Abstract
Epilepsy is associated with academic and neurocognitive disorders, with the latter often assumed to explain the former. We examined utilization of special education services (SpES) in relation to neurocognitive test scores in a case-matched sibling control study. In a follow-up assessment 8–9 years after entry into a prospective study of childhood-onset epilepsy, cases and siblings partook in an interview and standardized neurocognitive testing. Analyses included 142 pairs in which both had FSIQ≥80 and the case had normal exam and imaging. 64 (45%) of cases and 25 (17.6%) of controls reported SpES utilization, matched odds ratio (mOR)=5.3 (p<0.0001). Adjustment for neurocognitive test scores resulted in a mOR=4.58 (p<0.0001). Types and duration of services were similar in cases and controls. 24% of school-aged cases were already receiving services at the time of initial epilepsy diagnosis. Young people with epilepsy have academic difficulties that are not simply explained by cognitive test scores.
Keywords: Cognitive function, Special Education, Academic Difficulties, Case-Control Study, Epilepsy, Children
Introduction
Young people and adults with epilepsy have repeatedly been shown to be at increased risk of having a variety of cognitive difficulties. This is true even in individuals who do not have an intellectual disability per se and who have otherwise normal neurological status [1–4]. Further, these difficulties appear to be present right from the start and likely pre-date the onset of seizures. Children with epilepsy also have increased utilization of special education services [1, 2, 5]. It is easy to assume that relative cognitive impairments as indicated by standardized research batteries account for the differences in educational difficulties between children with epilepsy and appropriate controls.
Previously, we reported on the high proportion of children with epilepsy who had ever received special education or related services [5]. Here we examine the relationship between cognitive test scores and special education utilization within the context of a prospectively followed cohort, some members of which participated in standardized neurocognitive testing, with matched sibling controls who had been evaluated with the same neurocognitive battery.
Methods
The Connecticut Study of Epilepsy is a prospective, community-based cohort of young people recruited when first diagnosed with epilepsy within the state of Connecticut during 1993–1997. Eligible children were 1 month up through 15 years of age at the time of their initial onset of epilepsy. Their parents were interviewed and the families were contacted every 3–4 months to ascertain seizure occurrence. Medical records were reviewed at initial study entry and, with appropriate permissions, periodically during follow-up. Information about the underlying cause, imaging findings, the electroclinical syndrome, and other diagnostic testing was reviewed and the characterization of the epilepsy updated as necessary [6].
In 2002–2006, a comprehensive reassessment protocol was offered to cohort members. It included a questionnaire providing a detailed history of each child’s cumulative utilization of special education services and school placement. Respondents were asked about the agencies providing services, the specific types of services received, the age at which each service began, and whether the child was still receiving that service or the age at which the service ended. In addition, each child was offered a standardized neurocognitive assessment [7] and a research MRI. Determination of whether brain structure was normal or not was based on the best imaging information available as previously described [8]. Individuals could choose to participate at different levels in the assessment protocol. Minimum participation consisted of completing the interview only. More intense participation involved neurocognitive testing, and complete participation involved the preceding and a research MRI scan.
When available, a sibling control was recruited for matched comparisons with the cases, with the goal being to recruit a sibling who was as close as possible to the case’s age. The same questionnaires and neurocognitive testing procedures were used with the control.
For most of the analyses presented below, the analytic sample was limited to matched case-sibling control pairs in which the case did not have an underlying structural or metabolic condition as the cause of the epilepsy and also did not have a history of a syndrome characterized as an “epileptic encephalopathy” (e.g. West, or MAE) [9] (“complicated epilepsy”). In addition, both the case and control had participated in the neurocognitive assessment and had received full scale IQ (FSIQ) scores of ≥80.
All analyses were performed in SAS (SAS, 9.2). Common techniques for simple bivariate unmatched and matched comparisons were used to compare means (t-tests and paired t-tests) and frequencies (chi-squares and McNemar’s chi-square). Multivariable analysis was performed with conditional logistic regression to determine the independent correlates of the use of special education services while taking into account the matching between siblings [10].
All procedures used in this study were approved by the IRBs of the involved institutions and conformed with the intent of the Declaration of Helsinki and current applicable laws. Parents initially provided informed permission and children oral or written informed assent when possible depending on age and ability. When study subjects attained the age of majority, they were invited to participate as adults and, if they agreed, provided written informed consent. For controls, either written permission and informed assent or informed consent was obtained depending on age.
Results
Of the original 613 cohort members, 502 participated in at least the assessment interview, although one was subsequently excluded for confidential reasons. Of the remaining 501 who participated in the 9-year interview, 296 (59.1%) had received special education or related services (excluding gifted programs) and 105 (21.0%) had been retained in school. Consistent with a previous report on the entire cohort, 122 (24.4%) of participants who participated in the 9-year interview were considered to have some degree of cognitive deficit (full scale IQ (FSIQ) <80), if not frank intellectual disability [11]. All but 2 of 122 (98%) participants with measured or estimated FSIQ<80 had also received special services versus 176 of 379 (46.4%) of those with FSIQ≥80 (p<0.0001). Similarly, 46 (37.7%) of those with FSIQ<80 were retained in school (the denominator included severely impaired children who would not have been in graded programs and for whom the issue of retention would have been irrelevant) versus 59 (15.6%) of those with FSIQ≥80.
All further analyses were limited to cohort members who participated in neurocognitive testing as part of the assessment, whose FSIQ was measured as being ≥80, who had no known structural brain abnormalities or other conditions responsible for their epilepsy, and who also had a sibling control who underwent the same battery of testing (N=142 matched pairs). This is the same sample described previously [7] with the exclusion of one participant (with a control) as mentioned above (Figure 1).
Figure 1.
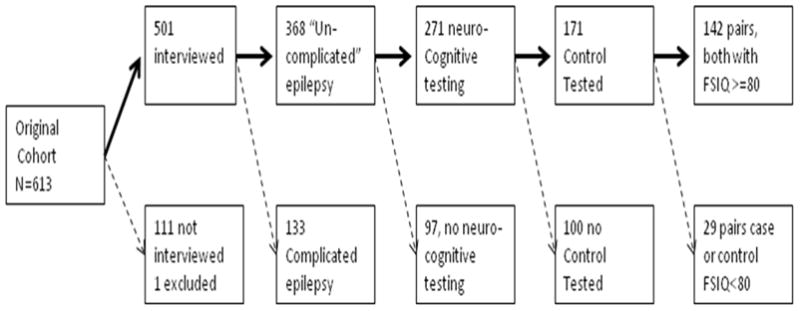
Derivation of the analyzed sample:
In the 142 matched pairs, 50% of cases and 42% of controls were male (p=0.17). At the time of participation in the interview and testing protocol, cases were on average 15.1 (SD=3.9) years old and controls were 15.6 (SD=5.2) years old (p=0.33).
Use of any special education or related services was reported in 64 (45.1%) of cases and 25 (17.6%) of controls (matched Odds Ratio (mOR)=5.3, 95% CI, 2.6, 10.9, p<0.0001). Being held back a year in school was reported in 23 (16.2%) of cases and 11 (8.5%) of controls (mOR=3.0, 95% CI, 1.2, 7.6, p=0.02). Of cases who had been held back, 20/23 (87%) received special services in contrast to 4/11 (36%) of controls who had been held back (p=0.003). Academic concerns were mentioned more often in cases who were held back than in controls (65% cases and 27% controls, p=0.04). This suggests that the reasons for academic retention may have been different for cases and controls, and that retention is not necessarily a direct marker of academic difficulties, especially in controls.
In cases and controls who reported receiving services, the mean age at onset of services was very similar (7.1y for cases and 6.8y for controls, p=0.76) and corresponds to the age at which academic difficulties tend to be noticed. The mean duration of services was also similar in the two groups (5.4y for cases and 4.8y for controls, p=0.42). Cases who reported receiving services were somewhat more likely still to be receiving services at the time of the interview than controls (57.8% vs. 36.0% p= 0.06). In cases and controls who received services, the proportions receiving specific types of services were comparable in the two groups (Table 1).
Table 1.
Types of special education services received by those cases and controls reporting any services.
| Type of services | Cases (N=64) | Control (N=25) * |
|---|---|---|
| Occupational therapy | 13 (20.3%) | 4 (16.0%) |
| Physical therapy | 10 (15.6%) | 4 (16.0%) |
| Speech therapy | 24 (37.5%) | 10 (40.0%) |
| Individual aide | 11 (17.2%) | 2 (8.0%) |
| Resource Room | 41 (64.1%) | 16 (64.0%) |
| Special classes | 18 (28.1%) | 10 (40.0%) |
| Psychological counseling | 21 (32.8%) | 4 (16.0%) |
| Pre-K intervention | 13 (20.3%) | 6 (24.0%) |
| Other services through special education | 15 (23.4%) | 5 (20.0%) |
| Services through birth-to-3 or Easter Seals | 4 (6.3%) | 1 (4.0%) |
| Multiple types of services | 44 (68.8%) | 15 (60.0%) |
No difference approached statistical significance. The smallest p-value was for psychological counseling services, p=0.11.
We considered initiation of services with respect to the case’s age at diagnosis of epilepsy. For controls, the case-sibling’s age at diagnosis was used as the referent age. Services were initiated in 26 (18.3%) cases prior to the diagnosis of epilepsy and in 12 (8.5%) of controls prior to the age at which their sibling was diagnosed with epilepsy (p=0.02). Of cases who were school-aged (≥6 years) at time of diagnosis, 20/82 (24%) were already receiving services when first diagnosed versus 6/60 (10%) of those who were younger (p=0.05). In controls, these figures were 7% (for <6 years) and 8% (for ≥ 6 years).
Multivariable analysis
In a conditional logistic regression analysis for matched pair data, we considered whether the association between special education services and case-control status might be explained partially or entirely by gender, overall IQ, and individual IQ subscale factor indices (verbal comprehension, perceptual organization, processing speed, and freedom from distractibility/working memory).
Processing speed was the only one of these variables to be significantly and independently associated with case-control status. Adjustment for processing speed, however, resulted in only a small reduction of the matched odds ratio for special services (mOR= 4.58, 95% CI,2.22, 9.47, p<0.0001). Other factors were not significantly associated with case-control status in this model.
To explore the multivariable results further, we stratified cases and controls first according to 10-point FSIQ intervals (80–89, 90–99, 100– 109, 110–119, >=120) and separately by 10-point intervals for the processing speed index which had been found to differ significantly between the groups. For processing speed, a category for <80 was necessary. Within the case and control groups separately, the proportion who reported special services was strongly and inversely correlated with IQ and with processing speed levels. At each score level, however, cases were more likely to have received services than controls (Tables 2&3 or Figure 2).
Table 2.
Services by IQ level in cases and in controls
| Cases | Controls | |||
|---|---|---|---|---|
| IQ | N | N (%) w/services | N | N (%) w/services |
| 80–89 | 20 | 16 (80.0%) | 10 | 3 (30.0%) |
| 90–99 | 39 | 23 (59.0%) | 28 | 9 (32.1%) |
| 100–109 | 31 | 13 (41.9%) | 51 | 11 (21.6%) |
| 110–119 | 31 | 7 (22.6%) | 24 | 1 (4.2%) |
| ≥120 | 21 | 5 (23.8%) | 29 | 1 (3.5%) |
| P<0.0001 (trend) | P<0.0001 (trend) | |||
Table 3.
Services by Processing Speed Index level in cases and in controls
| Cases | Controls | |||
|---|---|---|---|---|
| Processing Speed | N | N (%) w/services | N | N (%) w/services |
| <80 | 14 | 12 (85.7%) | 1 | 0 |
| 80–89 | 25 | 17 (68.0%) | 22 | 7 (31.8%) |
| 90–99 | 39 | 13 (33.3%) | 34 | 8 (23.5%) |
| 100–109 | 25 | 10 (40.0%) | 34 | 6 (17.7%) |
| 110–119 | 31 | 9 (34.6%) | 32 | 4 (12.5%) |
| ≥120 | 13 | 3 (23.1%) | 19 | 0 |
| P<0.0001 (trend) | P<0.0001 (trend) | |||
Figure 2.
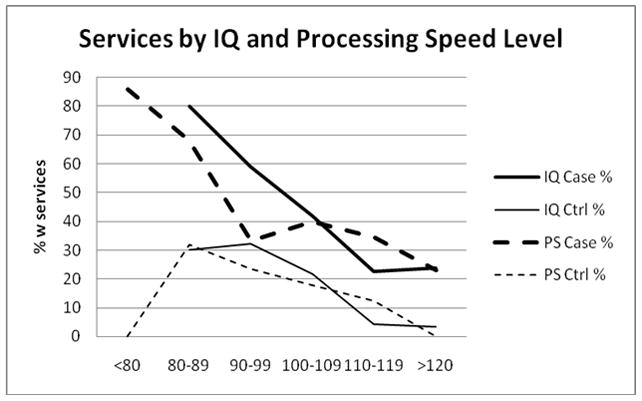
Receipt of services in cases and controls as a function of 10-point intervals for overall IQ and for processing speed.
To explore whether there was evidence of specific effects for specific subtypes of epilepsy, we examined case-control differences for Wechsler index scores, receipt of special services, and academic retention by subtypes of epilepsy (Table 4). The findings suggested that BECTS might be associated with a lesser impact on educational and cognitive difficulties than the other forms of epilepsy, although a small effect remained for processing speed. Aside from that, the other three epilepsy groups appeared associated with similar disadvantage relative to their sibling controls.
Table 4.
Case-Control means Wechsler scores differences and matched odds ratios by type of epilepsy
| BECTS (N=20) | NSE-UNK (N=70) | GGE (N=40) | Other (N=12) | |
|---|---|---|---|---|
| FSIQ | 0.4 | −2.8 | −3.4 | −9.4** |
| Verbal Comprehension | 2.7 | −2.0 | −2.5 | −11.0** |
| Perceptual Organization | 1.3 | 0.8 | −1.7 | −4.8 |
| Freedom from Distractibility | 3.6 | −1.1 | −4.9* | −9.3 |
| Processing speed | −2.8 | −6.8*** | −4.0 | −6.1 |
| Special Education services | 2.0 (0.5, 8.0) P=0.31 | 8.3 (2.5, 27.6) P<0.0001 | 6.5 (1.5, 28.8) P=0.003 | 4.0 (0.4, 35.8) P=0.17 |
| Academic retention | 0.3 (0.1, 3.2) P=0.30 | 6.0 (1.3, 26.8) P=0.02 | 8/0 | 2.0 (0.2, 22.1) P=0.56 |
BECTS=Benign Epilepsy with Central-Temporal Spikes
NSE-UNK=nonsyndromic epilepsy of unknown cause
GGE=genetic generalized epilepsies
FSIQ=full scale intelligence quotient
Most score differences were not statistically significant. For those that approached significance or were:
p<0.1
P,0.05
P<0.01
The remission status of cases did not influence the association between case-control status and use of services. The matched odds ratios were 5.8 (95% CI=2.25, 15.0, p=0.0003) if the case was in five-year remission and 4.8 (95% CI=1.62, 14.0, p=0.005) if not.
Mean cognitive scores were very similar in cases and controls who reported never having received special education services (all p-values >0.20, Table 5). They were also similar in cases and controls who reported receipt of such services (all p-values >0.30). Within cases, receipt of services was associated with significantly lower cognitive scores (p<0.001) on all Wechsler indices except for perceptual organization (p=0.07). Within the control group, receipt of services was similarly associated with lower cognitive scores (all p<0.03).
Table 5.
All cognitive scores in cases and controls as a function of receipt of special services
| Cases, − services N=78 a,c | Controls, − services N=117 a,d | Cases, + services N=64 b,c | Controls, + services, N=25 b,d | |
|---|---|---|---|---|
| FSIQ | 109.3 (12.5) | 109.0 (12.7) | 98.4 (13.3) | 100.0 (8.7) |
| Verbal comprehension | 112.8 (15.0) | 111.7 (15.4) | 102.6 (13.7) | 104.5 (11.4) |
| Perceptual organization | 108.4 (14.6) | 107.9 (14.4) | 103.6 (14.1) | 101.1 (13.0) |
| Processing speed | 103.8 (13.1) | 106.2 (13.7) | 93.7 (15.9) | 97.3 (10.7) |
| Freedom from distractability | 107.6 (12.9) | 105.4 (13.1) | 94.8 (13.9) | 97.3 (10.5) |
| WRAT-READING | 107.8 (10.0) | 106.8 (9.7) | 97.7 (13.9) | 100.6 (8.6) |
| WRAT-spelling | 107.8 (9.5) | 107.2 (10.0) | 96.8 (14.5) | 100.1 (11.0) |
| WRAT-arithmetic | 10.7 (19.7) | 102.3 (13.2) | 92.6 (14.2) | 94.0 (11.9) |
| CVLT-list | 55.4 (10.0) | 54.4 (11.5) | 49.2 (11.2) | 49.6 (9.3) |
| CVLT-short | 0.37 (0.86) | 0.21 (1.01) | −0.24 (1.05) | 0.04 (0.84) |
| CVLT-long | 0.36 (0.91) | 0.31 (0.92) | −0.16 (1.01) | 0.08 (0.86) |
| CPT-omissions | 47.6 (11.9) | 48.3 (9.9) | 52.4 (18.6) | 47.5 (5.9) |
| CPT-comissions | 48.8 (12.2) | 49.5 (10.8) | 52.7 (11.1) | 49.2 (15.3) |
| CPT-reaction time | 45.5 (10.4) | 46.3 (10.8) | 48.5 (10.5) | 47.7 (11.4) |
| DigitSym/coding | 11.6 (2.5) | 11.6 (2.7) | 9.8 (2.6) | 10.0 (2.1) |
Comparisons of cases and controls both without services. No difference was statistically significant, smallest p-value>0.2
Comparisons cases and controls both with services. No difference was statistically significant, smallest p-value=0.07 for CPT omissions. The next smallest was >0.20
Comparisons of cases with and without services. Most P-values <0.001. Perceptual organization, and all three CPT scores were of borderline significance only (0.05<p<0.10)
Comparisons of controls with and without services. FSIQ (p<0.0001), processing speed FREEDOM, digit-symbol, WRAT-A WRAT-B, WRATC (P<0.01) vcomp&porg (p<0.05), CVLTlist (p=0.05), Other CVLT and CPT scores not statistically significant (p>0.05)
FISQ=full scale intelligence quotient
WRAT=Wide Range Achievement Test
CVLT=California Verbal Learning Test
CPT=Continuous Performance Test
DigitSym/Coding = score from digit symbol or coding subtest of the Wechsler intelligence test
Discussion
In this group of young people with epilepsy and normal overall intellectual function, we found a high cumulative level of special education service utilization in comparison to matched sibling controls. Basic measures of cognitive function did not explain this rather large difference in receipt of services between the groups.
In a previous analysis from this cohort, we reported a high proportion of children who ever received services prior to and up through 5 years after diagnosis [5]. At the time, we had no controls, only overall statistics for the entire state of Connecticut. In addition, we did not have detailed information about levels of cognitive function, just what could be surmised from the neurological record. In the present report, we now have sibling controls and a standardized cognitive assessment performed in both cases and sibling-controls. The sibling control group allows for relatively tight control for family environment (which others have noticed to play a role in academic performance [2]), school system, and even specific school and staff. The standardized cognitive assessment allows a reasonably high degree of certainty regarding the children’s level of cognitive function.
Other studies have demonstrated that a large proportion of children receive services prior to their diagnosis of epilepsy [1, 2]. Those studies focused on children who were already school-aged when they first were diagnosed with epilepsy. They also limited analyses to children with a measured FSIQ of ≥70 [3] or specified that participants had to be developing normally and have no underlying brain condition as an explanation for their epilepsy [1, 2]. We chose a FSIQ of ≥80 as cutoff because the 70–79 range is generally considered to indicate “borderline” intellectual disability. Our study also included children with epilepsy of onset down to one month of age. In fact, our figures for school-aged children were only slightly lower than those reported by others [1] despite having limited our sample to children with measured IQ of at least 80. When we included those with lower IQ but who did not meet criteria for intellectual disability [7], and considered only children who were of school age at the time of initial diagnosis, then the proportion of children in our study who were already receiving services at the time they were diagnosed with epilepsy rose slightly to 29%.
Oostrom [2], Austin [12], Hermann [1], all did systematic testing in their cohorts and found children with epilepsy more likely to have cognitive difficulties from the outset. As with our results, however, it was unclear whether their neurocognitive test results explained the schools’ perceptions of the children’s academic needs and the administrative decision to provide special services.
Hermann et al. [13] reported that, in case-children who did not receive special services, cognitive test results were indistinguishable from those of controls without academic problems. Our findings are quite similar in that respect. Hermann et al. did not include controls with academic problems, so further comparisons with our findings in that particular subgroup cannot be made; however, in our study, we found no difference in cognitive test scores for cases and controls who did receive special services. This result is difficult to reconcile with the findings that cases were more likely controls to receive services even after adjustment for test scores. It suggests that other unmeasured factors may be playing a role such as neurocognitive domains that were not reflected in our particular cognitive battery. For example, the language disorders typically seen in BECTS [14] would likely not be detected on the types of tests we used. Further, other investigators have evaluated cognitive processing index domains much more extensively than we have. Several studies have suggested that executive functions and attention skills are particularly vulnerable in children as well as adults with epilepsy [1, 3, 4]. Our battery did not sample these domains as thoroughly as did other studies, and one could argue that we simply did not measure what was important. It is unlikely, however, that this is entirely the case as we did, in fact, sample these domains to some extent. Further, although cognitive domains are often discussed as though they are as distinct from each other, they are still inter-correlated, and we would expect that our measures would still be somewhat sensitive to variation in those areas although perhaps not adequately to detect differences that are significant but subtle.
Alternatively, it is possible that services are more likely to be provided to a child who carries the diagnosis of epilepsy. This might be because such children are seen as more vulnerable by parents or educators, and that fact lowers the threshold for providing services. While this may have occurred to a certain degree, it would not explain why in our study and in every other study that has examined the issue, a larger proportion of children with epilepsy than controls were already receiving services at the time of their diagnosis of epilepsy.
It is helpful to consider the general relationship between formal cognitive test results and participation in special education services. There has been longstanding controversy about the utility of psychometric approaches to determining academic need, with criticism extending back nearly three decades [15]. In fact, studies questioning the validity of discrepancy-based definitions of learning disabilities [16] [17] ultimately contributed to a reformulation of federal criteria for special education participation in IDEA 2004 [18]. In light of this literature, it is reasonable to posit that factors unrelated to the ability to complete psychometric tasks are important in determining special education participation. For example, Fastenau et al [19] found that other factors, specifically family mastery, moderate the relationship between neurocognitive functioning and academic achievement in childhood epilepsy. Recent research in non-epilepsy populations also suggests that personality factors such as self discipline[20] and motivation [21] may be operative as well. Whether such factors actually differ between young people with and without epilepsy is not known.
In interpreting the higher likelihood of being in special education for cases relative to controls after adjustment for cognitive scores, we must consider whether any cognitive impairment associated with epilepsy might have been greater at onset but lessened (at least to some degree) later once the epilepsy had largely resolved. This explanation would be hard to reconcile with the findings from studies that have followed patients over time and documented the decreases or stability in cognitive test performance. For example, one study showed that, over the course of two years, children with epilepsy had cognitive trajectories that were essentially the same as controls [13]. Oostrom et al [22] also found no evidence that children with epilepsy had trajectories that differed from control children over approximately 3.5 years. In newly diagnosed adults, statistically significant declines were observed over the course of a year for certain domains of cognitive function (memory, psychomotor speed, and higher executive function) compared to controls [4]. Finally, Hermann et al (2006) documented substantial declines in similar domains over the course of four years in adults with chronic temporal lobe epilepsy compared to controls [23], although this last study focused on a more severe and selected group than the group we studied. No studies, however, suggest that cognitive scores substantially improve over time. Thus, there is reason to believe that scores could decline but not to suspect that they would increase, which makes it difficult to argue that the neurocognitive function measured some years later, after the onset of epilepsy, would be substantially higher than it was at onset. Specifically, in our group who received services, we do not believe that their scores were actually lower at the beginning of their epilepsy and have risen since. At the same time, we are unaware of any studies that have actually measured cognitive function in patients at onset and then again 8–9 years later once epilepsy has resolved in many or been fully controlled for years.
Sogawa et al (2010) considered neurocognitive testing in a cohort of children followed years after a first unprovoked seizure [24]. Although by design, they included children who, at the time of presentation, had only had a single seizure and consequently excluded specific forms of epilepsy that virtually never present as a single seizure (e.g. absence epilepsies), their findings and ours are quite similar. Overall, they found few, if any, discrepancies in cognitive scores between cases and controls, yet their cases were much more likely to have ever received special services. While it remains to be seen what the long-term impact of school difficulties - as we have measured them -might be, one hoped for outcome is that early problems may have been appropriately identified and managed and therefore may not result in substantial disadvantage later in life. In fact, that is the goal of early detection and intervention. The persistent low neurocognitive test scores years later in those who received special services raise concerns that the cognitive co-morbidities of childhood epilepsy may be enduring, although this appears to be true in both cases and controls.
Whether children with school difficulties always receive services needed to address such difficulties is, in part, a function of jurisdiction. Results from our studies and those of others certainly highlight the potential need for careful evaluation in young people with epilepsy. Because there does not appear to be any single cognitive domain that is selectively affected, testing should encompass a broad range of cognitive abilities and likely also behavioral and emotional problems as well.
Highlights.
Children of normal intellect with epilepsy receive special education services more often than siblings.
Differences in receipt of services remain after adjustment for cognitive scores.
Services are often initiated before epilepsy is diagnosed.
Other factors are likely responsible and require more in-depth characterization.
Acknowledgments
Funding: This study was funded by a grant from the National Institutes of Health, NINDS-NS-R37-31146
We would like to thank our colleagues Drs. Susan Levy, Francine Testa, Francis DiMario, and Shlomo Shinnar for their participation in various phases of this study and to Christina Rios, Charles Hurst, and Lyla Johnson for their continued efforts in following this cohort. This work would not have been possible without the generous participation of the many families who have contributed to this study over the years or without the kind assistance of the physicians in Connecticut who referred their patients to our study.
Footnotes
Disclosures: Anne T. Berg: Travel funding and honoraria from Eisai, the British Pediatric Neurological Association, and the Epilepsy Research Center (Melbourne); travel funding from UCB the American Epilepsy Society and the International League Against Epilepsy; awards from the British Pediatric Neurological Association; and consulting fees from Dow Agro Science.
Dale Hesdorffer: Advisory board for Pfizer; travel funding from the International League Against Epilepsy
Frank Zelko: No disclosures
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain. 2006;129:2609–2619. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- 2.Oostrom KJ, Smeets-Schouten A, Kruitwagen CLJJ, Peters ACB, Jennekens-Schinkel A. Not only a matter of epilepsy: early problems of cognition and behavior in children with ‘epilepsy only’ - a prospective controlled study starting at diagnosis. Pediatrics. 2003;112:1338–1344. doi: 10.1542/peds.112.6.1338. [DOI] [PubMed] [Google Scholar]
- 3.Fastenau PS, Johnson CS, Perkins SM, Byars AW, deGrauw T, Austin JK, Dunn DW. Neuropsychological status at seizure onset in children: Risk factors for early cognitive deficits. Neurology. 2009;73:526–534. doi: 10.1212/WNL.0b013e3181b23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor J, Kolamunnage-Dona R, Marson AG, Smith PEM, Aldenkamp AP, Baker GA. Patients with epilepsy: Cognitively compromised before start of antiepileptic drug treatment? Epilepsia. 2010;51:48–56. doi: 10.1111/j.1528-1167.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- 5.Berg AT, Smith SN, Frobish D, Levy SR, Testa FM, Beckerman B, Shinnar S. Special education needs in children with newly diagnosed epilepsy. Dev Med Child Neurol. 2005;47:749–753. doi: 10.1017/S001216220500157X. [DOI] [PubMed] [Google Scholar]
- 6.Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 1999;40:445–452. doi: 10.1111/j.1528-1157.1999.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 7.Berg AT, Langfitt JT, Testa FM, Levy SR, DiMario F, Westerveld M, Kulas J. Residual cognitive effects of uncomplicated idiopathic and cryptogenic epilepsy. Epilepsy & Behavior. 2008;13:614–619. doi: 10.1016/j.yebeh.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Berg AT, Mathern GW, Bronen RA, Fulbright RK, DiMario F, Testa FM, Levy SR. Frequency, prognosis and surgical treatment of structural abnormalities seen with magnetic resonance imaging in childhood epilepsy. Brain. 2009:2785–2797. doi: 10.1093/brain/awp187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dulac O. Epileptic encephalopathy. Epilepsia. 2001;42 (Suppl 3):23–26. doi: 10.1046/j.1528-1157.2001.042suppl.3023.x. [DOI] [PubMed] [Google Scholar]
- 10.Breslow NE, Day NE. Statistical Methods In Cancer Research. Vol. 1. Lyon, France: International Agency for Research Cancer; 1980. Conditional logistic regression for matched sets; pp. 248–279. [Google Scholar]
- 11.Berg AT, Langfitt JT, Testa FM, Levy SR, DiMario F, Westerveld M, Kulas J. Global Cognitive Function in Children with Epilepsy: A community-based study. Epilepsia. 2008;49:608–614. doi: 10.1111/j.1528-1167.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- 12.Austin JK, Fastenau PS. Are seizure variables related to cognitive and behavior problems? Developmental Medicine & Child Neurology. 2010;52:4–9. doi: 10.1111/j.1469-8749.2009.03412.x. [DOI] [PubMed] [Google Scholar]
- 13.Hermann BP, Jone JE, Sheth R, Koehn M, Becker T, Fine J, Allen CA, Seidenberg M. Growing up with epilepsy: A two-year investigation of cognitive development in children with new onset epilepsy. Epilepsia. 2008;49:1847–1858. doi: 10.1111/j.1528-1167.2008.01735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lillywhite LM, Saling MM, Harvey AS, Abbott DF, Archer JS, Vears DF, Scheffer IE, Jackson GD. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia. 2009;50:2276–2284. doi: 10.1111/j.1528-1167.2009.02065.x. [DOI] [PubMed] [Google Scholar]
- 15.Ysseldyke JE. Current practices in making psycho-educational decisions about learning disabled students. J Learn Disabil. 1983;16:227–233. doi: 10.1177/002221948301600411. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher JM, Francis DJ, Rourke BP, Shaywitz SE, Shaywitz BA. The validity of discrepancy-based definitions of reading disabilities. J Learn Disabil. 1992;25:555–561. doi: 10.1177/002221949202500903. [DOI] [PubMed] [Google Scholar]
- 17.Vellutino FR, Scanlon DM, Lyon GR. Differentiating Between Difficult-to-Remediate and Readily Remediated Poor Readers: More Evidence Against the IQ-Achievement Discrepancy Definition of Reading Disability. J Learn Disabil. 2000;33:223. doi: 10.1177/002221940003300302. [DOI] [PubMed] [Google Scholar]
- 18.Pub. L. No. 108–445. 2. 2004. Individuals with Disability Education Improvement Act of 2004. [Google Scholar]
- 19.Fastenau PS, Shen J, Dunn DW, Perkins SM, Hermann BP, Austin JK. Neuropsychological Predictors of Academic Underachievement in Pediatric Epilepsy: Moderating Roles of Demographic, Seizure, and Psychosocial Variables. Epilepsia. 2004;45:1261–1272. doi: 10.1111/j.0013-9580.2004.15204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duckworth AL, Seligman ME. Self-discipline outdoes IQ in predicting academic performance of adolescents. Psychol Sci. 2005;16:939–944. doi: 10.1111/j.1467-9280.2005.01641.x. [DOI] [PubMed] [Google Scholar]
- 21.Duckworth AL, Quinn PD, Lynam DR, Loeber R, Stouthamer-Loeber M. Role of test motivation in intelligence testing. PNAS. 2011;19:7716–7720. doi: 10.1073/pnas.1018601108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oostom KJ, van Teeseling H, Smeets-Schouten A, Peters ACB, Jennekens-Schinkel A. Three to four years after diagnosis: cognition and behavior in children with ‘epilepsy only’. A prospective, controlled study. Brain. 2005;128:1546–1555. doi: 10.1093/brain/awh494. [DOI] [PubMed] [Google Scholar]
- 23.Hermann BP, Seidenberg M, Dow C, Jones J, Rutecki P, Bhattacharya A, Bell B. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006;60:80–87. doi: 10.1002/ana.20872. [DOI] [PubMed] [Google Scholar]
- 24.Sogawa Y, Masur D, O’Dell C, Moshe SL, Shinnar S. Cognitive outcome in children who present with a first unprovoked seizure. Epilepsia. 2010;51:2432–2439. doi: 10.1111/j.1528-1167.2010.02724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


