Abstract
Arterial spin labeling imaging (ASL) perfusion MRI is a relatively novel technique that can allow for quantitative measurement of cerebral blood flow (CBF) by using magnetically labeled arterial blood water as an endogenous tracer. Available data on resting CBF in schizophrenia primarily comes from invasive and expensive nuclear medicine techniques that are often limited to small samples and yield mixed results. The noninvasive nature of ASL offers promise for larger-scale studies. The utility of this approach was examined in 24 healthy controls and 30 patients with schizophrenia. Differences between groups in quantitative CBF were assessed, as were relationships between CBF and psychiatric symptoms. Group comparisons demonstrated greater CBF for controls in several regions including bilateral precuneus and middle frontal gyrus. Patients showed increased CBF in left putamen/superior corona radiata and right middle temporal gyrus. For patients, greater severity of negative symptoms was associated with reduced CBF in bilateral superior temporal gyrus, cingulate gyrus, and left middle frontal gyrus. Increased severity of positive symptoms was related to both higher CBF in cingulate gyrus and superior frontal gyrus and decreased CBF in precentral gyrus/middle frontal gyrus. These findings support the feasibility and utility of implementing ASL in schizophrenia research and expand upon previous results.
Keywords: ASL fMRI, positive symptoms, negative symptoms, superior temporal gyrus, middle frontal gyrus, white matter
1. Introduction
Studies of cerebral blood flow (CBF) in schizophrenia have been of interest since Kety and Schmidt first applied their nitrous oxide inhalation method to study the disorder (Kety et al., 1948). While Kety and Schmidt failed to find differences between patients and healthy individuals in CBF for the brain as a whole, they proposed that regional differences could provide clues to neural substrates of schizophrenia. Several years and technological advances later, this expectation was supported by the development of nuclear medicine techniques for measuring regional CBF such as positron emission tomography (PET) and single photon emission computerized tomography (SPECT), which have revealed more focal and nuanced abnormalities in the CBF of individuals with schizophrenia.
Although variable, results have largely highlighted decreased CBF in specific brain areas such as frontal and temporal regions (Erkwoh et al., 1999; Taylor et al., 1999; Esel et al., 2000; Malaspina et al., 2004). In regard to frontal regions, and perhaps contributing to inconsistencies in results, hypofrontality appears to be most pronounced during active engagement in cognitive tasks (for reviews, see (Andreasen et al., 1992; Chua and McKenna, 1995; R. C. Gur and Gur, 1995; Weinberger and Berman, 1996), and for a meta-analysis see (Hill et al., 2004). Additional findings include abnormal hemispheric laterality (Sheppard et al., 1983; R. E. Gur et al., 1985) and abnormal anterior posterior gradients that may contribute to findings of hypofrontality (Mathew et al., 1988); for reviews see (R. E. Gur, 1995; Bachneff, 1996). Utilization of these methods has also clarified the relationships between symptomatology and regional hyper- and hypoperfusion at rest. Specifically, increased severity of negative symptoms has been associated with reduced CBF in frontal and temporal regions as well as the thalamus (Lewis et al., 1992; Sabri et al., 1997; Min et al., 1999; Esel et al., 2000), and positive symptoms have been associated with increased CBF in temporal (Mathew et al., 1988; Parellada et al., 1998; Esel et al., 2000; Kohno et al., 2006; Horn et al., 2009), parietal (Mathew et al., 1988; Erkwoh et al., 1999; Esel et al., 2000; Franck et al., 2002), and frontal regions (Erkwoh et al., 1999; Horn et al., 2009), as well as decreased CBF in posterior cingulate gyrus and lingual gyrus (Liddle et al., 1992; Franck et al., 2002).
Despite the value of PET and SPECT in informing the neurobiology of schizophrenia, both are limited by invasiveness, reliance on radioactive tracer material, and expense. These limitations make PET and SPECT imaging difficult to implement in large scale, multi-site studies. A promising alternative can be found in arterial spin labeling (ASL) imaging. ASL measures CBF by using magnetically labeled arterial blood water as an endogenous tracer (Detre et al., 1992; Williams et al., 1992) and offers several advantages over other techniques. First, the noninvasive nature of ASL allows for repeated measurements with limited discomfort to participants aside from those typically associated with MRI such as loud noise, feelings of claustrophobia, and vibration. Work examining the reproducibility of ASL CBF measurements over time shows good stability and reliability across sessions with healthy individuals (Yen et al., 2002; Parkes et al., 2004; Hermes et al., 2007; Petersen et al., 2010; Pfefferbaum et al., 2010b; Y. Wang et al., 2011) and suggests that ASL may be particularly useful for longitudinal studies of schizophrenia that assess changes over time due to symptom remission/exacerbation or behavioral/psychopharamacological intervention. It should be noted, however, that a debate about the long-term reproducibility of ASL measurements is currently open, as some studies suggest that reproducibility estimates decline over longer time periods (Parkes et al., 2004; Gevers et al., 2009) whereas others do not (Hermes et al., 2007; Petersen et al., 2010). Second, as opposed to fMRI techniques such as blood oxygenation level dependent (BOLD) imaging that focus on task related activation, ASL provides quantitative measurement of CBF, an advantage that makes ASL optimal for assessing resting or baseline states. Third, ASL can be acquired quickly and easily in conjunction with other structural and functional MR information. These utilities offer a unique opportunity to provide a measurable and meaningful baseline to be integrated with blood oxygenation level dependent (BOLD) paradigms.
To our knowledge, two studies have utilized ASL to examine CBF in schizophrenia. Horn and colleagues (2009) found no group differences in CBF between patients and healthy controls but did report a significant correlation between severity of thought disorder and increased blood flow in left superior temporal gyrus, left anterior insula, and left inferior frontal gyrus. Additionally, in an examination of unmedicated patients, Scheef et al. (2010) found reduced perfusion in bilateral frontal and parietal lobes and middle and anterior cingulate gyrus in patients but increased perfusion in cerebellum, thalamus, and brainstem as compared to healthy controls. While informative, these early studies utilized small samples, and the examination of correlations with symptoms was limited to thought disorder.
Thus, given the benefits of ASL and the promising findings of these initial studies, we utilized this method to examine resting CBF in a relatively large sample of healthy control individuals and individuals with schizophrenia. Our goals were to: 1) quantify resting CBF in individuals with schizophrenia, 2) assess differences in resting CBF between patients and healthy individuals, and 3) examine the relationship between resting CBF and psychiatric symptoms. We expected that the quantitative CBF values obtained for both controls and patients would be comparable to those obtained with both nuclear medicine techniques and previous ASL investigations of healthy individuals. We also hypothesized that patients would show reduced CBF in frontal and temporal regions as compared to control participants; however, because participants were only assessed at rest, we expected these differences to be modest in comparison to those reported by previous studies that have utilized a cognitive task. Finally, consistent with previous reports reviewed above, we hypothesized that increased severity of negative symptoms would be related to reduced CBF in frontal and temporal regions and that increased severity of positive symptoms would be related to increased CBF in frontal, temporal, and parietal regions. Furthermore, in using a voxelwise analysis of these quantitative images, we intended to expand upon previous work by better localizing brain regions linked to more severe symptomatology.
2. Materials and Methods
2.1 Subjects
The original sample included 26 healthy control individuals and 31 individuals diagnosed with schizophrenia or schizoaffective disorder. Data from 2 controls and 1 patient were excluded due to excessive motion during scanning (>3mm). The final sample included 24 controls and 30 patients. Groups did not significantly differ in gender (χ2=0.19, p=0.67), handedness (χ2=0.18, p=0.94), ethnicity (χ2=1.35, p=0.51), age (t(52)=0.35, p=0.73), personal education (t(52)=1.71, p=0.09), parental education (maternal education: t(50)=0.52, p=0.61 and paternal education: t(47)=0.13, p=0.89) or nicotine dependence (t(52)=0.14, p=0.88) as assessed with the Fagerstrom Test for Nicotine Dependence (FTND; Heatherton et al., 1991). All participants were volunteers at the Schizophrenia Research Center (SRC) of the University of Pennsylvania Medical Center and were recruited via study advertisements and/or previous participation in SRC studies. After full description of all study procedures, each participant provided written informed consent, and the University of Pennsylvania ethics review board approved the study.
Diagnoses for individuals in the patient group were confirmed with the Diagnostic Interview for Genetics Studies (DIGS; Nurnberger et al., 1994), as well as self-reported demographic and medical history information. Following review of DIGS, Family Interview for Genetic Studies, and available medical records, lifetime Best Estimate Final Diagnoses (BEFD) for each participant was achieved via consensus review by at least two faculty clinicians. Only patients receiving a BEFD of schizophrenia or schizoaffective disorder depressed type with no other current Axis I or Axis II diagnoses were considered eligible for study participation. Control participants also completed the assessment procedures to ensure that they did not currently meet criteria for any Axis I or II disorders, never met criteria for a psychotic disorder, and did not have any first-degree family members with a psychotic illness. For both groups, exclusion criteria were current substance use, abuse, or dependence (except nicotine), history of head injury, or any medical conditions known to affect brain function (e.g. uncontrolled hypertension (BP>140/90), cardiac disease, diabetes mellitus, endocrine disorders, renal disease, chronic obstructive pulmonary disease, or history of seizures or head trauma).
For individuals in the patient group, severity of symptoms was assessed with the Scale for Assessment of Negative Symptoms (SANS) and the Scale for Assessment of Positive Symptoms (SAPS). At the time of the study, 1 patient was unmedicated, and all remaining patients were receiving stable doses: 5 received first generation antipsychotics (mean CPZ equivalent: 330.74 (±175.44) mg/day) and 24 received second generation antipsychotic medications (mean CPZ equivalent: 398.27 (±357.37) mg/day). Sample characteristics are provided in Table 1.
Table 1.
Sample Characteristics
|
Controls (n=24) Mean (SD) |
Patients (n=30) Mean (SD) |
|
|---|---|---|
| Gender | ||
| Male | 13 | 18 |
| Female | 11 | 12 |
| Ethnicity | ||
| Caucasian | 9 | 16 |
| African American | 14 | 13 |
| Asian | 1 | 1 |
| Handedness | ||
| Right | 20 | 26 |
| Left | 3 | 3 |
| Ambidextrous | 1 | 1 |
| Age | 35.73 (10.09) | 36.73 (10.35) |
| Education | 14.67 (2.24) | 13.53 (2.57) |
| Maternal Education | 13.04 (3.39) | 13.46 (2.84) |
| Paternal Education | 14.04 (3.39) | 13.92 (3.24) |
| FTND Score | 1.11 (1.69) | 1.19 (2.25) |
| Sum of SAPS Global Scores | 4.90 (4.02) | |
| Sums of SANS Global Scores | 4.97 (3.45) | |
| Chlorpromazine Equivalent | 373.74 (333.42) |
Abbreviations: FTND: Fagerstrom Test for Nicotine Dependence
Note: Maternal education was missing for 2 patients, and paternal education was missing for 5 patients.
2.2 Imaging Parameters and Procedure
Imaging was performed on a Siemens (Erlangen, Germany) 3T Trio MR scanner using the body coil for transmission and an eight-channel head coil for reception. A 5 minute magnetization-prepared, rapid acquisition gradient echo (MPRAGE: 160 slices, voxel size 1 × 1 × 1 mm, matrix=192 × 256, FOV=180 × 240, TR=1630 ms, TE=3.87 ms) image was first acquired to obtain a high-resolution, T1-weighted anatomical image for spatial normalization and overlays of functional data. Perfusion imaging was then conducted using a variant of flow-sensitive alternating inversion recovery imaging (FAIR; Kim, 1995) developed in our group. In this pulsed labeling scheme, a hyperbolic-secant inversion radio-frequency pulse was applied before the excitation pulse, and its frequency was tuned to match the frequency of the center slice to be imaged. The inversion band was 400mm for the non-selective (NS) image and 10mm thicker at both ends than the imaging region for the slice-selective (SS) image. NS and SS images were acquired in an interleaved fashion. Forty pairs of NS and SS images were obtained with the following parameters: FOV=22cm, matrix=64×64, TR=4s, TE=17ms, flip angle=90°, QUIPSS II (TI1 = 700 ms, TI2 = 1900 ms) (Wong et al., 1998), 20 slices (6mm thick with 1.2mm gap). Data readout was achieved using a single-shot gradient-echo echo-planar sequence. During scanning, participants were instructed to relax and lie still while keeping their eyes open.
2.3 Data Analysis
2.3.1 Primary Analyses
Pulsed arterial spin labeled (PASL) images were processed and quantified using SPM5 according to the optimization procedure detailed by Wang et al. (ASLtbx; Z. Wang et al., 2008). Input parameters included TI1 and TI2 after specifying the type of ASL method as PASL, while other parameters such as TR/TE and matrix size were automatically retrieved from the file header. Image pairs were realigned to the mean image to correct for motion. Forty perfusion-weighted images were generated by surround subtraction and then converted to quantitative CBF in units of ml/100ml/min, following the procedure summarized in Wong et al. (1998),
where ΔM was the difference between the SS and NS images, α was the tagging efficiency, and M0B was the fully relaxed magnetization of arterial blood, and T1B was the T1 of arterial blood. We assumed that T1B = 1600 ms (Greenman et al., 2003) and α = 0.97 (Wong et al., 1998), whereas M0B was estimated using white matter as an internal reference. In short, voxels fully occupied by white matter were identified on the average NS image, from which M0B was calculated based upon our imaging parameters and the literature T1 values for blood (1600 ms (Greenman et al., 2003)) and white matter (1100 ms (Stanisz et al., 2005)). M0 of white matter = 0.72 M0B (Wong et al., 1998).
Given that signal outliers can result from a mismatch of NS and SS images in spatial location or background suppression (Z. Wang et al., 2008), the 40 individual CBF maps for each participant were inspected for quality and any maps showing excessively low or high values (±2 SD beyond the mean for that individual across the time series) were discarded (8.5% of all CBF maps acquired). An average CBF map was then produced to yield one CBF image per participant. For inclusion in voxel-wise group-level analyses, averaged CBF and structural images were first coregistered, and averaged CBF images were then normalized to the MNI standard template provided by SPM and smoothed with a 6 mm full-width at half-maximum 3D isotropic Gaussian kernel.
To ensure that the CBF values obtained here were comparable to those of other methods and other ASL investigations, we first segmented each participant’s structural image to obtain individualized gray and white matter masks. These masks were then coregistered and applied to the averaged CBF image for that participant. Voxel values within each mask were extracted and averaged to yield estimates of the mean gray and white matter CBF for each individual. These analyses were performed in subject space prior to normalization so that the gray and white matter masks would be most accurate.
Next, to assess group differences and then the relationship between CBF and symptomatology within the patient group, normalized and smoothed CBF images were first used in a two-sample t-test and then in multiple regression analysis conforming to random effect analyses as implemented in SPM5. In the regression analysis, covariates of interest included the sum of global scores on the SANS and the sum of global scores on the SAPS. Gender and age were used as nuisance covariates in both analyses, and CPZ equivalent was also included as a nuisance covariate in the regression analysis. For all voxel-wise statistical results, Monte Carlo cluster correction (AlphaSim; Ward, 2000) at p<0.05 was used to correct for multiple comparisons. Additionally, due to variability in head position during scanning, the volume of the brain covered by group analyses was somewhat restricted, and the most inferior regions of the posterior occipital lobe and anterior temporal poles were not evaluated. The volume of coverage is shown in Supplemental Figure 1.
2.3.2 Supplemental Analyses
Two additional supplementary analyses were conducted. First, a voxel-based morphometry (VBM) analysis was completed to confirm that any emergent group differences in CBF were not due to morphological differences between patients and controls. Second, to facilitate a direct comparison with previously published studies, a region of interest (ROI) analysis using each ROI included in the AAL template (Tzourio-Mazoyer et al., 2002) was completed. Details of these analyses, including results, are provided in the online Supplementary Material.
Likewise, while the potential effects of CPZ equivalent were controlled for in the main analyses and not of primary interest here, the present study does allow for a general investigation of the effect of medication dosage on CBF within the patient group. These results are provided in Supplemental Table 3.
3. Results
3.1 Group Differences in CBF
Quantification of CBF produced gray matter values that on average were 2–3 times higher than white matter values, resulting in a mean gray to white matter ratio of 2.37 (±0.45) for controls and 2.32 (±0.68) for patients. Mean gray matter CBF (including cortical and subcortical gray matter) for controls and patients was 69.11 (±16.53) and 67.51 (±13.27) ml/100 g/min, respectively, and mean white matter CBF was 30.28 (±10.05) and 30.57 (±8.38) ml/100 g/min, respectively. These values did not significantly differ between groups (gray: t(52)=0.39, p=0.69, d=.11 and white: t(52)=0.62, p=0.91, d=.03).
Voxelwise comparisons, however, revealed several focal brain regions encompassing both gray and white matter in which controls showed greater resting CBF than patients and vice versa (Table 2 and Figure 1; perfusion weighted images for each group are provided in Supplemental Figure 2). Regions in which controls showed higher CBF than patients included bilateral precuneus extending into superior parietal white matter, left middle/inferior frontal gyrus (BA 10/46), right middle occipital gyrus, and left lingual gyrus (BA 18). Patients showed higher resting CBF than controls in left putamen extending into superior corona radiata and corpus callosum, right middle temporal gyrus (BA 39), right precentral gyrus (BA 4)/postcentral white matter, and right external capsule extending into dorsal putamen (x,y,z: 21,2,17; Z=2.00). All regions were significant when controlling for the effects of gender and age, and all survived cluster level correction at p<0.05. Importantly, none of the regions identified here overlapped with areas of morphological difference between the groups.
Table 2.
Brain regions showing significant group differences in CBF
| Cluster Sizea |
Anatomical Label and Brodmann Areab |
Talairach Coordinates x, y, z |
Peak Z Score |
Cohen’s dc |
|---|---|---|---|---|
| Controls > | Patients | |||
| 363 | L. precuneus (BA 7) | −22, −53, 44 | 3.07 | 0.92 |
| L. superior parietal WM | −16, −56, 49 | 2.79 | ||
| 443 | L. middle frontal gyrus (BA 10) | −42, 56, −4 | 2.89 | 0.76 |
| L. inferior frontal gyrus (BA 46) | −49, 45, 5 | 2.70 | ||
| L. inferior frontal gyrus (BA 46) | −49, 42, −14 | 2.26 | ||
| 506 | R. middle occipital gyrus | 24, −89, 10 | 2.68 | 0.75 |
| R. middle occipital gyrus WM | 28, −80, 2 | 2.49 | ||
| R. lingual gyrus (BA18) | 12, −83, −9 | 2.40 | ||
| 282 | R. superior parietal WM | 22, −50, 48 | 2.62 | 0.78 |
| R. precuneus (BA 7) | 19, −73, 41 | 2.37 | ||
| 306 | L. lingual gyrus (BA 18) | −8, −76, 2 | 2.49 | 0.73 |
| L. lingual gyrus (BA 18) | −18, −76, −7 | 2.26 | ||
| L. middle occipital WM | −26, −87, 3 | 2.08 | ||
| Patients > | Controls | |||
| 662 | L. putamen | −24, 1, 17 | 3.45 | 0.92 |
| L. superior corona radiata | −28, 13, 18 | 2.74 | ||
| L. corpus callosum | −5, 8, 19 | 2.46 | ||
| 373 | R. angular WM | 51, −55, 34 | 2.97 | 0.81 |
| R. middle temporal gyrus (BA 39) | 53, −65, 29 | 2.14 | ||
| R. middle temporal gyrus (BA 39) | 55, −65, 18 | 2.13 | ||
| 243 | R. postcentral WM | 50, −14, 34 | 2.89 | 0.80 |
| R. postcentral WM | 59, −5, 22 | 2.04 | ||
| R. precentral gyrus (BA 4) | 53, −4, 35 | 1.78 | ||
| 253 | R. external capsule | 26, 8, 14 | 2.46 | 0.67 |
| R. external capsule | 28, 1, 15 | 2.15 | ||
| R. external capsule | 32, −9, 15 | 2.00 |
R=right, L=left, WM=white matter
Cluster size is reported in voxels (2 × 2 × 2 mm), and coordinates for a maximum of 3 local maxima at least 8 mm apart are reported for each cluster.
Anatomical labels for gray matter were assigned according to Talairach and Tournoux (1988), and anatomical labels for white matter were assigned according to the JHU_MNI_SS_WMPM_TypeI atlas (Oishi et al., 2009).
Effect sizes for group differences were calculated by extracting CBF values from the peak voxel for each cluster.
Figure 1.
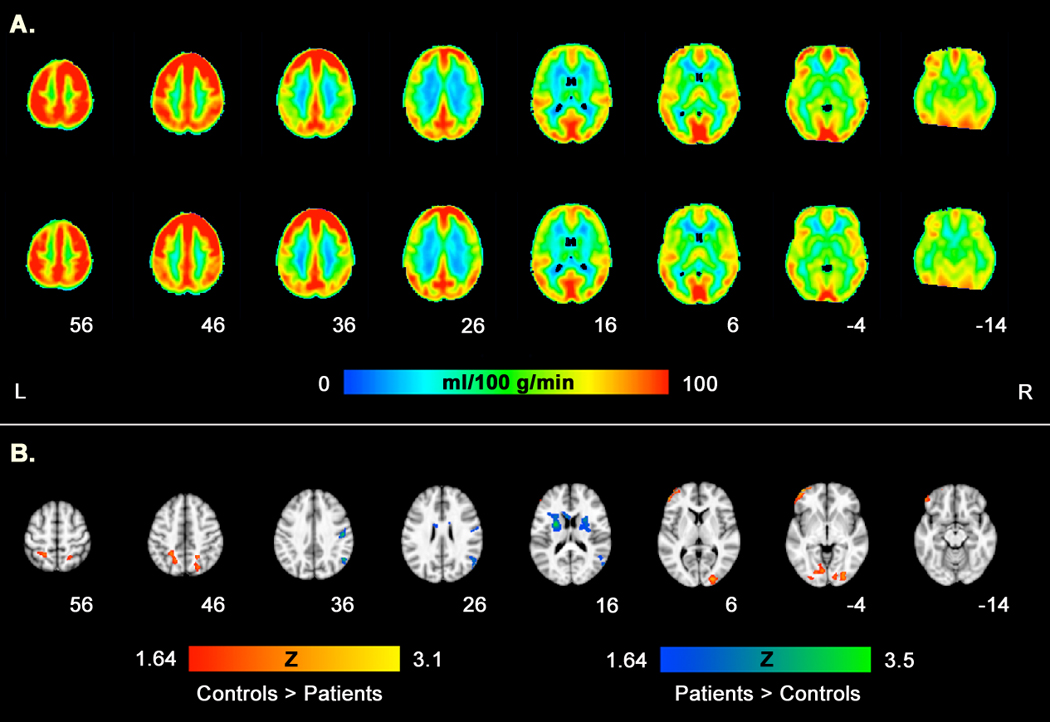
Group differences in resting CBF. Panel A shows mean CBF for controls and patients, respectively. Panel B shows areas of greater CBF (p<0.05, corrected) in controls relative to patients (red scale) and in patients relative to controls (blue scale).
3.2 Relationship between CBF and Symptomatology
The relationship between resting CBF and symptom severity was evaluated via a voxelwise multiple regression analysis that identified brain regions showing a significant correlation between CBF and symptom ratings (the sums of global scores on the SANS and SAPS). To avoid the potential confounding effects of overall illness severity, we first assessed the relationships between covariates. Severity of negative symptoms was not significantly correlated with severity of positive symptoms (r=0.03, p=0.89).
Several brain regions showed significant negative correlations with the sum of SANS global scores indicating that reduced resting CBF was associated with more severe negative symptoms (Table 3, Figure 2, and Supplemental Figure 3). As with the group differences in CBF, these regions spanned both gray and white matter. Areas included bilateral superior temporal gyrus, left angular white matter/middle occipital gyrus, left inferior frontal gyrus, anterior and posterior cingulate gyrus, and left middle frontal gyrus. No regions showed a positive correlation between resting CBF and negative symptom severity.
Table 3.
Brain regions showing significant correlations between resting CBF and symptomatology
| Cluster Sizea |
Anatomical Label and Brodmann Areab |
Talairach Coordinates x, y, z |
Peak Z Score |
Pearson’s rc |
|---|---|---|---|---|
| Negative Symptoms – Negative Correlation | ||||
| 9358 | R superior temporal gyrus (BA 22) | 55, −50, 10 | 4.07 | −0.71 |
| R superior temporal gyrus (BA 22) | 63, −2, 4 | 3.78 | ||
| R cuneus (BA 19) | 28, −88, 30 | 3.51 | ||
| 3800 | L angular WM | −40, −41, 31 | 3.79 | −0.68 |
| L middle occipital gyrus | −32, −75, 22 | 3.32 | ||
| L superior temporal gyrus (BA 39) | −61, −57, 23 | 3.16 | ||
| 240 | L inferior frontal gyrus (BA 45) | −59, 18, 8 | 2.84 | −0.54 |
| L inferior frontal gyrus (BA 45) | −53, 37, 2 | 2.26 | ||
| L superior temporal gyrus (BA 22) | −57, 11, −6 | 1.86 | ||
| 301 | Cingulate gyrus, anterior division (BA 24) | 0, 3, 27 | 2.77 | −0.53 |
| Cingulate gyrus, posterior division (BA 23) | 0, −14, 26 | 2.49 | ||
| 259 | L middle frontal gyrus | −32, 15, 36 | 2.34 | −0.45 |
| L middle frontal WM | −28, 25, 26 | 2.26 | ||
| L superior corona radiata | −24, 17, 29 | 2.15 | ||
| Positive Symptoms – Negative Correlation | ||||
| 6178 | R precentral gyrus (BA 9) | 44, 23, 43 | 3.45 | −0.63 |
| R middle frontal gyrus (BA 6) | 48, 2, 39 | 3.28 | ||
| R superior frontal WM | 18, 31, 32 | 3.25 | ||
| 415 | L superior frontal WM | −16, −7, −48 | 3.09 | −0.56 |
| L precentral WM | −14, −21, 47 | 2.53 | ||
| L paracentral lobule (BA 5) | −6, −30, 51 | 2.53 | ||
| Positive Symptoms - Positive Correlation | ||||
| 1187 | R cingulate gyrus (BA 31) | 20, −45, 26 | 3.47 | 0.63 |
| R superior corona radiata | 18, −12, 30 | 2.78 | ||
| R posterior corona radiata | 22, −28, 29 | 2.71 | ||
| 2028 | R superior frontal gyrus (BA 6) | 14, 20, 56 | 3.46 | 0.63 |
| R superior frontal gyrus (BA 6) | 6, 30, 54 | 3.37 | ||
| R superior frontal gyrus (BA 6) | −10, 24, 56 | 3.15 | ||
| 1725 | L superior parietal WM | −24, −51, 25 | 3.38 | 0.62 |
| L superior parietal WM | −22, −50, 39 | 3.02 | ||
| L precuneus (BA 31) | −18, −71, 21 | 2.75 | ||
R=right, L=left, WM=white matter
Cluster size is reported in voxels (2 × 2 × 2 mm), and coordinates for a maximum of 3 local maxima at least 8 mm apart are reported for each cluster.
Anatomical labels for gray matter were assigned according to Talairach and Tournoux (1988), and anatomical labels for white matter were assigned according to the JHU_MNI_SS_WMPM_TypeI atlas (Oishi et al., 2009).
Pearson’s r values were calculated by extracting CBF values from the peak voxel for each cluster.
Figure 2.
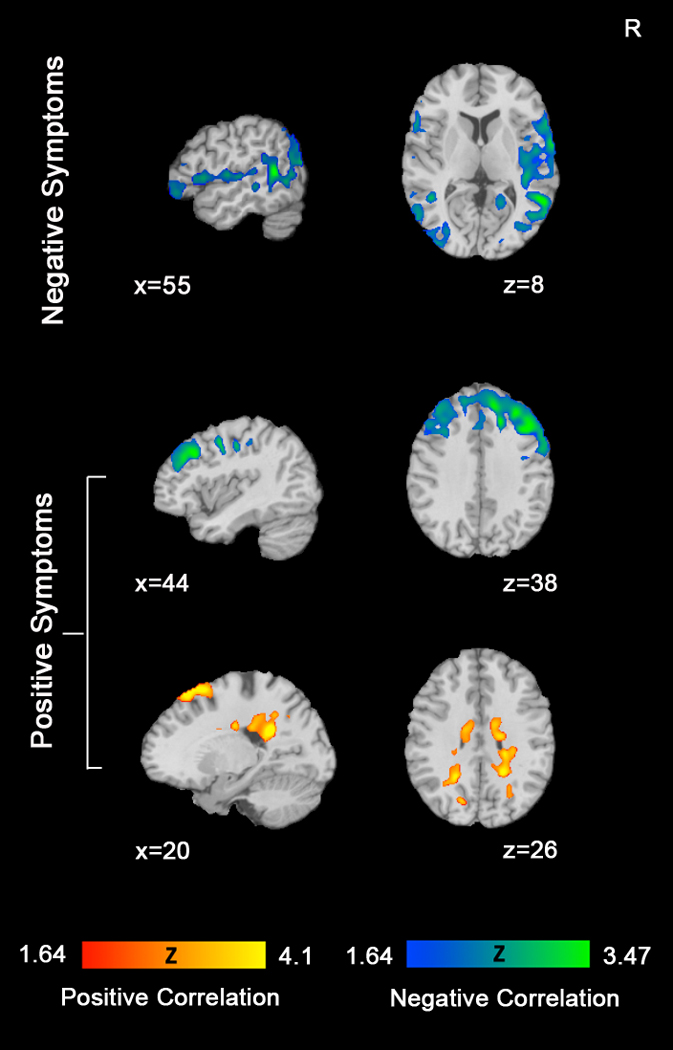
Correlations between CBF and Symptomatology. Top row shows brain regions in which CBF is negatively correlated with the severity of negative symptoms (blue scale). Middle and bottom rows show brain regions in which CBF is negatively correlated with positive symptom severity (blue scale) and positively correlated (red scale) with positive symptom severity. All images displayed with Monte Carlo cluster correction (AlphaSim) at p<0.05.
For positive symptoms, greater severity was associated with decreased resting CBF in right precentral gyrus, right middle frontal gyrus, and left superior frontal white matter extending into paracentral lobule. In contrast, right cingulate gyrus, right corona radiata, bilateral superior frontal gyrus, and left superior parietal white matter all showed a positive correlation with resting CBF indicating that greater severity of positive symptoms was related to increased blood flow in these regions (Table 3, Figure 2, and Supplemental Figure 3)1.
4. Discussion
Results from this study support the feasibility and utility of implementing ASL perfusion imaging in the study of schizophrenia. The quantitative CBF values reported here are consistent with those provided by nuclear medicine techniques (Ariel et al., 1983; R. E. Gur et al., 1983; Lassen, 1985; Leenders et al., 1990) and demonstrate the validity of ASL for providing quantitative and meaningful indices of CBF. Similarly, the findings from comparing CBF in patients with schizophrenia to healthy controls and from examining the relationships between symptom severity and CBF are comparable to PET, SPECT and ASL studies of individuals with schizophrenia and expand upon these previous findings.
First, in comparing CBF between patients and controls, we found no overall differences in whole brain values for gray or white matter perfusion. The lack of whole brain group differences has been reported by numerous studies at rest (Kety et al., 1948; R. E. Gur et al., 1983; Parellada et al., 1998; Liu et al., 2002). The majority of studies that do report widespread group differences in CBF have utilized cognitive challenges that appear to amplify group differences, particularly in frontal regions. Somewhat consistent with Scheef et al.(2010), our results revealed localized regions in which controls showed greater CBF than patients, including a small area of inferior and middle frontal gyri that suggests limited hypofrontality during rest. The pattern of differences generally suggests that, at a resting state, healthy controls show greater activity in cortical executive regions while patients activate somatosensory and sensory integration regions. Thus, in addition to frontal regions, higher resting activity in controls than patients was observed in precuneus, a region reported to be among those showing the highest resting metabolic rates in healthy individuals and to be related to self-processing and the “default mode of brain function” (Cavanna and Trimble, 2006; Fransson and Marrelec, 2008; Pfefferbaum et al., 2010a). This suggests that patients may be less able to engage in normative default modes of neural activation during non-goal-directed activities.
In contrast, patients showed greater CBF in right posterior middle temporal gyrus, a region linked to sensory integration (Beauchamp, 2005). While individuals were not given a task in the present study, the request that individuals keep their eyes open while being exposed to scanner noise and vibration may have created strong multisensory processing demands. Previous reports indicate that patients have difficulty processing multiple sensory modalities (de Gelder et al., 2003), and thus, while speculative, higher CBF in this region in patients may be reflective of the greater effort required to integrate various sensory cues, even passively while at rest. Alternatively, it is possible that increased CBF in this region may be related to treatment with antipsychotic medications. As noted in Supplemental Table 3, there was a significant positive correlation between CPZ equivalent and CBF in right middle temporal gyrus suggesting a dose dependent response, which may have contributed to group differences. Patients also showed greater resting CBF in the putamen. This finding is consistent with resting BOLD studies demonstrating over recruitment of the putamen at rest in schizophrenia (Huang et al., 2010; Ongur et al., 2010) and may also be related to use of antipsychotics. Previous work has demonstrated an association between increased perfusion/glucose metabolism in the putamen and typical antipsychotic treatment, particularly haloperidol (Holcomb et al., 1996; Miller et al., 1997; Lahti et al., 2009). However, given that we found no correlation between CPZ equivalent and CBF in the putamen and that the majority of our participants received atypical antipsychotics, this conclusion remains tentative.
We also found several white matter regions in which perfusion differed between patients and controls. Our findings of greater white matter CBF in patients are consistent with the work of Buchsbaum and colleagues (Buchsbaum et al., 2007) who reported higher relative glucose metabolic rates in patients in frontal and temporal white matter, corpus callosum, and superior longitudinal fasciculus. As noted by Buchsbaum et al., there are several hypotheses that may explain increased rates of white matter metabolism in schizophrenia, and among them is the possibility that transmission along axons may be ineffective due to redundant repetition, poor routing or decreased inhibition and synchrony. Likewise, the increases in white matter CBF reported here may be indicative of abnormal neuronal signaling at a resting state. It should be noted however that a few studies have argued that PASL has marginal sensitivity in detecting perfusion in deep white matter due to the low SNR of PASL as a consequence of the long transit time in white matter (van Gelderen et al., 2008). While this could be a confounding factor, the white matter regions reported here are generally not deep and mostly adjacent to cortical gray matter. The potential of partial volume effects must therefore be considered. With our spatial resolution, gray matter may account for as much as 78% of the perfusion in a voxel that appears to be white matter (van Gelderen et al., 2008). Given these potential confounds, we urge strong caution when interpreting the present findings in white matter and highlight the need for replication. Recent work by van Osch and colleagues (2009) suggests that use of pseudo-continuous labeling and background suppression can provide adequate signal detection in WM. Such strategies should be considered in future work, particularly given reports of abnormal white matter connectivity in frontal and temporal deep white matter in schizophrenia (Ellison-Wright and Bullmore, 2009).
Results regarding the relationships between CBF and symptom severity are also largely consistent with previous work. As anticipated, increased severity of negative symptoms was associated with decreased CBF in a number of frontal and temporal regions including bilateral superior temporal gyrus and left middle and inferior frontal gyrus. These findings are similar to those of Sabri et al. who reported negative correlations between affective flattening, emotional withdrawal and CBF in left frontal and temporal regions of interest (ROIs) and those of Esel and colleagues who reported a negative correlation between emotional withdrawal and CBF in bilateral temporal ROIs. The present findings further these reports by demonstrating that such correlations are strongest in posterior regions of right superior temporal gyrus and the left inferior frontal gyrus. Moreover, the association between reduced CBF and increased negative symptoms in frontal regions is in line with the longstanding body of work suggesting that hypofrontality is most prominent in individuals with more severe negative symptoms (Volkow et al., 1987; Andreasen et al., 1992; Wolkin et al., 1992). It is also noteworthy that no region showed positive correlations of increased activity associated with more severe symptoms. This absence supports the theoretical underpinnings of negative symptoms as reflecting diminished brain output capacities. Taken together, these findings may suggest that CBF at rest may have potential use as a predictive indicator of functional outcome (Milev et al., 2005; Brill et al., 2009).
Similarly, our findings concerning the relationships between positive symptom severity and CBF are in agreement with previous work demonstrating positive correlations between positive symptom severity and CBF in frontal regions (Erkwoh et al., 1999; Horn et al., 2009) and cingulate gyrus (Lahti et al., 2006). In contrast to previous work however, we found significant positive and negative correlations between positive symptom severity and CBF in several white matter regions. This difference between observations can most likely be attributed to the fact that we utilized a near whole brain voxelwise approach that allowed for the assessment of white matter instead of an ROI approach focusing only on gray matter (Parellada et al., 1998; Erkwoh et al., 1999; Esel et al., 2000) or methods that limit measurement to cortical gray matter (i.e. 133Xe inhalation technique). While relatively little is currently known about white matter functional abnormalities in schizophrenia, these correlations may provide valuable neuroimaging markers for this symptom dimension and may help explain the neural basis of positive symptoms in schizophrenia. Finally, in contrast to negative symptoms whose severity increased with reduced resting activity, greater severity of positive symptoms was associated with reduced activity in some regions but increased activity in others. This finding indicates that positive symptoms reflect an impaired balance in activation among regions both from reduced activation and compensatory over-activation of specific brain systems. This suggests different interventions for ameliorating positive and negative symptoms.
In directly comparing our results to those of the two previous studies that utilized ASL in schizophrenia, a number of considerations require mention. First, while both Horn et al. (2009) and Scheef et al. (2010) utilized relatively small samples, only the latter found significant differences between groups. We also report differential resting CBF between patients and controls; however, our results appear to be more focal than those of Scheef and colleagues. This may be due in part to the fact that their patient sample was unmedicated whereas the vast majority of our sample was taking medication. Given that the patients in Horn et al. were also medicated, this raises the possibility that pharmacological treatment may partially normalize CBF in patients. Second, in addition to differences in acquisition sequences, the volume of coverage differs between studies. In contrast to Scheef et al., the coverage obtained here did not encompass the cerebellum, which negated the possibility of replicating their finding of hyperperfusion in this region. Our coverage did however extended beyond that of Horn and colleagues to fully assess the most superior portions of the cortex. One of the most robust differences between patients and controls found here was in the precuneus, which was above their acquisition slab. Finally, our correlational analyses failed to replicate those of Horn and colleagues. While this could be due to the fact that we utilized only summary scores and did not specifically examine ratings for thought disorder, it should also be noted that only a subset of our patient sample (11 out of 30) displayed any sign of formal thought disorder, and the majority of these individuals (8 out of the 11) showed only very mild symptoms of thought disorder that were of questionable clinical significance.
As an initial investigation of the utility of ASL imaging for schizophrenia, the present study has several strengths including relatively large, well-matched samples, and high consistency with previous studies of CBF in both healthy and patient groups. Despite these strengths, several limitations are worthy of note. First, given this study’s focus on examining the feasibility of implementing ASL imaging in schizophrenia research, the present findings do not dramatically expand the existing literature. Establishing the validity of this method, however, is an essential first step, and our results do highlight a number of promising findings, specifically those in white matter, that require further study. Second, previous work with healthy populations has highlighted considerable variability in gray matter perfusion across individuals (Parkes et al., 2004; Y. Wang et al., 2011). As seen in Supplemental Table 2, some variability in CBF estimates was also evident in the present study, which may have limited the sensitivity of between group comparisons. Third, positive and negative symptoms were assessed broadly using the sums of global scores on the SANS and SAPS rather than examining each symptom individually. This was necessary in order to limit the number of statistical comparisons, but it is possible that looking at the relationship between each symptom and CBF may have been informative. Potential distinctions between patients with primary vs. secondary negative symptoms were also not assessed but may be meaningful for specifying the link between negative symptoms and reduced frontal perfusion (Lahti et al., 2001). Larger samples will be needed for such analyses. Finally, while the present study goes beyond the traditional ROI approach used in several previous studies to further localize brain regions associated with positive and negative symptoms, the spatial resolution implemented here is still somewhat coarse relative to more advanced BOLD methods. Likewise, PASL is advantageous in its relatively easy implementation without the need of a dedicated transmit/receive coil as required by its counterpart continuous ASL (Alsop and Detre, 1998); however, use of these alternative techniques, such as pseudo-continuous ASL (PCASL) (Wu et al., 2007) for further investigation of the white matter findings, may be informative. As the development of ASL methodology continues, future work will benefit from using more advanced sequences with improved spatial and temporal resolution and signal detection.
Notwithstanding these limitations, the agreement between the results reported here and those of previous studies of CBF in schizophrenia provides a solid rationale for incorporating ASL imaging into the study of neural substrates for schizophrenia. ASL offers unique advantages including a noninvasive approach and the ability to obtain quantitative estimates of CBF that can be used as a baseline measure. The availability of baseline assessments could be particularly informative for interpreting functional brain abnormalities in schizophrenia, and given that rest is so often used as a baseline in traditional BOLD studies, it is imperative that we gain a full understanding of the variability between patients and healthy individuals in quantitative CBF measures of the resting state. Finally, ASL offers particular promise for assessing the neural effects of behavioral and pharmacological interventions since it can be administered repeatedly with limited discomfort and inconvenience to participants.
Supplementary Material
Acknowledgments
This work was supported by the following grants from the National Institute of Mental Health (NIMH) at the University of Pennsylvania: T32 -MH019112, T32-NS054575, and R01-MH060722. We thank Drs. John Detre, Jiongjiong Wang, and Ze Wang from the Center for Functional Neuroimaging at the University of Pennsylvania for their assistance in protocol implementation and data analysis consultation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
To account for possible variation in global perfusion across the whole brain, each analysis was repeated using data that were scaled to a value of 50 ml/100 g/min. The results were largely identical to those presented here with the same regions of significance appearing in both sets of analyses.
Financial Disclosures
All authors report no conflicts of interest.
References
- Alsop DC, Detre JA. Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology. 1998;208(2):410–416. doi: 10.1148/radiology.208.2.9680569. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Rezai K, Alliger R, Swayze VW, 2nd, Flaum M, Kirchner P, Cohen G, O'Leary DS. Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia. Assessment with xenon 133 single-photon emission computed tomography and the Tower of London. Archives of general psychiatry. 1992;49(12):943–958. doi: 10.1001/archpsyc.1992.01820120031006. [DOI] [PubMed] [Google Scholar]
- Ariel RN, Golden CJ, Berg RA, Quaife MA, Dirksen JW, Forsell T, Wilson J, Graber B. Regional cerebral blood flow in schizophrenics. Tests using the xenon Xe 133 inhalation method. Archives of general psychiatry. 1983;40(3):258–263. doi: 10.1001/archpsyc.1983.01790030028003. [DOI] [PubMed] [Google Scholar]
- Bachneff SA. Regional cerebral blood flow in schizophrenia and the local circuit neurons hypothesis. Schizophrenia bulletin. 1996;22(1):163–182. doi: 10.1093/schbul/22.1.163. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS. See me, hear me, touch me: multisensory integration in lateral occipital-temporal cortex. Current opinion in neurobiology. 2005;15(2):145–153. doi: 10.1016/j.conb.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Brill N, Levine SZ, Reichenberg A, Lubin G, Weiser M, Rabinowitz J. Pathways to functional outcomes in schizophrenia: the role of premorbid functioning, negative symptoms and intelligence. Schizophrenia research. 2009;110(1–3):40–46. doi: 10.1016/j.schres.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Buchsbaum BR, Hazlett EA, Haznedar MM, Newmark R, Tang CY, Hof PR. Relative glucose metabolic rate higher in white matter in patients with schizophrenia. The American journal of psychiatry. 2007;164(7):1072–1081. doi: 10.1176/ajp.2007.164.7.1072. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chua SE, McKenna PJ. Schizophrenia--a brain disease? A critical review of structural and functional cerebral abnormality in the disorder. British Journal of Psychiatry. 1995;166(5):563–582. doi: 10.1192/bjp.166.5.563. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Vroomen J, Annen L, Masthof E, Hodiamont P. Audio-visual integration in schizophrenia. Schizophrenia research. 2003;59(2–3):211–218. doi: 10.1016/s0920-9964(01)00344-9. [DOI] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magnetic Resonance in Medicine. 1992;23(1):37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophrenia research. 2009;108(1–3):3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Erkwoh R, Sabri O, Willmes K, Steinmeyer EM, Bull U, Sass H. Active and remitted schizophrenia: psychopathological and regional cerebral blood flow findings. Psychiatry research. 1999;90(1):17–30. doi: 10.1016/s0925-4927(99)00005-0. [DOI] [PubMed] [Google Scholar]
- Esel E, Kula M, Gonul AS, Tutus A, Basturk M, Turan T, Sofuoglu S, Yilmaz S. Negative and positive symptoms: In relation to regional cerebral blood flow in drug-free schizophrenic patients. Bulletin of Clinical Psychopharmacology. 2000;20:57–63. [Google Scholar]
- Franck N, O'Leary DS, Flaum M, Hichwa RD, Andreasen NC. Cerebral blood flow changes associated with Schneiderian first-rank symptoms in schizophrenia. The Journal of neuropsychiatry and clinical neurosciences. 2002;14(3):277–282. doi: 10.1176/jnp.14.3.277. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. NeuroImage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Gevers S, Majoie CB, van den Tweel XW, Lavini C, Nederveen AJ. Acquisition time and reproducibility of continuous arterial spin-labeling perfusion imaging at 3T. American Journal of Neuroradiology. 2009;30(5):968–971. doi: 10.3174/ajnr.A1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Gur RE. Hypofrontality in schizophrenia: RIP. Lancet. 1995;345(8962):1383–1384. doi: 10.1016/s0140-6736(95)92591-0. [DOI] [PubMed] [Google Scholar]
- Gur RE. Functional brain-imaging studies in schizophrenia. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York, NY: Raven Press; 1995. pp. 1185–1192. [Google Scholar]
- Gur RE, Gur RC, Skolnick BE, Caroff S, Obrist WD, Resnick S, Reivich M. Brain function in psychiatric disorders. III. Regional cerebral blood flow in unmedicated schizophrenics. Archives of general psychiatry. 1985;42(4):329–334. doi: 10.1001/archpsyc.1985.01790270015001. [DOI] [PubMed] [Google Scholar]
- Gur RE, Skolnick BE, Gur RC, Caroff S, Rieger W, Obrist WD, Younkin D, Reivich M. Brain function in psychiatric disorders. I. Regional cerebral blood flow in medicated schizophrenics. Archives of general psychiatry. 1983;40(11):1250–1254. doi: 10.1001/archpsyc.1983.01790100096013. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hermes M, Hagemann D, Britz P, Lieser S, Rock J, Naumann E, Walter C. Reproducibility of continuous arterial spin labeling perfusion MRI after 7 weeks. Magnetic Resonance Materials in Physics, Biology and Medicine. 2007;20(2):103–115. doi: 10.1007/s10334-007-0073-3. [DOI] [PubMed] [Google Scholar]
- Hill K, Mann L, Laws KR, Stephenson CM, Nimmo-Smith I, McKenna PJ. Hypofrontality in schizophrenia: a meta-analysis of functional imaging studies. Acta psychiatrica Scandinavica. 2004;110(4):243–256. doi: 10.1111/j.1600-0447.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- Holcomb HH, Cascella NG, Thaker GK, Medoff DR, Dannals RF, Tamminga CA. Functional sites of neuroleptic drug action in the human brain: PET/FDG studies with and without haloperidol. The American journal of psychiatry. 1996;153(1):41–49. doi: 10.1176/ajp.153.1.41. [DOI] [PubMed] [Google Scholar]
- Horn H, Federspiel A, Wirth M, Muller TJ, Wiest R, Wang JJ, Strik W. Structural and metabolic changes in language areas linked to formal thought disorder. British Journal of Psychiatry. 2009;194(2):130–138. doi: 10.1192/bjp.bp.107.045633. [DOI] [PubMed] [Google Scholar]
- Huang XQ, Lui S, Deng W, Chan RC, Wu QZ, Jiang LJ, Zhang JR, Jia ZY, Li XL, Li F, Chen L, Li T, Gong QY. Localization of cerebral functional deficits in treatment-naive, first-episode schizophrenia using resting-state fMRI. NeuroImage. 2010;49(4):2901–2906. doi: 10.1016/j.neuroimage.2009.11.072. [DOI] [PubMed] [Google Scholar]
- Kety SS, Woodford RB, Harmel MH, Freyhan FA, Appel KE, Schmidt CF. Cerebral blood flow and metabolism in schizophrenia: The effects of barbiturate semi-narcosis, insulin coma, and electroshock. American Journal of Psychiatry. 1948;104:765–770. doi: 10.1176/ajp.104.12.765. [DOI] [PubMed] [Google Scholar]
- Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magnetic Resonance in Medicine. 1995;34(3):293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- Kohno T, Shiga T, Kusumi I, Matsuyama T, Kageyama H, Katoh C, Koyama T, Tamaki N. Left temporal perfusion associated with suspiciousness score on the Brief Psychiatric Rating Scale in schizophrenia. Psychiatry research. 2006;147(2–3):163–171. doi: 10.1016/j.pscychresns.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Medoff DR, Weiler MA, Tamminga CA, Carpenter WT., Jr Abnormal patterns of regional cerebral blood flow in schizophrenia with primary negative symptoms during an effortful auditory recognition task. The American journal of psychiatry. 2001;158(11):1797–1808. doi: 10.1176/appi.ajp.158.11.1797. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31(1):221–230. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Cropsey KL. Modulation of limbic circuitry predicts treatment response to antipsychotic medication: a functional imaging study in schizophrenia. Neuropsychopharmacology. 2009;34(13):2675–2690. doi: 10.1038/npp.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen NA. Normal average value of cerebral blood flow in younger adults is 50 ml/100 g/min. Journal of Cerebral Blood Flow & Metabolism. 1985;5(3):347–349. doi: 10.1038/jcbfm.1985.48. [DOI] [PubMed] [Google Scholar]
- Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, Gibbs JM, Wise RJ, Hatazawa J, Herold S, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113(Pt 1):27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- Lewis SW, Ford RA, Syed GM, Reveley AM, Toone BK. A controlled study of 99mTc-HMPAO single-photon emission imaging in chronic schizophrenia. Psychological medicine. 1992;22(1):27–35. doi: 10.1017/s0033291700032694. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RS. Patterns of cerebral blood flow in schizophrenia. British Journal of Psychiatry. 1992;160:179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- Liu Z, Tam WC, Xie Y, Zhao J. The relationship between regional cerebral blood flow and the Wisconsin Card Sorting Test in negative schizophrenia. Psychiatry and clinical neurosciences. 2002;56(1):3–7. doi: 10.1046/j.1440-1819.2002.00924.x. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Harkavy-Friedman J, Corcoran C, Mujica-Parodi L, Printz D, Gorman JM, Van Heertum R. Resting neural activity distinguishes subgroups of schizophrenia patients. Biological psychiatry. 2004;56(12):931–937. doi: 10.1016/j.biopsych.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Tant SR, Robinson L, Prakash R. Abnormal resting regional cerebral blood flow patterns and their correlates in schizophrenia. Archives of general psychiatry. 1988;45(6):542–549. doi: 10.1001/archpsyc.1988.01800300038004. [DOI] [PubMed] [Google Scholar]
- Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. The American journal of psychiatry. 2005;162(3):495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- Miller DD, Rezai K, Alliger R, Andreasen NC. The effect of antipsychotic medication on relative cerebral blood perfusion in schizophrenia: assessment with technetium-99m hexamethyl-propyleneamine oxime single photon emission computed tomography. Biological psychiatry. 1997;41(5):550–559. doi: 10.1016/s0006-3223(96)00110-2. [DOI] [PubMed] [Google Scholar]
- Min SK, An SK, Jon DI, Lee JD. Positive and negative symptoms and regional cerebral perfusion in antipsychotic-naive schizophrenic patients: a high-resolution SPECT study. Psychiatry research. 1999;90(3):159–168. doi: 10.1016/s0925-4927(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of general psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–844. [DOI] [PubMed] [Google Scholar]
- Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, Hsu JT, Miller MI, van Zijl PC, Albert M, Lyketsos CG, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Mazziotta J, Mori S. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer's disease participants. NeuroImage. 2009;46(2):486–499. doi: 10.1016/j.neuroimage.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry research. 2010;183(1):59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parellada E, Catafau AM, Bernardo M, Lomena F, Catarineu S, Gonzalez-Monclus E. The resting and activation issue of hypofrontality: a single photon emission computed tomography study in neuroleptic-naive and neuroleptic-free schizophrenic female patients. Biological psychiatry. 1998;44(8):787–790. doi: 10.1016/s0006-3223(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magnetic Resonance in Medicine. 2004;51(4):736–743. doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- Petersen ET, Mouridsen K, Golay X. The QUASAR reproducibility study, Part II: Results from a multi-center Arterial Spin Labeling test-retest study. NeuroImage. 2010;49(1):104–113. doi: 10.1016/j.neuroimage.2009.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Chanraud S, Pitel AL, Muller-Oehring E, Shankaranarayanan A, Alsop DC, Rohlfing T, Sullivan EV. Cerebral blood flow in posterior cortical nodes of the default mode network decreases with task engagement but remains higher than in most brain regions. Cerebral Cortex. 2010a;21(1):233–244. doi: 10.1093/cercor/bhq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Chanraud S, Pitel AL, Shankaranarayanan A, Alsop DC, Rohlfing T, Sullivan EV. Volumetric cerebral perfusion imaging in healthy adults: regional distribution, laterality, and repeatability of pulsed continuous arterial spin labeling (PCASL) Psychiatry research. 2010b;182(3):266–273. doi: 10.1016/j.pscychresns.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri O, Erkwoh R, Schreckenberger M, Cremerius U, Schulz G, Dickmann C, Kaiser HJ, Steinmeyer EM, Sass H, Buell U. Regional cerebral blood flow and negative/positive symptoms in 24 drug-naive schizophrenics. Journal of Nuclear Medicine. 1997;38(2):181–188. [PubMed] [Google Scholar]
- Scheef L, Manka C, Daamen M, Kuhn KU, Maier W, Schild HH, Jessen F. Resting-state perfusion in nonmedicated schizophrenic patients: a continuous arterial spin-labeling 3.0-T MR study. Radiology. 2010;256(1):253–260. doi: 10.1148/radiol.10091224. [DOI] [PubMed] [Google Scholar]
- Sheppard G, Gruzelier J, Manchanda R, Hirsch SR, Wise R, Frackowiak R, Jones T. 15O positron emission tomographic scanning in predominantly never-treated acute schizophrenic patients. Lancet. 1983;2(8365–66):1448–1452. doi: 10.1016/s0140-6736(83)90798-5. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planer Stereotaxic Atlas of the Human Brain. New York, NY: Thieme Medical Publishers; 1988. [Google Scholar]
- Taylor SF, Tandon R, Koeppe RA. Global cerebral blood flow increase reveals focal hypoperfusion in schizophrenia. Neuropsychopharmacology. 1999;21(3):368–371. doi: 10.1016/S0893-133X(98)00109-2. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Gelderen P, de Zwart JA, Duyn JH. Pittfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magnetic Resonance in Medicine. 2008;59(4):788–795. doi: 10.1002/mrm.21515. [DOI] [PubMed] [Google Scholar]
- van Osch MJ, Teeuwisse WM, van Walderveen MA, Hendrikse J, Kies DA, van Buchem MA. Can arterial spin labeling detect white matter perfusion signal? Magnetic Resonance in Medicine. 2009;62(1):165–173. doi: 10.1002/mrm.22002. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wolf AP, Van Gelder P, Brodie JD, Overall JE, Cancro R, Gomez-Mont F. Phenomenological correlates of metabolic activity in 18 patients with chronic schizophrenia. The American journal of psychiatry. 1987;144(2):151–158. doi: 10.1176/ajp.144.2.151. [DOI] [PubMed] [Google Scholar]
- Wang Y, Saykin AJ, Pfeuffer J, Lin C, Mosier KM, Shen L, Kim S, Hutchins GD. Regional reproducibility of pulsed arterial spin labeling perfusion imaging at 3T. NeuroImage. 2011;54(2):1188–1195. doi: 10.1016/j.neuroimage.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Aguirre GK, Rao H, Wang J, Fernandez-Seara MA, Childress AR, Detre JA. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magnetic resonance imaging. 2008;26(2):261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. 2000 http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf.
- Weinberger DR, Berman KF. Prefrontal function in schizophrenia: confounds and controversies. Philosophical transactions of the Royal Society of London. 1996;351(1346):1495–1503. doi: 10.1098/rstb.1996.0135. [DOI] [PubMed] [Google Scholar]
- Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(1):212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkin A, Sanfilipo M, Wolf AP, Angrist B, Brodie JD, Rotrosen J. Negative symptoms and hypofrontality in chronic schizophrenia. Archives of general psychiatry. 1992;49(12):959–965. doi: 10.1001/archpsyc.1992.01820120047007. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magnetic Resonance in Medicine. 1998;39(5):702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- Wu WC, Fernandez-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magnetic Resonance in Medicine. 2007;58(5):1020–1027. doi: 10.1002/mrm.21403. [DOI] [PubMed] [Google Scholar]
- Yen YF, Field AS, Martin EM, Ari N, Burdette JH, Moody DM, Takahashi AM. Test-retest reproducibility of quantitative CBF measurements using FAIR perfusion MRI and acetazolamide challenge. Magnetic Resonance in Medicine. 2002;47(5):921–928. doi: 10.1002/mrm.10140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


