Abstract
Accumulating laboratory studies have implicated the mobilization of bone marrow (BM)-derived stem cells in brain plasticity and stroke therapy. This mobilization of bone cells to the brain is an essential concept in regenerative medicine. Over the past ten years, mounting data have shown the ability of bone marrow–derived stem cells to mobilize from BM to the peripheral blood (PB) and eventually enter the injured brain. This homing action is exemplified in BM stem cell mobilization following ischemic brain injury. Various BM-derived cells, such as hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), endothelial progenitor cells (EPCs) and very small embryonic-like cells (VSELs) have been demonstrated to exert therapeutic benefits in stroke. Here, we discuss the current status of these BM-derived stem cells in stroke therapy, with emphasis on possible cellular and molecular mechanisms of action that mediate the cells’ beneficial effects in the ischemic brain. When possible, we also discuss the relevance of this therapeutic regimen in other central nervous system (CNS) disorders.
Keywords: stem cells, stroke, endogenous, homing, migration, transplantation, growth factor secretion
1. Introduction
Increasing evidence supports the capability of bone marrow (BM)-derived cells to mobilize from the marrow to the peripheral blood (PB) and home the injured tissue/organ. This homing action is exemplified in BM stem cell mobilization following ischemic brain injury. This article will review the accumulating laboratory supporting evidence of the ostensible feasibility to induce the therapeutic mobilization of transplanted BM stem cells for brain plasticity and remodeling following a stroke. There have been studies published involving similar research into the mobilization of BM-derived cells; however, where one paper has focused on malignancies and cancer, this paper is novel in its focus on CNS-disorders and stroke therapy (Hess and Allan, 2011).
Heterogeneous populations of stem and progenitor cells are found in the bone marrow (Herzog et al., 2003). The more developed research pertains to hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs). Additionally, endothelial progenitor cells (EPCs) and very small embryonic-like stem cells (VSELs) have also been isolated from the BM (Figure 1). Previous reports have discussed in vitro differentiation of BM-derived stem cells into neurons following exposure to various inducing regimens (Munoz-Elias et al., 2003), and their secretion of growth factors critical for neuronal survival (Hara et al., 2008; Hess and Borlongan, 2008a, 2008b). Interest in these stem cells as donors has increased as researchers look to use the BM-derived cells as therapy for neurological disorders, such as stroke. Although the concepts discussed here are derived primarily from stroke studies, they have a broad significance in treating other CNS diseases. BM-derived stem cells have been used in the laboratory as a potential therapeutic for various CNS disorders, such as epilepsy (Venturin et al., 2011), Parkinson’s disease (Khoo et al., 2011), and Alzheimer’s disease (Nikolic et al., 2008).
Figure 1.
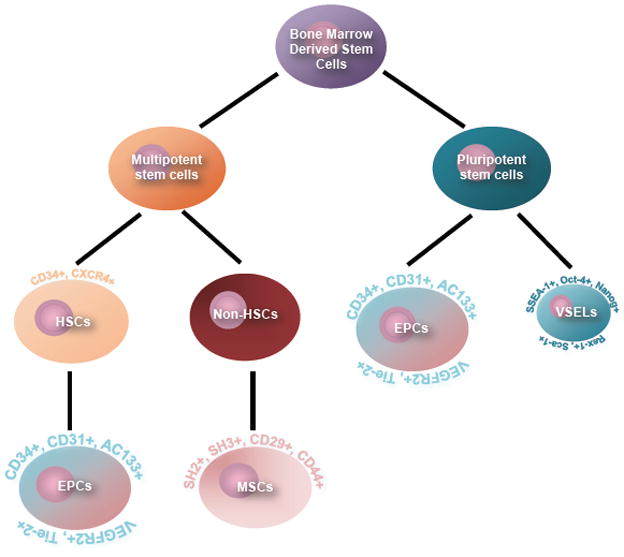
Bone Marrow-Derived Stem Cells. Schematic diagram shows subsets of bone marrow-derived stem cells, including HSCs, MSCs, EPCs, and VSELs, which have been examined in the laboratory and are rapidly being translated into clinical applications as efficacious stem cell source for transplantation therapy in stroke.
Stroke is a major cause of death in the US and around the world. Over the last decade, stem cell therapy has shown promise as an experimental treatment for stroke (Borlongan et al., 2008; Chopp et al., 2009; “Stem Cell Therapies”, 2009). The first clinical trial occurred in 1998 (Kondziolka et al, 2000; Meltzer et al., 2001; Nelson et al., 2002). Recently, there has been an increase in cell-based therapy clinical trials for stroke patients.
There are distinct therapeutic advantages in stroke to use a minimally invasive intra-arterial or intravenous transplantation. However, this peripheral route of cell injection requires mobilization of the cells and their secreted products proximal to the site of injury in order to induce brain plasticity and remodeling. A better understanding of mechanisms underlying the homing of cells from the periphery to the ischemic brain is likely to aid in optimizing cell therapy for stroke. Stem cells can be mobilized from various niches within the body. A stem cell niche is any microenvironment where stem cells reside. Various stem cell phenotypes are found within different niches. For example, neural stem cells are found in the dentate gyrus and subventricular zone of the brain, whereas HSCs can be found in the BM. Here, the specific (sometimes overlapping) mobilization pathways mediating the homing of HSCs, MSCs, EPCs and VSELs from BM (and other stem cell niches) to the intravascular space and into the ischemic brain are explored.
We are cognizant that no stroke is the same. The size of the stroke has a greater influence than the size of non-migrating transplanted cells. Heterogenity of stroke shape, size, and location complicate a surgical trial design. Intravenous and intra-arterial delivery requires an open blood brain barrier (BBB) and access to the penumbra, which limits the timing of therapy to a period when the BBB is compromised.
2. Blood is Thicker than Water: The Hematopoietic Stem Cells
HSCs have the ability to repopulate removed BM, a central characteristic feature demonstrating the proliferative capacity of HSCs (Lapidot and Kollet, 2010). The quiescent HSCs, while low in number during homeostasis, can quickly proliferate and become mobile with increased migration from their resident BM to blood circulation in response to injury (Lapidot et al., 2005; Nervi et al., 2006; Papayannopoulou and Scadden, 2008). A key chemokine implicated in HSCs’ quiescence within the BM is stromal derived factor-1 (SDF-1, also termed CXCL12) acting via its major receptor CXCR4 (Lapidot et al., 2005; Sugiyama et al., 2006). SDF-1 is also highly expressed in other stem cells niches, similarly playing the role of preserving HSC primitive status (Lapidot and Kollet, 2010; Suiyama et al., 2006). SDF-1 was originally found to be expressed by murine and human BM endothelial and endosteal bone lining stromal cells (Imai et al., 1999), and later also found in various tissues, including skin (Pablos et al., 1999), epithelial cells in human liver bile ducts (Kollet et al., 2003), and brain endothelium (Stumm et al., 2002). In the hematopoietic system, it is a crucial chemoattractant for CXCR4-expressing BM-derived cells, including HSCs. This SDF-1/CXCR 4 chemoattractant pathway is essential for HSC migration and seeding. The activation of SDF-1 stimulates the migration of the stem cells from the BM reservoir into the circulation (Dar et al., 2006).
In order to deploy from and re-enter the stem cell niches, HSC movement includes the adhesion to the vascular wall with mobilization across the endothelial blood-BM barrier (Lapidot and Kollet, 2010). The central nervous system (CNS) contributes to this HSC mobilization via cytokine production, and under stress condition (i.e., stroke), can amplify the recruitment of HSCs into the brain (Figure 3) (Lapidot et al., 2005; Lapidot and Kollet, 2010; Nervi et al., 2006; Papayannopoulou and Scadden, 2008). The quantity of endogenous BM-derived stem cells that migrate to the brain during the acute phase of injury is initially upregulated (Figure 3). However, the number of endogenous BM-derived stem cells that mobilize and home to the injured brain during the chronic phase of injury is decreased (Figure 3). This cytokine-mediated recruitment of HSCs from BM to the circulation is employed in clinical protocols, such as treatment with granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF), for the creation of ample supply of HSCs for brain repair (Nervi et al., 2006; Papayannopoulou and Scadden, 2008).
Figure 3.

Migration of Endogenous and Exogenous Stem Cells in the Acute and Chronic Phases of Stroke. Quinescent bone marrow-derived stem cells are mobilized and home to the site of injury via the CXCR4/SDF-1 signaling pathway during the acute phase of injury (Panel A). During the chronic stage of injury, the quantity of BM-derived stem cells that are mobilized and home to the ischemic site is greatly decreased (Panel B). Like endogenous stem cells, transplanted stem cells utilize the CXCR4/SDF-1 pathway to migrate to the site of injury during the acute phase of ischemia (Panel C) and the chronic phase of ischemia (Panel D).
2.1 Migrating Through the Blood Stream: The Role of the Microenvironment
As previously noted, cues by CNS-induced cytokines have the possibility of being migratory signals to HSCs. Recently, a crosstalk via the neurotransmitter catecholaminergic signaling pathway has been proposed to link the nervous system and the immune system (Lapidot and Kollet, 2010). The sympathetic system produces catecholamines, which are amplified during stress situations and secreted by activated leukocytes, including lymphocytes and lipopolysaccharide-stimulated macrophages, harnessing mobilization of BM-derived stem cells (Lapidot and Kollet, 2010). In particular, catecholamines, neurotransmitters secreted either from the sympathetic system into the blood circulation or directly from the nerve endings in the BM, acting via a paracrine fashion can modulate the migration of BM from its residence to the injured CNS (Kalinkovich et al., 2009). That this directed migration might entail a ligand-receptor mechanism is indicated by upregulated levels of catecholaminergic receptors in mobilized human CD34 HSCs, compared to tissue-anchored BM human CD34 HSCs. The catecholaminergic receptor expression is further increased by repeated stimulation with the mobilizing agents G-CSF and GM-CSF. The process of homing the BM-derived cells via the catecholamingergic neurotransmitter system involves multiple pathways, including signaling by Wnt and beta-catenin, and specific migratory molecules, such as membrane-bound enzymes MT1-MMP and SDF-1 (Aicher et al., 2008; Fleming et al., 2008; Kalinkovich et al., 2009; Spiegel et al., 2007) which altogether increase cell proliferation, motility and engraftment capabilities of human CD34 HSCs.
2.2 Regulation of Neural Function via Blood
The hematopoietic signaling contributes equally to the HSC regulation of CNS functions with the two-way neurotransmitter-mediated interaction between CNS and BM (Spiegel et al., 2008). Accumulating scientific evidence advances the concept that human HSCs can affect the nervous system and modulates its action (Kalinkovich et al., 2009). In the field of stroke, clinical data show that human acute stroke is followed by large and bursting mobilization of PB immature hematopoietic CD34+ cells, colony-forming cells and LTC-IC (Hennemann et al., 2008), and the extent of such mobilization is directly related to recovery of function (Dunac et al., 2007). The main postulated mechanism underlying HSC mobilization implicates the upregulation of SDF-1 within ischemic tissues of the brain, which promotes CXCR4+ HSC recruitment from PB to the site of injury (Kalinkovich et al., 2009). A multi-pronged neuroprotective and/or neurorestorative set of events closely precede HSC mobilization to the ischemic site, notably neoangiogenesis, which parallels the therapeutic window of G-CSF treatment for stroke therapy (Chang et al., 2007). The critical role of angiogenesis in HSC fate following ischemic injury is further supported by the observation that systemic administration of human CD34+ cells to SCID mice exposed to stroke 48 h earlier induces neovascularization in the ischemic zone (Taguchi et al., 2004a) thereby creating a conducive microenvironment for survival of both exogenous grafts and endogenous stem cells, which are pivotal for neuronal regeneration. Similarly, not only BM derived, but also cord blood (CB) derived CD34+ cells have replicated the homing event and subsequent therapeutic benefits of HSCs in brain disorders. Human CB CD34+ cells injected to rats before (Hwang et al., 2008) or during heat stress (Chen et al., 2007), significantly reduce symptoms of heatstroke and increase animal survival time by attenuating inflammatory, coagulatory and multiorgan dysfunction. HSC mobilization to the CNS may correspond to an early host endogenous repair mechanism. Mobilization is also seen in other neurological diseases. For example, elevated number of human BM CD34+ cells accompanies patients with chronic spinal injury (Chernykh et al., 2006). Transplantation of human CB CD34+ enriched cells into the injured spinal cords of rats produces a significant recovery of functional outcome and increases survival rate (Zhao et al., 2004; Nishio et al., 2006). Recent studies have shown that systemic injections of human CB mononuclear cells in Alzheimer’s disease animal models decrease parenchymal and cerebral vascular beta-amyloid deposits, and increase microglial phagocytic activity (Nikolic et al., 2008), while their transplantation in aged rats significantly enhances the hippocampal neurogenic niche characterized by rejuvenation of the aged neural stem/progenitor cells (Bachstetter et al., 2008). Taken together, the experiments lend support to the notion that human HSCs play key regulatory roles in the maintenance of homeostasis and the repair of the nervous system. The crosstalk between the hematopoietic and the nervous system, and the resulting HSC mobilization to the site of injury and subsequent observation of therapeutic benefits suggest their potential application for designing treatment strategies that can be utilized in the field of regenerative medicine. There has been impetus for HSCs to be the main donor graft source for cell therapy, especially those derived from BM, due to their feasibility, safety and efficacy profile in the clinic for other disease treatments such as cardiovascular, bone, cartilage, bladder, and liver dysfunctions (Bajada et al., 2008; Pai et al., 2008; Wojakowski et al., 2008).
3. A Sticky Situation with the Mesenchymal Stem Cells
Friedenstein and colleagues (1970) first defined mesenchymal stem cells (MSCs) as a population of plastic-adherent fibroblastic cells isolated by Percoll density centrifugation. Human MSCs (hMSCs) express CD105 (SH2), SH3, Stro-1, and CD13, but do not show the hematopoietic surface markers CD34 and CD45.
The potential lies in MSCs ability to differentiate into mesodermal cell lineages such as adipocytes, chrondroblasts, fibroblasts, osteoblasts, and skeletal myoblasts both in vitro and in vivo (Gronthos and Simmons, 1996; Haynesworth et al., 1992; Muraglia et al., 2000; Pereira et al., 1998; Prockop et al., 1997) yet hMSCs lack telomerase activity, with about 18 population doublings (PDs) (Zimmermann et al., 2003). A study conducted at the single cell level by Muraglia and colleagues demonstrated that MSCs are capable of differentiation into osteogenic, chondrogenic, and adipogenic phenotype up to 19 PDs, but lose their apparent proliferation potential at 22–23 PDs, in about 80 days in culture (Table 1) (Muraglia et al., 2000). Strikingly similar to this study, clonal hMSCs cultured in vitro by Banfi and coworkers were also found to gradually lose the differentiation potential when reaching 22–23 PDs after about 80 days of culture (Table 1) (Banfi et al., 2000). Kobune’s study reveals that MSCs exhibit a reduced mitogenic activity after about 5 PDs over the course of about 6 weeks and undergo crisis at 16 PDs (Kobune et al., 2003). Bruder and colleagues demonstrated that the growth rate of MSCs decreases with passaging, in that the PDs for hMSCs before degenerating are around 38, even though the osteogenic differentiation potential is preserved (Bruder et al., 1997). Cumulative population doubling level (CPDL) for hMSCs selected with serum-deprived medium is about 9 PDs at passage 10, while, for hMSCs selected with regular medium, CPDL is about 7 PDs at passage 8 (Pochampally et al., 2004). Although evidence on the limitation of MSCs’ differentiation and proliferation potential exists, a report from Pittenger and colleagues indicates that MSCs maintain telomerase activity in both early and late passage (Pittenger et al., 1999) (Table 1). This discrepancy might be due to the fact that cell phenotypes isolated from different laboratories are distinct. In order to override the senescence of MSCs, Hamada and colleagues transfected MSCs with the human telomerase gene using retroviral infection to generate stable cell clones with high efficiency and low cell mortality (Hamada et al., 2005; Honma et al., 2006; Kurozumi et al., 2005). These cells, regarded as hTERT-MSCs, can survive in culture for over 1 year and maintain their characteristic surface antigens as well as typical morphology (Table 1). In hTERT-MSC treated stroke animal models, functional outcome is improved and cerebral infarct volume is significantly reduced (Honma et al., 2006).
Table 1.
Bone-Marrow Derived Hematopoietic and Non-Hematopoietic Stem Cells.
| BM Cells | Target Disease | Differentiation Potential | Phenotype | Telomerase | Senescence Tendency | References |
|---|---|---|---|---|---|---|
| EPCs | Retinal Ischemia, Parkinson’s Disease, Malignant Tumors, Chronic Obstructive Pulmonary Disease, Ischemic Stroke, Traumatic Brain Injury, Alzheimer’s Disease | CD34+, CD31+, AC133+, VEGFR2+, Tle-2+ | Endothelial Cells | Positive | PD6 until 7–8 days, with Senescence augmented by estrogen or SDF-1 treatment | Asahara et al., 1997, 1999. Takahashi et al., 1999. Gehling et al., 2000. Lin et al., 2000. Heissig et al., 2002. Imanishi et al., 2006. Ingram et al., 2006. Kocher et al., 2006. Rustemeyer et al., 2006. Lapergue et al., 2007. McCarty, 2009. Li Carzi et al., 2010. Reimers et al., 2010. Zheng et al., 2010. Andres et al., 2011. Hesa and Allan, 2011. Huertas and Palange, 2011. Jiang and chen, 2011. Kong et al., 2011. |
|
| ||||||
| MSCs | Malignant Tumors and cancer | Not Addressed | OCA cells | N/A | Mean CPDL is about 38 PDs at passage 15 | Bruder et al., 1997. Ozawa et al., 2005. Sordi and Piemonti, 2010. Hamada et al., 2011. |
| Inflamation, Autoimmune, Asthma | Not Addressed | OCA cells | N/A | Lose MDP at 19–22 PDs. CPDL is around 22–23 in 80 days of culture | Banfi et al., 2000. Parekkadan et al., 2008, Nemeth et al., 2010. | |
| Neurological Damage and Regeneration (Stroke, Alzheimec, Parkinson, Tramatic Brain Injury, Spinal Cord Injury) | Not Addressed | OCA cells | N/A | Lose MDP at 19–22 PDs. CPDL is around 22–23 in 80 days of culture | Muraglia et al., 2000. Zwart et al., 2009. Bakhtiary et al., 2010. Cheng et al., 2010. Lee et al., 2010. Osaka 2010. Khoo et al., 2011. Zhou, 2011. | |
| Cardio-Related Disease (i.e., Ischemic Cardiomyopathy, Myocardial Infarction) | SH2+, SH3+, CD29+, CD44+, CD14−, CD34− and CD45− | OCA cells | Negative | CPDL is around 18 PDs, at about 80 days | Zimmerman et al., 2003. Wang et al., 2010. Liang et al., 2011. | |
| Inflamatory Bowel | Not Addressed | OCA cells | N/A | CPDL is 15 PDs of about 40 days of culture | Kobune et al., 2003. Ko et al., 2010. | |
| Multiple Sclerosis and Liver Regeneration | Not Addressed | OCA cells | N/A | CPDL in serum-deprived hMSCs is about 9 PDs at passage 10. CPDL for control hMSCs is about 7 PDs at passage 8 | Pochampally et al., 2004. Witherick et al., 2010., Sokal 2011. | |
|
| ||||||
| hTERT-MSCs | Cerebral Ischemia and Spinal Cord Injury | Not Addressed | OCA cells | N/A | PP remains until 80 PDs of about 400 days of culture | Kobune et al., 2003. Honma et al., 2006. |
|
| ||||||
| HSCs | Alzheimer’s Disease, Traumatic Brain Injury, Myeloid Leukaemia, Sickle Cell Anemia, Cutaneous Repair | Blood cells | CD34+, CXCR4+ | Positive | Up for debate, thought to be dysfunctional in aging HSCs, but even reduced telemere expression neither shorten telemere nor limit PP | Lapidot and Kollet, 2005. Nervi et al., 2006. Papayannopoulou and Scadden, 2008. Sanchez-Ramos et al., 2006. Zimmerman and Martens, 2008. Geiger and Rudolph, 2009. Guo et al., 2009. Lapidot and Kollet, 2010. Drukala et al., 2011. Klinakis et al., 2011. McNeer et al., 2011. |
|
| ||||||
| VSEL Cells | Tissue Rejuvertation, Stroke, Myocardial Infarctions, Diabetes, Cutaneous Repair | SSEA-1+, Oct-4+, Nanog+, Rex-1+, Sca-1+, CD45− | Three germ lineages | Positive | Expand for about 7 PDs (personal communication with author) | Kucia et al., 2006b, 2007. Ratajczak et al., 2006. Zuba-Surma et al., 2005. Paczkowska et al., 2009. Shin et al., 2009. Wojakowski et al., 2009. Hocking et al. 2010. Huang et al., 2010. Shin et al., 2010. Drukala et al., 2011. Sovalat et al., 2011. Wojakowski et al., 2011. |
OCA cells: osteogenic, chondrogenic and adipogenic cells; PDs: population doublings; CPDL: cumulative population doubling level; PP: proliferation potential; MDP: multiple lineages differentiate potential; Not Addressed: authors did not describe this topic in their study; Negative/positive: the activity of telomerase is negative or positive.
3.1 Bench to Bedside: Transplantation of MSCs in Stroke
MSCs utilized in experimental stroke models have improved the functional recovery of neurological deficits caused by cerebral ischemia (Bang et al., 2005; Chen et al., 2001a, 2001b; Chop and Li, 2002; Li et al., 2001, 2002a, 2005; Rempe et al., 2002; Song et al., 2004; Tang et al., 2007). Clinical reports of MSC transplantation in stroke patients reveal that MSCs may improve the functional recovery of patients without adverse side effects (Bang et al., 2005). The underlying mechanism remains unclear. What follows here are some potential mechanisms for mediation of the effects of MSC implantation in stroke.
3.2 Regulating Traffic: Mediation of Trophic Factor Secretion via MSCs
There has been little addition to the mechanism of MSC-derived trophic factor induction of neuronal survival and differentiation in disease. Moreover, transplantation of MSCs, via intravenous, intracarotid, or even intracerebral delivery, leads to very low, or at best a modest, graft survival rate (Shen et al., 2007). A more reasonable explanation for the graft-derived beneficial effects is that MSCs secrete neurotrophic factors that may induce the host ischemic brain to activate endogenous repair mechanisms. Chopp and colleagues (2002) showed that MSCs produce hepatocyte growth factor (HGF) (Chen et al., 2002a), vascular endothelial growth factor (VEGF) (Chen et al., 2003a), nerve growth factor (NGF) (Li et al., 2002b), brain-derived neurotrophic factor (BDNF) (Chen, et al., 2002b; Li et al., 2002b), basic fibroblast growth factor (bFGF, FGF-2) (Chen et al., 2003a) and insulin growth factor-1 (IGF-1) (Zhang J., et al. 2004). These neurotrophic cytokines have been implicated to play an important role in the process of neurogenesis and angiogenesis. For instance, the upregulation of VEGF and the VEGF receptor 2 (VEGFR2) at the impaired site could increase the number of enlarged and thin walled blood vessels as well as newly formed capillaries at the ischemia border zone (IBZ) (Imai et al., 1999). The early increase (1 h after stroke) of VEGF could increase blood brain–barrier (BBB) leakage and hence increase ischemia hemorrhagic transformation and exacerbate ischemic cell damage, when administered 48 h after stroke. However, VEGF could enhance angiogenesis in the IBZ and significantly improve neurological recovery (Zhang et al., 2000). The time course from hMSC administration to the secretion of VEGF by the graft cells exceeds 1 h after stroke; hence, VEGF here could function as the angiogenesis promoter and improve functional recovery after stroke. Reducing intracranial pressure, induction of angiogenesis, and amelioration of BBB destruction have all been implicated as characteristics of HGF.
There has also been evidence that neurogenesis is promoted by grafted MSCs. The subventricular zone (SVZ) and subgranular zone (SGZ) are remarkable brain neurogenic sites (Cameron et al., 2003; García-Verdugo et al., 1998). The presence of MSCs in the brain has been shown to promote the induction and migration of new cells from these primary sources within the SVZ and the SGZ into the injured brain (Chen et al., 2003a; Li et al., 2001, 2002a). The newly differentiated cells differentiate into progenitor-like neurons and astrocytes. The process of neurogenesis is highly dependent upon the neurotrophic factors secreted by MSCs (Chen et al., 2003a). Upregulation of BDNF levels is capable of recruiting neural progenitors from the endogenous progenitors of the forebrain (Benraiss et al. 2001). Recent reports show that bFGF is capable of enhancing the proliferation of neuronal progenitors as well as exerting neuroprotective or vasodilating effects (Ay et al., 2001; Rosenblatt et al., 1994). To enhance the efficacy of MSCs, gene-modified MSCs have been used as donor cells for transplantation in stroke models. In these experiments, specific genes such as FGF-2/HGF/BDNF, are transfected into the cells before they are transplanted into the brain. These grafted cells subsequently are able to express the target gene in the brain. Kurozumi and coworkers (Kurozumi et al., 2004, 2005) transfected telomerized human MSCs with the BDNF gene using a fiber-mutant F/RGD adenovirus vector and transplanted these cells in stroke rats. BDNF production by MSC-BDNF cells is 23-fold greater than that seen in unmodified MSCs. Rats that underwent middle cerebral artery occusion (MCAo) and received MSC-BDNF display significantly better functional recovery than do control stroke rats. The authors also conducted a parallel study in which they transfected MSCs with glial cell line-derived neurotrophic factor (GDNF), ciliary neurotrophic factor (CNTF), and neurotrophin 3 (NT3) genes (Kurozumi et al., 2005). MCAo rats that received MSC-GDNF, but not those treated with CNTF and NT3, showed significantly greater functional improvement than control stroke rats. A study from Ikeda et al. (2005) demonstrates that stroke animals that received bFGF-MSCs show significantly better attenuation of behavioral impairments and infarction volumes than those that received non-transfected MSCs. These data suggest that bFGF enhances the beneficial effects of MSCs in animal models of stroke. A relatively new addition to the list of growth factors exerting benefits in stroke is HGF. Ischemia animals treated both with and without HGF-modified MSCs exhibit improvement of neurological deficits, yet those that received HGF-MSCs treatment display superior behavioral effects to the non-modified MSC-treated group (Zhao et al., 2006). Even though graft survival rate or neuronal differentiation appear to play minor roles in the MSC transplantation behavioral effect, studies demonstrate that transplantation with MSCs that are committed to differentiate into a neuronal phenotype show better therapeutic effects. MSCs transfected with Notch intracellular domain and subsequently treated with bFGF, forskolin, and CNTF have the potential to differentiate into neurons, termed by the authors as BM stromal cell-derived neuronal cell (MSDNC) (Dezawa et al., 2005). Transplantation of MSDNCs in stroke models reveals better functional improvement than those treated with MSCs (Dezawa et al., 2005). Although gene-based strategies appear to improve MSC graft functional effects, the use of viral vectors poses additional clinical problems, especially with uncontrolled gene replications that may cause neoplasm, tumors, and even death. An alternative approach to circumvent adverse side effects associated with genetic manipulation, but still facilitating the therapeutic effects of MSCs, is to exogenously deliver neurotrophic factors. Studies show that BDNF delivered along with MSCs significantly improves the recovery of the stroke animals (Chen et al., 2000). In an effort to further improve MSC efficacy, MSC transplantation is combined with adjunctive treatment of specific reagents. A cell-permeable inhibitor of caspases, Z-Val-Ala-DL-Asp-fluoromethylketone (Z-VAD), is found to enhance graft survival and behavioral recovery (Chen et al., 2002a) when intracerebrally infused together with MSCs into the ischemic region. Nitric oxide has been proven to play an important role in cell proliferation and neurogenesis. Intravenous infusion of MSCs with a nitric oxide donor, (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl) aminio] diazen-1-ium-1,2-diolate (DETA/NONOate) significantly improves functional recovery in stroke animals, with accompanying enhancement of vessel perimeter and endothelial cell proliferation. In addition, when compared to single treatments the grafts of combined DETA/NONOate and MSC increase neurogenesis in the SVZ, as well as VEGF and bFGF expression in the ischemic area (Chen et al., 2004a).
3.3 Rebuilding the Foundation: Axonal Remodeling and Cell Proliferation Induced by MSCs
Grafted MSCs can induce both neuron remyelination and synaptogenesis and modulate glial cell proliferation (Shen et al., 2006). Oligogenesis and astrocytogenesis are markedly enhanced in the SVZ in the ischemia animal models treated with MSCs (Li et al., 2005; Shen et al, 2006, 2007). In addition to enhancing gliogenesis, reducing apoptosis is shown to be another critical effect of MSCs. Using an anaerobic chamber to duplicate the in vivo ischemia conditions in in vitro experiments, Gao and colleagues demonstrated that astrocytes, when cocultured with MSCs, undergo reduced cell death and apoptosis (Gao et al., 2005a). In vivo studies indicate that intravenously administration of MCSs in stroke models could reduce apoptosis in the penumbral zone of the lesion (Chen et al., 2003a; Li et al., 2002a). A recent study demonstrated that MSCs significantly increase astrocytic expression of connexin-43 (Cx43) and growth associated protein-43 (GAP-43) (Li et al., 2005) This Cx43 upregulation is concomitant with altered gap junction intercellular communication (GJIC) with the participation of the PI3K signaling pathway (Gao et al., 2005b). In vivo data confirm that transplanted MSCs enhance bone morphogenetic protein 2/bone morphogenetic protein 4 (BMP2/4) and Cx43 expression in astrocytes (Zhang, C et al., 2006). Cx43 is the primary component of intercellular channels in astrocytes, while BMP2/4 belongs to a subgroup of the transforming growth factor-β superfamily. BMP2/4 maintains extensive gap junction communication through Cx43 and hence mediates communication between astrocytes and increases synaptogenesis in the IBZ (Zhang, C et al., 2006). Areas of the corpus callosum and the numbers of white matter bundles in the striatum are larger in the IBZ with MSC-treated ischemic animals compared to nontransplanted ischemic animals (Li et al., 2006; Shen et al., 2004). At the same time, the axons and myelin thickness are increased along with scar thickness being reduced (Li et al., 2005; Shen et al., 2007). In contrast, the numbers of microglia and macrophages within the scar wall are reduced (Li et al., 2005). Further investigation is required to determine whether microglia inhibit axonal regeneration.
3.4 The Great Journey: Homing of MSCs to the Site of Injury via the SDF-1/CXCR4 Pathway
As discussed previously, a necessary chemoattractant for CXCR4-expressing BM-derived cells in the hematopoietic system (i.e., HSCs) is SDF-1 (Figure 2). In the nonhematopoietic system, CXCR4-positive cells similarly respond to SDF-1 signals secreted by injured organs, and migrate into these areas (Figure 3). Such SDF-1 upregulation and cell migration are found to subsequently lead to upregulation of various trophic factors either secreted by the mobilized stem cells or by endogenous cells. In stroke rats, the chemokine SDF-1 level is significantly increased in the injured hemisphere compared to the uninjured hemisphere (Shen et al., 2007). The upregulation of SDF-1 is mostly manifested in the IBZ and maintained for up to 30 days after the injury. Of note, CXCR4 is detected in the transplanted MSCs. The interaction between SDF-1 and CXCR4 serves as the chemoattractant guide for MSC migration towards the impaired site (Shen et al., 2007). SDF-1 gene expression is regulated by the hypoxia-responsive transcription factor, hypoxia-inducible factor 1 (HIF-1) (Ceradini et al., 2005), and follows a gradient pattern consistent with the hypoxia gradient in the penumbra of stroke animals. In general, a significant signal following brain injury is the expression of SDF-1, attracting the BM-derived stem cells to migrate into the damaged region, crucial for functional recovery after stroke.
Figure 2.
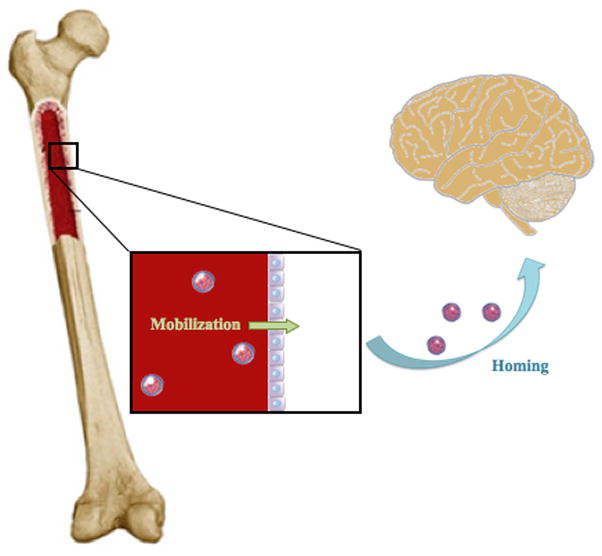
Migration of Bone-Marrow Derived Stem Cells in Non-Pathologic Conditions. Under non-pathologic conditions, minute quantities of quinescent bone marrow-derived stem cells mobilize and utilize the CXCR4/SDF-1 signaling pathway to migrate to the brain.
Of all the cells used for beneficial applications MSCs are one of most studied, yet the mechanisms of action of MSCs are still poorly understood. Two recent FDA-approved clinical trials have been initiated via intravenous autologous MSCs in acute stroke (University of Texas at Houston with Dr. Sean Savitz) and stereotactic transplantation of allogeneic Notch-induced MSCs in ischemic stroke (Stanford University with Dr. Gary Steinberg).
4. Every Cloud has a Silver Lining, and So Does the Vasculature: The Endothelial Progenitor Cells
As initially described by Asahara et al. (1997), endothelial progenitor cells (EPC), are young, immature endothelial cells circulating in the PB. EPCs mature as endothelial cells, and are important components of the vascular system (Asahara et al., 1997, 1999; Heissig et al., 2002; Lin et al., 2000; McCarty, 2009). In their pioneering study (Asahara et al., 1997), transplanted EPCs isolated from human UCB were found in the endothelium of newly formed vessels in ischemic regions, indicating that a discrete cell population within the human blood participates in the formation of new vessels after ischemia. Griese et al. (2003) also found that grafted EPCs populated the endothelium in animals with experimentally induced endothelial damage (Shi et al., 1998), further advancing the notion that EPCs contribute to the repair of damaged endothelium. Although EPCs are hematopoietic in origin and they can be found in PB of adults; a population of cells with similar characteristics can also be derived from human umbilical cord blood (UCB) (Griese et al., 2003; Murohara et al., 2000). There have been implications from several lines of investigations from both animal and human studies that EPCs principally participate in re-endothelialization during the neovascularization of ischemic organs. This suggests that EPCs modulation could be focused toward the therapy of cerebrovascular diseases (Asahara et al., 1997, 1999; Griese et al., 2003; McCarty, 2009; Murohara et al., 2000; Lapergue et al., 2007; Peichev et al, 2000).
4.1 Characterizing the Silver Lining: Phenotype of EPCs
To determine cell characteristics, immunological surface markers and functional profiling via colony formation capacity of these cells have been employed (Gehling et al., 2000; Ingram et al., 2005; Lapergue et al., 2007; Rustemeyer et al., 2006; Takahashi et al., 1999). EPCs were first isolated from human PB, which expressed shared markers with HSCs, angioblasts and receptors for vascular endothelial growth factor (VEGFR2/KDR) (Asahara et al., 1997). Cultured EPCs could differentiate into endothelial cells and be incorporated into sites of active angiogenesis in animal models of ischemia (Asahara et al., 1997). Isolation of EPCs remains controversial, in that different markers such as CD31, VE-cadherine, E-selectine, eNOS, von Willebrand factor, among others (Asahara et al., 1997; Lapergue et al., 2007; Ingram et al., 2005; Rustemeyer et al., 2006; Schatteman and Awad, 2004) have been employed to harvest EPCs, whereas equally solid evidence indicates that only CD34 positive EPCs isolated from BM or UCB possessed the potency to differentiate into mature endothelial cells (Hristov and Weber, 2004; Khakoo and Finkel, 2005; McCarty et al., 2009). In parallel, recent studies have used the HSC specific AC133 as a surface marker to isolate EPCs (Lapergue et al., 2007; Takahashi et al., 1999). Varying developmental stages of EPCs or the presence of residual cells derived from the mature vascular wall may influence to the heterogeneity of tissue sources of EPCs. Double labeling with CD34/VEGFR2 is the prevailing marker of choice for obtaining highly homogenous EPCs (Kocher et al., 2006; Lapergue et al., 2007; Takahashi et al., 1999).
Another approach via functional profiling to further define the phenotypic feature of EPCs has been used. This is performed by counting the number of EPC colonies formed after 7 days of culture. A colony consists of a central cluster of rounded cells with surrounding radiating thin, flat cells (Asahara et al., 1997; Cines et al., 1998; Ghani et al., 2005; Hill et al., 2003). These colonies exhibit many endothelial characteristics including expression of CD31, VEGFR2 and Tie-2 (Asahara et al., 1997; Hill et al., 2003). Another method is to measure the uptake of Dil-labeled acLDL, or binding of specific lectins. This method has also been useful in defining endothelial cells; however, these characteristics are also displayed by most macrophages in a culture (Asahara et al., 1997; Gehling et al., 2000; Ito et al., 1999; Kreipe et at al., 1986). These characteristics are mostly considered to be functional in nature and do not represent the number of EPCs present in the culture. Several sources of EPCs have been identified, including PB, BM and umbilical cord blood (Asahara et al., 1997, 1999; Lin et al., 2000; McCarty, 2009; Griese et al., 2003). Recently, it has been reported that EPCs may even originate from the area between smooth muscle and the adventitial layer of the human adult vascular wall (Lougheed et al., 1999). EPCs remain extremely rare in adult PB (0.01% of mononuclear cells, under steady state conditions), which may be a contributing factor for the lack of clearly defined methods for cell isolation and definition (Gehling et al., 2000). Whereas BM-derived stem/progenitor cells have been widely considered as a source of EPCs, the BM contains heterogeneous groups of cells with at least two major progenitor populations, namely MSCs and hematopoietic EPCs, with both populations capable of inducing neovascularization through vasculogenesis thus making them good candidates for cell therapy in cerebrovascular diseases (Borlongan et al., 2004; Chopp and Li, 2002; Griese et al., 2003; Hess et al., 2002; Nan et al., 2005; Taguchi et al., 2004b; Vendrame et al., 2004; Willing et al., 2003; Zhang et al., 2002; Zengin et al., 2006) Although characterization of EPCs in cell culture remains challenging, the unique properties of these cells as described by Finkel and co-workers (Hristov and Weber, 2004) may suffice as a starting point in delineating this novel BM cell population. Circulating BM-derived EPCs are distinct from mature endothelial cells through the EPCs ability to differentiate into endothelial cells. This can be assessed by expression profiles, functional characteristics, and regenerative capacity, especially for promoting vasculogenesis and/or vascular homeostasis (Hristov and Weber, 2004).
4.2 Repairing the Broken Pipes: The Role of EPCs in Angiogenesis and Vasculogenesis
Until recently, it was understood that neovascularization, or the formation of new blood vessels, exclusively resulted from proliferation and migration of pre-existing endothelial cells, a process referred as to angiogenesis (Carmeliet, 2005). Furthermore, vasculogenesis or vascularization, defined as in situ differentiation of vascular endothelial cells from endothelial precursor cells, was thought to occur only in the embryo during vascular development. However, recent evidence has now established that circulating BM-derived EPCs are capable of homing to neovascularization sites, proliferating and differentiating into endothelial cells (Masuda and Asahara, 2003; Shi et al., 1998). EPCs have been identified mainly in the mononuclear cell fraction of PB, leukapheresis products and in umbilical cord blood (Asahara et al., 1997; Bompais et al., 2004; Zheng et al., 2010), but can also be harvested from BM. Over the last few years, EPC have been studied as biomarkers to assess the risk of cardiovascular disease in human subjects. For example, a low EPC count predicts severe functional impairments in several disorders such as diabetes (Fadini, 2008), hypercholesterolemia (Chen et al., 2004b), hypertension (Pirro et al., 2007; Umemura et al., 2008), scleroderma (Del Papa et al., 2006; Kuwana et al., 2004), aging (Fadini, 2008; Heiss et al., 2005), cigarettes smoking (Fadini, 2008; Kondo et al., 2004; Michaud et al., 2006), and coronary artery disease (Kunz et al., 2006). In addition, EPC have been examined as potent donor graft cells for transplantation therapy. Transplantation of EPC into ischemic tissues has emerged as a promising approach in the treatment of diseases with blood vessels disorders (Botta et al., 2004; Kawamoto et al., 2001; Madeddu et al., 2004). In mouse models of ischemia, neovascularization in hind limb ischemia was improved by EPC injection (Botta et al., 2004; Kawamoto et al., 2001; Madeddu et al., 2004).
4.3 Utilizing the Silver Lining: Potential of EPCs for Cell Therapy
Because of laboratory findings suggesting vasculogenic and angiogenic potential of EPCs, clinical studies have been initiated to determine whether patients with lower numbers of EPCs are at an increased risk of atherosclerotic events, and whether patients with ischemic events may benefit from EPC administration (Rouhl et al., 2008). Clinical studies to date support the therapeutic potential of EPC transplantation, although this assumption should be approached with much caution due to being open label trials, observational and/or anecdotal accounts, and a limited number of patients. Ex vivo expanded EPCs, isolated from PB mononuclear cells, can incorporate into the foci of myocardial neovascularization (Erbs et al., 2005; Li et al., 2007), and intracoronary infusion of PB or BM-derived progenitors in patients with acute myocardial infarction was associated with significant benefits in post-infarction remodeling (Fernandez-Aviles et al., 2004; Meluzín et al., 2006, 2008; Mocini et al., 2006; Perin et al., 2003, 2004; Strauer et al., 2005). In observational studies in patients with myocardial infarction, higher numbers of EPC correlate with better prognosis, more myocardial salvage (Dobert et al., 2004), viability and perfusion (Numaguchi et al., 2006), and more collaterals in the ischemic zone (Lev et al., 2005). Randomized clinical trials on autologous BM-derived cells are mixed. Whereas transplanted coronary artery disease patients display improved left ventricular function, at least in the short term (Hristov et al., 2006), transplanted patients with chronic ischemic heart failure exhibit modest to no effects on left ventricular function (Dimmeler et al., 2005). Similar randomized trials of autologous BM-derived cells have been carried out in patients with peripheral artery disease and showed improved endothelium-dependent vasodilation (Higashi et al., 2004), ankle brachial index, rest pain, and pain-free walking time (Tateishi-Yuyama, et al., 2002), but the degree of functional recovery was not as robust as seen in animal models. These results, obtained from autologous BM-derived cells, show that the cells are heterogenous with sparse numbers of EPCs.
Available clinical studies of EPC in neurovascular disease are much more limited with only 3 observational studies in patients with stroke. In 25 patients with an ischemic stroke, CD34+-cells peaked 7 days after stroke but generally reverted to baseline after 30 days (Taguchi et al., 2004b). Interestingly, higher CD34+-cell levels at 30 days related to higher numbers of infarcts on magnetic resonance imaging and also to worse cerebrovascular function as measured with positron emission tomography scanning (cerebral metabolic rate of oxygen, and cerebral blood flow). On the other hand, decreased numbers of clusters of rapidly adhering cells were seen after stroke and in “stable cerebrovascular disease,” compared to controls free of vascular disease (Ghani et al., 2005). Higher age and the presence of cerebrovascular disease in general independently related to lower EPC numbers. These discrepancies in results could be from mismatched controls for age of patients and/or the lack of methodological design for testing specific hypotheses on the causal role of EPC in cerebrovascular disease (Ghani et al., 2005).
There is evidence showing immunological attack upon the brain and/or its vasculature, though the primary mitigating mechanisms underlying stroke pathogenesis and its abrogation by cell therapy are still uncertain. Widespread inflammatory reactions in stroke may trigger a cascade of events, which alter the integrity of the BBB, resulting in migration of leukocytes into the CNS. Leukocyte transmigration across the BBB during stroke-mediated immune/inflammatory processes could influence inter-endothelial junctional complex function leading to vascular endothelium damage and BBB breakdown. A key component of Manaenko et al.’s (2011) mechanism-based hypothesis is that disruption or dysfunction of the BBB, preceding entry of harmful substances into the brain parenchyma, could be a key initial factor in stroke pathogenesis. Thus, the study highlights the need for EPCs as transplantable cells for stroke therapy as the restoration of barrier integrity could play a critical role in the prevention of stroke progression.
5. Sizing Up the Competition: The Very Small Embryonic-Like Stem Cells
As previously mentioned, the numbers of various types of stem cells in PB, both in experimental animals and patients, are acutely increased by stress conditions such as ischemic injury or stroke (Komitova et al., 2005; Kucia et al., 2006a; Ratajczak et al., 2010a; Zhang et al., 2001). Stem cells, including HSCs (Levesque et al., 2010), MSCs (Salem and Thiemermann, 2010), and EPCs (Mund, et al., 2009) are mobilized into PB from BM and probably other non-hematopoietic niches as well, likely contributing to an endogenous regenerative mechanism (Gharib et al., 2010; Kucia et al., 2006a; Ratajczak et al., 2010a; Szczot et al., 2010). However, since regions of secondary damage after stroke leads to irreversible brain damage, these cells circulating in PB are not sufficient to halt the stroke damage. A hostile microenvironment ensues during stroke progression that alters chemotaxis and homing of circulating PB cells to the ischemic brain. In addition, the BBB may limit the entry of circulating stem cells from periphery to the brain thereby further blocking the therapeutic benefits of this endogenous repair mechanism. Treatment strategies designed to increase the number of circulating stem cells in PB (i.e., after administration of mobilizing agents such as G-CSF and/or CXCR4 antagonists), maintain the chemoattractant pathway to provide migratory cues for circulating stem cells to home to the site of injury, and enhance permeability of the BBB. This would facilitate stem cell entry into the brain or stereotactic delivery of stem cells into the peri-infarct area in order to foster an effective regenerative regimen against stroke (Burns et al., 2009; Dwain et al., 2006; Farin et al., 2009; Morancho et al., 2010). Along this line, the acute endogenous stem cell mobilization following ischemic injury can be enhanced in both mice and humans after G-CSF treatment during acute cardiovascular diseases e.g., myocardial infarction and stroke (Ratajczak et al., 2010a; Szczot et al., 2010). Accordingly, stem cell-based strategies aimed at regeneration of the stroke brain stands as a potent therapeutic modality. Ratajczak’s research team recently discovered a unique population of stem cells called very small embryonic-like stem cells (VSELs). Following experimental stroke, the number of circulating VSELs in PB increases in mice (Kucia et al., 2006a) as well as in stroke patients (Ratajczak et al., 2010a), suggesting that the VSELs which reside in adult tissues and mobilized into PB are a potent source of adult tissue-derived stem cells that can be utilized for regenerative processes, specifically for neural repair after stroke.
Ratajczak and co-workers showed that following stroke VSELs could be mobilized into PB in patients similar to movement after acute myocardial infarction in humans and mice (Kucia et al., 2006a; Wojakowski et al., 2009). They observe an increase in mRNA for both pluripotent (Oct-4 and Nanog) and neural (GFAP, Nestin, b-IIItubulin, Olig1, Olig2, Sox2, and Musashi-1) stem cell markers in PB-borne nucleated cells (PBMNCs) circulating in stroke patients. This resembled the general phenotypic patterns that they previously noted in an experimental murine model of stroke (Kucia et al., 2006a), with the exception that maximally increased expression of neural stem cell markers in humans was delayed by two days (i.e., 1 day for mice vs. 3 days for human). Further analyses using computer tomography (CT) scans reveal differences in VSEL mobilization in that patients with posterior circulation infarcts (POCI), had the best chance of recovery. In contrast, partial anterior circulation infarcts (PACI) was associated with the highest risk of early recurrence of stroke (i.e., within 3 months), although not associated with high mortality and significant disability. Whether stroke morbidity or mortality correlates with location of the stroke area in relation to the neurogenic regions of subventricular zone and subgranular zone warrants further examination. Additionally, increases in the number of these cells by administration of mobilization-promoting agents (e.g., G-CSF or AMD3100) represents as a logical extension of this therapy for clinical application. Nonetheless, studies have indicated a link between the mobilization of VSELs and tissue-committed progenitor cells expressing early neural markers into PB and ischemic stroke (Paczkowska et al., 2009; Ratajczak et al., 2011a). Further research on this close association could prove to be useful as a prognostic tool.
5.1 Small Cells, Big Genes: Genomic Imprinting and Phenotypic Characterization of VSELs
VSELs are present in a variety of adult organs expressing several progenitor stem cell (PSC) markers (Kucia et al., 2006b), Specifically the brain contains a relatively high number of cells that display the VSEL phenotype (Kucia et al., 2005; Zuba-Surma et al., 2008). Initially separated from murine BM (Hocking and Gibran, 2010), VSELs are smaller than erythrocytes (3–5 μm in diameter in mice) with a corresponding human population of small cells (5–7 μm in diameter) purified from human UCB and mobilized PB (Kucia et al., 2006b). In the Ratajczak reports, well-validated protocols for VSEL identification in human PB using flow cytometric methods have been established. Following tissue and organ injuries, human VSELs are mobilized into PB of patients and are recognized as very small cells belonging to the non-hematopoietic fraction of leukocytes (Lin–/CD45− cells) expressing CD34, CD133, and CXCR4 antigens (Kucia et al., 2006a, 2007). The absolute numbers of circulating VSELs in PB are exceptionally low (1–2 cells in 1μl of blood under steady-state conditions) and thus special flow cytometric protocols have to be applied for their identification. Phenotypic markers used to identify VSELs include negative expression of CD45 (mouse and human), positive expression of Sca-1 (mouse), CXCR4, CD133, and CD34+(mouse and human), positive expression for PSC markers (i.e., Oct-4, Nanog, and SSEA) (Kucia et al., 2007; Ratajczak et al., 2011b; Shin et al., 2009; Wojakowski et al., 2009) and expression of several markers characteristic of epiblast/germ line stem cells (Shin et al., 2010a). Based on a multi-analytical flow cytometric approach the identity and confirmation of the presence of VSELs among blood leukocytes, in addition to a quantitative determination of the absolute numbers of these rare cells circulating in the blood of patients with various tissue/organ injuries and disorders could be ascertained (Kucia et al., 2006a; Wojakowski et al., 2009). VSELs have been successfully purified both from human mobilized PB as well as BM by independent groups (Shin et al., 2010b; Sovalat et al., 2011).
While studies have shown that there are stem cells in the intestinal region (Rao and Wang, 2011), there has been little progress or discussion on VSEL availability. Many cells mentioned in this paper have been shown to not only to be present, but also to be an aid in regeneration. The details on VSEL have remained somewhat obscure (Zuba-Surma et al., 2009); however, studies do show that VSELs may be present in the liver (Chan et al., 2009).
A notable feature when deciphering the genomic imprint of VSELs, is that most of the homeodomain-containing developmental transcription factors in VSELs are repressed by specific epigenetic marks, called bivalent domains. These represent a state of the DNA structure characteristic of PSCs, where transcriptionally antagonistic histone codes physically co-exist within the same promoter (Ratajczak et al., 2011a). Murine Oct-4+ VSELs do not proliferate spontaneously in vitro if cultured alone and the quiescence of these cells is regulated by genomic imprinting through the aid of DNA methylation. This is an epigenetic program that ensures the parent-specific monoallelic transcription of some developmentally important genes such as those implicated in embryogenesis, fetal growth. This maintains the totipotential state of the zygote, and maintains the pluripotency of developmentally early stem cells (Shin et al., 2009). The expression of imprinted genes is regulated by DNA methylation in freshly isolated VSELs from murine BM, deleting the paternally methylated imprints, but hypermethylating the maternally methylated imprints. Because paternally expressed imprinted genes (Igf2 and Rasgrf1) enhance embryo growth, while those that are maternally expressed (H19, p57KIP2, and Igf2R) inhibit cell proliferation (Shin et al., 2009), the unique genomic imprinting pattern observed in VSELs demonstrates a growth-repressive program in this developmentally early stem cell. Although the quiescent pattern of genomic imprinting is largely influenced by DNA methylation, cell culture conditions, such as the formation of spheres by VSELs in co-cultures with myoblastic C2C12 cells (Ratajczak et al., 2010b), could prime VSELs to proliferate and differentiate. Taken together, these findings suggest that the epigenetic reprogramming of genomic imprinting is capable of maintaining quiescence of the most primitive pluripotent adult stem cells (i.e., Oct-4+ VSELs) deposited in the adult body and protect them from premature aging and tumor formation, but that specific external micro-environmental signals can influence the epigenetic state of this imprinting and force VSELs to differentiate (Ratajczak et al., 2010b). What remains to be determined is whether human VSELs, as well as cells isolated from non-hematopoietic tissues (e.g., the CNS), which display the VSEL phenotype, also manifest such a DNA methylated genomic imprint
5.2 Finite Size, Infinite Possibilities: Therapeutic Potential of VSELs
The population of VSELs has an important role in physiological tissue rejuvenation and regeneration, as suggested by the hypothesis that VSELs are epiblast-derived PSCs deposited early during embryonic development in immature organs, as a potential reserve pool of precursors for tissue-committed stem cells (TCSCs). The observation that microglial VSELs have the potential to differentiate into neurons, oligodendrocytes and microglia, further advances the utility of these cells as donor grafts for regeneration of a damaged CNS. In contemplating clinical application of the cells, the ease of harvesting VSELs should be considered. With autologous transplantation in mind, the patient’s own BM, stored UCB, and mobilized PB appear as readily accessible sources of VSELs for regenerative therapies. For allotransplantation, VSELs could be harvested from histocompatible-related or unrelated donors as is currently done with HSC allotransplantation (Ratajczak et al., 2010b). A potentially limiting factor for clinical application is the small number of VSELs that could be harvested, requiring the need for efficient ex vivo expansion strategies, especially if the goal is to generate an ample supply of neural stem cells for stroke therapy. Another caveat in using VSELs in the clinic is the observation that the number of VSELs decreases with age, implying that enhanced regeneration potential of human patients may be correlated with higher number of these cells deposited during embryogenesis in the adult tissues (Ratajczak et al., 2010c). Of note, VSELs level in adulthood may also be decreased by the calorie uptake-related increase in plasma level of insulin and insulin-like growth factors (Ratajczak et al., 2011a).
5.3 Finding the Needle in the Haystack: Isolating VSELs
While considering stroke therapy treatments, the best time period to initiate therapeutic intervention seems to be during the acute and sub-acute stage (time 0 to one week post-injury) in order to immediately abrogate the rapidly deteriorating ischemic brain. Due to this limited therapeutic window, it may not be feasible and practical to purify these rare cells from BM aspirates, UCB, or mobilized PB by employing multiparametrer staining and regular high speed sorting only. The Ratajczak group calculated that by employing only one cell FACS sorter, isolation of all VSELs present in 100 ml of UCB would take up to 4 working days (Zuba-Surma et al., 2010a, 2010b). For efficient cell isolation, they propose a relatively short and economical three-step isolation protocol that allows recovery of ~60% of the initial number of Lin–/CD45−/CD133+ UCB− VSELs. The rationale for this novel strategy takes into account lysis of erythrocytes in a hypotonic ammonium chloride solution, CD133+ cell selection by immunomagnetic beads, and sorting of Lin–/CD45−/CD133+ cells by FACS with size-marker bead controls. The entire isolation procedure takes 2–3 hours per UCB unit (but should also be applicable using BM aspirates and mobilized PB) and isolated cells are highly enriched for an Oct-4+ and SSEA-4+ population of small, highly primitive Lin–/CD45−/CD133+ cells. Either VSELs promptly isolated from BM, PB, or UCB, or VSELs pre-committed to the neurological lineage in ex vivo cultures would be the envisioned clinical product (Ratajczak et al., 2011b).
6. Future Directions
The major impetus for advancing regenerative therapies in many neurological disorders, including stroke, has been the discovery of stem cell plasticity in the adult. Bone marrow-derived HSCs, MSCs, EPCs and VSELs have been proposed as potential sources of adult stem cells for regeneration and repair of the CNS. However, the rationale for employing these non-neuronal stem cells remains controversial and, most recently, MSCs have been reported to be a possible source of tumor formation following their deposition into the brain (Snyder, 2011). Whereas BM-, PB-, or UCB-derived HSCs for regeneration of neural tissues have been explored, the postulated stem cell plasticity of HSCs, such that these cells may become neural stem cells (Kucia et al., 2005), has been not confirmed in recently published studies. On the contrary, the transdifferentiation may be explained as a transient change in HSC phenotype induced by neural tissue-derived, spherical membrane fragments called microvesicles (or exosomes) that may transfer neural cell-surface receptors, mRNA, and miRNA to the HSCs employed for regeneration (Ratajczak et al., 2006). Although there are similar technical obstacles for HSCs, MSCs can be isolated from BM, PB, or UCB by expansion of an adherent population of fibroblast-like cells (Sadan et al., 2009), and it is widely accepted that MSCs contribute to the regeneration of mesenchymal tissues (i.e., bone, cartilage, muscle, ligament, tendon, adipose tissue, and stromal support structures). MSCs could be also obtained from several other tissues (e.g., PB, UCB, adipose tissue, dental pulp, menstrual blood) (Borlongan et al., 2010; Kadar et al., 2009). An unexpected discovery a few years ago suggested that MSCs are able to give rise to neuronal cells (Jeong et al., 2004). Like HSCs, this rare transdifferentiation event of MSCs was challenged, pointing to the possibility of in vitro contamination in the cell culture media (Krabbe et al., 2005; Lu et al., 2004) that could alter the morphology of MSCs. Under these same cell culture conditions, fibroblasts could shrink, elongate, and mimic neurons, but this proved to be only an in vitro morphological artifact (Krabbe et al., 2005; Lu et al., 2004). Thus, the rationale behind applying MSCs, just like HSCs, in brain regeneration is not well supported. On other hand, modest functional recovery occurs in animal models and possibly in patients after treatment with HSCs or MSCs (Burns et al., 2009; Dwain et al., 2006; Farin et al., 2009). The mechanism of action, however, likely does not involve differentiation of these cells into neurons, because such cells are rapidly eliminated after local delivery (Burns et al., 2009; Dwain et al., 2006; Farin et al., 2009). Instead, the most plausible explanation currently receiving much support from the literature is that HSCs or MSCs employed for regeneration could involve a by-stander effect. This would involve the release of growth factors or cytokines which facilitates neo-vascularization of damaged tissues leading to neurogenesis, as well as affording anti-inflammatory, anti-apoptotic, and anti-oxidative stress effects among other reparative responses (Baraniak et al., 2010; Hocking and Gibran, 2010; Ratajczak et al., 2010d; Zoladz et al., 2008). More recently, my group has taken a keen interest in repairing the BBB after stroke via EPC transplantation. Our overarching hypothesis is that BBB breakdown accompanies stroke and may be exacerbated by tissue-type plasminogen activator (tPA). To date, most stroke therapies have not considered the repair of this BBB damage after stroke. If BBB restoration via EPC transplantation alone, or in combination with tPA, is proven effective, we believe that this treatment could help a large population of ischemic stroke patients who otherwise would have missed the limited 3-hour tPA window. In addition, we envision that this EPC transplantation can supplement other stroke therapeutics that require BBB manipulation in order to afford beneficial effects, and can be extended to other neurological disorders characterized by BBB breakdown. Finally, the advent of VSELs is equally appealing for cell therapy in stroke. A major caveat in delivery of stem cells into the brain, whether via stereotactic local implantation or peripheral delivery (i.e., intravascular or inta-arterial) is the possibility of creating embolism with the high number of stem cells required to exert therapeutic effects. In this regard, the very small size of VSELs is one of the cells’ most unique properties that could avoid the potential adverse effects of embolism associated with cell therapy. That the VSEL isolation and expansion may require tedious steps and longer days to acquire ample supply of the cells can be circumvented by pursuing allogeneic rather than autologous transplants. Since stroke episodes cannot be predicted, an off-the-shelf treatment intervention is likely optimal, thus allogeneic transplant therapy may very well be more suitable than autotransplantation. Harvesting VSELs from young donors (i.e., PB) is a rich source of cells, noting that aging or disease processes yield a lower number of healthy, viable stem cells.
Although the preceding sections focus on BM-derived cell therapy for stroke, extending similar cell transplantation regimens to other neurological disorders may prove beneficial. In particular, the gating items outlined for translating cell therapy from the laboratory to clinic in adult stroke (Chopp et al., 2009; Borlongan, 2009) may also apply to neonatal brain injury (Borlongan and Weiss, 2011). In addition, the recognition of BBB alterations in other brain diseases shown to be candidate targets for cell therapy solicits parallel strategies in identifying the optimal cell type, dose, route and timing of transplantation.
7. Conclusions
The preclinical results have been encouraging, and the use of HSCs, MSCs, EPCs, and VSELs as donor sources is rapidly being translated into clinical trials. Over the last 5 years, recommendations to better guide the successful entry of stem cell therapy into the clinic have received continuing critical assessments from meetings involving leaders from academia, industry, NIH and the FDA (Borlongan, 2009; Borlongan et al., 2008; Chopp et al., 2009; Stem Cell Therapies as an Emerging Paradigm in Stroke Participants, 2009). The mechanism of action underlying cell therapy remains to be fully determined, and this persisting gap in our knowledge should be seriously considered when evaluating the risk-to-benefit ratio of this novel, experimental treatment for stroke. Although the preclinical trials have been encouraging, little efficacy has been seen in subsequent clinical trials. The optimal timing, route of injection, and dosage have yet to be determined. All of these factors may contribute to the limited efficacy of BM-derived stem cell transplantation in the clinic. In order for the transplanted stem cells to enter the injured brain, the BBB must be compromised. Due to this transient BBB leakage, the optimal timing for cell transplantation in the clinical setting may be within the first few days of stroke. A similar leaky BBB accompanies other CNS diseases, suggesting that the therapeutic window when cell delivery to the brain is being contemplated should consider the status of the BBB. In the end, the initiation of clinical trials needs to be preceded by systematically designed translational preclinical studies to provide rigorous inquiry of the safety and efficacy profile of these adult BM-derived stem cells for cell therapy in stroke and other neurological disorders.
Highlights.
Bone marrow-derived hematopoietic and non-hematopoietic stem cells are an attractive transplantable cell source for stroke.
Bone marrow-derived stem cells most likely exert their benefits via the bystander effect, not transdifferentiation.
Bone marrow-derived stem cells are mobilized from the bone marrow and home to the ischemic brain.
Molecular signaling pathways and host cellular microenvironment guide bone marrow-derived stem cell migration in stroke.
Acknowledgments
The authors extend appreciation to Nathan Weinbren and Samuel Metzen who provided excellent technical assistance in the final preparation of the manuscript.
Abbreviations
- BM
bone marrow
- PB
peripheral blood
- HSCs
hematopoietic stem cells
- MSCs
mesenchymal stem cells
- EPCs
endothelial progenitor stem cells
- VSELs
very small embryonic-like stem cells
- BBB
blood brain barrier
- SDF-1
stromal derived factor-1
- CNS
central nervous system
- G-CSF
granulocyte-colony stimulating factor
- GM-CSF
granulocyte-macrophage colony stimulating factor
- CB
cord blood
- HGF
hepatocyte growth factor
- VEGF
vascular endothelial growth factor
- NGF
nerve growth factor
- BDNF
brain-derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
- IGF-1
insulin growth factor-1
- IBZ
ischemia border zone
- SVZ
subventricular zone
- SGZ
subgranular zone
- MCAo
middle cerebral artery occlusion
- GDNF
glial cell line-derived neurotrophic factor
- CNTF
ciliary neurotrophic factor
- NT3
neurotrophin-3
- Cx43
connexin-43
- UCB
umbilical cord blood
- PSC
progenitor stem cells
Footnotes
Disclosures/Conflict of Interests: CVB is supported by James and Esther King Foundation for Biomedical Research Program, USF Signature Program in Interdisciplinary Neuroscience, SanBio Inc., Celgene Cellular Therapeutics, KMPHC, NeuralStem Inc., NIH NINDS UO15U01NS055914-04, and NIH NINDS RO1 1R01NS071956-01.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aicher A, Kollet O, Heeschen C, Liebner S, Urbich C, Ihling C, Orlandi A, Lapidot T, Zeiher AM, Dimmel S. The Wnt antagonist Dickkopf-1 mobilizes vasculogenic progenitor cells via activation of the bone marrow endosteal stem cell niche. Circ Res. 2008;103:796–803. doi: 10.1161/CIRCRESAHA.107.172718. [DOI] [PubMed] [Google Scholar]
- Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL, Schaar BT, Svendsen CN, Bliss TM, Steinberg GK. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain. 2011;134:1777–89. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- Ay I, Sugimori H, Finklestein SP. Intravenous basic fibroblast growth factor (bFGF) decreases DNA fragmenintation and prevents downregulation of Bcl-2 expression in the ischemic brain following middle cerebral artery occlusion in rats. Brain Res Mol Brain Res. 2001;87:71–80. doi: 10.1016/s0169-328x(00)00285-0. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Pabon MM, Cole MJ, Hudson CE, Sanberg PR, Wiling AE, Bickford PC, Gemma C. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC Neurosci. 2008;9:22. doi: 10.1186/1471-2202-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajada S, Mazakova I, Richardson JB, Ashammakhi N. Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Regen Med. 2008;2:169–183. doi: 10.1002/term.83. [DOI] [PubMed] [Google Scholar]
- Bakhtiary M, Marzban M, Mehizadeh M, Joghataei MT, Khoei S, Pirhajati Mahabadi V, Laribi B, Tondar M, Moshforoush A. Comparison of transplantation of bone marrow stromal cells (BMSC) and stem cell mobilization by granulocyte colony stimulating factor after traumatic brain injury in rat. Iran Biomed J. 2010;14:142–9. [PMC free article] [PubMed] [Google Scholar]
- Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000;28:707–715. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bompais H, Chagraoui J, Canron X, Crisan M, Liu XH, Anjo A, Tolla-Le Port C, Leboeuf M, Charbord P, Bikfalvi A, Uzan G. Human endothelial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood. 2004;103:2577–2584. doi: 10.1182/blood-2003-08-2770. [DOI] [PubMed] [Google Scholar]
- Borlongan CV. Cell therapy for stroke: remaining issues to address before embarking on clinical trials. Stroke. 2009;40:S146–148. doi: 10.1161/STROKEAHA.108.533091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Ling JG, Dillon-Carter O, Yu G, Hadman M, Cheng C, Carroll J, Hess DC. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res. 2004;1010:108–116. doi: 10.1016/j.brainres.2004.02.072. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Chopp M, Steinberg GK, Bliss TM, Li Y, Lu M, Hess DC, Kondziolka D. Potential of stem/progenitor cells in treating stroke: the missing steps in translating cell therapy from laboratory to clinic. Regen Med. 2008;3:249–250. doi: 10.2217/17460751.3.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Kaneko Y, Maki M, Yu SJ, Ali M, Allickson JG, Sanberg CD, Kuzmin Nichols N, Sanberg PR. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev. 2010;19:439–52. doi: 10.1089/scd.2009.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Weiss MD. Baby STEPS: A giant leap for cell therapy in neonatal brain injury. Pediatr Res. 2011;70:3–9. doi: 10.1203/PDR.0b013e31821d0d00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta R, Gao E, Stassi G, Bonci D, Relosi E, Zwas D, Patti M, Colonna L, Baiocchi M, Cappola S, Ma X, Condorelli G, Peschle C. Heart infarct in NOD-SCID mice: therapeutic vasculogenesis by transplantation of human CD34+ cells and low dose CD34+KDR+ cells. FASEB J. 2004;18:1392–1394. doi: 10.1096/fj.03-0879fje. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Burns TC, Verfaillie CM, Low WC. Stem cells for ischemic brain injury: a critical review. J Comp Neurol. 2009;515:125–144. doi: 10.1002/cne.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–44. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Ceradini DJ, Gurtner GC. Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med. 2005;15:57–63. doi: 10.1016/j.tcm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Chan CK, Chen CC, Luppen CA, Kim JB, DeBoer AT, Wei K, Helms JA, Kuo CJ, Kraft DL, Weissman IL. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–4. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Shyu WC, Lin SZ, Li H. Regenerative therapy for stroke. Cell Transplant. 2007;16:171–181. [PubMed] [Google Scholar]
- Chen J, Li Y, Chopp M. Intracerebral transplantation of bone marrow with BDNF after MCAo in rat. Neuropharmacology. 2000;39:711–716. doi: 10.1016/s0028-3908(00)00006-x. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001a;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001b;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Lu M, Chopp M. Caspase inhibition by Z-VAD increases the survival of grafted bone marrow cells and improves functional outcome after MCAo in rats. J Neurol Sci. 2002a;199:17–24. doi: 10.1016/s0022-510x(02)00075-8. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003a;73:778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003b;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Zhang R, Katakowski M, Gautam SC, Xu Y, Lu M, Zhang Z, Chopp M. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Res. 2004a;1005:21–28. doi: 10.1016/j.brainres.2003.11.080. [DOI] [PubMed] [Google Scholar]
- Chen JZ, Zhang FR, Tao QM, Wang XX, Zhu JH, Zhu JH. Number and activity of endothelial progenitor cells from peripheral blood in patients with hypercholesterolaemia. Clin Sci. 2004b;107:273–280. doi: 10.1042/CS20030389. [DOI] [PubMed] [Google Scholar]
- Chen SH, Chang FM, Chang HK, Chen WC, Huang KF, Lin MT. Human umbilical cord blood-derived CD34+ cells cause attenuation of multiorgan dysfunction during experimental heatstroke. Shock. 2007;27:663–671. doi: 10.1097/01.shk.0000248593.71388.40. [DOI] [PubMed] [Google Scholar]
- Chen X, Li Y, Katakowski M, Zhang L, Chen J, Xu Y, Gautam SC, Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002b;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- Cheng JL, Yang YJ, Li HL, Wang J, Wang MH, Zhang Y. In vivo tracing of superparamagnetic iron oxide-labeled bone marrow mesenchymal stem cells transplanted for traumatic brain injury by susceptibility weighted imaging in a rat model. Chin J Traumatol. 2010;13:173–7. [PubMed] [Google Scholar]
- Chernykh ER, Shevela EY, Leplina OY, Tikhonova MA, Ostanin AA, Kulagin AD, Pronkina NV, Muradov ZhM, Stupak W, Kozlov VA. Characteristics of bone marrow cells under conditions of impaired innervation in patients with spinal trauma. Bull Exp Biol Med. 2006;141:117–120. doi: 10.1007/s10517-006-0109-0. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- Chopp M, Steinberg GK, Kondziolka D, Lu M, Bliss TM, Li Y, Hess DC, Borlongan CV. Who’s in favor of translational cell therapy for stroke: STEPS forward please? Cell Transplant. 2009;18:691–693. doi: 10.3727/096368909X470883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34:967–975. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Del Papa N, Quirici N, Soligo D, Scavulo C, Cortiana M, Borsotti C, Maglione W, Comina DP, Vitali C, Fraticelli P, Gabrielli A, Cortelezzi A, Lambertenghi-Deliliers G. Bone marrow endothelial progenitors are defective in systemic sclerosis. Arthritis Rheum. 2006;54:2605–2615. doi: 10.1002/art.22035. [DOI] [PubMed] [Google Scholar]
- Dezawa M, Hoshino M, Ide C. Treatment of neurodegenerative diseases using adult bone marrow stromal cell derived neurons. Expert Opin Biol Ther. 2005;5:427–435. doi: 10.1517/14712598.5.4.427. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Zelher AM, Schneider MD. Unchain my heart: The scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobert N, Britten M, Assmus B, Berner U, Menzel C, Lehmann R, Hamscho N, Schächinger V, Dimmeler S, Zeiher AM, Grünwald F. Transplantation of progenitor cells after reperfused acute myocardial infarction: Evaluation of perfusion and myocardial viability with fdg-pet and thallium spect. Eur J Nucl Med Mol Imaging. 2004;31:1146–1151. doi: 10.1007/s00259-004-1490-4. [DOI] [PubMed] [Google Scholar]
- Drukala J, Paczkowska E, Kucia M, Młyńska E, Krajewski A, Machaliński B, Madeja Z, Ratajczak MZ. Stem cells, including a population of very small embryonic-like stem cells, are mobilized into peripheral blood in patients after skin burn injury. Stem Cell Rev. 2011 doi: 10.1007/s12015-011-9272-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Dunac A, Frelin C, Popolo-Blondeau M, Chatel M, Mahagne MH, Philip PJ. Neurological and functional recovery in human stroke are associated with peripheral blood CD34+ cell mobilization. J Neurol. 2007;254:327–332. doi: 10.1007/s00415-006-0362-1. [DOI] [PubMed] [Google Scholar]
- Dwain I, Xiangpeng Y, Zeng Z, Patricia T, Joh SY. Neural stem cells - a promising potential therapy for brain tumors. Curr Stem Cell Res Ther. 2006;1:79–84. doi: 10.2174/157488806775269070. [DOI] [PubMed] [Google Scholar]
- Erbs S, Linke A, Adams V, Lenk K, Thiele H, Diederich KW, Emmrich F, Kluge R, Kendziorra K, Sabri O, Schuler G, Hambercht R. Transplantation of blood-derived progenitor cells after recanalization of chronic coronary artery occlusion: first randomized and placebo-controlled study. Circ Res. 2005;97:756–762. doi: 10.1161/01.RES.0000185811.71306.8b. [DOI] [PubMed] [Google Scholar]
- Fadini GP. An underlying principle for the study of circulating progenitor cells in diabetes and its complications. Diabetologia. 2008;51:1091–1094. doi: 10.1007/s00125-008-1021-0. [DOI] [PubMed] [Google Scholar]
- Farin A, Liu CY, Langmoen IA, Apuzzo ML. Biological restoration of central nervous system architecture and function: part 3-stem cell- and cell-based applications and realities in the biological management of central nervous system disorders: traumatic, vascular, and epilepsy disorders. Neurosurgery. 2009;65:831–859. doi: 10.1227/01.NEU.0000351721.81175.0B. [DOI] [PubMed] [Google Scholar]
- Fernandez-Aviles F, San Roman JA, Garcia-Frade J, Fernandez ME, Penarrubia MJ, de la Fuente L, Gómez-Bueno M, Cantalapiedra A, Fernández J, Gutierrez O, Sánchez PL, Hernández C, Sanz R, García-Sancho J, Sánchez A. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res. 2004;95:742–748. doi: 10.1161/01.RES.0000144798.54040.ed. [DOI] [PubMed] [Google Scholar]
- Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Gao Q, Li Y, Chopp M. Bone marrow stromal cells increase astrocyte survival via upregulation of phosphoinositide 3-kinase/threonine protein kinase and mitogen activated protein kinase kinase/extracellular signal-regulated kinase pathways and stimulate astrocyte trophic factor gene expression after anaerobic insult. Neuroscience. 2005a;136:123–134. doi: 10.1016/j.neuroscience.2005.06.091. [DOI] [PubMed] [Google Scholar]
- Gao Q, Katakowski M, Chen X, Li Y, Chopp M. Human marrow stromal cells enhance connexin43 gap junction intercellular communication in cultured astrocytes. Cell Transplant. 2005b;14:109–117. doi: 10.3727/000000005783983205. [DOI] [PubMed] [Google Scholar]
- García-Verdugo JM, Doetsch F, Wichterle H, Lim DA, Alvarez-Buylla A. Architecture and cell types of the adult subventricular zone: in search of the stem cells. J Neurobiol. 1998;36:234–48. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Gehling UM, Ergün S, Schumacher U, Wagener C, Pantel K, Otem M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schäfer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- Geiger H, Rudolph KL. Aging in the lympho-hematopoietic stem cell compartment. Trends Immunol. 2009;230:360–365. doi: 10.1016/j.it.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Ghani U, Shuaib A, Salam A, Nasir A, Shuaib U, Jeerakathil T, Sher F, O’Rourke F, Nasser AM, Schwindt B, Todd K. Endothelial progenitor cells during cerebrovascular disease. Stroke. 2005;36:151–153. doi: 10.1161/01.STR.0000149944.15406.16. [DOI] [PubMed] [Google Scholar]
- Gharib SA, Dayyat EA, Khalyfa A, Kim J, Clair HB, Kucia M, Gozal D. Intermittent hypoxia mobilizes bone marrow-derived very small embryonic - like stem cells and activates developmental transcriptional programs in mice. Sleep. 2010;33:1–8. doi: 10.1093/sleep/33.11.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: Implications for cell-based vascular therapy. Circulation. 2003;108:2710–2715. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Simmons PJ. The biology and application of human bone marrow stromal cell precursors. J Hematother. 1996;5:15–23. doi: 10.1089/scd.1.1996.5.15. [DOI] [PubMed] [Google Scholar]
- Guo X, Liu L, Zhang M, Bergeron A, Cui Z, Dong JF, Zhang J. Correlation of CD34+ cells with tissue angiogenesis after traumatic brain injury in a rat model. J Neurotrauma. 2009;26:1337–44. doi: 10.1089/neu.2008.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Kobune M, Nakamura K, Kawano Y, Kato K, Honmou O, Houkin K, Matsunaga T, Niitsu Y. Mesenchymal stem cells (MSC) as therapeutic cytoreagents for gene therapy. Cancer Sci. 2005;96:149–156. doi: 10.1111/j.1349-7006.2005.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S, Satoh K, Hirota M, Kanno A, Umino J, Ito H, Masamune A, Kikuta K, Kume K, Shimosegawa T. The homeobox gene MSX2 determines chemosensitivity of pancreatic cancer cells via the regulation of transporter gene ABCG2. J Cell Physiol. 2011 doi: 10.1002/jcp.22781. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hara K, Yasuhara T, Maki M, Matsukawa N, Masuda T, Yu SJ, Ali M, Yu G, Kim SU, Hess DC, Borlongan CV. Neural progenitor NT2N cell lines from teratocarcinoma for transplantation therapy in stroke. Prog Neurobiol. 2008;85:318–334. doi: 10.1016/j.pneurobio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69–80. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. JACC. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennemann B, Ickenstein G, Sauerbruch S, Luecke K, Haas S, Horn N, Andreesen R, Bogdahn U, Winkler J. Mobilization of CD34+ hematopoietic cells, colony-forming cells and long-term culture-initiating cells into the peripheral blood of patients with an acute cerebral ischemic insult. Cytotherapy. 2008;10:303–311. doi: 10.1080/14653240801949994. [DOI] [PubMed] [Google Scholar]
- Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- Hess DA, Allan AL. Migratory strategies of normal and malignant stem cells. Methods Mol Biol. 2011;750:25–44. doi: 10.1007/978-1-61779-145-1_2. [DOI] [PubMed] [Google Scholar]
- Hess DC, Hill WD, Martin-Studdard A, Carroll J, Brailer J, Carothers J. Bone marrow as a source of endothelial cells and NeuN-expressing cells after stroke. Stroke. 2002;33:1362–1368. doi: 10.1161/01.str.0000014925.09415.c3. [DOI] [PubMed] [Google Scholar]
- Hess DC, Borlongan CV. Cell-based therapy in ischemic stroke. Expert Rev Neurother. 2008;8:1193–1201. doi: 10.1586/14737175.8.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DC, Borlongan CV. Stem cells and neurological diseases. Cell Prolif. 2008;1:94–114. doi: 10.1111/j.1365-2184.2008.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y, Kimura M, Hara K, Noma K, Jitsuki D, Nakagawa K, Oshima T, Chayama K, Sueda T, Goto C, Matsubara H, Murohara T, Yoshumi M. Autologous bone-marrow mononuclear cell implantation improves endothelium-dependent vasodilation in patients with limb ischemia. Circulation. 2004;109:1215–1218. doi: 10.1161/01.CIR.0000121427.53291.78. [DOI] [PubMed] [Google Scholar]
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316:2213–2219. doi: 10.1016/j.yexcr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol. 2006;199:56–66. doi: 10.1016/j.expneurol.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov M, Weber C. Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med. 2004;8:498–508. doi: 10.1111/j.1582-4934.2004.tb00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov M, Heussen N, Schober A, Weber C. Intracoronary infusion of autologous bone marrow cells and left ventricular function after acute myocardial infarction: A meta-analysis. J Cell Mol Med. 2006;10:727–733. doi: 10.1111/j.1582-4934.2006.tb00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Kucia M, Hussain LR, Wen Y, Xu H, Yan J, Ratajczak MZ, Ildstad ST. Bone marrow transplantation temporarily improves pancreatic function in streptozotocin-induced diabetes: potential involvement of very small embryonic-like cells. Transplantation. 2010;89:677–85. doi: 10.1097/TP.0b013e3181c9dc7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huertas A, Palange P. Circulating endothelial progenitor cells and chronic pulmonary diseases. Eur Respir J. 2011;37:426–31. doi: 10.1183/09031936.00034810. [DOI] [PubMed] [Google Scholar]
- Hwang WS, Chen SH, Lin CH, Chang HK, Chen WC, Lin MT. Human umbilical cord blood-derived CD34+ cells can be used as a prophylactic agent for experimental heatstroke. J Pharmacol Sci. 2008;106:46–55. doi: 10.1254/jphs.fp0071567. [DOI] [PubMed] [Google Scholar]
- Ikeda N, Nonoguchi N, Zhao MZ, Watanabe T, Kajimoto Y, Furutama D, Kimura F, Dezawa M, Coffin RS, Otsuki Y, Kuroiwa T, Miyatake S. Bone marrow stromal cells that enhanced fibroblast growth factor-2 secretion by herpes simplex virus vector improve neurological outcome after transient focal cerebral ischemia in rats. Stroke. 2005;36:2725–2730. doi: 10.1161/01.STR.0000190006.88896.d3. [DOI] [PubMed] [Google Scholar]
- Imai K, Kobayashi M, Wang J, Shinobu N, Yoshida H, Hamada J, Shindo M, Higashino F, Tanaka J, Asaka M, Hosokawa M. Selective secretion of chemoattractants for haemopoietic progenitor cells in bone marrow endothelial cells: a possible role in homing of haemoatopoietic progenitor cells to bone marrow. Br J Haematol. 1999;106:905–911. doi: 10.1046/j.1365-2141.1999.01644.x. [DOI] [PubMed] [Google Scholar]
- Imanishi T, Hano T, Nishio I. Estrogen reduces endothelial progenitor cell senescence through augmentation of telomerase activity. J Hypertens. 2005;23:1699–1706. doi: 10.1097/01.hjh.0000176788.12376.20. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525–1531. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- Ito H, Rovira II, Bloom ML, Takeda K, Ferrans VJ, Quyyumi AA, Finkel T. Endothelial progenitor cells as putative targets for angiostatin. Cancer Res. 1999;59:5875–5877. [PubMed] [Google Scholar]
- Jeong JA, Gang EJ, Hong SH, Hwang SH, Kim SW, Yang IH, Ahn C, Han H, Kim H. Rapid neural differentiation of human cord blood-derived mesenchymal stem cells. Neuroreport. 2004;15:1731–1734. doi: 10.1097/01.wnr.0000134846.79002.5c. [DOI] [PubMed] [Google Scholar]
- Kadar K, Kiraly M, Porcsalmy B, Molnar B, Racz GZ, Blazsek J, Kallo K, Szabo EL, Gera I, Gerber G, Varga G. Differentiation potential of stem cells from human dental origin promise for tissue engineering. J Physiol Phramacol. 2009;60:167–175. [PubMed] [Google Scholar]
- Kalinkovich A, Spiegel A, Shivtiel S, Kollet O, Jordaney N, Piacibello W, Lapidot T. Blood-forming stem cells are nervous: direct and indirect regulation of immature human CD34+ cells by the nervous system. Brain Behav Immun. 2009;23:1059–1065. doi: 10.1016/j.bbi.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- Khakoo AY, Finkel T. Endothelial progenitor cells. Annu Rev Med. 2005;56:79–101. doi: 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- Khoo ML, Tao H, Meedeniya AC, Mackay-Sim A, Ma DD. Transplantation of neuronal-primed human bone marrow mesenchymal stem cells in hemiparkinsonian rodents. PLoS One. 2011;6:e19025. doi: 10.1371/journal.pone.0019025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinakis A, Lobry C, Abdel-Wahab O, Oh P, Haeno H, Buonamici S, van De Walle I, Cathelin S, Trimarchi T, Araldi E, Liu C, Ibrahim S, Beran M, Zavadil J, Efstratiadis A, Taghon T, Michor F, Levine RL, Aifantis I. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. 2011;473:230–3. doi: 10.1038/nature09999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko IK, Kim BG, Awadallah A, Mikulan J, Lin P, Letterio JJ, Dennis DE. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther. 2010;18:1365–72. doi: 10.1038/mt.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobune M, Kawano Y, Ito Y, Chiba H, Nakamura K, Tsuda H, Sasaki K, Dehari H, Uchida H, Honmou O, Takahashi S, Bizen A, Takimoto R, Matsunaga T, Kato J, Kato K, Houkin K, Niitsu Y, Hamada H. Telomerized human multipotent mesenchymal cells can differentiate into hematopoietic and cobble- stone area-supporting cells. Exp Hematol. 2003;31:715–722. doi: 10.1016/s0301-472x(03)00177-2. [DOI] [PubMed] [Google Scholar]
- Kocher AA, Schuster MD, Bonaros N, Lietz K, Xiang G, Martens TP, Kurlansky PA, Sondermeijer H, Witkowki P, Boyle A, Homma S, Wang SF, Itescu S. Myocardial homing and neovascularization by human bone marrow angioblasts is regulated by IL-8/Gro CXC chemokines. J Mol Cell Cardiol. 2006;40:455–464. doi: 10.1016/j.yjmcc.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S, Goichberg P, Kalinkovich A, Arenzana-Seisdedos F, Nagler A, Hardan I, Revel M, Shafritz DA, Lapidot T. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, Mattsson B, Johansson BB, Eriksson PS. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005;36:1278–1282. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24:1442–1447. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, Gebel J, Jannetta P, DeCesare S, Elder EM, McGrogan M, Reitman MA, Bynum L. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55:565–569. doi: 10.1212/wnl.55.4.565. [DOI] [PubMed] [Google Scholar]
- Kong XD, Zhang Y, Liu L, Sun N, Zhang MY, Zhang JN. Endothelial progenitor cells with Alzheimer’s disease. Chin Med J (Engl) 2011;124:901–6. [PubMed] [Google Scholar]
- Krabbe C, Zimmer J, Meyer M. Neural transdifferentiation of mesenchymal stem cells-a critical review. APMIS. 2005;113:831–844. doi: 10.1111/j.1600-0463.2005.apm_3061.x. [DOI] [PubMed] [Google Scholar]
- Khoo ML, Tao H, Meedeniva AC, Mackay-Sim A, Ma DD. Transplantation of neuronal-primed human mesenchymal stem cells in hemiparkinsonian rodents. PLoS One. 2011;6:c19025. doi: 10.1371/journal.pone.0019025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreipe H, Radzun HJ, Schumacher U, Parwaresch MR. Lectin binding and surface glycoprotein pattern of human macrophage populations. Histochemistry. 1986;86:201–206. doi: 10.1007/BF00493388. [DOI] [PubMed] [Google Scholar]
- Kucia M, Ratajczak J, Ratajczak MZ. Are bone marrow stem cells plastic or heterogenous - that is the question. Exp Hematol. 2005;33:613–623. doi: 10.1016/j.exphem.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Kucia M, Zhang YP, Reca R, Wysoczynski M, Machalinski B, Majka M, Ildstad ST, Ratajczak J, Shields CB, Ratajczak MZ. Cells enriched in markers of neural tissue-committed stem cells reside in the bone marrow and are mobilized into the peripheral blood following stroke. Leukemia. 2006a;20:18–28. doi: 10.1038/sj.leu.2404011. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006b;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiul M, Moldenhawer S, Zuba-Surma E, Czajka R, Wojakowski W, Machalinski B, Ratajczak MZ. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia. 2007;21:297–303. doi: 10.1038/sj.leu.2404470. [DOI] [PubMed] [Google Scholar]
- Kunz GA, Liang G, Cuculi F, Gregg D, Vata KC, Shaw LK, Goldschmidt-Clermont PJ, Dong C, Taylor DA, Person ED. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J. 2006;152:190–195. doi: 10.1016/j.ahj.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9:189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11:96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Kuwana M, Okazaki Y, Yasuoka H, Kawakami Y, Ikeda Y. Defective vasculogenesis in systemic sclerosis. Lancet. 2004;364:603–610. doi: 10.1016/S0140-6736(04)16853-0. [DOI] [PubMed] [Google Scholar]
- Lapergue B, Mohammad A, Shuaib A. Endothelial progenitor cells and cerebroascular diseases. Prog Neurobiol. 2007;83:349–362. doi: 10.1016/j.pneurobio.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Kollet O. The brain-bone-blood triad: traffic lights for stem-cell homing and mobilization. Hematology Am Soc Hematol Educ Program. 2010;2010:1–6. doi: 10.1182/asheducation-2010.1.1. [DOI] [PubMed] [Google Scholar]
- Lee JK, Jin HK, Endo S, Schuchman EH, Carter JE, Bae JS. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer’s disease mice by modulation of immune responses. Stem Cells. 2010;28:329–43. doi: 10.1002/stem.277. [DOI] [PubMed] [Google Scholar]
- Lev EI, Kleiman NS, Birnbaum Y, Harris D, Korbling M, Estrov Z. Circulating endothelial progenitor cells and coronary collaterals in patients with non-st segment elevation myocardial infarction. J Vasc Res. 2005;42:408–414. doi: 10.1159/000087370. [DOI] [PubMed] [Google Scholar]
- Levesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979–1992. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chopp M. Adult bone marrow transplantation after stroke in adult rats. Cell Transplant. 2001;10:31–40. [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: Neurotrophins and functional recovery. Neurology. 2002a;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2002b;56:1666–1672. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- Li Y, McIntosh K, Chen J, Zhang C, Gao Q, Borneman J, Raginski K, Mitchell J, Sen L, Zhang J, Lu D, Chopp M. Allogeneic bone marrow stromal cells promote glial-axonal remodeling without immunologic sensitization after stroke in rats. Exp Neurol. 2006;198:313–325. doi: 10.1016/j.expneurol.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Li ZQ, Zhang M, Jing YZ, Zhang WW, Liu Y, Cui LJ, Yuan L, Liu XY, Hu TS. The clinical study of autologous peripheral blood stem cell transplantation by intracoronary infusion in patients with acute myocardial infarction (AMI) Int J Cardiol. 2007;115:52–56. doi: 10.1016/j.ijcard.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Li Calzi S, Neu MB, Shaw LC, Grant MB. Endothelial progenitor dysfunction in the pathogenesis of diabetic retinopathy: treatment concept to correct diabetes-associated deficits. EPMA J. 2010;1:88–100. doi: 10.1007/s13167-010-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang B, Kan Z, Zhang B, Yang Z, Chen J, Wang D, Wei H, Zhang JN, Jiang R. Progesterone increases circulating endothelial progenitor cells and induces neural regeneration after traumatic brain injury in aged rats. J Neurotrauma. 2011 doi: 10.1089/neu.2011.1807. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Hou H, Yi W, Yang G, Gu C, Lau WB, Gao E, Ma X, Lu Z, Wei X, Pei J, Yi D. Increased expression of pigment epithelium-derived factor in aged mesenchymal stem cells impairs their therapeutic efficacy for attenuating myocardial infarction injury. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr131. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lougheed M, Moore ED, Scriven DR, Steinbrecher UP. Uptake of oxidized LDL by macrophages differs from that of acetyl LDL and leads to expansion of an acidic endolysosomal compartment. Arterioscler Thromb Vasc Biol. 1999;19:1881–1890. doi: 10.1161/01.atv.19.8.1881. [DOI] [PubMed] [Google Scholar]
- Lu P, Blesch A, Tuszynski MH. Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact? J Neurosci Res. 2004;77:174–191. doi: 10.1002/jnr.20148. [DOI] [PubMed] [Google Scholar]
- Madeddu P, Emanueli C, Pelosi E, Salis MB, Cerio AM, Bonanno G, Patti M, Stassi G, Condorelli G, Peschle C. Transplantation of low dose CD34+KDR+ cells promotes vascular and muscular regeneration in ischemic limbs. FASEB J. 2004;18:1737–1739. doi: 10.1096/fj.04-2192fje. [DOI] [PubMed] [Google Scholar]
- Manaenko A, Chen H, Kammer J, Zhang JH, Tang J. Comparison Evans Blue injection routes: Intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. J Neurosci Methods. 2011;195:206–10. doi: 10.1016/j.jneumeth.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Asahara T. Post-natal endothelial progenitor cells for neovascularization in tissue regeneration. Cardiovasc Res. 2003;58:390–398. doi: 10.1016/s0008-6363(02)00785-x. [DOI] [PubMed] [Google Scholar]
- McCarty JH. Cell adhesion and signaling networks in brain neurovascular units. Curr Opin Hematol. 2009;16:209–214. doi: 10.1097/MOH.0b013e32832a07eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeer NA, Schleifman EB, Glazer PM, Saltzman WM. Polymer delivery systems for site-specific genome editing. J Control Release. 2011 doi: 10.1016/j.jconrel.2011.05.011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer CC, Kondziolka D, Villemangne VL, Wechsler L, Goldstein S, Thulborn KR, Gebel J, Elder EM, DeCesare S, Jacobs A. Serial [18F] fluorodeoxyglucose positron emission tomography after human neuronal implantation for stroke. Neurosurgery. 2001;49:586–591. doi: 10.1097/00006123-200109000-00011. [DOI] [PubMed] [Google Scholar]
- Meluzín J, Mayer J, Groch L, Janousek S, Hornácek I, Hlinomaz O, Kala P, Panovský R, Prásek J, Kamínek M, Stanícek J, Klabusay M, Korístek Z, Navrátil M, Dusek L, Vinklárková J. Autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction: the effect of the dose of transplanted cells on myocardial function. Am Heart J. 2006;152:9–15. doi: 10.1016/j.ahj.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Meluzín J, Janousek S, Mayer J, Groch L, Hornácek I, Hlinomaz O, Kala P, Panovský R, Prásek J, Kamínek M, Stanícek J, Klabusay M, Korístek Z, Navrátil M, Dusek L, Vinklárková J. Three-, 6-, and 12-month results of autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction. Int J Cardiol. 2008;128:185–192. doi: 10.1016/j.ijcard.2007.04.098. [DOI] [PubMed] [Google Scholar]
- Michaud SE, Dussault S, Haddad P, Groleau J, Rivard A. Circulating endothelial progenitor cells from healthy smokers exhibit impaired functional activities. Atherosclerosis. 2006;187:423–432. doi: 10.1016/j.atherosclerosis.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Mocini D, Staibano M, Mele L, Giannantoni P, Menichella G, Colivicchi F, Sordini P, Salera P, Tubaro M, Santini M. Autologous bone marrow mononuclear cell transplantation in patients undergoing coronary artery bypass grafting. Am Heart J. 2006;151:192–197. doi: 10.1016/j.ahj.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Morancho A, Rosell A, Barcia-Bonilla L, Montaner J. Metalloproteinase and stroke size; role for anti-inflammatory treatment. Ann NY Acad Sci. 2010;1207:123–133. doi: 10.1111/j.1749-6632.2010.05734.x. [DOI] [PubMed] [Google Scholar]
- Mund JA, Ingram DA, Yoder MC, Case J. Endothelial progenitor cells and cardiovascular cell-based therapies. Cytotherapy. 2009;11:103–113. doi: 10.1080/14653240802714827. [DOI] [PubMed] [Google Scholar]
- Munoz-Elias G, Woodbury D, Black IB. Marrow stromal cells, mitosis, and neuronal differentiation: stem cell and precursor function. Stem Cells. 2003;21:437–448. doi: 10.1634/stemcells.21-4-437. [DOI] [PubMed] [Google Scholar]
- Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113:1161–1166. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Egucji H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan Z, Grande A, Sanberg CD, Sanberg PR, Low WC. Infusion of human umbilical cord blood ameliorates neurologic deficits in rats with hemorrhagic brain injury. Ann NY Acad Sci. 2005;1049:84–96. doi: 10.1196/annals.1334.009. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Kondziolka D, Wechsler L, Goldstein S, Gebel J, DeCesare S, Elder EM, Zhang PJ, Jacobs A, McGrogan M, Lee VM, Trojanowski JQ. Clonal human (hNT) neuron grafts for stroke therapy: neuropathology in a patient 27 months after implantation. Am J Pathol. 2002;160:1201–1206. doi: 10.1016/S0002-9440(10)62546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, Hodges MG, Jelinek I, Madala S, Karpati S, Mezey E. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci USA. 2010;107:5652–7. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690–70. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- Nikolic WV, Hou H, Touan T, Zhu Y, Giunta B, Sanberg CD, Tan J. Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice. Stem Cells Dev. 2008;17:423–439. doi: 10.1089/scd.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio Y, Koda M, Kamada T, Someya Y, Yoshinaga K, Okada S, Harada H, Okawa A, Moriya H, Yamazaki M. The use of hemopoietic stem cells derived from human umbilical cord blood to promote restoration of spinal cord tissue and recovery of hindlimb function in adult rats. J Neurosurg Spine. 2006;5:424–433. doi: 10.3171/spi.2006.5.5.424. [DOI] [PubMed] [Google Scholar]
- Numaguchi Y, Sone T, Okumura K, Ishii M, Morita Y, Kubota R, Yokouchi K, Imai H, Harada M, Osanai H, Kondo T, Murohara T. The impact of the capability of circulating progenitor cell to differentiate on myocardial salvage in patients with primary acute myocardial infarction. Circulation. 2006;114:114–119. doi: 10.1161/CIRCULATIONAHA.105.000588. [DOI] [PubMed] [Google Scholar]
- Osaka M, Honmou O, Murakami T, Nonaka T, Houkin K, Hamada H, Kocsis JD. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010;1343:226–35. doi: 10.1016/j.brainres.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Sato K, Oh I, Ozaki K, Uchibori R, Obara Y, Kikuchi Y, Ito T, Okada T, Urabe M, Mizukami H, Kume A. Cell and gene therapy using mesenchymal stem cells (MSCs) J Autoimmun. 2008;30:121–7. doi: 10.1016/j.jaut.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Pablos JL, Amara A, Bouloc A, Santiago B, Caruz A, Galindo M, Delaunay T, Virelizier JL, Arenzana-Seisdedos F. Stromal-cell derived factor is exoressed by dendritic cells and endothelium in human skin. Am J Pathol. 1999;155:1577–1586. doi: 10.1016/S0002-9440(10)65474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paczkowska E, Kucia M, Koziarska D, Halasa M, Safranow K, Masiuk M, Karbicka A, Nowik M, Nowacki P, Ratajczak MZ, Machalinski B. Clinical evidence that very small embryonic-like stem cells are mobilized into peripheral blood in patients after stroke. Stroke. 2009;4:1237–1244. doi: 10.1161/STROKEAHA.108.535062. [DOI] [PubMed] [Google Scholar]
- Pai M, Zacharoulis D, Milicevic MN, Helmy S, Jiao LR, Levicar N, Tait P, Scott M, Marley SB, Jestice K, Glibetic M, Bansi D, Khan SA, Kyriakou D, Rountas C, Thillainayagam A, Nicholls JP, Jensen S, Apperley JF, Gordon MY, Habib NA. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103:1952–1958. doi: 10.1111/j.1572-0241.2008.01993.x. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T, Scadden DT. Stem-cell ecology and stem cells in motion. Blood. 2008;111:3923–3930. doi: 10.1182/blood-2007-08-078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekkadan B, Tillies AW, Yarmush ML. Bone marrow-derived mesenchymal stem cells ameliorate autoimmune enteropathy independently of regulatory T cells. Stem Cells. 2008;26:1913–9. doi: 10.1634/stemcells.2007-0790. [DOI] [PubMed] [Google Scholar]
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- Pereira RF, O’Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Carvalho AC, Dutra HS, Dohmann HJ, Silva GV, Belém L, Vivacqua R, Rangel FO, Esporcatte R, Geng YJ, Vaughn WK, Assad JA, Mesquita ET, Willerson JT. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Silva GV, Mesquita CT, Belém L, Vaughn WK, Rangel FO, Assad JA, Carvalho AC, Branco RV, Rossi MI, Dohmann HJ, Willerson JT. Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation. 2004;110:213–218. doi: 10.1161/01.CIR.0000138398.77550.62. [DOI] [PubMed] [Google Scholar]
- Pirro M, Schillaci G, Menecali C, Bagalia F, Paltriccia R, Vaudo G, Mannarino MR, Mannarino E. Reduced number of circulating endothelial progenitors and HOXA9 expression in CD34+ cells of hypertensive patients. J Hypertens. 2007;25:2093–2099. doi: 10.1097/HJH.0b013e32828e506d. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay MA, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Pochampally RR, Smith JR, Ylostalo J, Prockop DJ. Serum deprivation of human marrow stromal cells (hMSCs) selects for a subpopulation of early progenitor cells with enhanced expression of OCT-4 and other embryonic genes. Blood. 2004;103:1647–1652. doi: 10.1182/blood-2003-06-1967. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Shin DM, Wan W, Liu R, Masternak MM, Piotrowska K, Wiszniewska B, Kucia M, Bartke A, Ratajczak MZ. Higher number of stem cells in bone marrow of circulating Igf-1 level low Laron dwarf mice - novel view on Igf-1, stem cells and aging. Leukemia. 2011a;25:729–33. doi: 10.1038/leu.2010.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Zuba-Surma E, Paczkowska W, Kucia M, Nowacki P, Ratajczak MZ. Stem cells for neural regeneration - a potential application of very small embryonic-like stem cells. J Physiol Pharmacol. 2011b;62:3–12. [PubMed] [Google Scholar]
- Ratajczak MZ, Zuba-Surma EK, Shin DM, Ratajczak J, Kucia M. Very small embryonic-like (VSEL) stem cells in adult organs and their potential role in rejuvenation of tissues and longevity. Exp Gerontol. 2008;43:1009–17. doi: 10.1016/j.exger.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Kim CH, Wojakowski W, Janowska-Wieczorek A, Kucia M, Ratajczak J. Innate immunity as orchestrator of stem cell mobilization. Leukemia. 2010a;24:1667–1675. doi: 10.1038/leu.2010.162. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Shin DM, Liu R, Marlicz W, Tarnowski M, Ratajczak J, Kucia M. Epiblast/germ line hypothesis of cancer development revisited: lesson from the presence of Oct-4+ cells in adult tissues. Stem Cell Rev. 2010b;6:307–316. doi: 10.1007/s12015-010-9143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Shin DM, Ratajczak J, Kucia M, Bartke A. A novel insight into aging: are there pluripotent very small embryonic-like stem cells (VSELs) in adult tissues overtime depleted in an Igf-1-dependent manner Aging. 2010c;2:875–883. doi: 10.18632/aging.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, Kucia M, Janowska-Wieczorek A, Ratajczak J. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010d;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers D, Osuna C, Gonzalo-Gobernado R, Herranz AS, Díaz-Gil JJ, Jiménez-Escrig A, Asensio MJ, Miranda C, Rodríguez-Serrano M, Banzán E. Liver growth factor promotes the survival of grafted neural stem cells in a rat model of parkinson’s disease. Curr Stem Cell Res Ther. 2010 doi: 10.2174/157488812798483421. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Rempe DA, Kent TA. Using bone marrow stromal cells for treatment of stroke. Neurology. 2002;59:486–487. doi: 10.1212/wnl.59.4.486. [DOI] [PubMed] [Google Scholar]
- Rosenblatt S, Irikura K, Caday CG, Finklestein SP, Moskowitz MA. Basic fibroblast growth factor dilates rat pial arterioles. J Cereb Blood Flow Metab. 1994;14:70–74. doi: 10.1038/jcbfm.1994.11. [DOI] [PubMed] [Google Scholar]
- Rouhl RP, van Oostenbrugge RJ, Damoiseaux J, Tervaert JW, Lodder J. Endothelial progenitor cell research in stroke: a potential shift in pathophysiological and therapeutical concepts. Stroke. 2008;39:2158–2165. doi: 10.1161/STROKEAHA.107.507251. [DOI] [PubMed] [Google Scholar]
- Rustemeyer P, Wittkowski W, Jurk K, Koller A. Optimized flow cytometric analysis of endothelial progenitor cells in peripheral blood. J Immunoassay Immunochem. 2006;27:77–88. doi: 10.1080/15321810500403789. [DOI] [PubMed] [Google Scholar]
- Sadan O, Melamed E, Offen D. Bone-marrow-derived mesenchymal stem cell therapy for neurodegenerative diseases. Expert Opin Biol Ther. 2009;9:1487–1497. doi: 10.1517/14712590903321439. [DOI] [PubMed] [Google Scholar]
- Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ramos J, Song S, Cao C, Arendash G. The potential of hematopoietic growth factors for treatment of Alzheimer’s disease: a mini-review. BMC Neurosci. 2008;9:S3. doi: 10.1186/1471-2202-9-S2-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatteman GC, Awad O. Hemangioblasts, angioblasts, and adult endothelial cell progenitors. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:13–21. doi: 10.1002/ar.a.10131. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, Chopp M. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137:393–399. doi: 10.1016/j.neuroscience.2005.08.092. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, Lu M, Raginski K, Vanguri P, Smith A, Chopp M. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–7. [PubMed] [Google Scholar]
- Shin DM, Zuba-Surma EK, Wu W, Ratajczak J, Wysoczynski M, Ratajczak MZ, Kucia M. Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4(+) very small embryoniclike stem cells. Leukemia. 2009;23:2042–2051. doi: 10.1038/leu.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Liu R, Klich I, Wu W, Ratajczak J, Kucia M, Ratajcza MZ. Molecular signature of adult bone marrow-purified very small embryonic-like stem cells supports their developmental epiblast/germ line origin. Leukemia. 2010;24:1450–1461. doi: 10.1038/leu.2010.121. [DOI] [PubMed] [Google Scholar]
- Shin DM, Liu R, Klich I, Ratajczak J, Kucia M, Ratajczak MZ. Molecular characterization of isolated from murine adult tissues very small embryonic/epiblast like stem cells (VSELs) Mol Cells. 2010;29:533–538. doi: 10.1007/s10059-010-0081-4. [DOI] [PubMed] [Google Scholar]
- Snyder EY. The risk of putting something where it does not belong: Mesenchymal stem cells produce masses in the brain. Exp Neurol. 2011;230:75–77. doi: 10.1016/j.expneurol.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Sokal EM. From hepatocytes to stem and progenitor cells for liver regenerative medicine: advances and clinical perspectives. Cell Prolif. 2011;44:39–43. doi: 10.1111/j.1365-2184.2010.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Kamath S, Mosquera D, Zigova T, Sanberg P, Vesely DL, Sanchez-Ramos J. Expression of brain natriuretic peptide by human bone marrow stromal cells. Exp Neurol. 2004;185:191–197. doi: 10.1016/j.expneurol.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Sordi V, Piemonti L. Mesenchymal stem cells as feeder cells for pancreatic islet transplants. Rev Diabet Stud. 2010;7:132–43. doi: 10.1900/RDS.2010.7.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovalat H, Scrofani M, Eidenschenk A, Pasquet S, Rimelen V, Hénon P. Identification and isolation from either adult human bone marrow or G-CSF mobilized peripheral blood of CD34+/CD133+/CXCR4+/ Lin-CD45− cells, featuring morphological, molecular and phenotypic characteristics of very small embryonic-like (VSEL) stem cells. Exp Hematol. 2011;39:495–505. doi: 10.1016/j.exphem.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Spiegel A, Shivtiel S, Kalinkovich A, Lundin A, Netzer N, Goichberg P, Azaria Y, Resnick I, Hardan I, Ben-Hur H, Nagler A, Rubinstein M, Lapidot T. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34(+) cells through Wnt signaling. Nat Immunol. 2007;8:1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- Spiegel A, Kalinkovich A, Shivtiel S, Kollet O, Lapidot T. Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell. 2008;3:484–492. doi: 10.1016/j.stem.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Stem Cell Therapies as an Emerging Paradigm in Stroke Participants. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–515. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- Strauer BE, Brehm M, Zeus T, Bartsch T, Schannwell C, Antke C, Sorg RV, Kögler G, Wernet P, Müller HW, Köstering M. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: the IACT Study. J Am Coll Cardiol. 2005;46:1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Höllt V, Schulz S. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: Isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22:5865–5878. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Szczot M, Wojtowicz T, Mozrzymas JW. GABAergic and glutamatergic currents in hippocampal slices and neuronal cultures show profound differences: a clue to a potent homeostatic modulation. J Physiol Pharmacol. 2010;61:501–506. [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004a;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Matsuyama T, Moriwaki H, Hayashi T, Hayashida K, Nagatsuka K, Todo K, Mori K, Stern DM, Soma T, Naritomi H. Circulating cd34-positive cells provide an index of cerebrovascular function. Circulation. 2004b;109:2972–2975. doi: 10.1161/01.CIR.0000133311.25587.DE. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Tang Y, Yasuhara T, Hara K, Matsukawa N, Maki M, Yu G, Xu L, Hess DC, Borlongan CV. Transplantation of Bone Marrow-Derived Stem Cells:A Promising Therapy for Stroke. Cell Transplant. 2007;16:159–169. [PubMed] [Google Scholar]
- Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet. 2002;360:427. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- Umemura T, Soga J, Hidaka T, Takemoto H, Nakamura S, Jitsuiki D, Nishioka K, Goto C, Teragawa H, Yoshizumi M, Chayama K, Higashi Y. Aging and hypertension are independent risk factors for reduced number of circulating endothelial progenitor cells. Am J Hypertens. 2008;21:1203–1209. doi: 10.1038/ajh.2008.278. [DOI] [PubMed] [Google Scholar]
- Vendrame MG, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T, Sanberg CD, Sanberg PR, Willing AE. Infusion of human umbilical cord blood cells in a rat model of stroke dose dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- Venturin GT, Greggio S, Marinowic DR, Zanirati G, Machado DC, Dacosta JC. Bone marrow mononuclear cells reduce seizure frequency and improve cognitive outcome in chronic epileptic rat. Life Sci. 2011;15:229–234. doi: 10.1016/j.lfs.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Wang D, Shen W, Zhang F, Chen M, Chen H, Cao K. Connexin43 promotes survival of mesenchymal stem cells in ischaemic heart. Cell Biol Int. 2010;34:415–23. doi: 10.1042/CBI20090118. [DOI] [PubMed] [Google Scholar]
- Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S, Hart C, Sanchez-Ramos J, Sanberg PR. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res. 2003;73:296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- Witherick J, Wilkins A, Scolding N, Kemp K. Mechanisms of oxidative damage in multiple sclerosis and a cell therapy approach to treatment. Autoimmune Dis. 2010;2011:164608. doi: 10.4061/2011/164608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojakowski W, Kucia M, Kazmierski M, Ratajczak MZ, Tendera M. Circulating progenitor cells in stable coronary heart disease and acute coronary syndromes: relevant reparatory mechanism? Heart. 2008;94:27–33. doi: 10.1136/hrt.2006.103358. [DOI] [PubMed] [Google Scholar]
- Wojakowski W, Tendera M, Kucia M, Zuba-Surma E, Paczkowska E, Ciosek J, Halasa M, Król M, Kazmiersi M, Buszman P, Ochala A, Ratajczak J, Machaliński B, Ratajcza MZ. Mobilization of bone marrow-derived Oct-4+ SSEA-4+ very small embryonic-like stem cells in patients with acute myocardial infarction. J Am Coll Cardiol. 2009;53:1–9. doi: 10.1016/j.jacc.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojakowski W, Kucia M, Liu R, Zuba-Surma E, Jadczyk T, Bachowski R, Nabialek E, KaŸmierski M, Ratajczak MZ, Tendera M. Circulating very small embryonic-like stem cells in cardiovascular disease. J Cardiovasc Transl Res. 2011;4:138–44. doi: 10.1007/s12265-010-9254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergün S. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133:1543–1551. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- Zhang C, Li Y, Chen J, Gao Q, Zacharek A, Kapke A, Chopp M. Bone marrow stromal cells upregulate expression of bone morphogenetic proteins 2 and 4, gap junction protein connexin-43 and synaptophysin after stroke in rats. Neuroscience. 2006;141:687–695. doi: 10.1016/j.neuroscience.2006.04.054. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Chen J, Yang M, Katakowski M, Lu M, Chopp M. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 2004;1030:19–27. doi: 10.1016/j.brainres.2004.09.061. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90:284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- Zhao ZM, Li HJ, Liu HY, Lu SH, Yang RC, Zhang QJ, Han ZC. Intraspinal transplantation of CD34+ human umbilical cord blood cells after spinal cord hemisection injury improves functional recovery in adult rats. Cell Transplant. 2004;13:113–122. doi: 10.3727/000000004773301780. [DOI] [PubMed] [Google Scholar]
- Zhao MZ, Nonoguchi N, Ikeda N, Watanabe T, Furutama D, Miyazawa D, Funakoshi H, Kajimoto Y, Nakamura T, Dezawa M, Shibata MA, Otsuki Y, Coffin RS, Liu WD, Kuroiwa T, Miyatake S. Novel therapeutic strategy for stroke in rats by bone marrow stromal cells and ex vivo HGF gene transfer with HSV-1 vector. J Cereb Blood Flow Metab. 2006;26:1176–1188. doi: 10.1038/sj.jcbfm.9600273. [DOI] [PubMed] [Google Scholar]
- Zheng H, Shen CJ, Qiu FY, Zhao YB, Fu GS. Stromal cell-derived factor 1 alpha reduces senescence of endothelial progenitor subpopulation in lectin-binding and DiLDL-uptaking cell through telomerase activation and telomere elongation. J Cell Physiol. 2010;223:757–763. doi: 10.1002/jcp.22086. [DOI] [PubMed] [Google Scholar]
- Zhou S. From bone to brain: Human skeletal stem cell therapy for stroke. Cent Nerv Syst Agents Med Chem. 2011 doi: 10.2174/187152411796011376. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Voss M, Kaiser S, Kapp U, Waller CF, Martens UM. Lack of telomerase activity in human mesenchymal stem cells. Leukemia. 2003;17:1146–1149. doi: 10.1038/sj.leu.2402962. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Martens UM. Telomeres, senescence, and hematopoietic stem cells. Cell Tissue Res. 2008;331:79–90. doi: 10.1007/s00441-007-0469-4. [DOI] [PubMed] [Google Scholar]
- Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol. 2008;59:119–132. [PubMed] [Google Scholar]
- Zuba-Surma EK, Kucia M, Wu W, Klich I, Lillard JW, Jr, Ratajczak J, Ratajczak MZ. Very small embryonic-like stem cells are present in adult murine organs: ImageStream-based morphological analysis and distribution studies. Cytometry A. 2008;73A:1116–1127. doi: 10.1002/cyto.a.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuba-Surma EK, Kucia M, Rui L, Shin DM, Wojakowski W, Rarajczak J, Rarajczak MZ. Fetal liver very small embryonic/epiblast like stem cells follow developmental migratory pathway of hematopoietic stem cells. Ann NY Acad Sci. 2009;1176:205–18. doi: 10.1111/j.1749-6632.2009.04562.x. [DOI] [PubMed] [Google Scholar]
- Zuba-Surma EK, Klich I, Greco N, Laughlin MJ, Ratajczak J, Ratajczak MZ. Optimization of isolation and further characterization of umbilical-cordblood-derived very small embryonic/epiblast-like stem cells (VSELs) Eur J Haematol. 2010;84:34–46. doi: 10.1111/j.1600-0609.2009.01352.x. [DOI] [PubMed] [Google Scholar]
- Zuba-Surma EK, Ratajczak MZ. Overview of very small embryonic-like stem cells (VSELs) and methodology of their identification and isolation by flow cytometric methods. Curr Protoc Cytom. 2010;Chapter 9(Unit 9–29) doi: 10.1002/0471142956.cy0929s51. [DOI] [PubMed] [Google Scholar]
- Zwart I, Hill AJ, Al-Allaf F, Shah M, Girdlestone J, Sanusi AB, Mehmet H, Navarrete R, Navarrete C, Jen LS. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Exp Neurol. 2009;216:439–4. doi: 10.1016/j.expneurol.2008.12.028. [DOI] [PubMed] [Google Scholar]


