Abstract
Background
The RIFLE criteria (Risk, Injury, Failure, Loss, End stage) are new consensus definitions for acute kidney injury (AKI) associated with increased mortality, however they have not been applied in lung transplantation (LTx). Using the RIFLE criteria we examined the impact of AKI on outcomes and cost in LTx.
Methods and Materials
We retrospectively reviewed all LTx patients at our institution since the lung allocation score (LAS) system (5/2005–8/2010). Using the Modification of Diet in Renal Disease (MDRD) formula, we assigned appropriate RIFLE class (R, I, F) comparing baseline creatinine to peak levels in first 7 days post-op. Generalized linear models assessed the effect of AKI on in-hospital and 1-year (yr) mortality. The financial impact of AKI was examined with hospital charges.
Results
106 LTx were performed during the study. Excluding patients bridged to LTx with ECMO, 84(86%) lived 1-yr. Median LAS was 37.1(IQR34.1-45.2). 39(36.7%) were RIFLE-I or F, and 14(13.2%) required renal replacement therapy (RRT). After adjusting for LAS, RIFLE-F had an increased relative rate (RR) of inhospital mortality (RR=4.76, 95% CI 1.65–13.7, p=0.004) and 1-yr mortality (RR=3.17, 95% CI 1.55–6.49, p=0.002). RIFLE-R and I were not associated with higher in-hospital or 1-yr mortality. Post-op RRT was associated with increased in-hospital (RR=28.2, 95% CI 6.18–128.1, p<0.001) and 1-yr mortality (RR=4.97, 95% CI 1.54–16.0, p<0.001). AKI patients had higher median hospital charges (AKI:$168,146 vs no AKI:$143,551, p=0.02).
Conclusions
This is the first study to show high rates of AKI using the new RIFLE criteria in LTx. RIFLE-F is associated with higher in-hospital mortality and 1-yr mortality. Less severe degrees of AKI are not associated with increased mortality. The financial burden associated with AKI is significant.
Keywords: Lung Transplantation, Acute Kidney Injury, RIFLE criteria
Background
Prior to 2004, numerous definitions for acute kidney failure were used in the medical literature, hindering comparisons between studies of kidney injury.1 To redress this issue, the Acute Dialysis Quality Initiative Workgroup set forth a new international consensus definition for acute kidney injury(AKI), known as the RIFLE criteria.2 This definition stratifies AKI into three grades of increasing severity(R-risk, I-injury, F-failure) and two outcome classes(L-loss, and E-end stage kidney disease). The three grades of severity for AKI are determined according to changes in either serum creatinine or urine output from baseline. The RIFLE criteria have since been validated in various patient populations including cardiac surgery patients undergoing routine coronary artery bypass. These studies found higher mortality rates with increasing RIFLE classification.3,4 Although many studies have examined the risk factors for chronic renal failure after LTx, the only major study to examine AKI after LTx used a pre-LAS cohort and focused only on RIFLE-I.5 Therefore, we applied the RIFLE criteria in an institutional cohort of LTx recipients to examine the impact of AKI on outcomes and cost in the post-LAS era.
Methods
Patient Data
After institutional review board approval, we performed a retrospective review of the prospectively maintained LTx database at the Johns Hopkins Hospital. The study period included the post-LAS era only(5/2005–8/2010). Children(<18yrs) and patients with prior LTx were excluded. Patients undergoing re-transplantation were excluded because prior national cohort studies examining the impact of LAS on survival have only used primary LTx recipients.6,7 All pertinent demographic, donor, and operative information was extracted from the LTx database. For all patients, LAS was calculated as described by Egan et al.8 The LAS value immediately prior to LTx was used for patients with multiple LAS values.
RIFLE classification
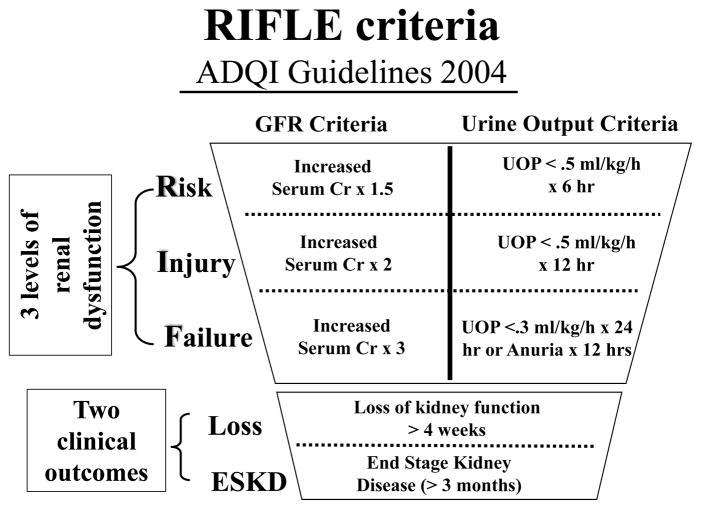
Patients were classified according to the maximum RIFLE class reached in the first seven postoperative days: (1)Risk when estimated glomerular filtration rate(eGFR) decreases by more than 25% from baseline; (2)Injury when eGFR decreases by more than 50% from baseline; and (3)Failure when eGFR decreases by more than 75% from baseline(Figure 1). The eGFR was calculated using the Modification of Diet in Renal Disease formula: eGFR = 175 × (Scr)−1.154 × (Age) −0.203 in milliliters per minute per 1.73 m2 of body surface area (the product was multiplied by 0.742 for women or by 1.212 for African-American patients).9 Changes from baseline serum creatinine levels were used to calculate percent change in eGFR and assign the appropriate RIFLE classification.4 Urine output criteria were not used, and the preoperative record was examined to ensure that baseline serum creatinine values(the last value before the day of surgical intervention) accurately reflected the patient’s baseline serum creatinine. Any patient who met criteria for RIFLE-R, I, or F was categorically defined as experiencing AKI.
Figure 1.
Detailed breakdown of the RIFLE criteria as defined according to the ADQI workshop. The RIFLE system also includes a differentiation between increasing severity of kidney injury (RIFLE R, I, or F) and two outcome categories L(loss, temporary dialysis) and E(endstage, permanent dialysis). GFR criteria (based on serum creatinine) and urine output criteria can be used to assign patients the appropriate RIFLE class.
Outcomes
The primary endpoints were in-hospital mortality and 1-year mortality. Additional postoperative data were collected and are depicted in (Table 2). Standardized definitions for primary graft dysfunction(PGD) grade 3 at 72 hours were used.10 We supplemented hospital records with the Social Security Death Index to ascertain survival status.
Table 2.
Pulmonary Outcomes and Resource Utilization According to RIFLE Classification
| Variable | Rifle-0 N=39 | Risk N=28 | Injury N=24 | Failure N=15 | P-value* |
|---|---|---|---|---|---|
| Vent time, hours (SD) | 46 (75) | 65 (80) | 76 (103) | 112 (114) | 0.2 |
| ICU LOS, days (SD) | 4 (7) | 11 (19) | 14 (40) | 18 (20) | 0.2 |
| Hospital LOS, days (SD) | 19 (18) | 38 (60) | 29 (36) | 38 (24) | 0.2 |
| Re-intubation, N (%) | 4 (11%) | 5 (18%) | 3 (13%) | 7 (46%) | 0.02 |
| Primary graft dysfunction, N (%) | 1 (4%) | 3 (14%) | 2 (10%) | 1 (13%) | 0.6 |
| Tracheostomy, N(%) | 5 (12%) | 4 (14%) | 4 (17%) | 7 (46%) | 0.048 |
| % Predicted FEV1 at 1 year, (SD) | 68% (19) | 78% (20) | 61% (31) | 55% (23) | 0.1 |
SD=standard deviation; FEV1=forced expiratory volume in 1 second; ICU=intensive care unit; LOS=length of stay
P-value for continuous variables based on one-way analysis of variance with post-hoc comparisons performed using the Tukey Kramer method, or chi-square analysis for categorical variables
Perioperative Care
LTx was performed using standardized techniques. Cardiopulmonary bypass was utilized only in patients intolerant of single lung ventilation or those with pulmonary hypertension. Inhaled nitric oxide was routinely initiated intraoperatively and weaned on the first postoperative day. Induction therapy was utilized at the transplant surgeon’s discretion. Maintenance immune-suppression was initiated in the early postoperative period and consisted of calcineurin-inhibitor based therapy with tacrolimus in all patients. Our algorithm consists of tacrolimus 0.5–1.0mg sublingual twice daily and is titrated to effect for a target serum level of 8–12ng/ml in the early post-operative period and a goal of 15ng/ml in the outpatient setting. Standardized care pathways for the post-operative period were used in all patients and provided by a multidisciplinary team in the ICU and hospital wards. The transplant surgeon guides diuretic and immune-suppression regimens, with aggressive diuretic therapy beginning on the first postoperative day.
Charge Data
Hospital charges are obtained through the billing department as reported to the Maryland State authorities, and represent index admission charges only, including charges incurred during the operation. The charges for RRT are included within total hospital charges. Financial information was inflation-adjusted according to the US Department of Labor Consumer Price Index in US dollars for 2010.
Statistical Analysis
Patients were stratified according to RIFLE class. Differences in demographic and operative variables among the RIFLE classes were compared using one-way analysis of variance(ANOVA) for normally distributed continuous variables. Chi-square analysis was used for categorical variables. Continuous variables are presented with the mean±standard deviation(SD). Categorical variables are shown in whole numbers and percentages. All charge data are presented as medians with interquartile ranges(IQR), as these data were not normally distributed. To confirm a non-parametric distribution, the data were visually inspected in graphical form and checked for skewness. Comparisons of charges were performed with nonparametric rank-sum analysis, and log transformed charges were analyzed using linear regression analysis. Postoperative complications were compared among RIFLE class using ANOVA or chi-square analysis.
To examine the impact of RIFLE class on in-hospital mortality and 1-year mortality, we estimated relative rate(RR) by constructing risk-adjusted generalized linear models(controlling for LAS values) with a Poisson distribution and log link as described by Zou.11 Multivariable regression was then performed for in-hospital and 1-year mortality. In addition to variables associated with each outcome on exploratory univariate analysis (p<0.2), those with biological plausibility were incorporated in a forwards and backwards stepwise fashion into the multivariable model. The likelihood ratio test and Akaike’s information criterion were used in a nested model approach to identify which covariates increased the explanatory power of the model. This method gives preference to more parsimonious models. Coefficients are presented with 95% confidence intervals(CI). The final model included: LAS, pre-LTx GFR, AKI, recipient age, donor cigarette use, postoperative tracheostomy, and RRT.
One-year survival was estimated using the Kaplan-Meier method, and the log-rank test compared survival curves among patients with AKI versus no AKI. A comparison was also performed between patients requiring RRT versus no RRT. P-values ≤0.05 were deemed significant. Analysis was performed using Stata v9.2 (StataCorp, College Station, Texas).
Results
Cohort Statistics
Between 5/2005 and 8/2010, 106 patients underwent LTx at our institution. The mean age was 49±13 years with 49% females(n=52). The distribution of recipient race was:81% Caucasian(n=86), 17% African American(n=18), and 2% Hispanic(n=2). Diagnoses were: Chronic obstructive pulmonary disease(COPD) in 33(31.3%), idiopathic pulmonary fibrosis(IPF) in 22(20.7%), cystic fibrosis(CF) in 21(19.8%), primary pulmonary hypertension in 4(3.7%), bronchiolitis obliterans syndrome in 6(5.6%), sarcoidosis in 7(6.6%), and other in 13(12.3%) of patients. Average LAS was 43.0(±14.8), and 93(87.7%) patients received bilateral LTx(BLTx). Twenty-two(21%) patients’ required ICU care pre-LTx. The majority of patients(67%) received induction immunotherapy with daclizumab(Zenapax, Hoffmann-LaRoche Inc, Nutley, NJ). Median wait-list time was 59(IQR:19-412) days. During the study, the number of adult LTx’s remained constant, ranging from 14–21 LTx’s annually.
RIFLE breakdown
During the first seven postoperative days, 67 patients(63.2%) had an episode of AKI: 28(26.4%) had RIFLE-R, 24(22.6%) RIFLE-I, and 15(14.2%) RIFLE-F. Fourteen(13.2%) patients needed RRT, and 8 of these patients died(57.1%). All patients requiring RRT underwent hemodialysis. Analysis of demographics, transplant diagnosis, and markers of clinical acuity according to RIFLE classification did not reveal any significant differences, though there was a trend toward patients with RIFLE-I or RIFLE-F requiring ICU care pre-LTx(Table 1).
Table 1.
Demographics According to RIFLE Classification
| Variable | Rifle-0 N=39 | Risk N=28 | Injury N=24 | Failure N=15 | P-value* |
|---|---|---|---|---|---|
| Age, years (SD) | 50 (15) | 51 (12) | 47 (13) | 50 (13) | 0.8 |
| Female Gender, N (%) | 20 (52%) | 12 (43%) | 11 (46%) | 9 (60%) | 0.7 |
| BMI | 24.6 (5.2) | 22.6 (4.2) | 24.1 (3.9) | 24.4 (5.4) | 0.4 |
| Days on Wait List, days (IQR) | 55 (19–149) | 86 (17–386) | 53 (20–777) | 285 (25–533) | 0.5 |
| Pre-op FEV1, % pred (SD) | 34 (19) | 34 (30) | 26 (17) | 37 (27) | 0.5 |
| ICU before LTx, N (%) | 7 (18%) | 5 (18%) | 7 (29%) | 3 (21%) | 0.7 |
| Bilateral LTx, N (%) | 30 (77%) | 27 (96%) | 22 (92%) | 14 (93%) | 0.1 |
| GFR <90mL/min/1.73 m2, N(%) | 13 (33%) | 11(39%) | 6 (25%) | 5 (33%) | 0.8 |
| LAS, (SD) | 43.1 (15.1) | 41.6 (10.6) | 44.9 (18.7) | 42.5 (14.9) | 0.9 |
SD=standard deviation; IQR=interquartile range; BMI=body mass index; FEV1=forced expiratory volume in 1 second; ICU=intensive care unit; GFR=glomerular filtration rate; LAS=lung allocation score
P-value for normally distributed continuous variables based on one-way analysis of variance with post-hoc comparisons performed using the Tukey Kramer method, or chi-square analysis for categorical variables. Wait list time is not normally distributed and the non-parametric Kruskall-Wallis rank test was used for group comparisons.
Outcomes and Survival
Average follow-up was 23±18 months. Median ICU LOS was 3 days(IQR: 2–6), and median hospital LOS was 17 days(IQR: 11–28). Breakdown of LOS data according to RIFLE class is shown in Table 2. Regarding pulmonary outcomes, the only differences were greater need for re-intubation and tracheostomy among RIFLE-F patients. The remaining pulmonary outcomes, including duration of mechanical ventilation, PGD, and % predicted forced expiratory volume in 1 second(FEV1) at one year, were equivalent among the four groups (Table 2). Although there was no statistical difference in the proportion of patients receiving bilateral LTx by RIFLE class, we performed a subgroup analysis examining FEV1 at one year among bilateral LTx recipients only, and no differences were detected.
During the study, 35(33.0%) patients died and 21(19.8%) patients did not survive one year. Inhospital mortality rates were roughly equivalent for RIFLE-0, R, and I, with higher in-hospital mortality for RIFLE-F. Three of 39(7.7%) RIFLE-0, 2 of 28(7.1%) RIFLE-R, 2 of 24(8.3%) RIFLE-I, and 4 of 15(26.7%) RIFLE-F patients died, respectively. Breakdown of patients according to RIFLE severity score and mortality data are presented in Table 3.
Table 3.
RRT and Mortality Rates According to RIFLE Classification
| RIFLE Category | No (%) | Dialysis No. (% of RIFLE category) N=14 | Hospital mortality No. (% of RIFLE Category) N=11 |
|---|---|---|---|
| Rifle-0 | 39 (36.8%) | 1 (2.5%) | 3 (7.7%) |
| Risk | 28 (26.4%) | 3 (10.7%) | 2 (7.1%) |
| Injury | 24 (22.6%) | 2 (8.3%) | 2 (8.3%) |
| Failure | 15 (14.1%) | 8 (53.3%)* | 4 (26.7%) |
P-value <0.05 by chi-square analysis and Bonferroni adjustment for multiple groups comparisons
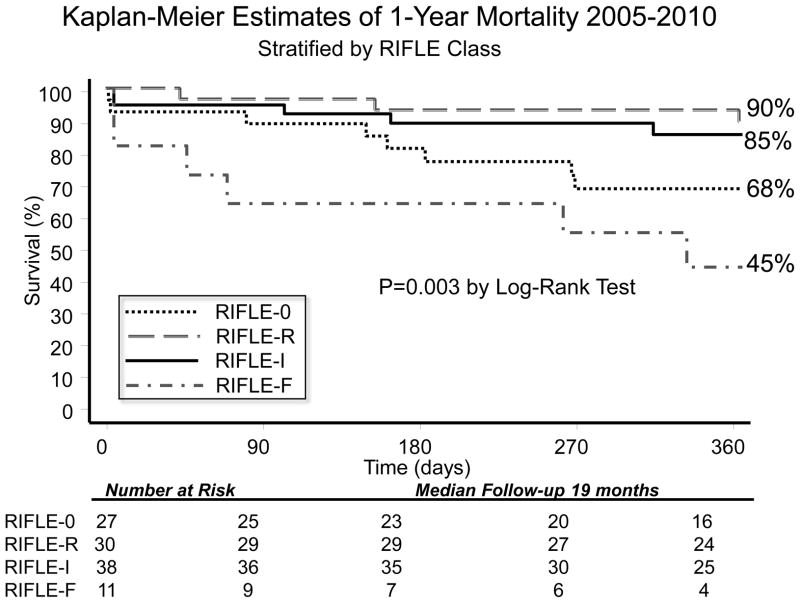
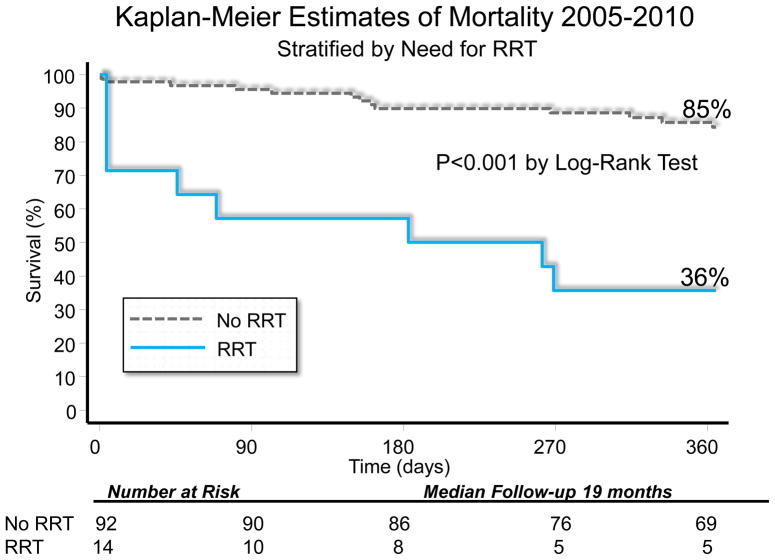
When the entire cohort was analyzed together without stratification, overall KM 1-year survival was 78.6%. After stratification by AKI versus no AKI, there was no difference in 1-year KM survival(AKI:78.7% versus no AKI:78.3%, p=0.7). When individual RIFLE categories were examined with KM analysis, there was worse 1-year survival for RIFLE-F patients(Figure 2). One-year survival was worse among patients requiring RRT(35.7% versus 85.8%, p<0.001)(Figure 3).
Figure 2.
Kaplan Meier Estimates of 1-Year Survival Stratified by RIFLE class. By Log-Rank analysis, there were significant differences in survival curves, with worse 1-yr survival among RIFLE-F (45% 1-yr survival), p<0.01.
Figure 3.
Kaplan Meier Estimates of 1-Year Survival Stratified by renal replacement therapy (RRT). By Log-Rank analysis, survival was worse in patients needing RRT (36% 1-yr survival) compared to no RRT (p<0.05).
Hospital charges
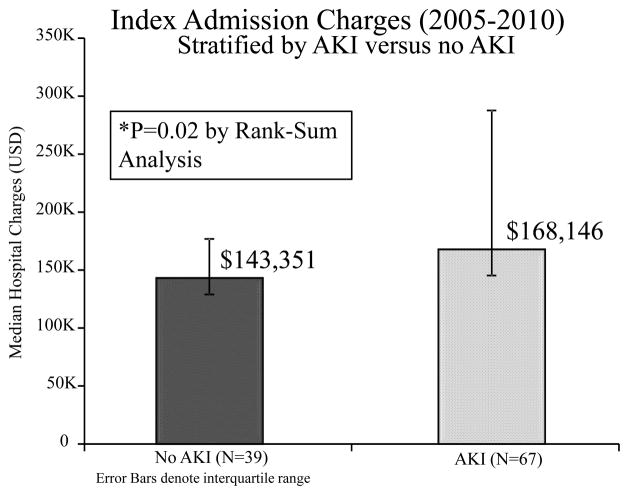
Median index admission hospital charges for the cohort overall was $165,236(IQR:136,155–245,805). By rank-sum analysis, patients with AKI had higher index admission charges compared with those without AKI($168,146 versus $143,551, p=0.02). The difference in hospital charges was more pronounced when examining patients who required RRT postoperatively(RRT:$391,917 versus no RRT:$159,331, p<0.001). A comparison of hospital charges among patients who survived to hospital discharge revealed no difference when compared to non-survivors(survivors:$162,134 IQR136,060–233,888 versus non-survivors:$188,755 IQR158,194–587,649, p=0.2). There was more variability among non-survivors, however.
Linear regression of charge data after logarithmic transformation revealed a positive association between AKI and index admission charges(coefficient:0.3, 95% CI 0.06–0.54, p=0.01), with a Spearman r-value of 0.26. A separate regression analysis revealed a more pronounced association between RRT and index admission charges(coefficient:0.8, 95% CI 0.50–1.03, p<0.001), with a Spearman r-value of 0.41.
Relative Rate Estimations
When analyzing in-hospital mortality after controlling for LAS, we observed that patients with RIFLE-F had a significant increase in relative mortality rates(RR:4.76, 95% CI 1.65–13.7, p=0.004). RIFLE-R, and I had no significant increase in relative rate of in-hospital mortality. Patients who required postoperative RRT had a more pronounced increase in relative rates of in-hospital mortality(RR:16.7, 95% CI 4.77–58.77, p<0.001). We also examined 1-yr mortality after controlling for LAS, and the increases in relative rates of mortality for RIFLE-F and RRT persisted in this analysis(Table 4).
Table 4.
Generalized Linear Model Regression Analysis Adjusted for LAS
| In-Hospital Mortality | 1-Year Mortality | |||||
|---|---|---|---|---|---|---|
| Relative Rate | 95% CI | P-value | Relative Rate | 95% CI | P-value | |
| RIFLE-0 | --- | Reference | --- | --- | Reference | --- |
| Risk | 0.27 | 0.04–2.07 | 0.2 | 0.57 | 0.20–1.63 | 0.3 |
| Injury | 0.61 | 0.17–2.24 | 0.5 | 0.63 | 0.26–1.48 | 0.3 |
| Failure | 4.76 | 1.65–13.7 | 0.004 | 3.17 | 1.55–6.49 | 0.002 |
| RRT | 16.7 | 4.77–58.77 | <0.001 | 4.41 | 2.27–8.54 | <0.001 |
On multivariate analysis, we found that AKI did not have a significant increase in the relative rate of either in-hospital or 1-yr mortality. As well, the other variables examined(age, pre-LTx GFR, LAS, donor cigarette use, postoperative tracheostomy) did not independently increase the risk of mortality. After risk-adjustment, only need for postoperative RRT had significantly increased relative rates of inhospital mortality or 1-yr mortality(Table 5).
Table 5.
Regression Analysis with Risk-adjusted Multivariable Generalized Linear Model
| In-Hospital Mortality | 1-Year Mortality | |||||
|---|---|---|---|---|---|---|
| Relative Rate | 95% CI | P-value | Relative Rate | 95% CI | P-value | |
| LAS | 1.01 | 0.98–1.04 | 0.4 | 1.00 | 0.98–1.02 | 0.8 |
| Pre-LTx GFR | 0.99 | 0.97–1.02 | 0.5 | 1.01 | 0.99–1.02 | 0.4 |
| AKI (RIFLE-R, I, or F) | 0.48 | 0.13–1.71 | 0.3 | 0.47 | 0.20–1.14 | 0.1 |
| Recipient Age | 1.00 | 0.96–1.03 | 0.9 | 1.01 | 0.99–1.02 | 0.7 |
| Donor cigarette use | 1.08 | 0.38–3.03 | 0.9 | 0.66 | 0.27–1.62 | 0.4 |
| Postoperative tracheostomy | 0.58 | 0.18–1.95 | 0.4 | 1.65 | 0.54–5.04 | 0.4 |
| RRT | 28.2 | 6.18–128.1 | <0.001 | 4.97 | 1.54–16.0 | 0.007 |
LAS=Lung Allocation Score; GFR=glomerular filtration rate; AKI=acute kidney injury; RRT=renal replacement therapy
Discussion
In this study, we found that only severe AKI(RIFLE-F) was independently associated with increased in-hospital or 1-yr mortality, whereas mild AKI(RIFLE-R or I) was not associated with increased risk of mortality. We also detected a marked increase in risk of in-hospital and 1-yr mortality for patients needing post-LTx RRT, and this effect was more pronounced for in-hospital mortality. Sixty-three percent of patients experienced AKI(as a binary variable RIFLE-R, I, or F), whereas 13% of patients required postoperative RRT. Our observed AKI rate is similar to the 56% AKI rate observed by Rocha et al. in the pre-LAS era, although they only incorporated RIFLE-I.5 There are several potential mechanisms specific to LTx that in part explain such high rates of AKI.
First, several authors have postulated “lung biotrauma,” whereby lung injury influences distant effects on the kidney.12–14 Imai et al. observed renal epithelial apoptosis in a mouse model of lung injury, while Gurkan et al. detected elevations in the inflammatory marker interleukin-6 in mouse kidney tissue in a similar model.12,14 Second, patients with respiratory failure are noted to have renal hypoperfusion, and this relative hypoperfusion may aggravate the injury attributable to hemodynamic instability during the perioperative period.15,16 Third, calcineurin inhibitors are a routine part of immune-suppression regimens, and are known to decrease renal perfusion due to vasoconstriction.17,18 Finally, LTx recipients are often subjected to aggressive diuresis in the immediate postoperative period to reduce pulmonary edema and protect allograft function, and they are also exposed to nephrotoxic antibiotics.
We used the highest serum creatinine level within the first seven postoperative days to detect perturbations in renal function that were due to insults incurred during the intraoperative and immediate postoperative period. In addition, all RIFLE classes were incorporated into our definition of AKI in order to assess the presence of a graded association with mortality; however, we did not observe such an association. Instead, there appeared to be an abrupt breakpoint, as only RIFLE-F was associated with a significant increase in mortality rates. We speculate this finding may be due, in part, to the aggressive fluid management strategy that we employ in the postoperative period. LTx recipients are routinely administered an intravenous loop diuretic within twelve hours postoperatively, and maintained on an aggressive diuretic regimen to achieve negative fluid balance, likely leading to decreased renal perfusion. The use of nephrotoxic agents(antifungals, antibacterials, and calcineurin inhibitors) may work in synergy with this renal hypoperfusion to cause kidney injury. Although we do not have information regarding serum levels of calcineurin inhibitors, exposure to these agents was uniform across all patients in the study.
Large single and multi-institution series confirm the high mortality risk associated with PGD, particularly grade 3 at any of the reported time points.19–21 In 1000 consecutive LTx, Kreisel et al. report a 15% absolute decrease in 1-yr survival among patients who developed PGD.20 Currey et al. described a protocol for post-LTx management aimed at achieving negative fluid balance, and found that average PGD grades were lower at 48 and 72 hours in the negative fluid balance group, suggesting a protective effect on the allograft.22 It is difficult to assess the iatrogenic component to the development of AKI(diuretic use, nephrotoxic agents) in our study. Our findings suggest that mild AKI(RIFLE-R or I) is well-tolerated, and our aggressive diuresis regimen is likely justified given the serious consequences of PGD. However, a proper balance between aggressive diuresis and adequate organ perfusion must be achieved. It is critical to emphasize that when AKI progresses to RIFLE-F or RRT, patients suffer markedly higher mortality and thus clinicians should bear caution in overly aggressive diuresis in the postoperative period.
After adjusting for LAS values only, we detected an independent effect of AKI on mortality for only RIFLE-F and RRT. When incorporating additional postoperative variables in the multivariable analysis, only RRT had significantly elevated risk of in-hospital and 1-yr mortality, and RRT has previously been found to independently predict mortality in cardiac surgery.23 Baseline GFR did not predict increased mortality, but this likely reflects the well-preserved baseline renal function of patients in this series. Except in dire situations, we strive to select patients for LTx with normal renal function. When examining patients according to individual RIFLE class, we did not observe a statistically significant difference in survival curves, although our power to detect differences is likely hampered by small sample size in each group. Comparing our study findings to those of Rocha et al. also suggests that implementation of the LAS has not dramatically altered the incidence of AKI, although to confirm this claim larger sample size is necessary. Unfortunately, large national registries lack post-LTx creatinine data needed to conduct this type of analysis.
The development of AKI after LTx was associated with an absolute difference of approximately $25,000 in additional hospital charges. AKI did have a positive relationship with increasing charges, albeit the r-value indicated low predictive ability of the regression model. The positive relationship between RRT and charges on regression analysis was more pronounced, and the association between LAS values, RRT, and cost has been shown in previous studies.24,25 When grouping all RIFLE classes collectively as AKI (data not shown), patients with AKI had longer ICU and hospital LOS, thus explaining the increased hospital charges.
Limitations
Data on RIFLE-E (endstage permanent dialysis) were not included because this information is not present in our database. In addition, it is unclear whether increasing severity of RIFLE score corresponds to a lower likelihood of recovery of renal function. It is unknown from these data whether those who only partially recover from their episode of AKI after LTx have worse long-term outcomes when compared to patients with complete recovery, and is a subject of future study. The urine output criteria, which also can be used to calculate the RIFLE class(Figure 1), pose another difficulty with employing the RIFLE system during retrospective analysis as these data are not readily available. Future prospective studies that employ the RIFLE system will be able to identify patients that meet the urine output criteria for improved RIFLE classification, as well as identify patients with temporary(L-loss) or Permanent (E-endstage) requirements for RRT.
An added limitation of this study is the use of hospital charges as a surrogate index for cost. However, the unique system for medical reimbursement in the State of Maryland offsets this issue. To contain costs, the Maryland Health Services Cost Review Commission(HSCRC) defines payment rates for both private and public insurers, including Medicare and Medicaid, within Maryland hospitals. Therefore, “cost shifting” by overcharging privately insured patients is absent. The authors’ institution HSCRC rate for charge payment has been cost + 1–3% over the study period. Our study is further limited by the retrospective design and relatively small sample size.
Conclusion
In summary, incorporating the RIFLE criteria as a consensus definition for AKI in LTx recipients, we found that mild degrees of AKI(RIFLE-R or I) were not associated with increased mortality risk, whereas severe AKI(RIFLE-F) was associated with worse 1-year mortality.
Figure 4.
Bar graph depicting median hospital charges by AKI versus no AKI. P<0.001 by Rank-sum analysis. Error bars denote interquartile range (IQR). Charges data adjusted for inflation according to consumer price index in US dollars for 2009.
Acknowledgments
No author has any relevant financial relationships to disclose. Dr. Arnaoutakis is the Irene Piccinini Investigator in Cardiac Surgery and Dr. George is the Hugh R. Sharp Cardiac Surgery Research Fellow.
Footnotes
Presented at: Poster Session, Thursday April 14th, 2010, Thirty-First annual meeting for International Society of Heart and Lung Transplantation, San Diego, California
Conflicts: The authors have no conflicts of interest to disclose.
References
- 1.Bellomo R, Kellum J, Ronco C. Acute renal failure: time for consensus. Intensive Care Med. 2001;27:1685–1688. doi: 10.1007/s00134-001-1120-6. [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuitunen A, Vento A, Suojaranta-Ylinen R, Pettila V. Acute renal failure after cardiac surgery: evaluation of the RIFLE classification. Ann Thorac Surg. 2006;81:542–546. doi: 10.1016/j.athoracsur.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 5.Rocha PN, Rocha AT, Palmer SM, Davis RD, Smith SR. Acute renal failure after lung transplantation: incidence, predictors and impact on perioperative morbidity and mortality. Am J Transplant. 2005;5:1469–1476. doi: 10.1111/j.1600-6143.2005.00867.x. [DOI] [PubMed] [Google Scholar]
- 6.Merlo CA, Weiss ES, Orens JB, Borja MC, Diener-West M, Conte JV, et al. Impact of U.S. Lung Allocation Score on survival after lung transplantation. J Heart Lung Transplant. 2009;28:769–775. doi: 10.1016/j.healun.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Weiss ES, Allen JG, Merlo CA, Conte JV, Shah AS. Lung allocation score predicts survival in lung transplantation patients with pulmonary fibrosis. Ann Thorac Surg. 2009;88:1757–1764. doi: 10.1016/j.athoracsur.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6:1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 9.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 10.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 11.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 12.Gurkan OU, O’Donnell C, Brower R, Ruckdeschel E, Becker PM. Differential effects of mechanical ventilatory strategy on lung injury and systemic organ inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2003;285:L710–718. doi: 10.1152/ajplung.00044.2003. [DOI] [PubMed] [Google Scholar]
- 13.Hoag JB, Liu M, Easley RB, Britos-Bray MF, Kesari P, Hassoun H, et al. Effects of acid aspiration-induced acute lung injury on kidney function. Am J Physiol Renal Physiol. 2008;294:F900–908. doi: 10.1152/ajprenal.00357.2007. [DOI] [PubMed] [Google Scholar]
- 14.Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289:2104–2112. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- 15.Kilburn KH, Dowell AR. Renal function in respiratory failure. Effects of hypoxia, hyperoxia, and hypercapnia. Arch Intern Med. 1971;127:754–762. [PubMed] [Google Scholar]
- 16.Navis G, Broekroelofs J, Mannes GP, van der Bij W, de Boer WJ, Tegzees AM, et al. Renal hemodynamics after lung transplantation. A prospective study. Transplantation. 1996;61:1600–1605. doi: 10.1097/00007890-199606150-00009. [DOI] [PubMed] [Google Scholar]
- 17.Puschett JB, Greenberg A, Holley J, McCauley J. The spectrum of ciclosporin nephrotoxicity. Am J Nephrol. 1990;10:296–309. doi: 10.1159/000168123. [DOI] [PubMed] [Google Scholar]
- 18.Zaltzman JS, Pei Y, Maurer J, Patterson A, Cattran DC. Cyclosporine nephrotoxicity in lung transplant recipients. Transplantation. 1992;54:875–878. doi: 10.1097/00007890-199211000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124:1232–1241. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 20.Kreisel D, Krupnick AS, Puri V, Guthrie TJ, Trulock EP, Meyers BF, et al. Short- and long-term outcomes of 1000 adult lung transplant recipients at a single center. J Thorac Cardiovasc Surg. 2011;141:215–222. doi: 10.1016/j.jtcvs.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Christie JD, Bellamy S, Ware LB, Lederer D, Hadjiliadis D, Lee J, et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2011;29:1231–1239. doi: 10.1016/j.healun.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Currey J, Pilcher DV, Davies A, Scheinkestel C, Botti M, Bailey M, et al. Implementation of a management guideline aimed at minimizing the severity of primary graft dysfunction after lung transplant. J Thorac Cardiovasc Surg. 2010;139:154–161. doi: 10.1016/j.jtcvs.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 23.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 24.Arnaoutakis GJ, Allen JG, Merlo CA, Sullivan BE, Baumgartner WA, Conte JV, et al. Impact of the lung allocation score on resource utilization after lung transplantation in the United States. J Heart Lung Transplant. 2011;30:14–21. doi: 10.1016/j.healun.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srisawat N, Lawsin L, Uchino S, Bellomo R, Kellum JA. Cost of acute renal replacement therapy in the intensive care unit: results from The Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) study. Crit Care. 2010;14:R46. doi: 10.1186/cc8933. [DOI] [PMC free article] [PubMed] [Google Scholar]