Abstract
Neurogenesis in the dentate gyrus of the hippocampus of adult laboratory animals has been widely reported to be vulnerable to many psychological and physical stressors. However, we have found no effects of acute restraint stress, acute or subchronic tailshock stress, or acute, subchronic, or chronic resident-intruder stress on neural progenitor cell (NPC) proliferation, short or long term survival of newborn cells, or brain-derived neurotrophic factor (BDNF) mRNA expression in adult rats. In addition, we did not observe any effect of chronic resident-intruder stress on NPC proliferation in adolescent rats. A selectively bred stress-sensitive line was also found to exhibit no alterations in NPC proliferation following tailshock stress, although this line did exhibit a lower proliferation rate under baseline (unstressed) conditions when compared with non-selected rats. These results challenge the prevailing hypothesis that any stressor of sufficient intensity and duration has a marked negative impact upon the rate of hippocampal neurogenesis, and suggest that some yet unidentified factors related to stress and experimental conditions are crucial in the regulation of neurogenesis.
Keywords: Neurogenesis, dentate gyrus, brain-derived neurotrophic factor, stress, tailshock, resident-intruder
Psychological or physical stressors have been reported to impair many aspects of hippocampal neurogenesis in adult laboratory animals, including decreasing proliferation rate of neural progenitor cells (NPCs), decreasing survival of neuroblasts and immature neurons, and decreasing growth and development of new neurons. Restraint or immobilization is a classic laboratory stressor which has been reported to inhibit proliferation, even after only a single session in some publications but requiring repeated treatment in others, and also to reduce survival rate of new cells (Pham et al., 2003, Vollmayr et al., 2003, Duric and McCarson, 2006, Koo and Duman, 2008). The resident-intruder model (also called the social defeat model, in reference to often-seen changes in social behavior resulting from exposure to this stressor) has been reported to reduce both cell proliferation and neuronal survival after chronic daily sessions (Czeh et al., 2002), whereas a single session reduces short- and long-term survival, but not proliferation (Thomas et al., 2007). A single session of footshock, either inescapable or as a component of avoidance conditioning, has been reported to be sufficient in some cases to reduce NPC proliferation by 50%, irrespective of whether the animals develop failure to perform avoidance-escape responses (called “learned helplessness” behavior; Malberg and Duman, 2003, Vollmayr et al., 2003). These results suggest that a multitude of stressors can inhibit neurogenesis when administered at sufficient intensity and duration. In contrast, one report involving tailshock found that a single session reduced proliferation only in those rats developing failure to escape (Chen et al., 2006). One paradigm which has yielded contradictory results is the study of yoked pairs, in which one animal controls the duration of shock for both partners. In separate experiments, both escapable and inescapable conditions resulted in decreased proliferation, only the inescapable condition did so, or neither had any effect on proliferation (Westenbroek et al., 2004, Bland et al., 2006, Shors et al., 2007).
The initial objective of these experiments was to establish a stress paradigm which would reliably and robustly decrease the rate of NPC proliferation in the dentate gyrus in adult animals, by direct replication of paradigms previously reported as successful in the literature. A subsequent experiment included animals from a selectively bred line of hyperactive rats (called “HYPER”; Weiss et al., 2008). As young adults, rats of this line react to stress by developing nocturnal hyperactivity for several days, but later in life (such as the ten month old animals used in this experiment), they respond to stress not with hyperactivity but with a depression of ambulatory activity that is much longer-lasting than that seen in non-selected rats. A final experiment sought to apply the chronic resident-intruder stressor to adolescent animals, because adolescents have been reported to be more sensitive than adults to another experimental treatment (i.e., alcohol consumption) shown to have deleterious effects on neurogenesis (Crews et al., 2006, Morris et al., 2009).
Although NPC proliferation rate was the major target of interest, assessment of other neurogenesis-related processes was performed in many of these experiments. The effect of resident-intruder stress on rates of short-term and long-term survival of immature neurons was measured. Because brain-derived neurotrophic factor (BDNF) has been strongly implicated in the downregulation of neurogenesis by stress (reviewed in Duman and Monteggia, 2006), its expression was measured in the tailshock experiments.
Methods
Animals
Adult male Sprague-Dawley rats (Charles River, Wilmington MA) were obtained one week prior to the initiation of the experimental protocols. To partially control for age, animals in acute studies were approximately ten weeks old and animals in chronic studies were approximately eight weeks old at the experiment initiation, so that ages would be comparable when the brains were harvested. For the adolescent experiment, animals were three weeks old at the experiment initiation and six weeks old at the conclusion. The acute tailshock experiment was an exception to this: animals in this experiment were male Sprague-Dawley rats, approximately ten months old, chosen from an in-house breeding colony originally established in the 1980s using lines from Charles River, including animals from the selectively bred HYPER line and from a non-selected line. Older animals were used in order to capture the depressive behavior only seen later in life in the HYPER line, as described in the introduction (Weiss et al., 2008). Resident animals for the resident-intruder procedures were male Long-Evans retired breeders (Charles River), pair-housed with sexually mature females.
In all cases, animals were maintained in standard laboratory conditions: pair-housed (except as noted for resident-intruder cohorts) in polycarbonate cages (40 × 28 × 20 cm for trained residents; 30 × 20 × 20 cm for all others) with corn cob bedding, on a 12:12 light cycle with lights on at 0700 hrs, with ad libitum access to food and water. All procedures used were approved by the Institutional Animal Care and Use Committee (IACUC) of Emory University and are in compliance with National Institutes of Health (NIH) guidelines for the care and use of laboratory animals.
HYPER rating
Several months prior to their inclusion in the stress experiment described here, animals of the HYPER line were exposed to a single session of intermittent grid shock. Shock intensity varied randomly between 0.50 and 1.25 mA and shock intervals were randomized with a mean interval of 1.0 minute (thus, an animal will receive about 60 shocks in a 60-minute session). Following the shock session, animals were removed from the chambers and individually housed in standard rodent cages where ambulatory motor activity was recorded for 14 days. Their nocturnal ambulatory activity was counted by number of photocell beam-breaks. Individual rats were graded as to the extent of hyperactivity resulting from exposure to the stress: rats were classified as “good” or “poor” on the basis of hyperactive response.
Stress procedures
Restraint stress
Between 1000 and 1200 hrs, animals in the restraint group were transported to another room, removed from their cages, and restrained in flexible plastic DecapiCones (Braintree Scientific, Braintree MA) for two hours. During this time, control animals remained in their home cages.
Tailshock stress
Tailshock experiments were conducted under two different protocols, one subchronic (three daily sessions) and one acute, with different sets of equipment in different locations.
In the subchronic experiment, between 1000 and 1200 hrs, animals in the tailshock group were transported to another room, removed from their cages, and restrained in individual wire mesh cylinders. Two electrodes were taped to either side of the tails of four animals concurrently and connected in series to ensure that each animal received an equal current. Each tailshock session consisted of 60 1.0 mA shocks of 1.0 s duration, delivered on a variable interval schedule averaging one shock per minute, for a one hour period. Tailshock sessions took place once daily on three consecutive days. Blood samples were collected via tail nick 20 minutes after the start of the session and again at the conclusion of the session. At the end of each session, the animals were returned to their home cages. During this time, control animals remained in their home cages and a tail blood sample was also collected at the same time of day as the tailshock animals, but each control animal was only subjected to this procedure on one of the three days in order to minimize stress.
In the acute experiment, between 1000 and 1200 hrs, animals in the tailshock group were transported to another room, removed from their cages, and placed into plexiglass containers. Two electrodes were taped to either side of the tails of eight animals concurrently and connected in series to ensure that each animal received an equal current. The tailshock session lasted for three hours, within which shocks gradually increased from 1.0 to 2.5 mA, delivered on a variable interval schedule averaging one shock per minute, with shocks of variable duration ranging from 0.1 to 3.0 s. During this time, control animals remained in their home cages.
Resident-Intruder stress
Animals subjected to resident-intruder stress were housed individually, in order to maximize the impact of the stressor by removing the soothing effects of non-hostile conspecifics and to eliminate the additional variable of social dominance or subordinance in the home cage pair. Control animals were housed in pairs.
Several weeks in advance of the resident-intruder experiment, resident animals were trained for aggressive territorial behavior by challenging them multiple times with smaller intruder animals in the resident-intruder procedure detailed below. Only those animals which met criteria for sufficiently aggressive and dominant behaviors were used as residents in the experiments. These criteria included minimum latency to first attack and minimum latency to pin.
Between 1400 and 1600 hrs, resident animals were transported to another room, where the female cage mates were removed, and were left undisturbed for one hour. Experimental animals in the intruder group were then transported from the colony and each one placed into the home cage of a resident, separated by a wire mesh barrier which allowed visual and olfactory contact only for the first 2 minutes of the encounter. The barrier was then removed and the animals allowed to interact for 20 minutes. If the intruder was injured during the encounter, the barrier was replaced for the remainder of the session. For those animals subjected to repeated sessions, these took place on Mondays, Wednesdays, and Fridays, with resident-intruder pairs systematically shuffled for each session to equalize the defeat experience between experimental animals. Behavior was monitored during these sessions to ensure that all intruders displayed sufficient signs of defeat. This experiment was repeated identically using adolescent animals.
Serum corticosterone analysis
Blood samples obtained from the subchronic tailshock experiment were centrifuged to obtain serum, and corticosterone concentrations measured by radioimmunoassay. A 100 μl aliquot of 1:200 rat serum in assay buffer (MP Biomedicals, Solon OH) was incubated with 200 μl of highly specific antibody and 200 μl *125I]-corticosterone at room temperature for 2 hours. The bound complexes were separated via addition of 500 μl of secondary antibody, followed by centrifugation at 2500 × g for 25 minutes, and the unbound complexes were decanted to waste. The residual pellets were counted for 3 minutes in a LKB Clin-Gamma gamma counter. Quantification was achieved via log/logit data reduction.
BrdU administration and sacrifice
Bromodeoxyuridine (BrdU, 200 mg/kg in 0.9% saline, Sigma-Aldrich, St. Louis MO) was administered via a single ip injection. For assessment of cell proliferation rate, BrdU injection was given immediately following the final stress session (for all stressors) and animals were sacrificed 24 hours later; for assessment of newborn cell survival, BrdU injection was given before the first stress session and animals were sacrificed 1 (short-term survival) or 3 (long-term survival) weeks later. At the time of sacrifice, animals were deeply anesthesized with pentobarbital and transcardially perfused with cold 0.1 M phosphate buffered saline (PBS) followed by 4% paraformaldehyde. The brains were removed, post-fixed overnight, and equilibrated in a 30% sucrose solution. Sections of 25 μm through the entire hippocampus (bregma −2.4 to −6.2 mm) were cut on a cryostat and mounted on slides for immunocytochemical and in situ hybridization procedures.
Immunocytochemistry
Immunocytochemical staining was performed on every twelfth section throughout the hippocampus. For BrdU staining, antigen retrieval was performed by incubation in 50% formamide/SSC at 65° C, followed by 2M HCl at 37° C, and then neutralization in 0.1 M boric acid (pH 8.5). After quenching in 2% hydrogen peroxide, sections were blocked in 3% normal horse serum (Vector, Burlingame, CA) and incubated overnight in 1:100 mouse monoclonal anti-BrdU antibody (Becton-Dickinson, Franklin Lakes, NJ). The next day, they were treated with 1:200 horse anti-mouse rat-adsorbed secondary antibody, then treated with a Vectastain ABC kit and visualized with DAB substrate (all Vector).
Stained sections were examined at 40x magnification under a light microscope. Labeled cells were counted if they were within the subgranular zone of the dentate gyrus or within one cell width from its edge. A total number of positively labeled cells was acquired for each animal and multiplied by 12 to approximate the number of labeled cells throughout the entire dentate gyrus. Additionally, cell counts were divided into dorsal and ventral parts of the dentate gyrus. This procedure follows established guidelines for unbiased rare event stereology (Mouton, 2002). All cell counting was performed by the same individual under treatment-blind conditions.
A Student’s t-test or one-way ANOVA (depending on whether multiple levels of stress were included in each experiment) was performed on each of the resulting measures, with total dentate gyrus and dorsal and ventral regions considered separately. The criterion for significance was p < .05. If justified by ANOVA, post hoc comparisons were made between individual groups and control using Dunnett’s test.
In situ hybridization
In situ hybridization was utilized with an [35S]-labeled riboprobe to quantify mRNA expression. As in the immunocytological protocol above, every twelfth section was treated and analyzed. A BDNF riboprobe was constructed from a cDNA insert including the full coding region plus some flanking sequence, ligated into a pR1112-8 plasmid. Radiolabeled antisense cRNA was synthesized by incorporating [35S]-CTP into the probe. The transcription reaction was performed utilizing an Ambion MAXIscript kit with T3 RNA polymerase (Applied Biosystems, Foster City, CA), according to the instructions provided. Following transcription and removal of the cDNA template with DNase, the cRNA probe was recovered through gel filtration using a G-50 Sephadex Quick Spin column (GE Healthcare, Piscataway NJ).
The slides underwent 10 minutes of acetylation (0.5% acetic anhydride in 0.1 mol/L triethanolamine, pH 8.0), two rinses in 2X SSC, and dehydration through a graded ethanol series. The sections were then air-dried for at least one hour prior to hybridization. The brain sections were hybridized overnight at 60°C with 1–2 × 106 cpm of [35S]-labeled cRNA probe diluted into hybridization buffer (50% formamide, 10% dextran sulfate, 0.3 M NaCl, 1X Denhardt’s solution, 10 mM Tris, 1 mM ethylenediaminetetraacetic acid (EDTA), 2 mg/ml yeast tRNA, 10 mM dithiothreitol [DTT]) in humidified Nunc trays.
The next day, slides were allowed to cool to room temperature before being washed four times in 4X saline-sodium citrate buffer (SSC). The sections were then treated with 250 μg/ml RNase A for 30 minutes at 37°C. Subsequently, the slides underwent a series of SSC washes (supplemented with 1 mM DTT) with salt concentrations decreasing from 2X to 0.5X, followed by a 60 minute high stringency wash with 0.1X SSC + 1mM DTT at 60°C, then dehydration through a graded ethanol series. The slides were air-dried and then apposed to Kodak Biomax MR film until signal reached the desired intensity.
Images on film were digitized with a CCD-72 camera and image analysis system (Dage-MTI, Michigan City IN). Semiquantitative analysis was performed using AIS software. Messenger RNA expression levels were calculated by subtracting the neutral background density from the specific signal. Sections were matched for rostrocaudal level and analyzed for values representing the regions CA1/2, CA3, and dentate gyrus, which were then averaged between sections to produce a single value for each region for each animal.
A Student’s t-test or one-way ANOVA (depending on whether multiple levels of stress were included in each experiment) was performed on each of the resulting measures, with CA1/2, CA3, and dentate gyrus regions considered separately. The criterion for significance was p < .05. If justified by ANOVA, post hoc comparisons were made between individual groups and control using Dunnett’s test.
Results
Acute restraint stress
A single two hour session of restraint stress had no significant effect on cell proliferation rate, either in the entire dentate gyrus or in the dorsal or ventral parts (Figure 1, p = 0.90,0.98, and 0.77 respectively).
Figure 1.
Acute restraint stress did not alter cell proliferation rate in the dentate gyrus (A) or in its dorsal (B) or ventral parts (C). Data are presented as mean ± SEM. n = 8 per group.
Acute and subchronic tailshock stress
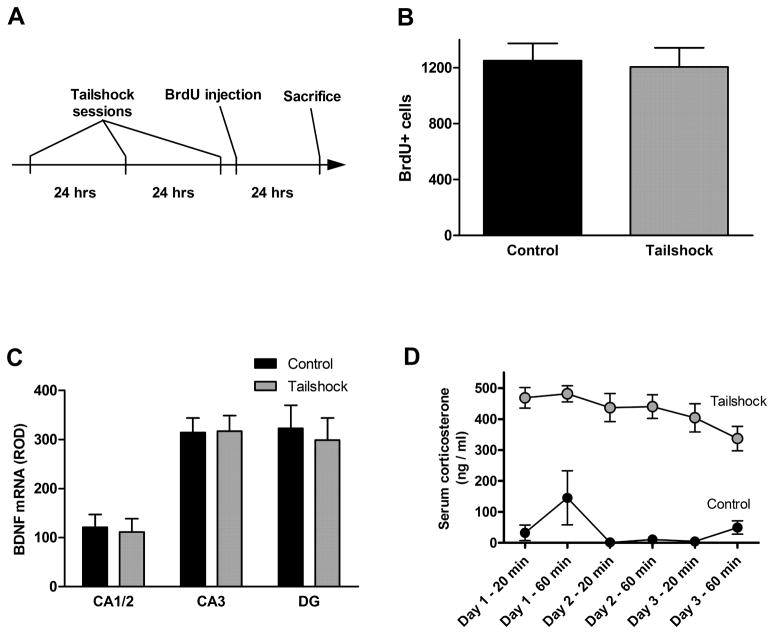
No significant effect on cell proliferation was seen of either the acute procedure, consisting of a single three-hour session (Figure 2B, p = 0.3446) or the subchronic procedure, consisting of one daily one-hour session performed on each of three consecutive days (Figure 3B, p = 0.82). As noted in the Methods section above, the animals used in the acute procedure were aged approximately ten months, in contrast to the standard “young adult” age of ten weeks used in the other experiments. The baseline (non-stressed) rate of cell proliferation is dramatically lower, consistent with reports of decreased neurogenesis in aged animals (Kuhn et al., 1996).
Figure 2.
Acute tailshock stress (experimental timeline shown in A) did not alter cell proliferation rate in the dentate gyrus (B), and did not alter expression of BDNF mRNA in any hippocampal subfield (C). Data are presented as mean ± SEM. n = 8 per group.
Figure 3.
Subchronic tailshock stress (experimental timeline shown in A) did not alter cell proliferation rate in the dentate gyrus (B), and did not alter expression of BDNF mRNA in any hippocampal subfield (C). Serum corticosterone measurements confirmed intensity of tailshock stressor and minimal stress of control conditions (D). Data are presented as mean ± SEM. n = 12 per group.
No significant effect of either tailshock procedure was seen on BDNF mRNA expression in the CA1/2, CA3, or dentate gyrus (Figure 2C [acute], p = 0.67 [CA1/2], 0.49 [CA3], and 0.39 [DG]; Figure 3C [subchronic], p = 0.80 [CA1/2], 0.95 [CA3], and 0.72 [DG]).
The subchronic stress procedure was verified by measurements of serum corticosterone taken twice during each stress session (Figure 3D). Elevated concentrations (over 400 ng/ml) at the 20 minute timepoint confirmed the intensity of the stressor, and the still elevated corticosterone concentrations at the 60 minute timepoint indicate that this stress intensity was maintained throughout the entire session. Moreover, elevated corticosterone concentrations during the second and third sessions of the subchronic experiment reveal that animals did not appreciably habituate to the tailshock stress over the course of the experiment. Low concentrations in the control group (less than 50 ng/ml, with the exception of one animal at the session 1 – 60 minute timepoint) establish a minimally stressful baseline housing condition and show that the tail nick procedure itself does not result in elevations in corticosterone.
Because the acute tailshock procedure has been previously validated as reliably producing behavioral depression, no additional verification of the stress level of the protocol was performed (Weiss et al., 1980, Simson et al., 1986).
Tailshock of HYPER line
A cohort of animals from the selectively bred HYPER line was included in the acute tailshock experiment. These rats showed no effect of the stress procedure on cell proliferation rate (Figure 4A, p = 0.36). However, post-experimental analysis of the effect of HYPER rating showed that control animals rated “good” had a significantly lower proliferation rate than control animals rated “poor” (p=0.0004). The proliferation rate of “poor” HYPER animals was similar to that of non-selected animals.
Figure 4.
In the HYPER line, cell proliferation rate in the dentate gyrus differed significantly according to hyper rating of unshocked control animals (A, p = 0.0004), but was not altered by acute tailshock stress. BDNF mRNA expression did not differ between hyper ratings and was not altered by acute tailshock stress (B). Data are presented as mean ± SEM. n = 4 for the “good” control group; n = 7 for the “good” tailshock group; n = 4 for the “poor” control group.
No significant effect of the tailshock procedure (p = 0.75) or of HYPER rating (p = 0.50) was seen on BDNF mRNA expression in any area examined (Figure 4B).
Resident-Intruder stress
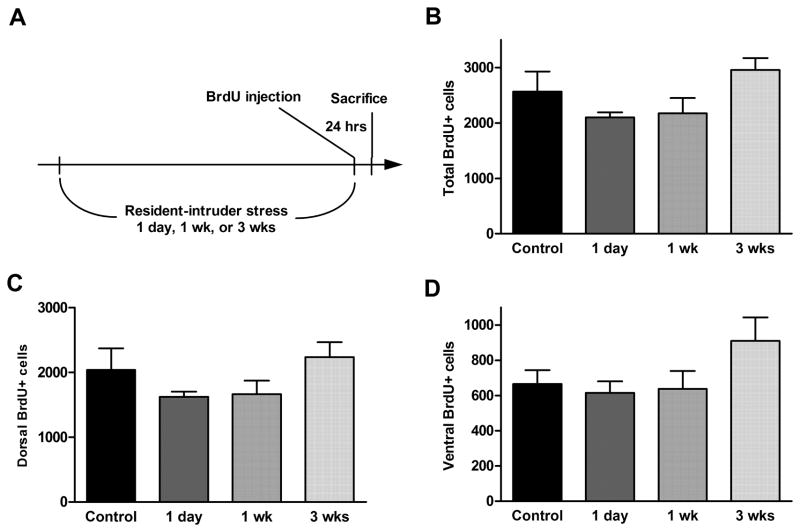
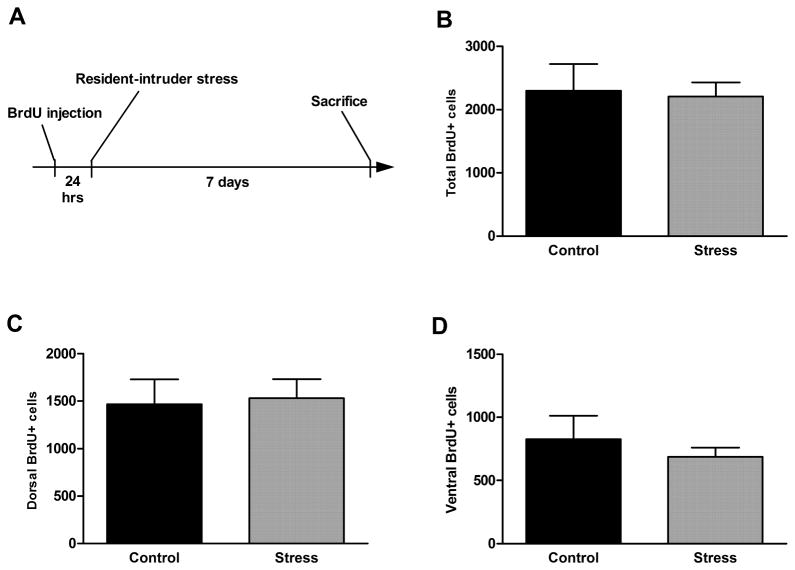
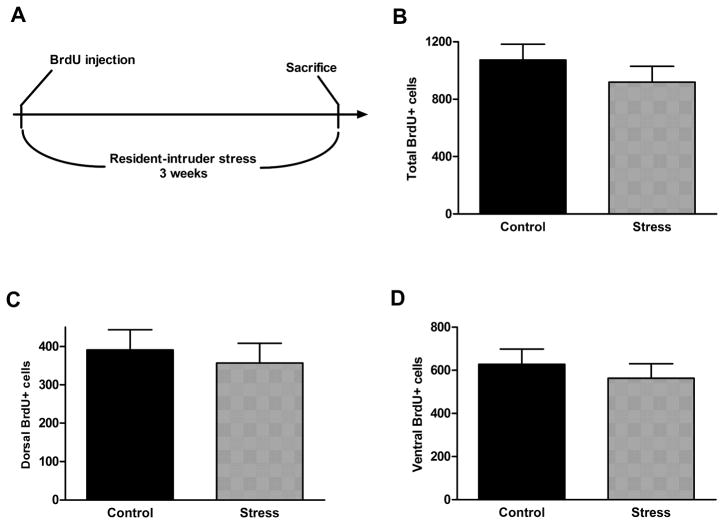
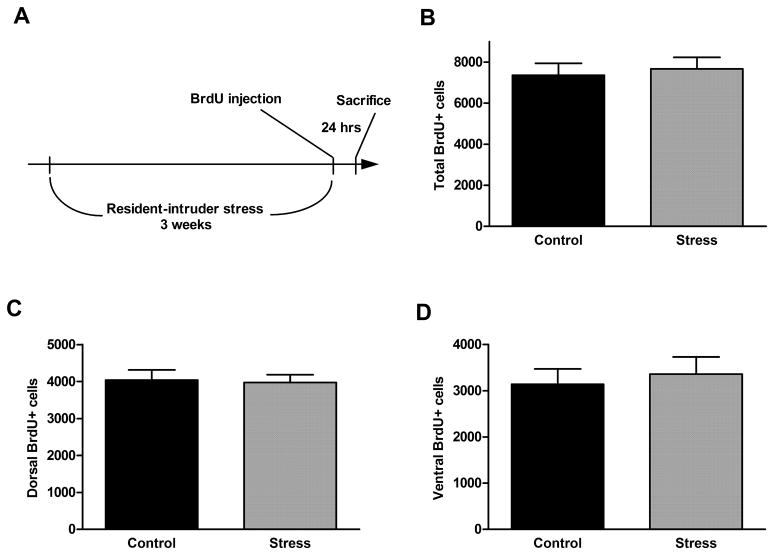
No significant effect on cell proliferation rate was seen after either 1 day (1 session), 7 days (3 sessions), or 21 days (9 sessions) of resident-intruder stress, either in the entire dentate gyrus or in the dorsal or ventral parts (Figure 5, p = 0.39, 0.65, and 0.13, respectively). In addition, there was no effect of resident-intruder stress during either a short survival period (7 days, Figure 6, p = 0.86 [total], 0.85 [dorsal], and 0.53 [ventral]) or a long survival period (21 days, Figure 7, p = 0.34 [total, 0.65 [dorsal], and 0.51 [ventral]).
Figure 5.
Acute (1 session), subchronic (3 sessions weekly for 1 week), or chronic (3 sessions weekly for 3 weeks) resident-intruder stress (experimental timeline shown in A) did not alter cell proliferation rate in the dentate gyrus (B) or in its dorsal (C) or ventral (D) parts. Data are presented as mean ± SEM. n = 6 for each stress group; n = 8 for control group.
Figure 6.
Acute resident-intruder stress (experimental timeline shown in A) did not alter short term survival of newborn cells in the dentate gyrus (B) or in its dorsal (C) or ventral (D) parts. Data are presented as mean ± SEM. n = 12 per group.
Figure 7.
Chronic resident-intruder stress (experimental timeline shown in A) did not alter long term survival of newborn cells in the dentate gyrus (B) or in its dorsal (C) or ventral (D) parts. Data are presented as mean ± SEM. n = 12 per group.
Additionally, no effect of chronic resident-intruder stress on cell proliferation rate was seen in adolescent animals (Figure 8, p = 0.70 [total], 0.86 [dorsal], and 0.68 [ventral]). The baseline rate of cell proliferation in the adolescent animals was found to be much higher than in adult animals, consistent with reports in the literature (Schlessinger et al., 1975).
Figure 8.
In adolescent animals, chronic resident-intruder stress (experimental timeline shown in A) did not alter cell proliferation rate in the dentate gyrus (B) or in its dorsal (C) or ventral (D) parts. Data are presented as mean ± SEM. n = 12 per group.
Discussion
Based upon the extant literature, the complete inability to induce a decrease in NPC proliferation in the dentate gyrus of normal adult rats with any of three different stressors was unexpected. Given that a variety of stressors have been reported to produce substantial deficits in neurogenesis, provided the stress intensity was sufficient, it is very surprising that increasing the severity and length of exposure of the tailshock and resident-intruder protocols to the upper boundaries of acceptable laboratory procedure led to no decrease in NPC proliferation.
Because the original goal of this project was to explore novel pharmacological regulation of neurogenesis under both stressed and unstressed conditions, rather than undertaking a comprehensive study of the relation between stress and neurogenesis, the experiments presented here were not designed de novo but were intended as a necessary first step, namely replications of specific successful experiments previously reported in the literature. The mildest stressor, a single period of restraint, was modeled after a successful reduction of cell proliferation of approximately 30% reported by Vollmayr et al. (2003).
After failure to confirm this result, tailshock was chosen as the most intense stressor available and familiar to this lab. The first tailshock experiment (subchronic) was performed multiple times, initially according to the single session footshock protocols reported as successful by Vollmayr et al. (2003) and Malberg and Duman (2003) and, after initial failure to replicate previously reported results, using increasing shock current over multiple sessions also failed to replicate previously reported results (only the final, most intense, version of the experiment is included in this article). Even application of a long-standing tailshock procedure with behavioral and endocrine validation (the acute experiment; Figures 2 and 4) yielded no success.
Finally, resident-intruder stress, thought by some to be a more salient and naturalistic stressor and therefore potentially a more valid model mimicking human depression, was explored in both acute and chronic versions, according to the previous work of Yap, et al. (2006) and Pulliam et al. (2009) (figures 5 and 8). After Thomas et al. (2007) reported that survival of neuroblasts through maturation might be affected by the stressor while cell proliferation was not, alternate experimental timelines were attempted, assessing survival of BrdU-labeled cells over one or three weeks of continued resident-intruder stress sessions, but again, no stress effect was observed (Figures 6–7).
In these cell survival experiments, it is possible that the longer periods (up to 3 weeks) between BrdU labeling and sacrifice mean that cell counts within a designated area (in and near the SGZ) reflect not only the number of surviving cells but also the migration rate of these cells. Thus, had we found a difference in the number of labeled cells remaining several weeks after BrdU labeling, this difference might be attributed to a change in rate of cell survival, migration, or a combination of both.
The present results clearly demonstrate that a severe stressor is not itself sufficient to alter the proliferation rate of neural progenitor cells, findings discordant with the commonly accepted hypothesis that hippocampal neurogenesis is finely regulated by stress. Perhaps there is an as yet unidentified component of stress or some factor concurrent with it which is responsible for the reported effects of “stress” on neurogenesis, rather than the stress per se. A number of possible speculative factors include: 1) subtle characteristics of the stress experiences, such as accessible coping mechanisms, auditory or visual cues, or handling; 2) differences in housing environments such as ambient noise level or staff activity; 3) prior experiences in breeding facilities or shipping; etc. Because each different stress procedure was conducted in a different room and animals for the acute tailshock experiment were housed in a different colony, it is unlikely that some condition unique to our environment is responsible for the lack of effects seen. Additionally, because our lab has extensive experience in stress neurobiology and behavioral experiments, the personnel responsible for contact with the animals are very experienced in minimizing stressful confounds in housing and experimental conditions, and other (non-neurogenesis-related) effects of stress were observed in other experiments concurrently using the same facilities and animal suppliers. Moreover, given that significant differences in cell proliferation have been detected by our lab within the HYPER line (here) and following chronic lithium administration (Hanson et al., 2011), it is very unlikely that the failure to demonstrate a stress effect is due to a problem with our assessment techniques. In other experiments, we have used Ki-67 immuncytochemistry in addition to BrdU; these two methods have shown highly correlated results, providing further evidence that our measurements of proliferation rate are valid. Although differences in levels of neurogenesis between rat strains do exist (Husum et al., 2006, Kronenberg et al., 2007, Alahmed and Herbert, 2008), the strain used in these experiments (Sprague-Dawley) is by far the most commonly utilized in the neurogenesis literature and is used in the particular experiments cited as examples for the stress procedures performed here, suggesting that strain differences are not a consideration in the failure to replicate the work of others.
We did not perform behavioral testing to assess the learned helplessness in these animal cohorts, although our tailshock protocols are similar to those resulting in learned helplessness in other studies, and our social defeat protocol is likewise similar to those reported to result in social avoidance behavior. Indeed, these authors (cf. Weiss et al. 1980; Simson et al. 1986) were instrumental in developing and characterizing rat models of stress-induced behavioral depression (also called learned helplessness) involving tail shock stressors such as those used here. According to the reports of Vollmayr et al. (2003) and Malberg and Duman (2003), decreased cell proliferation is caused by inescapable stress itself (which typically produces learned helplessness in many but not all animals), regardless of whether this stress leads to the development of learned helplessness. Thus, we felt that behavioral testing would be superfluous, although presumably many of our stressed animals would have exhibited learned helplessness had we performed those tests.
It is also possible that individual variation, specifically a predisposition to depression as a result of hereditary factors or of early life experience, plays an important role in stress-induced decreases in neurogenesis. There is some support for this hypothesis, because altered neurogenesis has been reported in the Flinders sensitive rat, a selectively bred line with some characteristics of depression (Bjornebekk et al., 2005, Husum et al., 2006), and in adult rats subjected to maternal separation as pups (Mirescu et al., 2004, Karten et al., 2005). The HYPER line was used here in the hope that these animals’ enhanced behavioral responsiveness to stress might be linked to an enhanced response of neurogenesis to stress, and that therefore we might be able to reduce neurogenesis using an experimental stressor that was unsuccessful in non-selected rats. This did not turn out to be the case, as HYPER rats, like their non-selected counterparts, showed no effect of tailshock on either cell proliferation or hippocampal BDNF expression. Analysis of those with high HYPER ratings (which can be considered “true” hypers, as the hyper phenotype is not fully penetrant without continued selective breeding in every generation) did show a significantly lower rate of baseline (unstressed) proliferation, although the possibility exists that this altered baseline is due to an interaction of the hyper phenotype with the stress of the earlier screening procedure experienced by all HYPER animals (described in the “HYPER rating” subsection of Methods), rather than being a function of the phenotype itself. This finding (altered baseline neurogenesis without altered stress responsivity) is similar to results obtained in other rat models of depression, such as the Flinders sensitive line and maternally separated pups, noted above.
Another possibility is that the experience of stress has been conflated in many experiments with the acquisition of fear learning. Some reports allude to this by assessing the development of poor escape-avoidance behavior (learned helplessness) following a stressor. Vollmayr et al. (2003) reported that only those animals which became helpless following an inescapable shock showed decreased cell proliferation, while those which were exposed to the shock but did not become helpless maintained normal proliferation levels. Malberg and Duman (2003) eliminated animals which failed a post-shock test criterion establishing helplessness from their cohorts, such that their results comprise not strictly an effect of inescapable shock but of learned helplessness, in concordance with Vollmayr et al. (2003). This hypothesis is supported by results from Pham et al. (2005), who reported a shock-induced decrease in proliferation only when animals had previous exposure to the shock chamber, allowing them to develop normal fear conditioning. Animals shocked immediately upon introduction to the chamber did not develop fear conditioning and did not exhibit decreased cell proliferation, despite highly elevated corticosterone levels. Because our experimental protocols involved initiating stressors quickly enough to prevent context-dependent fear conditioning, the failure of our animals to demonstrate reduced cell proliferation is consistent with these observations.
The lack of effect of these stress procedures on BDNF mRNA expression was likewise surprising. Although BDNF has sometimes been shown to increase immediately following acute stress, a detailed study of the time-course of expression revealed that it reaches a nadir 24 hours post-stress (Marmigere et al., 2003, Pizarro et al., 2004). Other studies of repeated stress show BDNF expression is lowest immediately following the final stress, but still significantly lower than control 24 hours post-stress (Murakami et al., 2005, Bland et al., 2007). Based on this, 24 hours is an appropriate time point at which we would expect to see decreased BDNF mRNA expression following a stress session. However, the strong link between BDNF expression and regulation of neurogenesis makes it perhaps less surprising to observe no change in BDNF expression than it would be to observe a change in BDNF in the absence of a change in patterns of neurogenesis.
In summary, our results challenge the prevailing view that adult hippocampal neurogenesis, including the rate of NPC proliferation, is negatively regulated by any stressor of sufficient intensity. The procedures used cover a range of stress modalities and were surely profoundly stressful, yet they failed to alter any measure of neurogenesis assessed. The complex nature of stress makes reproducibility of results a common problem in the field, because it is impossible to monitor and report every variable contributing to the stress level in achieved in a laboratory animal, but these experiments have credibly negated such subtle environmental factors by varying procedures, locations, and even animal populations, while consistently producing very intense levels of stress in the experimental groups and minimal levels in the controls. The simple “stress decreases neurogenesis” maxim has been challenged by several other hypotheses, as presented briefly above, and these findings strongly support one of these more complex explanations for this relationship.
Acknowledgments
Role of the funding source
This work was supported by NIH MH-42088 and DA-15040 (NDH). Previous support for the development of selectively bred lines was provided by the Theodore and Vada Stanley Foundation of the National Alliance for the Mentally Ill (NAMI). These sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Serum corticosterone analysis was performed by Robert Bonsall, Ph.D. and Milburn Emery, M.S. The authors are also grateful to the valuable training and insight provided by Ronald Duman, Ph.D., Yale University.
Footnotes
Conflict of interest
Charles B. Nemeroff, M.D., Ph.D., served on the Board of Directors of NovaDel Pharma and Mt. Cook Pharma. He served on the Scientific Advisory Board of, AstraZeneca Pharmaceuticals, CeNeRx BioPharma, NovaDel Pharma, PharmaNeuroboost, the American Foundation for Suicide Prevention (AFSP) and NARSAD. He holds stock, stock options or equity in CeNeRx BioPharma, Corcept, NovaDel Pharma, PharmaNeuroboost, and Revaax Pharma. He holds two US patents: Method and devices for transdermal delivery of lithium, and Method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters.
Michael J. Owens, Ph.D., has received research grants from Eli Lilly, Lundbeck A/S, Cyberonics, Ortho-McNeil Janssen, AstraZeneca, and Dainippon Sumitomo Pharma. He serves as a consultant for H. Lundbeck A/S. He holds one US patent: Method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters (US 7,148,027 B2).
Nicola D. Hanson, Ph.D., Jay M. Weiss, Ph.D., and Katherine A. Boss-Williams, Ph.D., have no financial conflicts of interest.
Contributors
Nicola D. Hanson, Ph.D. played the primary role in experimental design and execution, data analysis, and manuscript preparation. Katherine A. Boss-Williams, Ph.D. and Jay M. Weiss, Ph.D. assisted in the design and execution of the tailshock experiments. Michael J. Owens, Ph.D. and Charles B. Nemeroff, Ph.D. performed advisory functions in all stages of experimental design and interpretation. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alahmed S, Herbert J. Strain differences in proliferation of progenitor cells in the dentate gyrus of the adult rat and the response to fluoxetine are dependent on corticosterone. Neuroscience. 2008;157:677–682. doi: 10.1016/j.neuroscience.2008.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornebekk A, Mathe AA, Brene S. The antidepressant effect of running is associated with increased hippocampal cell proliferation. Int J Neuropsychopharmacol. 2005;8:357–368. doi: 10.1017/S1461145705005122. [DOI] [PubMed] [Google Scholar]
- Bland ST, Schmid MJ, Greenwood BN, Watkins LR, Maier SF. Behavioral control of the stressor modulates stress-induced changes in neurogenesis and fibroblast growth factor-2. Neuroreport. 2006;17:593–597. doi: 10.1097/00001756-200604240-00008. [DOI] [PubMed] [Google Scholar]
- Bland ST, Tamlyn JP, Barrientos RM, Greenwood BN, Watkins LR, Campeau S, Day HE, Maier SF. Expression of fibroblast growth factor-2 and brain-derived neurotrophic factor mRNA in the medial prefrontal cortex and hippocampus after uncontrollable or controllable stress. Neuroscience. 2007;144:1219–1228. doi: 10.1016/j.neuroscience.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Pandey GN, Dwivedi Y. Hippocampal cell proliferation regulation by repeated stress and antidepressants. Neuroreport. 2006;17:863–867. doi: 10.1097/01.wnr.0000221827.03222.70. [DOI] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Czeh B, Welt T, Fischer AK, Erhardt A, Schmitt W, Muller MB, Toschi N, Fuchs E, Keck ME. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry. 2002;52:1057–1065. doi: 10.1016/s0006-3223(02)01457-9. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Duric V, McCarson KE. Persistent pain produces stress-like alterations in hippocampal neurogenesis and gene expression. J Pain. 2006;7:544–555. doi: 10.1016/j.jpain.2006.01.458. [DOI] [PubMed] [Google Scholar]
- Hanson ND, Nemeroff CB, Owens MJ. Lithium, but not fluoxetine or the CRF1 receptor antagonist R121919, increases cell proliferation in the adult dentate gyrus. J Pharm Exp Ther. 337:180–186. doi: 10.1124/jpet.110.175372. http://jpet.aspetjournals.org/content/early/2011/01/10/jpet.110.175372.full.pdf+html. [DOI] [PMC free article] [PubMed]
- Husum H, Aznar S, Hoyer-Hansen S, Larsen MH, Mikkelsen JD, Moller A, Mathe AA, Wortwein G. Exacerbated loss of cell survival, neuropeptide Y-immunoreactive (IR) cells, and serotonin-IR fiber lengths in the dorsal hippocampus of the aged flinders sensitive line “depressed” rat: Implications for the pathophysiology of depression? J Neurosci Res. 2006;84:1292–1302. doi: 10.1002/jnr.21027. [DOI] [PubMed] [Google Scholar]
- Karten YJ, Olariu A, Cameron HA. Stress in early life inhibits neurogenesis in adulthood. Trends Neurosci. 2005;28:171–172. doi: 10.1016/j.tins.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Lippoldt A, Kempermann G. Two genetic rat models of arterial hypertension show different mechanisms by which adult hippocampal neurogenesis is increased. Dev Neurosci. 2007;29:124–133. doi: 10.1159/000096217. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Marmigere F, Givalois L, Rage F, Arancibia S, Tapia-Arancibia L. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus. 2003;13:646–655. doi: 10.1002/hipo.10109. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: A mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2009;20:596–607. doi: 10.1002/hipo.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton P. Principles and practices of unbiased stereology: An introduction for bioscientists. Johns Hopkins; Baltimore, MD: 2002. [Google Scholar]
- Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005;53:129–139. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Pham K, McEwen BS, Ledoux JE, Nader K. Fear learning transiently impairs hippocampal cell proliferation. Neuroscience. 2005;130:17–24. doi: 10.1016/j.neuroscience.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Pizarro JM, Lumley LA, Medina W, Robison CL, Chang WE, Alagappan A, Bah MJ, Dawood MY, Shah JD, Mark B, Kendall N, Smith MA, Saviolakis GA, Meyerhoff JL. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res. 2004;1025:10–20. doi: 10.1016/j.brainres.2004.06.085. [DOI] [PubMed] [Google Scholar]
- Pulliam JV, Dawaghreh AM, Alema-Mensah E, Plotsky PM. Social defeat stress produces prolonged alterations in acoustic startle and body weight gain in male Long Evans rats. J Psychiatr Res. 2009;44:106–111. doi: 10.1016/j.jpsychires.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975;159:149–175. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Mathew J, Sisti HM, Edgecomb C, Beckoff S, Dalla C. Neurogenesis and helplessness are mediated by controllability in males but not in females. Biol Psychiatry. 2007;62:487–495. doi: 10.1016/j.biopsych.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simson PG, Weiss JM, Ambrose MJ, Webster A. Infusion of a monoamine oxidase inhibitor into the locus coeruleus can prevent stress-induced behavioral depression. Biol Psychiatry. 1986;21:724–734. doi: 10.1016/0006-3223(86)90237-4. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Hotsenpiller G, Peterson DA. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J Neurosci. 2007;27:2734–2743. doi: 10.1523/JNEUROSCI.3849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmayr B, Simonis C, Weber S, Gass P, Henn F. Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry. 2003;54:1035–1040. doi: 10.1016/s0006-3223(03)00527-4. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Bailey WH, Pohorecky LA, Korzeniowski D, Grillione G. Stress-induced depression of motor activity correlates with regional changes in brain norepinephrine but not in dopamine. Neurochem Res. 1980;5:9–22. doi: 10.1007/BF00964456. [DOI] [PubMed] [Google Scholar]
- Weiss JM, West CH, Emery MS, Bonsall RW, Moore JP, Boss-Williams KA. Rats selectively-bred for behavior related to affective disorders: proclivity for intake of alcohol and drugs of abuse, and measures of brain monoamines. Biochem Pharmacol. 2008;75:134–159. doi: 10.1016/j.bcp.2007.09.027. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull. 2004;64:303–308. doi: 10.1016/j.brainresbull.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Takase LF, Kochman LJ, Fornal CA, Miczek KA, Jacobs BL. Repeated brief social defeat episodes in mice: effects on cell proliferation in the dentate gyrus. Behav Brain Res. 2006;172:344–350. doi: 10.1016/j.bbr.2006.05.027. [DOI] [PubMed] [Google Scholar]