Abstract
Epithelial ovarian cancer (EOC) remains the most lethal gynecological malignancy in the US. Thus, there is an urgent need to develop novel therapeutics for this disease. Cellular senescence is an important tumor suppression mechanism that has recently been suggested as a novel mechanism to target for developing cancer therapeutics. Wnt5a is a non-canonical Wnt ligand that plays a context-dependent role in human cancers. Here, we investigate the role of Wnt5a in regulating senescence of EOC cells. We demonstrate that Wnt5a is expressed at significantly lower levels in human EOC cell lines and in primary human EOCs (n = 130) compared with either normal ovarian surface epithelium (n = 31; p = 0.039) or fallopian tube epithelium (n = 28; p < 0.001). Notably, a lower level of Wnt5a expression correlates with tumor stage (p = 0.003) and predicts shorter overall survival in EOC patients (p = 0.003). Significantly, restoration of Wnt5a expression inhibits the proliferation of human EOC cells both in vitro and in vivo in an orthotopic EOC mouse model. Mechanistically, Wnt5a antagonizes canonical Wnt/β-catenin signaling and induces cellular senescence by activating the histone repressor A (HIRA)/promyelocytic leukemia (PML) senescence pathway. In summary, we show that loss of Wnt5a predicts poor outcome in EOC patients and Wnt5a suppresses the growth of EOC cells by triggering cellular senescence. We suggest that strategies to drive senescence in EOC cells by reconstituting Wnt5a signaling may offer an effective new strategy for EOC therapy.
Keywords: Ovarian Cancer, Cellular senescence, Wnt5a, HIRA, PML
Introduction
Cellular senescence is an important tumor suppression mechanism in vivo (1). In primary mammalian cells, cellular senescence can be triggered by various inducers including critically shortened telomeres and activated oncogenes (such as oncogenic-RAS) (1). Senescent cells are viable but non-dividing (2). Senescent cells also exhibit several distinctive morphological characteristics and molecular markers, including a large flat cellular morphology and expression of senescence-associated β-galactosidase (SA-β-gal) activity (3). In murine liver carcinoma and sarcoma models, reactivation of the tumor suppressor p53 induces senescence and is associated with tumor regression (4, 5). Hence, driving cancer cells to undergo cellular senescence represents a novel mechanism for developing cancer therapeutics (6, 7).
Over 85% of ovarian cancers are of epithelial origin (8). Epithelial ovarian cancers (EOC) are classified into distinct histological types including serous, mucinous, endometrioid, and clear cell (9). The most common histology of EOC is serous (~60% of all cancers) and less common histologies include endometrioid, clear cell, and mucinous (9). Recently, an alternative classification has been proposed, in which EOC is broadly divided into two types (10). Type I EOC includes endometrioid, mucinous, low-grade serous, and clear cell carcinomas, and type II EOC includes high-grade serous carcinomas (10). EOC remains the most lethal gynecological malignancy in the United States (8). Thus, there is an urgent need to better understand the etiology of EOC in order to develop novel therapeutics for this devastating disease.
Wnt signaling is initiated by binding of the Wnt ligand to its cognate Frizzled receptor (11). Canonical Wnt signaling results in stabilization of the key transcription factor β-catenin, which then translocates into the nucleus and drives expression of its target genes such as CCND1 (cyclin D1), FOSL1 and c-MYC (12, 13). Canonical Wnt signaling is active in the putative somatic stem/progenitor cells of the coelomic epithelium of the mouse ovary (14). Underscoring the importance of Wnt signaling in EOC, in a murine ovarian cancer model, activation of canonical Wnt signaling cooperates with inactivation of the tumor suppressor PTEN in driving ovarian carcinogenesis (15). However, the role of Wnt signaling in EOC is not fully understood.
Wnt5a is a non-canonical Wnt ligand that plays opposing roles in different types of cancer and has variable expression dependent on the cancer context (16). Specifically, in EOC the role of Wnt5a remains unclear. Thus, in this study, we investigated Wnt5a expression and its potential function in human EOC cells. We discovered that Wnt5a was expressed at significantly lower levels in primary human EOC compared with either primary human ovarian surface epithelium or fallopian tube epithelium. Notably, loss of Wnt5a expression was associated with tumor stage and predicted shorter overall survival in EOC patients. Significantly, Wnt5a reconstitution inhibited the growth of EOC cells both in vitro and in vivo in an orthotopic EOC mouse model by promoting cellular senescence. These studies demonstrate, for the first time, a functional role of the non-canonical Wnt ligand, Wnt5a, in promoting senescence. Importantly, they also suggest that promoting EOC cells to undergo senescence represents a potential novel strategy for developing urgently needed EOC therapeutics.
Materials and Methods
Cells and culture conditions
Primary HOSE cells were isolated and cultured as previously described (17). Human EOC cell lines were obtained from ATCC and were passaged for fewer than six months. EOC cell line identification was further confirmed by DNA Diagnostic Center (www.dnacenter.com). EOC cell lines were cultured according to ATCC in RPMI1640 medium supplemented with 10% FBS. 5-Aza-cytadine (Sigma) was used at working concentration of 5 μM (18).
Human ovarian specimens and immunohistochemistry
The protocol to evaluate de-identified human tissue specimens was approved by Fox Chase Cancer Center (FCCC) institutional review board (IRB). Ovarian tumor microarray and normal human ovary and fallopian tube specimens were obtained from the FCCC Biosample Repository Core Facility (BRCF). Histopathology of the selected specimens on the tumor microarrays was provided by BRCF. Immunohistochemistry was performed using goat anti-Wnt5a polyclonal antibody (R&D Systems) and mouse anti-Ki67 (Dako) with a Dako Envision Tm+ System and the Peroxidase (DAB) kit (DAKO Corporation) following the manufacturer’s instructions and as previously described (17). Wnt5a staining was scored in a double-blinded manner by Dr. Qi Cai at the BRCF and a proportion of the cases were independently confirmed by Dr. Hong Wu, a board-certified pathologist, at the FCCC Department of Pathology.
Anchorage-independent soft agar colony formation assay
Soft agar assay were performed as previously described (17). Briefly, 3,500 cells were resuspended in 0.35% low melt agarose dissolved in RPMI1640 medium supplemented with 10% FBS, and inoculated on top of 0.6% low melt agarose base in 6-well plates. After 2 weeks in culture, the plates were stained with 0.005% crystal violet and the number of colonies was counted using a dissecting microscope.
Retrovirus production, infection and drug-selection
The following retrovirus constructs were utilized: pBabe-puro was obtained from Addgene, hygro-pWZL-Luciferase was a kind gift of Dr. Denise Connolly, and pBABE-Wnt5a was generated using standard cloning protocol. Retrovirus packaging was performed as previously described using Phoenix packaging cells (19, 20). To increase infection efficacy, double virus infection was performed. For drug-selection, 3 μg/ml of puromycin was used for the OVCAR5 human EOC cell line.
RT-PCR, qRT-PCR and immunoblotting
RNA from cultured primary HOSE cells or human EOC cell lines was isolated using Trizol (Invitrogen) according to manufacturer’s instruction. For qRT-PCR, Trizol-isolated RNA was further purified using an RNeasy kit (Qiagen) following manufacture’s instruction. The Wnt5a, CCND1, FOSL1 and c-MYC primers used for qRT-PCR were purchased from SA Biosciences. mRNA expression of the housekeeping gene β-2-microglobulin was used to normalize mRNA expression. Soluble β-catenin was extracted using a buffer that consisting of 10mM pH7.5 Tris-HCl, 0.05% NP-40, 10mM NaCl, 3mM MgCl2, 1mM EDTA and proteinase inhibitors (Roche) as previously described (21, 22). The following antibodies were used for immunoblotting from the indicated suppliers, goat anti-Wnt5a (R&D Systems), mouse β-actin (Sigma), mouse anti-GAPDH (Millipore), mouse anti-β-catenin, mouse anti-Rb (BD Biosciences), and rabbit anti-pRBpS780 (Cell Signaling).
Immunofluorescence and SA-β-gal staining
Indirect immunofluorescence staining was performed as previously described (19, 20, 22). The following antibodies were used for immunofluorescence: a cocktail of mouse anti-HIRA monoclonal antibodies (WC19, WC117 and WC119, 1:10) (20) and a rabbit anti-PML antibody (Chemicon, 1:5000). Images were captured using a DS-Qilmc camera on a Nikon Eclipse 80i microscope, and processed using NIS-Elements BR3.0 software (Nikon). SA-β-gal staining was performed as described previously (3, 23). For SA-β-gal staining in sections from xenografted tumors, eight separate fields were examined from two individual tumors for each of the groups.
In vivo orthotopic xenograft tumorigenesis study
The protocol was approved by the FCCC Institutional Animal Care and Use Committee (IACUC). OVCAR5 cells were infected with a luciferase encoding retrovirus (hygro-pWZL-Luciferase) and infected cells were selected with 50 μg/ml hygromycin. Drug-selected cells were then infected with control or Wnt5a encoding retrovirus and subsequently selected with 3 μg/mL puromycin and 50 μg/mL hygromycin. 3 × 106 drug-selected cells were unilaterally injected into the ovarian bursa sac of immuno-compromised mice (6 mice per group) (24). From day 10 post infection, tumors were visualized by injecting luciferin (i.p., 4 mg/mice) resuspended in PBS and imaged with an IVIS Spectrum imaging system every 5 days until day 30. Images were analyzed using Live Imaging 4.0 software. At day 30, tumors were surgically dissected and either fixed in 10% formalin or fresh-frozen in Optimal Cutting Temperature compound (Tissue-Tek). Sections of the dissected tumors were processed by the FCCC Histopathology Core Facility.
Statistical analysis
Quantitative data are expressed as mean ± SD, unless otherwise indicated. Analysis of variance (ANOVA) with Student t test was used to identify significant differences in multiple comparisons. The Pearson chi-squared test was used to analyze the relationship between categorical variables. Overall survival was defined as the time elapsed from the date of diagnosis and the date of death from any cause or the date of last follow-up. Kaplan-Meier survival plots were generated and comparisons were made using the log-rank sum statistic. For all statistical analyses, the level of significance was set at 0.05.
Results
Wnt5a is expressed at significantly lower levels in human EOC cell lines and primary human EOCs compared with normal human ovarian surface epithelium or fallopian tube epithelium
To determine Wnt5a expression in human EOC cell lines and primary human ovarian surface epithelial (HOSE) cells, we examined the relative Wnt5a mRNA levels by performing semi-quantitative RT-PCR. We observed that Wnt5a mRNA levels were greatly diminished in human EOC cell lines compared with primary HOSE cells (Figure 1A). This finding was further confirmed through quantitative RT-PCR (qRT-PCR) analysis of Wnt5a mRNA in multiple isolations of primary HOSE cells and human EOC cell lines, showing that the levels of Wnt5a mRNA were significantly lower in human EOC cell lines compared with primary HOSE cells (Figure 1B; p = 0.008). Consistently, we observed that Wnt5a protein levels were also lower in human EOC cell lines compared with primary HOSE cells as determined by immunoblotting (Figure 1C). Based on these results, we conclude that Wnt5a is expressed at lower levels in human EOC cell lines compared with primary HOSE cells.
Figure 1. Wnt5a is expressed at significantly lower levels in human EOC cells compared with normal human ovarian surface or fallopian tube epithelial cells, and a lower level of Wnt5a expression predicts shorter overall survival in human EOC patients.
(A) Expression of Wnt5a mRNA in primary human ovarian surface epithelial (HOSE) cells and the indicated human EOC cell lines was determined by semi-quantitative RT-PCR. Expression of GAPDH mRNA was used a loading control. (B) Wnt5a mRNA levels were quantified by quantitative RT-PCR in six individual isolations of primary HOSE cells and seven different EOC cell lines. Expression of β-2-microglobulin was used to normalize Wnt5a mRNA expression. * p = 0.008 compared with human EOC cells. (C) Same as (A), but examined for Wnt5a and GAPDH protein expression by immunoblotting. (D) Examples of Wnt5a IHC staining in normal human ovarian surface epithelium, fallopian tube epithelium, and EOC of indicated histological subtypes. Bar = 50 μm. Arrows point to examples of positively stained human ovarian surface epithelial cells and fallopian tube epithelial cells. (E) Representative images from tissue microarray depicting low Wnt5a expression correlated with high Ki67, a cell proliferation marker. Bar = 50 μm. (F) Loss of Wnt5a expression is an independent poor prognosis marker in human EOC patients. A lower level of Wnt5a expression correlates with shorter overall survival in human EOC patients. The univariate overall survival curve (Kaplan-Meier method) for EOC patients (n = 123) with high or low Wnt5a expression as determined by immunohistochemical analysis.
We next determined if the loss of Wnt5a expression found in human EOC cell lines was also observed in primary human EOCs. We examined Wnt5a expression in 130 cases of primary human EOC specimens and 31 cases of normal human ovary with surface epithelium by immunohistochemistry (IHC) using an antibody against Wnt5a (Table 1). Additionally, there is recent evidence to suggest that a proportion of high-grade serous EOC may arise from distant fallopian tube epithelium (25). Thus, we also included 28 cases of normal human fallopian tube specimens in our IHC analysis (Table 1). The specificity of the anti-Wnt5a antibody was confirmed in our study (Figure S1). A single band at predicted molecular weight (~ 42 kDa) was detected in OVCAR5 cells with ectopically expressed Wnt5a and was absent after expression of a shRNA to the human Wnt5a gene (shWnt5a), which effectively knocked down Wnt5a mRNA expression (Figure S1A and data not shown). In addition, Wnt5a staining was lost when primary anti-Wnt5a antibody was replaced with an isotype matched IgG control (Figure S1B).
Table 1.
Wnt5a expression in primary human EOCs and correlation of its expression with clinicopathological variables.
| Patient characteristics | Wnt5a Protein Expression
|
p | |||
|---|---|---|---|---|---|
| Low (n) | High (n) | Total (n) | High (%) | ||
| Age (23–85yrs, mean 59.2yrs) | |||||
| ≤55 | 24 | 16 | 40 | 40.0% | |
| >55 | 52 | 33 | 85 | 38.8% | 0.900 |
| Unknown | 5 | 0 | 5 | ||
| Laterality | |||||
| Left | 22 | 14 | 36 | 38.9% | |
| Right | 12 | 9 | 21 | 42.9% | 0.957 |
| Bilaterality | 35 | 24 | 59 | 40.7% | |
| Undetermined | 12 | 2 | 14 | ||
| Histotype | |||||
| Epithelial ovarian cancer | 81 | 49 | 130 | 37.7% | |
| Type I | 16 | 21 | 37 | 56.8% | |
| Low Grade Serous | 1 | 1 | 2 | 50.0% | |
| Endometrioid | 4 | 9 | 13 | 69.2% | |
| Mucinous | 2 | 3 | 5 | 60.0% | |
| Clear Cell | 5 | 4 | 9 | 44.4% | |
| Others | 4 | 4 | 8 | 50.0% | |
| Type II | |||||
| High Grade Serous | 65 | 28 | 93 | 30.1% | 0.005 # |
| Normal epithelium | |||||
| Ovarian Surface | 13 | 18 | 31 | 58.1% | 0.039 * |
| Fallopian Tube | 5 | 23 | 28 | 82.1% | <0.001 * |
| Ki67 | |||||
| Low | 22 | 23 | 44 | 52.3% | |
| High | 51 | 24 | 75 | 32.0% | 0.038 |
| Undetermined | 7 | 3 | 11 | ||
| Tumor grade | |||||
| 1 | 3 | 7 | 10 | 70.0% | |
| 2 | 12 | 8 | 20 | 40.0% | |
| 3 | 64 | 31 | 95 | 32.6% | |
| Undetermined | 2 | 3 | 5 | ||
| Tumor stage | |||||
| Stage 1/2 | 12 | 18 | 30 | 60.0% | |
| Stage 3/4 | 67 | 29 | 96 | 30.2% | 0.003 ** |
| Undetermined | 2 | 2 | 4 | ||
Compared with Type I epithelial ovarian cancer
Compared with epithelial ovarian cancer;
compared with Stage 1/2
As shown in Figure 1D, in normal human ovarian surface epithelial cells and fallopian tube epithelial cells, both cytoplasm and cell membrane were positive for Wnt5a IHC staining (black arrows, Figure 1D). In contrast, Wnt5a staining in EOC cells was dramatically decreased (Figure 1D). We scored expression of Wnt5a as high (H-score ≥ 30) or low (H-score < 30) based on a histological score (H-score) (26), which considers both intensity of staining and percentage of positively stained cells, as previously described (17). Wnt5a expression was scored as high in 58.1% (18/31) cases of normal human ovarian surface epithelium and 82.1% (23/28) cases of normal human fallopian tube epithelium (Table 1). In contrast, Wnt5a expression was scored as high in 37.7% (49/130) cases of primary human EOCs (Table 1). Statistical analysis revealed that Wnt5a was expressed at significantly lower levels in primary human EOCs compared with either normal human ovarian surface epithelium (p = 0.039) or normal human fallopian tube epithelium (p < 0.001) (Table 1). On the basis of these studies, we conclude that Wnt5a is expressed at significantly lower levels in primary human EOCs compared with either normal human ovarian surface epithelium or fallopian tube epithelium.
Wnt5a expression negatively correlates with tumor stage and lower Wnt5a expression predicts shorter overall survival
We next examined the correlation between Wnt5a expression and clinical and pathological features of human EOCs. Significantly, there was a negative correlation between Wnt5a expression and tumor stage (p = 0.003) (Table 1). Notably, the majority of examined cases are high-grade serous subtypes that are usually of stage 3/4. In addition, we examined the correlation between expression of Wnt5a and a marker of cell proliferation, Ki67 (27) (Figure 1E). There was a significant negative correlation between Wnt5a expression and Ki67 (p = 0.038) (Table 1). We next assessed whether Wnt5a expression based on H-score might predict prognosis of EOC patients (High, H-score ≥ 30; Low, H-score < 30) (n = 123), for which long-term follow-up data were available. Significantly, lower Wnt5a expression correlated with shorter overall survival in the examined EOC patients (p = 0.003) (Figure 1F). Together, we conclude that a lower level of Wnt5a expression correlates with tumor stage and predicts shorter overall survival in human EOC patients.
Wnt5a gene promoter hypermethylation contributes to its downregulation in human EOC cells
Wnt5a gene promoter hypermethylation has been implicated as a mechanism underlying its silencing in several types of human cancers (16). Consistently, we also observed Wnt5a gene promoter hypermethylation in a number of human EOC cell lines (Figure 2A and Table S1). Further supporting a role of promoter hypermethylation in suppression of Wnt5a expression, treatment with a DNA demethylation drug, 5-Aza-cytadine (28), in PEO1 EOC cells resulted in a significant increase in levels of both Wnt5a mRNA and protein (Figure 2B–C). We conclude that Wnt5a gene promoter hypermethylation contributes to its downregulation in human EOC cells.
Figure 2. Promoter DNA CpG island hypermethylation contributes to Wnt5a downregulation in human EOC cells.
(A) Schematic structure of the human Wnt5a gene transcript and its promoter CpG islands. Locations of exon 1 (open rectangle), CpG sites (vertical lines) and coding exons (filled rectangle) and the transcription start site (curved arrow) are indicated. Flat arrows indicated the positions of primers used for PCR amplification, and the size of PCR product is also indicated. (B) PEO1 cells were treated with 5 μM AzaC for 4 days, and mRNA was isolated from control and AzaC treated cells and examined for Wnt5a mRNA expression by qRT-PCR. Mean of three independent experiments with SD. (C) Same as (C) but examined for Wnt5a protein expression by immunoblotting.
Wnt5a restoration inhibits the growth of human EOC cells by antagonizing the canonical Wnt/β-catenin signaling
We next sought to determine the effects of Wnt5a reconstitution in human EOC cells. Wnt5a expression was reconstituted in the OVCAR5 EOC cell line via retroviral transduction. Ectopically expressed Wnt5a was confirmed by both qRT-PCR and immunoblotting in OVCAR5 cells stably expressing Wnt5a or a vector control (Figure 3A–B). Of note, the levels of ectopically expressed Wnt5a in OVCAR5 cells are comparable to the levels observed in primary HOSE cells (Figure 3B). Interestingly, Wnt5a reconstitution in OVCAR5 human EOC cells significantly inhibited both anchorage-dependent and anchorage-independent growth in soft-agar compared with vector controls (Figure 3C–D). In addition, similar growth inhibition by Wnt5a reconstitution was also observed in the PEO1 human EOC cell line (Figure S2A–C), suggesting that this effect is not cell line specific. Based on these results, we conclude that Wnt5a reconstitution inhibits the growth of human EOC cells in vitro.
Figure 3. Wnt5a restoration inhibits the growth of human EOC cells by antagonizing canonical Wnt/β-catenin signaling.
(A) OVCAR5 cells were transduced with a control or Wnt5a encoding puromycin resistant retrovirus. The infected cells were drug-selected with 3 μg/ml puromycin. Expression of Wnt5a mRNA in drug-selected cells was determined by qRT-PCR. (B) Same as (A), but examined for expression of Wnt5a and β-actin in control or Wnt5a-infected OVCAR5 and primary HOSE cells by immunoblotting. Relative levels of Wnt5a expression was indicated based on densometric analysis using NIH Image J software. (C) Same as (A), but equal number (5000) of drug-selected control (open triangles and solid line) or Wnt5a-infected cells (open circles and dotted line) were cultured on plastic plates for 4 days, and the number of cells was counted (Control ± SD or Wnt5a ± SD (n=3); student t-test was used for calculating p value) at day 1 (6,666±1,258 vs. 5,000±1,000; p = 0.1469), day 2 (14,583±954 vs. 9,583±3,463; p = 0.084), day 3 (41,250±6,538 vs. 14,750±2,787; *p = 0.0038) and day 4 (83,055±8,978 vs. 35,416±2,055; **p = 0.001). Mean of three independent experiments with SD and linear regression. (D) Same as (C), but grown under anchorage-independent condition in soft-agar. The number of colonies was counted 2 weeks after initial inoculation. Mean of three independent experiments with SD. (E) Same as (A), but examined for the levels of soluble β-catenin and β-actin expression by immunoblotting (NT = non-treated). (F) Same as (A), but examined for expression of indicated β-catenin target genes by qRT-PCR. Expression of β-2- microglobulin was used to normalize the expression of indicated genes. * p = 0.0095, ** p = 0.0012 and *** p = 0.0286 compared with controls.
Canonical Wnt signaling promotes cell proliferation and Wnt5a has been demonstrated to antagonize the canonical Wnt/β-catenin signaling in certain cell contexts (16, 29–31). Since Wnt5a expression inversely correlated with expression of Ki67 (Figure 1E and Table 1), a cell proliferation marker, we hypothesized that Wnt5a would suppress the growth of human EOC cells by antagonizing canonical Wnt/β-catenin signaling. To test our hypothesis, we examined the effect of Wnt5a reconstitution on expression of markers of active Wnt/β-catenin signaling in human EOC cells, namely the levels of “active” soluble β-catenin (21, 22, 32) and expression of β-catenin target genes such as CCND1, c-MYC and FOSL1 (12, 13). Indeed, we observed a decrease in soluble β-catenin in Wnt5a reconstituted OVCAR5 cells compared with vector controls (Figure 3E). Consistently, we also observed a significant decrease in the levels of β-catenin target genes in these cells, namely CCND1 (p = 0.0095), FOSL1 (p = 0.0012) and c-MYC (p = 0.0286) (Figure 3F). Similar effects of Wnt5a reconstitution on expression of markers of active Wnt/β-catenin signaling (such as decreased levels of soluble β-catenin) were also observed in PEO1 human EOC cells (Figure S2D), suggesting that this is not cell line specific. Based on these results, we conclude that Wnt5a suppresses the growth of human EOC cells by antagonizing canonical Wnt/β-catenin signaling in human EOC cells.
Wnt5a reconstitution drives cellular senescence in human EOC cells
Next, we sought to determine the cellular mechanism whereby Wnt5a inhibits the growth of human EOC cells. We have previously shown that suppression of canonical Wnt signaling promotes cellular senescence in primary human fibroblasts by activating the senescence-promoting histone repressor A (HIRA)/promyelocytic leukemia (PML) pathway (22). PML bodies are 20–30 dot-like structures in the nucleus of virtually all human cells. PML bodies are sites of poorly defined tumor suppressor activity, and are disrupted in acute promyelocytic leukemia (33). PML has been implicated in regulating cellular senescence. For example, the foci number and size of PML bodies increase during senescence (33, 34) and inactivation of PML suppresses senescence (35). Activation of the HIRA/PML pathway is reflected by the recruitment of HIRA into PML bodies (36).
To determine whether Wnt5a reconstitution activates the HIRA/PML senescence pathway and induces senescence in EOC cells, we first sought to determine whether the HIRA/PML pathway is conserved in human ovarian epithelial cells. Ectopically expressing activated oncogenes (such as oncogenic-RAS) is a standard approach for inducing senescence in a synchronized manner in primary human cells (1, 2, 19, 20). Indeed, ectopic expression of oncogenic H-RASG12V induced senescence of primary HOSE cells, as evident by an increase in SA-β-gal activity, a universal marker of cellular senescence (Figure S3A–B). Notably, the HIRA/PML pathway was activated during senescence of primary HOSE cells induced by oncogenic RAS, as evident by the relocalization of HIRA into PML bodies (Figure S3C–D). This result demonstrates that the senescence-promoting HIRA/PML pathway is conserved in human ovarian epithelial cells. In addition, primary HOSE cells with HIRA foci displayed a marked decrease in BrdU incorporation, a marker of cell proliferation, compared with HIRA foci negative cells (Figure S3E–F). This result is consistent with the idea that activation of the HIRA/PML pathway is directly correlated with senescence-associated cell growth arrest (37).
We next asked whether Wnt5a expression is regulated during natural senescence of primary HOSE cells. Indeed, we observed an increase in the levels of Wnt5a mRNA in senescent primary HOSE cells compared with young cells (Figure 4A–C). In addition, we found that ectopic Wnt5a induces senescence of primary HOSE cells (Figure 4D–F). Together, we conclude that Wnt5a plays a role in regulating senescence of primary HOSE cells.
Figure 4. Wnt5a promotes senescence of primary HOSE cells.
(A) Young proliferating primary HOSE cells were passaged to senescence (after seven population doublings). Expression of SA-β-gal activity was measured in young and naturally senescent primary HOSE cells. (B) Same as (A). Quantitation of SA-β-gal positive cells (**p < 0.001). (C) Same as (A), but mRNA was isolated and examined for Wnt5a expression by qRT-PCR. Expression of β-2-microglobulin (B2M) was used as a control. (*p = 0.003). (D) Young primary HOSE cells were transduced with retrovirus encoding human Wnt5a gene or a control. Expression of Wnt5a in indicated cells was determined by qRT-PCR. Expression of β-2-microglobulin (B2M) was used as a control. (E) Same as (D), but stained for expression of SA-β-gal activity in drug-selected cells. (F) Quantitation of (E). Mean of three independent experiments with SD. * p<0.05.
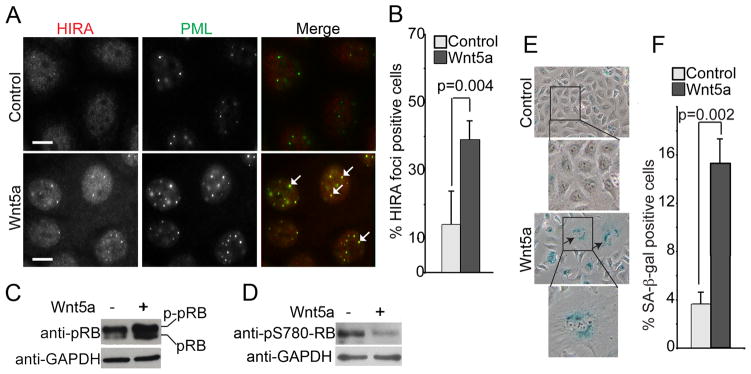
As Wnt5a antagonizes canonical Wnt signaling in human EOC cells (Figure 3E–F), we sought to determine whether Wnt5a restoration might activate the senescence-promoting HIRA/PML pathway and induce senescence in human EOC cells. Towards this goal, we examined the localization of HIRA in OVCAR5 EOC cells reconstituted with Wnt5a or vector control. Notably, there was a significant increase in the percentage of cells with HIRA localized to PML bodies in Wnt5a restored human EOC cells compared with controls (Figure 5A–B, p = 0.004). In addition, we also observed an increase in the number and size of PML bodies in the Wnt5a restored OVCAR5 EOC cells (Figure 5A), which are also established markers of cellular senescence (35, 38). Similarly, we observed activation of the HIRA/PML pathway by Wnt5a restoration in PEO1 human EOC cells (Figure S4A–B), suggesting that the observed effects are not cell line specific. Together, we conclude that Wnt5a reconstitution activates the HIRA/PML senescence pathway.
Figure 5. Wnt5a restoration triggers cellular senescence in human EOC cells.
(A) Control and Wnt5a expressing OVCAR5 EOC cells were stained with antibodies to HIRA and PML. Arrows point to examples of co-localized HIRA and PML bodies. Bar = 10 μm. (B) Quantitation of (A). 200 cells from control and Wnt5a expressing cells were examined for HIRA and PML co-localization. Mean of three independent experiments with SD. (C) Same as (A), but examined for pRB and GAPDH expression. (D) Same as (C), but examined for pRBpS780 and GAPDH expression. (E) Same as (A), but examined for senescence-associated β-galactosidase (SA-β-gal) activity. (F) Quantitation of (E). Mean of three independent experiments with SD.
The p53 and pRB tumor suppressor pathways play a key role in regulating senescence (1). Thus, we sought to determine whether activation of the HIRA/PML pathway depends upon the p53 and pRB pathways. Interestingly, p16INK4a, the upstream repressor of pRB, is deleted in OVCAR5 human EOC cell line (39). In addition, the levels of total phosphorylated pRB were not decreased by Wnt5a, while the levels of cyclin D1/CKD4-mediated Serine 780 phosphorylation on pRB (pRBpS780) were decreased by Wnt5a (40) (Figure 5C–D). Further, p53 is null in OVCAR5 cells (41). We conclude that activation of the HIRA/PML pathway is independent of the p53 and p16INK4a.
We next sought to determine whether Wnt5a restoration induces SA-β-gal activity, a universal marker of cellular senescence (1). Indeed, SA-β-gal activity was notably induced by Wnt5a reconstitution in both OVCAR5 and PEO1 human EOC cells compared with controls (Figure 5E–F and S4C–D, respectively). Based on these results, we concluded that Wnt5a restoration induced senescence of human EOC cells by activating the HIRA/PML senescence pathway.
Wnt5a inhibits the growth of human EOC cells in vivo by inducing cellular senescence
We next sought to determine whether Wnt5a would mediate growth inhibition and induce senescence in vivo in an orthotopic EOC model in immuno-compromised mice. A luciferase gene was retrovirally transduced into control or Wnt5a reconstituted OVCAR5 cells to monitor the cell growth in vivo via non-invasive imaging. These cells were injected unilaterally into the bursa sac covering the ovary in female immuno-compromised mice (n = 6 for each of the groups) (Figure S5). Tumor growth was monitored every 5 days starting at day 10 post-injection by measuring luciferase activity, and the growth of the tumor was followed for a total of 30 days. (Figure 6A). Wnt5a significantly suppressed the growth of xenografted OVCAR5 human EOC cells compared with controls (Figure 6B, p < 0.03). Consistently, following general pathological examination during surgical dissection at day 30, we observed that tumor sizes were notably smaller from mice injected with Wnt5a reconstituted OVCAR5 cells compared with controls (data not shown). The expression of ectopic Wnt5a was confirmed by IHC staining in sections from dissected tumors (Figure 6C).
Figure 6. Wnt5a restoration inhibits tumor growth and promotes senescence of human EOC cells in vivo.
(A) OVCAR5 cells were transduced with luciferase encoding hygromycin resistant retrovirus together with a control or Wnt5a encoding puromycin-resistant retrovirus. Drug-selected cells were unilaterally injected into the peri-ovarian bursa sac of the female immuno-compromised mice (n = 6 for each of the groups). The radiance of luciferase bioluminescence, an indicator of the rate for tumor growth, was measured every 5 day from day 10 until day 30 using the IVIS imaging system. Shown are images taken at day 10 and day 30, respectively. (B) Quantitation of tumor growth from injected OVCAR5 cells expressing Wnt5a or control at indicated time points. * p = 0.038 compared with controls. (C) Following tumor dissection, expression of Wnt5a in tumors formed by control or Wnt5a expressing OVCAR5 EOC cells was determined by immunohistochemical staining against Wnt5a (40X). Bar = 50 μm. (D) Same as (C), but examined for expression of Ki67, a marker of cell proliferation (40X). Bar = 50 μm. (E) Quantitation of (D). * p = 0.008 compared with controls. (F) Expression of SA-β-gal activity was examined on sections of fresh-frozen tumors formed by OVCAR5 cells expressing control or Wnt5a (40X). Bar = 100 μm. (G) Quantitation of (F). * p = 0.003 compared with controls.
We next sought to determine whether cell proliferation was suppressed by Wnt5a reconstitution in dissected tumors. Towards this goal, we examined the expression of Ki67 by IHC. We observed there was a significant decrease in the number of Ki67 positive cells in tumors formed by Wnt5a reconstituted OVCAR5 cells compared with controls (Figure 6D–E). In addition, intensity of Ki67 staining was also notably weaker in Ki67 positive Wnt5A reconstituted OVCAR5 cells compared with control Ki67 positive cells (Figure 6D). On the basis of these results, we conclude that Wnt5a reconstitution inhibits the proliferation of human EOC cells in vivo in an orthotopic xenograft EOC model.
We next investigated whether the growth inhibition observed by Wnt5a reconstitution in vivo was due to induction of cellular senescence. Towards this goal, we examined the expression of SA-β–gal activity in fresh sections of dissected tumors formed by OVCAR5 cells reconstituted with Wnt5a or control cells. Indeed, we observed a significant increase in the number of cells positive for SA-β-gal activity in OVCAR5 cells reconstituted with Wnt5a compared with control tumors (Figure 6F–G; p = 0.003). Together, we conclude that Wnt5a reconstitution inhibits the growth of human EOC cells in vivo by inducing cellular senescence.
Discussion
Driving cancer cells to undergo cellular senescence has recently been proposed to be a novel mechanism to target for developing cancer therapeutics (1, 6). For example, pharmacological inhibitor of PTEN drives senescence and, consequently, inhibits tumorigenesis in vivo in xenograft models of PTEN heterozygous prostate cancer cells (42, 43). Compared with apoptosis, therapeutics that drive cellular senescence are proposed to have less cytotoxic side effects (6), which makes pro-senescence therapy attractive. Herein, we describe that restoration of Wnt5a signaling drives senescence of human EOC cells both in vitro and in vivo in an orthotopic mouse model of EOC (Figure 5 and 6). Restoring gene expression by gene therapy has had limited success. Therefore, restoring Wnt5a signaling via exogenous ligand could prove to be an alternative approach. Interestingly, it has been previously reported that a Wnt5a-derived hexapeptide is sufficient to restore Wnt5a signaling both in vitro and in vivo in xenograft models of breast cancer (44). It would be interesting to test whether the Wnt5a-derived hexapeptide will be sufficient to reconstitute Wnt5a signaling and drive senescence of EOC cells. Our data suggest that cellular senescence is a potential target for developing EOC therapeutics. In addition, these data imply that restoration of Wnt5a signaling represents a potential novel strategy to drive senescence of EOC cells.
This study is the first to demonstrate a role for Wnt5a in regulating senescence. We demonstrated that Wnt5a activated the senescence-promoting HIRA/PML pathway in human EOC cells (Figure 5A and S4A). In primary human cells, activation of HIRA/PML pathway is sufficient to drive senescence by facilitating epigenetic silencing of proliferation-promoting genes (such as E2F target genes) (19). Herein, we reported for the first time that the key HIRA/PML senescence pathway can be reactivated to drive senescence of human cancer cells. Further studies are warranted to elucidate the molecular basis by which Wnt5a restoration and activation of HIRA/PML pathway drive cellular senescence in human EOC cells.
Interestingly, senescence induced by Wnt5a restoration in human EOC cells was independent of both the p53 and p16INK4a tumor suppressors, which implies that EOC cells that lack p53 and p16INK4a retain the capacity to undergo senescence via HIRA/PML pathway through suppressing the canonical Wnt signaling. This is consistent with previous reports showing that cancer cells that lack p53 and pRB retain the capacity to undergo senescence when treated with anticancer agents or ionizing radiation (6). Notably, although the levels of total phosphorylated pRB were not decreased by Wnt5a, we observed a decrease in the levels of pRBpS780 that is mediated by cyclin D1/CDK4 (Figure 5C–D). Future studies will determine whether the decrease in pRBpS780 levels plays a role in regulating senescence of human EOC cells.
Expression of Wnt5a is altered in many types of cancers (45). For example, in melanoma, Wnt5a overexpression correlates with cancer progression and a higher tumor stage (16). However, in colorectal and esophageal squamous cell carcinomas, Wnt5a has been described to be a tumor suppressor and was frequently silenced by promoter hypermethylation (16, 46). Consistently, we also observed Wnt5a promoter hypermethylation in a number of human EOC cell lines in which Wnt5a is downregulated (Figure 2 and Table S1). This result is consistent with the idea that Wnt5a promoter hypermethylation contributes to Wnt5a downregulation in human EOC cells.
Wnt5a function is highly dependent on cellular context (45). For example, the cellular Wnt receptor/co-receptor context dictates the downstream signaling pathways upon the binding of Wnt5a, which include activating non-canonical Wnt signaling or antagonizing canonical Wnt/β-catenin signaling (47). These reports illustrate that Wnt5a expression and its resulting activity are cell type and context dependent. The Wnt receptor/co-receptor profile in EOC cells is currently unknown, and our future studies will elucidate the mechanism by which Wnt5a antagonizes Wnt/β-catenin signaling in human EOC cells. Regardless, our data show that Wnt5a downregulation is an independent predictor for overall survival in EOC patients. In contrast, two other studies showed that higher Wnt5a expression predicts poor survival in EOC patients (48, 49). The basis for this discrepancy remains to be elucidated. An explanation may be that our study included more cases than the other two studies (130 EOC cases in our study vs. 38 cases in the study by Badiglian et al or 63 cases in the study by Peng et al). It may also be due to the difference in the composition of Type I and Type II cases in this study compared to the other two studies. The vast majority of EOC cases in this study are of Type II high-grade serous subtypes. Consistently, our data showed that there is a difference in Wnt5a expression between Type I and Type II EOC (Table 1, p=0.005). Further, it has been demonstrated in microarray analysis that Wnt5a is expressed at lower levels in laser capture and microdissected high-grade serous EOC compared with normal primary HOSE cells (50).
In summary, the data reported here show that Wnt5a is often expressed at lower levels in human EOCs compared with either normal human ovarian surface epithelium or fallopian tube epithelium. A lower level of Wnt5a expression correlates with tumor stage and predicts shorter overall survival in EOC patients. Reconstitution of Wnt5a signaling inhibits the growth of human EOC cells both in vitro and in vivo. In addition, Wnt5a reconstitution suppresses the proliferation-promoting canonical Wnt/β-catenin signaling in human EOC cells. Significantly, Wnt5a reconstitution drives cellular senescence in human EOC cells and this correlates with activation of the senescence-promoting HIRA/PML pathway. Together, our data imply that reconstitution of Wnt5a signaling to drive senescence of human EOC cells is a potential novel strategy for developing EOC therapeutics.
Supplementary Material
Acknowledgments
Grant Support: R.Z. is an Ovarian Cancer Research Fund (OCRF) Liz Tilberis Scholar. This work was supported in part by a NCI FCCC-UPenn ovarian cancer SPORE (P50 CA083638) pilot project and SPORE career development award (to R.Z.), a DOD ovarian cancer academy award (OC093420 to R.Z.), an OCRF program project (to R.Z., M.B. and A.K.G.) and a generous gift from Catherine and Peter Getchell. B.G.B is supported by a NCI postdoctoral training grant (CA-009035-35).
We thank Dr. Denise Connolly for reagent, and Dr. Harvey Hensley for technical assistance. We thank Drs. Katherine Aird and Maureen Murphy for critical reading of the manuscript.
References
- 1.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–79. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams PD. Healing and hurting: molecular mechanisms, functions, and pathologies of cellular senescence. Mol Cell. 2009;36:2–14. doi: 10.1016/j.molcel.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–5. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 5.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewald JA, Desotelle JA, Wilding G, Jarrard DF. Therapy-induced senescence in cancer. J Natl Cancer Inst. 2010;102:1536–46. doi: 10.1093/jnci/djq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peeper DS. PICS-ure this: prosenescence therapy? Cancer Cell. 2010;17:219–20. doi: 10.1016/j.ccr.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 8.Ozols RF, Bookman MA, Connolly DC, Daly MB, Godwin AK, Schilder RJ, et al. Focus on epithelial ovarian cancer. Cancer Cell. 2004;5:19–24. doi: 10.1016/s1535-6108(04)00002-9. [DOI] [PubMed] [Google Scholar]
- 9.Farley J, Ozbun LL, Birrer MJ. Genomic analysis of epithelial ovarian cancer. Cell Res. 2008;18:538–48. doi: 10.1038/cr.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–8. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 12.Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–5. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- 13.Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, et al. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci U S A. 1999;96:1603–8. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szotek PP, Chang HL, Brennand K, Fujino A, Pieretti-Vanmarcke R, Lo Celso C, et al. Normal ovarian surface epithelial label-retaining cells exhibit stem/progenitor cell characteristics. Proc Natl Acad Sci U S A. 2008;105:12469–73. doi: 10.1073/pnas.0805012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu R, Hendrix-Lucas N, Kuick R, Zhai Y, Schwartz DR, Akyol A, et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell. 2007;11:321–33. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 16.McDonald SL, Silver A. The opposing roles of Wnt-5a in cancer. Br J Cancer. 2009;101:209–14. doi: 10.1038/sj.bjc.6605174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Cai Q, Godwin AK, Zhang R. Enhancer of zeste homolog 2 promotes the proliferation and invasion of epithelial ovarian cancer cells. Mol Cancer Res. 2010;8:1610–8. doi: 10.1158/1541-7786.MCR-10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibanez de Caceres I, Dulaimi E, Hoffman AM, Al-Saleem T, Uzzo RG, Cairns P. Identification of novel target genes by an epigenetic reactivation screen of renal cancer. Cancer Res. 2006;66:5021–8. doi: 10.1158/0008-5472.CAN-05-3365. [DOI] [PubMed] [Google Scholar]
- 19.Zhang R, Chen W, Adams PD. Molecular dissection of formation of senescence-associated heterochromatin foci. Mol Cell Biol. 2007;27:2343–58. doi: 10.1128/MCB.02019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang R, Poustovoitov MV, Ye X, Santos HA, Chen W, Daganzo SM, et al. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev Cell. 2005;8:19–30. doi: 10.1016/j.devcel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Cheyette BN, Waxman JS, Miller JR, Takemaru K, Sheldahl LC, Khlebtsova N, et al. Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev Cell. 2002;2:449–61. doi: 10.1016/s1534-5807(02)00140-5. [DOI] [PubMed] [Google Scholar]
- 22.Ye X, Zerlanko B, Kennedy A, Banumathy G, Zhang R, Adams PD. Downregulation of Wnt signaling is a trigger for formation of facultative heterochromatin and onset of cell senescence in primary human cells. Mol Cell. 2007;27:183–96. doi: 10.1016/j.molcel.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol. 2007;371:21–31. doi: 10.1007/978-1-59745-361-5_3. [DOI] [PubMed] [Google Scholar]
- 24.Connolly DC, Hensley HH. Xenograft and Transgenic Mouse Models of Epithelial Ovarian Cancer and Non Invasive Imaging Modalities to Monitor Ovarian Tumor Growth In situ-Applications in Evaluating Novel Therapeutic Agents. Curr Protoc Pharmacol. 2009;45:14 12 1–14 12 26. [PMC free article] [PubMed] [Google Scholar]
- 25.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarty KS, Jr, Szabo E, Flowers JL, Cox EB, Leight GS, Miller L, et al. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res. 1986;46:4244s–4248s. [PubMed] [Google Scholar]
- 27.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–5. [PubMed] [Google Scholar]
- 28.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–96. [PubMed] [Google Scholar]
- 29.Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A, et al. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 2003;4:349–60. doi: 10.1016/s1535-6108(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 30.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 33.Bernardi R, Pandolfi PP. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol. 2007;8:1006–16. doi: 10.1038/nrm2277. [DOI] [PubMed] [Google Scholar]
- 34.Mallette FA, Goumard S, Gaumont-Leclerc MF, Moiseeva O, Ferbeyre G. Human fibroblasts require the Rb family of tumor suppressors, but not p53, for PML-induced senescence. Oncogene. 2004;23:91–9. doi: 10.1038/sj.onc.1206886. [DOI] [PubMed] [Google Scholar]
- 35.Ferbeyre G, de Stanchina E, Querido E, Baptiste N, Prives C, Lowe SW. PML is induced by oncogenic ras and promotes premature senescence. Genes Dev. 2000;14:2015–27. [PMC free article] [PubMed] [Google Scholar]
- 36.Salomoni P, Pandolfi PP. The role of PML in tumor suppression. Cell. 2002;108:165–70. doi: 10.1016/s0092-8674(02)00626-8. [DOI] [PubMed] [Google Scholar]
- 37.Ye X, Zerlanko B, Zhang R, Somaiah N, Lipinski M, Salomoni P, et al. Definition of pRB- and p53-dependent and -independent steps in HIRA/ASF1a-mediated formation of senescence-associated heterochromatin foci. Mol Cell Biol. 2007;27:2452–65. doi: 10.1128/MCB.01592-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson M, Carbone R, Sebastiani C, Cioce M, Fagioli M, Saito S, et al. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature. 2000;406:207–10. doi: 10.1038/35018127. [DOI] [PubMed] [Google Scholar]
- 39.Watson JE, Gabra H, Taylor KJ, Rabiasz GJ, Morrison H, Perry P, et al. Identification and characterization of a homozygous deletion found in ovarian ascites by representational difference analysis. Genome Res. 1999;9:226–33. [PubMed] [Google Scholar]
- 40.Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–61. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yaginuma Y, Westphal H. Abnormal structure and expression of the p53 gene in human ovarian carcinoma cell lines. Cancer Res. 1992;52:4196–9. [PubMed] [Google Scholar]
- 42.Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC, et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest. 2010;120:681–93. doi: 10.1172/JCI40535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collado M. Exploring a ‘pro-senescence’ approach for prostate cancer therapy by targeting PTEN. Future Oncol. 2010;6:687–9. doi: 10.2217/fon.10.39. [DOI] [PubMed] [Google Scholar]
- 44.Safholm A, Tuomela J, Rosenkvist J, Dejmek J, Harkonen P, Andersson T. The Wnt-5a-derived hexapeptide Foxy-5 inhibits breast cancer metastasis in vivo by targeting cell motility. Clin Cancer Res. 2008;14:6556–63. doi: 10.1158/1078-0432.CCR-08-0711. [DOI] [PubMed] [Google Scholar]
- 45.Pukrop T, Binder C. The complex pathways of Wnt 5a in cancer progression. J Mol Med. 2008;86:259–66. doi: 10.1007/s00109-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 46.Li J, Ying J, Fan Y, Wu L, Ying Y, Chan AT, et al. WNT5A antagonizes WNT/beta-catenin signaling and is frequently silenced by promoter CpG methylation in esophageal squamous cell carcinoma. Cancer Biol Ther. 2007;10:617–24. doi: 10.4161/cbt.10.6.12609. [DOI] [PubMed] [Google Scholar]
- 47.Nishita M, Enomoto M, Yamagata K, Minami Y. Cell/tissue-tropic functions of Wnt5a signaling in normal and cancer cells. Trends Cell Biol. 2010;20:346–54. doi: 10.1016/j.tcb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Badiglian Filho L, Oshima CT, De Oliveira Lima F, De Oliveira Costa H, De Sousa Damiao R, Gomes TS, et al. Canonical and noncanonical Wnt pathway: a comparison among normal ovary, benign ovarian tumor and ovarian cancer. Oncol Rep. 2009;21:313–20. [PubMed] [Google Scholar]
- 49.Peng C, Zhang X, Yu H, Wu D, Zheng J. Wnt5a as a predictor in poor clinical outcome of patients and a mediator in chemoresistance of ovarian cancer. Int J Gynecol Cancer. 2011;21:280–8. doi: 10.1097/IGC.0b013e31820aaadb. [DOI] [PubMed] [Google Scholar]
- 50.Mok SC, Bonome T, Vathipadiekal V, Bell A, Johnson ME, Wong KK, et al. A gene signature predictive for outcome in advanced ovarian cancer identifies a survival factor: microfibril-associated glycoprotein 2. Cancer Cell. 2009;16:521–32. doi: 10.1016/j.ccr.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.