA, Fujimoto S. Activation of NADPH oxidase in Alzheimer’s disease brains. Biochem. Biophys. Res. Commun. 2000;273:5–9. doi: 10.1006/bbrc.2000.2897. [DOI] [PubMed] [Google Scholar]
- Siman R, Reaume AG, Savage MJ, Trusko S, Lin YG, Scott RW, Flood DG. Presenilin-1 P264L knock-in mutation: differential effects on abeta production, amyloid deposition, and neuronal vulnerability. J. Neurosci. 2000;20:8717–8726. doi: 10.1523/JNEUROSCI.20-23-08717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid. Redox Signal. 2009;11:2481–504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- Spangler E, Chachich M, Curtis N, Ingram D. Age-related impairment in complex maze learning in rats: relationship to neophobia and cholinergic antagonism. Neurobiol. Aging. 1989;10:133–41. doi: 10.1016/0197-4580(89)90022-5. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–18. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Pistell PJ, Nelson CM, Readal N, Miller MG, Spangler EL, Ingram DK, Mattson MP. Accelerated cognitive aging in diabetic rats is prevented by lowering corticosterone levels. Neurobiol. Learn. Mem. 2008;90:479–83. doi: 10.1016/j.nlm.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Tang XN, Cairns B, Cairns N, Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 2008;154:556–62. doi: 10.1016/j.neuroscience.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu GY, Quinn MT, Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol. Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. beta-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–71. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- Vinters HV, Farag ES. Amyloidosis of cerebral arteries. Adv. Neurol. 2003;92:105–112. [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Wang J, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. J. Neurochem. 2006;96:825–32. doi: 10.1111/j.1471-4159.2005.03615.x. [DOI] [PubMed] [Google Scholar]
- Wang JM, Singh C, Liu L, Irwin RW, Chen S, Chung EJ, Thompson RF, Brinton RD. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U S A. 2010;107:6498–503. doi: 10.1073/pnas.1001422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006;1090:182–9. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie J. Of mice and mazes: similarities between mice and rats on dry land but not water mazes. Physiol. Behav. 1996;60:1191–7. doi: 10.1016/s0031-9384(96)00176-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson BL, Landreth GE. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. J. Neuroinflam. 2006;3:30. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol. Aging. 2006;27:1094–9. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]