Abstract
DAX1 (dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1; also known as NROB1, nuclear receptor subfamily 0, group B, member 1) encodes a nuclear receptor that is expressed in embryonic stem (ES) cells, steroidogenic tissues (gonads, adrenals), the ventromedial hypothalamus (VMH), and pituitary gonadotropes. Humans with DAX1 mutations develop an X-linked syndrome referred to as adrenal hypoplasia congenita (AHC). These boys typically present in infancy with adrenal failure but later fail to undergo puberty because of hypogonadotropic hypogonadism (HHG). The adrenal failure reflects a developmental abnormality in the transition of the fetal to adult zone, resulting in glucocorticoid and mineralocorticoid deficiency. The etiology of HHG involves a combined and variable deficiency of hypothalamic GnRH secretion and/or pituitary responsiveness to GnRH resulting in low LH, FSH and testosterone. Treatment with exogenous gonadotropins generally does not induce spermatogenesis. Animal models indicate that DAX1 also plays a critical role in testis development and function. As a nuclear receptor, DAX1 has been shown to function as a transcriptional repressor, particularly of pathways regulated by other nuclear receptors, such as steroidogenic factor 1 (SF1). In addition to reproductive tissues, DAX1 is also expressed at high levels in ES cells and plays a role in the maintenance of pluripotentiality. Here we review the clinical manifestations associated with DAX1 mutations as well as the evolving information about its function based on animal models and in vitro studies.
Human Studies
Clinical Features of AHC
X-linked AHC is a disorder that presents in infancy with adrenal insufficiency and occurs in fewer than 1:12, 500 live births (Sikl, 1948, Mitchell and Rhaney, 1959, Dacou and Di George, 1968, Uttley, 1968, Petersen, Bille, Jacobsen et al., 1982, Kelch, Virdis, Rapaport et al., 1984). The age at presentation has a bimodal distribution: the majority of patients present within the first 2 months of life; the remainder present insidiously later in childhood (Yu, Ito, Saunders et al., 1998, Reutens, Achermann, Ito et al., 1999, Lin, Gu, Ozisik et al., 2006). Histologically, the disorder is characterized by absence of the adrenal cortex permanent zone, with residual cytomegalic cells (Uttley, 1968, Kerenyi, 1961, Mamelle, David, Riou et al., 1975).
Clinical signs and symptoms include typical features of adrenal insufficiency: hyperpigmentation, vomiting, poor feeding, failure to thrive, convulsions, vascular collapse and sudden death. Biochemical findings include hyponatremia, hyperkalemia, hypoglycemia, reduced serum cortisol and aldosterone, and increased plasma ACTH (Kelch et al., 1984). The diagnosis of adrenal insufficiency can be made on the basis of baseline low cortisol and elevated ACTH levels or after ACTH stimulation testing (Achermann, Silverman, Habiby et al., 2000). Some patients have residual cortisol or mineralocorticoid production but their circulating levels are inadequate to prevent the clinical manifestations. In some pedigrees, unexplained deaths of male infants may be attributed to X-linked AHC before recognition of the disorder in the family.
Treatment with glucocorticoids and mineralocorticoids allows affected individuals to survive into adolescence at which point hypogonadotropic hypogonadism (HHG) is manifest and distinguishes AHC from other causes of adrenal insufficiency (Petersen et al., 1982, Prader, Zachmann and Illig, 1975, Golden, Lippe and Kaplan, 1977, Zachmann, Illig and Prader, 1980, Kelly, Joplin and Pearson, 1977).
Genetics of AHC
Prior to the identification of the DAX1 gene, the underlying cause of AHC was unknown. The observation that some patients with X-linked AHC have a contiguous gene syndrome, and present with various combinations of glycerol kinase deficiency, Duchenne muscular dystrophy, ornithine transcarbamoyltransferase deficiency, and mental retardation allowed the locus to be narrowed to Xp21.3-p21.2 (Hammond, Howard, Brookwell et al., 1985, Bartley, Patil, Davenport et al., 1986, Francke, Harper, Darras et al., 1987, Mandel, Willard, Nussbaum et al., 1989, Goonewardena, Dahl, Ritzen et al., 1989, Worley, Ellison, Zhang et al., 1993). Subsequently, mutations were identified in DAX1, confirming that it is the causative gene for AHC (Muscatelli, Strom, Walker et al., 1994, Zanaria, Muscatelli, Bardoni et al., 1994).
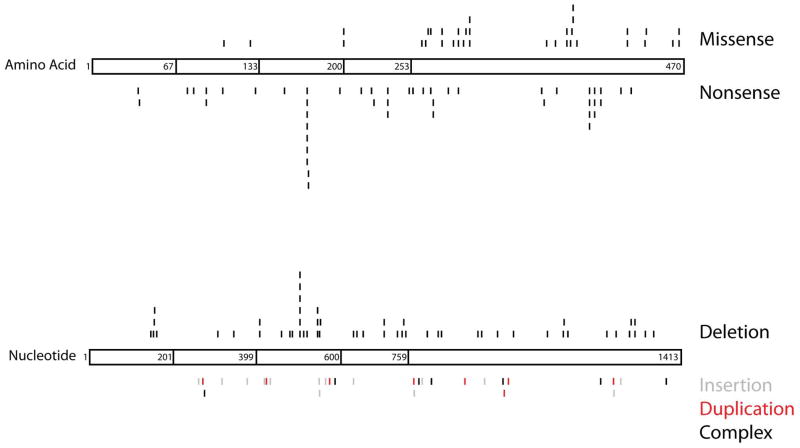
Since the initial identification of DAX1 as the gene responsible for AHC, numerous additional mutations have been discovered including deletions, alterations of splice-sites, missense mutations, nonsense mutations and frameshift mutations (Fig. 1) (Reutens et al., 1999, Lin et al., 2006, Achermann et al., 2000, Muscatelli et al., 1994, Zanaria et al., 1994, Guo, Mason, Stone et al., 1995, Takahashi, Shoji, Haraguchi et al., 1997, Meloni, Cao and Rosatelli, 1996, Nakae, Abe, Tajima et al., 1997, Schwartz, Blichfeldt and Muller, 1997, Peter, Viemann, Partsch et al., 1998, Seminara, Achermann, Genel et al., 1999, Bassett, O’Halloran, Williams et al., 1999, Caron, Imbeaud, Bennet et al., 1999, Merke, Tajima, Baron et al., 1999, Tabarin, Achermann, Recan et al., 2000, Domenice, Latronico, Brito et al., 2001, Wiltshire, Couper, Rodda et al., 2001, Sekiguchi, Hara, Matsuoka et al., 2007, Bernard, Ludbrook, Queipo et al., 2006, Verrijn Stuart, Ozisik, de Vroede et al., 2007, Landau, Hanukoglu, Sack et al., 2010, Li, Liu, Zhang et al., 2010, Sykiotis, Hoang, Avbelj et al., 2010, Abe, Nakae, Yasoshima et al., 1999, Achermann, Meeks and Jameson, 2001, Ahmad, Paterson, Lin et al., 2007, Argente, Ozisik, Pozo et al., 2003, Balsamo, Antelli, Baldazzi et al., 2005, Brown, Scobie, Townsend et al., 2003, Calliari, Longui, Rocha et al., 2007, Frapsauce, Ravel, Legendre et al., 2011, Garcia-Malpartida, Gomez-Balaguer, Sola-Izquierdo et al., 2009, Guo, Burris, Zhang et al., 1996, Habiby, Boepple, Nachtigall et al., 1996, Hamaguchi, Arikawa, Yasunaga et al., 1998, Kinoshita, Yoshimoto, Motomura et al., 1997, Krone, Riepe, Dorr et al., 2005, Lam, Cheng, Poon et al., 2006, Mantovani, Ozisik, Achermann et al., 2002, Mericq, Ciaccio, Marino et al., 2007, Nakae, Tajima, Kusuda et al., 1996, Ozisik, Mantovani, Achermann et al., 2003, Salvi, Gomez, Fiaux et al., 2002, Tsai and Tung, 2005, Wang, Killinger and Hegele, 1999, Wu, Zhang, Zhou et al., 2011, Yanase, Takayanagi, Oba et al., 1996, Zhang, Guo, Wagner et al., 1998, Zhang, Huang, Anyane-Yeboa et al., 2001, Franzese, Brunetti-Pierri, Spagnuolo et al., 2005, Laissue, Copelli, Bergada et al., 2006, Okuhara, Abe, Kondo et al., 2008). After other known causes (i.e., congenital adrenal hyperplasia, CAH) have been excluded, it is estimated that as many as 50% of boys with idiopathic primary adrenal insufficiency may have mutations in DAX1 (Lin et al., 2006). Because females are typically silent carriers of AHC, a careful family history is required to detect the X-linked pattern of transmission.
Figure 1.
Schematic of known DAX1 mutations. The DAX1 protein structure is divided into three and a half amino-terminal repeats (amino acids 1–67, 68–133, 134–200 and 201–253) and one nuclear receptor-like domain (amino acids 254–470). In the top diagram, missense (top) and nonsense (bottom) mutations are shown at specific amino acids along the length of DAX1. The bottom figure depicts deletions (black above protein), insertions (gray below protein), duplications (red below protein) and complex deletions/insertions (black below protein), which are assigned based on nucleotide number. Visualization of all naturally occurring mutations demonstrates clustering at specific points along the DAX1 protein. Numbering of mutations began at the A of the ATG translational initiation codon (Ensembl transcript ID ENST00000378970 and protein ID ENSP00000368253). The locations of the frameshift mutations (deletions, insertions, duplications and complex deletions/insertions) are designated by the first nucleotide altered. References are found above in the section “Genetics of AHC.” This represents a comprehensive list of DAX1 mutations to the best of our knowledge. Please refer to the original articles for details.
About one-third of AHC patients harbor DAX1 deletions, which can range from microdeletions within the coding sequence or promoter, to very large deletions involving adjacent genes (Lin et al., 2006). Deletions are important to recognize because DAX1 PCR reactions in an affected male will yield no product and the DAX1 sequence of an unaffected carrier will be normal, reflecting amplification of the normal X-chromosomal allele.
The locations of naturally occurring DAX1 mutations provide insights into the structure-function relationships in the protein (Fig. 1). Truncation of as little as 39 amino acids from the carboxy-terminus is sufficient to eliminate transcriptional repression by DAX1 (Ito, Yu and Jameson, 1997). Missense mutations account for about one-quarter of DAX1 mutations and tend to cluster in the carboxy-terminal ligand binding-like domain (Lin et al., 2006). These frequently occur in conserved amino acids and structural models suggest that most alter protein folding, thereby impairing nuclear localization and cofactor recruitment (Lehmann, Wurtz, Renaud et al., 2003, Lehmann, Lalli and Sassone-Corsi, 2002). It is notable that relatively few missense mutations occur in the amino-terminal region of the protein, possibly because of redundant function of the repeated LXXLL protein interaction domains (Verrijn Stuart et al., 2007, Kawajiri, Ikuta, Suzuki et al., 2003, Suzuki, Kasahara, Yoshioka et al., 2003).
Phenotypic Expression
The phenotypes of patients with AHC are heterogeneous. The type or location of a DAX1 mutation does not consistently predict the age of onset of adrenal insufficiency or disease severity (Muscatelli et al., 1994, Peter et al., 1998, Merke et al., 1999, Wiltshire et al., 2001, Sekiguchi et al., 2007, Bernard et al., 2006, Verrijn Stuart et al., 2007). Phenotypic heterogeneity occurs within a family with the same mutation, as well as with different DAX1 mutations, suggesting an effect of modifier genes or environmental effects on the expression of clinical manifestations (see the Overview Section and Chapter 1).
Although AHC usually presents during infancy, some patients are not diagnosed until later in life. One individual was diagnosed at 28 years of age. He presented with isolated adrenal insufficiency and undiagnosed partial HHG, as there was sufficient testosterone to produce some masculinization at puberty (Tabarin et al., 2000). Of note, this mutation (I439S) resulted in incomplete transcriptional repression in cell-based assays. Another patient presented at 28 years of age with HHG and was shown to have compensated adrenal insufficiency (Mantovani et al., 2002). He had a Y380D mutation that also caused partial loss of function in transfection assays. A 20 year old male presented with adrenal insufficiency and was found to have hypogonadism with low testosterone and inappropriately normal LH, but elevated FSH. He had an unusual amino-terminal (Q37X) frameshift mutation predicted to cause a severe truncation of the DAX1 protein (Ozisik et al., 2003). However, utilization of a downstream alternate translation site allowed production of an amino-terminally truncated protein, presumably accounting for his milder phenotype. A patient with a DAX1 missense mutation (W105C) had isolated mineralocorticoid deficiency, without evidence of glucocorticoid deficiency or HHG (Verrijn Stuart et al., 2007). This same amino acid substitution was present in 3 unaffected male relatives. This mutation was located in the amino-terminal repeat region of the DAX1 protein and the mild phenotype was suggested to be caused by redundant function of the other repeat domains.
Although the majority of AHC patients are male, mutations with phenotypic expression in females have been reported. The first was a homozygous nonsense mutation (codon 172) in a woman with isolated HHG. This mutation was proposed to involve gene conversion (Merke et al., 1999). The second was a heterozygous missense mutation in a female with late-onset AHC; of note, her hemizygous father and heterozygous sister were asymptomatic (Bernard et al., 2006). In some pedigrees with affected males, delayed puberty has been reported in carrier females (Seminara et al., 1999). A female with a contiguous gene deletion including DAX1 and DMD presented with adrenal insufficiency in infancy (Shaikh, Boyes, Kingston et al., 2008). The deletion was located on the maternal X-chromosome and she was found to have skewed X-inactivation, resulting in relatively low expression of paternal genes in some tissues. Thus, while most female carriers of DAX1 mutations are asymptomatic, some have been reported with clinical features of AHC. Like other X-linked disorders, this may reflect variable gene expression or oligogenicity (Sykiotis et al., 2010).
The onset of puberty is also variable in AHC. Most commonly, boys fail to enter puberty and gonadotropin levels are low and unresponsive to GnRH. Rarely, there is delayed or incomplete puberty associated with partial gonadotropin deficiency. Surprisingly, some affected boys develop gonadotropin-independent precocious puberty, including pubic hair, penile growth, increased testicular volume and advanced bone age (Domenice et al., 2001, Landau et al., 2010). Although testosterone levels are elevated, gonadotropin levels are usually low. There is variable responsiveness to GnRH stimulation. When present, the features of precocious puberty wane over time. The mechanism of precocious puberty is unclear and may be multifactorial. Possible causes include Leydig cell autonomy, persistent androgen production by the fetal adrenal, ACTH-stimulation of steroidogenic cells in the testis, and impaired gonadal steroid negative feedback of the HPG axis (Peter et al., 1998, Domenice et al., 2001, Landau et al., 2010).
Unilateral or bilateral cryptorchidism may occur, likely because of low gonadotropin production in utero (Zachmann et al., 1980, Muscatelli et al., 1994, Peter et al., 1998, Kruse, Sippell and Schnakenburg, 1984). As adults, affected patients typically have oligospermia or azoospermia that is unresponsive to gonadotropin therapy, raising the possibility of Sertoli, peritubular myoid, or germ cell dysfunction. In a limited number of cases in which biopsies are available, there is evidence of Sertoli cell only syndrome (Seminara et al., 1999) or testicular dysgenesis (Ozisik et al., 2003). As noted below, mice with Dax1 mutations exhibit gonadal dysgenesis, including abnormal testis cord development (Yu et al., 1998, Jeffs, Meeks, Ito et al., 2001, Meeks, Weiss and Jameson, 2003).
The phenotypic heterogeneity of AHC may be due to multiple factors including compensation from other genes (Dipple and McCabe, 2000, Scriver and Waters, 1999), epigenetic or non-genetic factors (Peter et al., 1998, Sekiguchi et al., 2007), and modulation of DAX1 localization, and therefore potentially function, in the fetal adrenal gland by components of the extracellular matrix and hormones such as ACTH (Battista, Otis, Cote et al., 2005) As noted above, some DAX1 mutations cause incomplete loss of function. There is also evidence of skewed X-inactivation in some female carriers (Shaikh et al., 2008). In addition, the symptoms of adrenal insufficiency can be nonspecific and the diagnosis may be delayed until adrenal crisis is precipitated by other illnesses. A family history of AHC and access to medical care can also lead to earlier recognition of the disorder (Reutens et al., 1999).
The Hypothalamic-Pituitary Axis in AHC
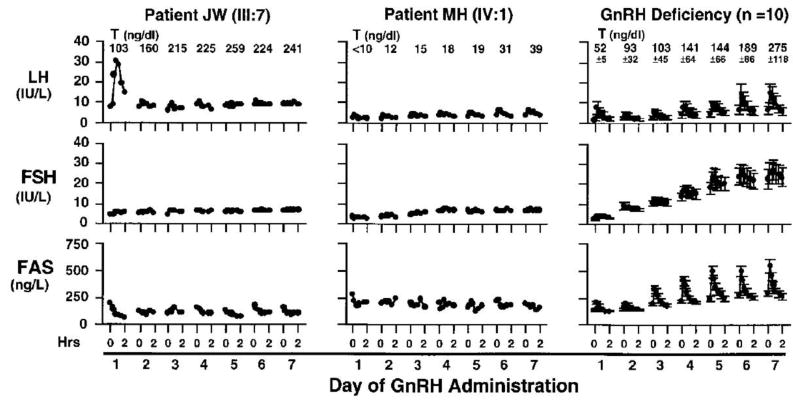
Due to the heterogeneity of the phenotype in AHC, it has been challenging to pinpoint the site of deficiency within the hypothalamic-pituitary axis (Golden et al., 1977, Hay, Smail and Forsyth, 1981). Early studies suggested that HHG resulted from either a primary pituitary (Gordon, Cohen, Beastall et al., 1984, Kikuchi, Kaji, Momoi et al., 1987, Bovet, Reymond, Rey et al., 1988, Pitteloud, Thambundit, Dwyer et al., 2009) or a primary hypothalamic defect (Peter et al., 1998, Kruse et al., 1984, Kletter, Gorski and Kelch, 1991, Partsch and Sippell, 1989). The current theory is based largely on a detailed study of two kindreds with HHG, each with mutations that truncated the protein, albeit at different amino acids (Habiby et al., 1996). In the first kindred, the proband (MH) had low baseline LH levels but normal free alpha subunit (FAS) levels (Fig. 2). In the second kindred, the proband (JW) had normal LH and FSH but 24h profiles of pulsatile LH secretion were erratic, without a clear pulsatile pattern. After administration of exogenous pulsatile GnRH, MH failed to mount a gonadotropin response, consistent with a pituitary defect. In contrast, JW responded to the first pulse but did not respond to subsequent GnRH pulses. This response contrasts with GnRH deficient patients (e.g., Kallmann syndrome), who exhibit a progressive increase in GnRH-stimulated gonadotropin responses as the pituitary is primed over several days. The lack of a gonadotropin response to GnRH in AHC patients with HHG confirms a defect at the level of the pituitary (Seminara et al., 1999, Habiby et al., 1996). However, because the initial bolus of exogenous GnRH stimulated LH release in JW, it is postulated that he had a low level of pre-existing GnRH, sufficient for priming, but insufficient for maintaining normal gonadotropin secretion (Habiby et al., 1996). Thus, the hormonal responses in JW are consistent with a hypothalamic defect in GnRH production, as well as a pituitary defect in GnRH responsiveness and gonadotropin production. Unanticipated pituitary and gonadal defects have been identified in other subsets of patients with isolated GnRH deficiency receiving pulsatile GnRH (Sykiotis et al; JCEM, 2011), suggesting that, like DAX1, mutations in genes expressed in the hypothalamus, pituitary, and gonads may reveal phenotypes that are masked by abnormalities in other components of the reproductive axis.
Figure 2.
Gonadotropin levels after 1 week of GnRH administration to two probands with HHG and ten patients with Kallmann Syndrome (GnRH deficiency). Intravenous pulsatile GnRH (25ng/kg) was administered every 2 hours after baseline secretory studies. Daily testosterone levels are shown above each graph.
Diagnosis and Treatment of AHC
Molecular genetic techniques now provide the opportunity for genetic testing, early diagnosis, and steroid replacement (Schwartz et al., 1997, Wiltshire et al., 2001). Sequencing of DAX1 has demonstrated the value of presymptomatic diagnosis of AHC, allowing close clinical monitoring and early treatment before an Addisonian crisis as well as appropriate hormonal therapy at the time of puberty (Achermann et al., 2000). In families known to harbor DAX1 mutations, it is reasonable to perform measurements of plasma cortisol and aldosterone before and after ACTH stimulation, as well as plasma ACTH and plasma renin activity, pending genetic testing (Peter et al., 1998). If the location of a DAX1 mutation is known, and is absent in a potentially affected boy, the diagnosis has been excluded, although it may be reasonable to confirm the DNA sequence independently before eliminating all monitoring. If the location of a mutation is not known, a normal coding sequence does not necessarily exclude the disorder, as some mutations may alter promoter activity or DAX1 mRNA splicing (Goto and Katsumata, 2009). Submicroscopic deletions upstream of DAX1, resulting in loss of regulatory sequences, has been reported to result in sex-reversal, reminiscent of duplication of the DAX1 locus (Smyk, Berg, Pursley et al., 2007).
The treatment of adrenal insufficiency in AHC is similar to that of other causes. Most patients require replacement of both glucocorticoids and mineralocorticoids. The treatment of HHG includes several options. Testosterone replacement therapy has been used to directly stimulate virilization (Prader et al., 1975, Hay et al., 1981). Human chorionic gonadotropin has also been used to stimulate testosterone production (Kelly et al., 1977, Tabarin et al., 2000, Hay et al., 1981). Gonadotropins have been administered in an attempt to induce spermatogenesis but without success, possibly pointing to an independent DAX1-mediated defect in the gonad (Seminara et al., 1999). As noted above, pulsatile GnRH is ineffective in these patients. It remains to be seen whether earlier interventions or ICSI might be used to achieve fertility.
Differentiating AHC from Other Causes of Congenital Adrenal Insufficiency
AHC must be differentiated from a variety of other adrenalopathies as the clinical manifestations, treatment, and prognosis vary considerably. Congenital adrenal hyperplasia (CAH) can be caused by mutations in several genes encoding steroidogenic enzymes involved in glucocorticoid synthesis (CYP21A2, CYP17A1, HSD3B2, CYP11B1) or in the co-factor enzyme P450 oxidoreductase (POR) that serves as an electron donor to CYP21A2 and CYP17A1. 21-hydroxylase (CYP21A2) deficiency is by far the most common cause of neonatal adrenal insufficiency. It is characterized by increased levels of 17-hydroxyprogesterone and other steroids upstream of the enzymatic block. Mutation of CYP11A1 (side-chain cleavage enzyme) is a rare cause of adrenal insufficiency and undervirilization. XY patients with StAR mutations (Lipoid Congenital Adrenal Hyperplasia, Lipoid CAH) also present with adrenal insufficiency and severe undervirilization (female phenotype) because they lack the StAR cholesterol transport protein and the resulting lipid accumulation is ultimately toxic to steroidogenic cells (Tee, Lin, Sugawara et al., 1995). XY patients with heterozygous SF1 mutations exhibit a range of features that can include gonadal dysgenesis and adrenal insufficiency (Achermann et al., 2001). Adrenoleukodystrophy can be excluded by measurement of serum long chain fatty acids, which are elevated in adrenoleukodystrophy but normal in AHC (Reutens et al., 1999). Aldosterone deficiency precedes overt glucocorticoid deficiency in some AHC infants. (Wiltshire et al., 2001, Sills, Voorhess, MacGillivray et al., 1983). Thus, AHC should be considered in patients initially diagnosed with primary hypoaldosteronism who later develop cortisol deficiency. Not uncommonly, AHC is not recognized as the cause of adrenal insufficiency until patients fail to enter puberty.
Mouse Models of Dax1 Function
Mapping and Structure of Dax1
The human and mouse Dax1 genes are structurally similar with two exons and about 75% similarity in the coding region (Burris, Guo and McCabe, 1996). Exon 1 encodes the amino-terminal region with three and one-half repeated sequences containing hydrophobic LXXLL motifs, which are thought to be involved in protein-protein interactions (Suzuki et al., 2003, Iyer and McCabe, 2004) (Fig. 1). A region homologous to the ligand binding domain (LBD) of other nuclear receptors is encoded by portions of exons 1 and 2. Notably, the DAX1 protein lacks the zinc-finger DNA binding domain that characterizes other members of the nuclear receptor superfamily. The carboxy-terminus contains transcriptional regulatory sequences (Iyer and McCabe, 2004). To date, no ligand has been found for DAX1 (Iyer and McCabe, 2004).
Dax1 Expression
Dax1 is expressed in the adrenal and in all regions of the HPG axis during development and in the adult. In situ hybridization demonstrates Dax1 expression at embryonic day 10.5 (E10.5) in the urogenital ridge (Swain, Zanaria, Hacker et al., 1996). Dax1 expression is sexually dimorphic in the developing gonads. By E12.5, Dax1 expression is extinguished in the testis although it persists in the ovaries. In the testis, both Sertoli cells and Leydig cells stain positive for DAX1; in the ovaries, the granulosa and theca cells show DAX1 expression. In the adrenal gland, Dax1 is expressed predominantly in the zona glomerulosa (Ikeda, Takeda, Shikayama et al., 2001). Studies using RT-PCR reveal that Dax1 is expressed in the adult ovary, testis, adrenal gland, hypothalamus, cerebral cortex, spinal cord, thymus, heart, lung, kidney, and spleen (Bae, Schaefer, Partan et al., 1996).
Dax1 Mouse Models
Multiple Dax1 mouse models have been generated to characterize its biological function and its role in AHC. The dosage-sensitive sex-reversal (DSS) phenotype was first replicated in mice by overexpression of Dax1 in the Poschiavinus mouse strain (Swain, Narvaez, Burgoyne et al., 1998, Eicher, Washburn, Schork et al., 1996). Dax1 was overexpressed using multiple copies of an 11kb genital ridge specific region of the gene on the genetic background of a weak Sry allele in this carefully selected murine model. XY sex-reversal occurred in a subset of mice. Although this model confirmed the important role of Dax1 in sex determination, the phenotype was not replicated on backgrounds other than the Poschiavinus mouse, presumably due to modifying effects of the genetic background.
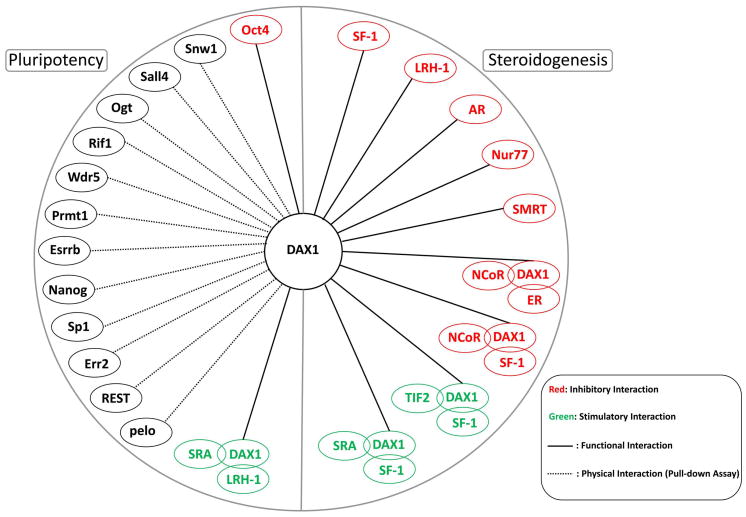
When Dax1 was deleted from ES cells in an effort to generate a Dax1 knock-out mouse, rapid ES cell differentiation was observed (Yu et al., 1998), precluding reimplantation of the cells into blastocysts. Subsequent studies showed that Dax1 is expressed in the blastocyst and is important for maintenance of ES cell pluripotency (Clipsham, Niakan and McCabe, 2004, Niakan, Davis, Clipsham et al., 2006). DAX1 is part of a protein network of molecules that includes OCT4 and other regulators of pluripotency (Kelly and Hammer, 2010, Kelly, Xu, Kuick et al., 2010, Kim, Chu, Shen et al., 2008) (Fig. 3). DAX1 is also highly expressed in certain poorly differentiated malignancies, such as Ewing’s sarcoma (Garcia-Aragoncillo, Carrillo, Lalli et al., 2008).
Figure 3.
DAX1 protein interactions. DAX1 acts in either an inhibitory (red) or a stimulatory (green) manner by interacting with proteins involved in pluripotency and steroidogenesis. These protein complexes may contain other transcription factors, coactivators, and corepressors. Physical interactions (dotted line) have been determined using mutagenesis studies and pull-down assays.
A Dax1 knock-out model was developed by Cre-mediated deletion of exon 2 (Yu et al., 1998). This approach allowed integration of the floxed version of Dax1 during ES cell manipulation, followed by deletion of exon 2 in female carriers, and transmission of the mutant gene to XY offspring. Deletion of Dax1 was hypothesized to impair ovarian development, largely because excess doses of Dax1 (i.e., duplication) caused XY sex reversal, suggesting that Dax1 might be an ovary-determination gene. Unexpectedly, ovarian development was normal, and instead, the mice had testis dysgenesis and a spermatogenesis defect. Gonadotropin and testosterone levels were normal, suggesting a non-hormonal testis defect. The dysgenetic testis was determined to be a result of disorganized testis cord formation, apparently the result of impaired differentiation of peritubular myoid and Sertoli cells (Jeffs et al., 2001, Meeks et al., 2003, Meeks, Crawford, Russell et al., 2003). Additional genetic crosses substantiated the role of Dax1 in testis development. Dax1-deficient mice were crossed with the Poschiavinus strain. If Dax1 was an ovary-determining gene, the absence of Dax1 in this cross should have favored normal testis development. On the other hand, if Dax1 played a role in testis development, a combined deficiency of Dax1 with a weakened Sry allele could accentuate intersex phenotypes or result in sex reversal. All XY mice in this cross were phenotypic females with activation of the ovary pathway, confirming the importance of Dax1 in testis development (Meeks et al., 2003). Levels of Sry expression were normal, indicating that Dax1 acted downstream of Sry in testis development. Similarly, crosses of Dax1-deficient mice onto C57BL/6J and other genetic backgrounds resulted in varying degrees of sex reversal (Bouma, Albrecht, Washburn et al., 2005, Park, Lee, Emge et al., 2008).
These Dax1-deficient mice do not develop adrenal insufficiency, although they do have expanded X-zones, which correspond to the fetal zone in humans (Yu et al., 1998, Beuschlein, Keegan, Bavers et al., 2002). Thus, Dax1-deficient mice lack some of the classic features of AHC, such as adrenal insufficiency and gonadotropin deficiency. However, they reveal new aspects of DAX1 function in the testis. It should also be noted that Dax1 function is very sensitive to genetic background, with phenotypic expression being dramatically modified by mouse strain (Bouma et al., 2005, Park et al., 2008). It is also possible that the deletion of exon 2 does not eliminate all aspects of Dax1 function, although analogous mutations in exon 2 have been shown in humans to cause AHC.
Molecular Mechanisms of DAX1 Action
DAX1 is structurally related to another nuclear receptor, SF1 (NR5A1). SF1 is essential for the development of the HPA and HPG axes and the expression of various steroidogenic enzyme genes (Worley et al., 1993). Disruption of the Sf1 gene in mice causes complete agenesis of the gonads and adrenals, as well as hypothalamic and pituitary defects (Luo, Ikeda and Parker, 1994). Dax1 and Sf1 are also known to have overlapping expression patterns during development and in several adult tissues(Ikeda et al., 2001, Ikeda, Swain, Weber et al., 1996). These observations suggested the possibility of a functional interaction between DAX1 and SF1. In addition, because many nuclear receptors interact as homodimers or heterodimers, their coexpression raised the possibility that Sf1 and Dax1 might interact directly as transcription factors. As described below, there is convincing evidence that DAX1 modulates the transcriptional activity of SF1 (Ito et al., 1997). However, this action appears to involve direct protein-protein interactions and intermediary factors rather than traditional binding of SF1-DAX1 heterodimers to DNA.
Nuclear receptors either repress or activate target genes, dependent in part on the recruitment of cofactor proteins (Lonard, Lanz and O’Malley, 2007). In many cases, receptors switch from a repressor to an activator in response to ligand-mediated conformational changes. DAX1 has been found to function largely as a transcriptional repressor. It possesses basal repressive activity, even when fused to other transcription factors (Ito et al., 1997). Moreover, DAX1 is a potent inhibitor of SF1-mediated transcriptional activation (Ito et al., 1997, Clipsham and McCabe, 2003). Corepressor recruitment, and the resulting transcriptional silencing by DAX1, occurs through a C-terminal LBD-like (LBD-L) domain (Ito et al., 1997). DAX1 recruits the nuclear receptor corepressor (N-CoR) to SF1 through two conserved regions within the LBD-L domain of DAX1 (Crawford, Dorn, Sadovsky et al., 1998). DAX1 is also known to interact with the corepressor Alien through its C-terminal domain (Altincicek, Tenbaum, Dressel et al., 2000). The ability of DAX1 to repress SF1-mediated transcription has been used to assess the function of naturally occurring human DAX1 mutations (Lin et al., 2006).
It remains somewhat puzzling how this inhibitory activity of DAX1 in vitro is related to its function in vivo. SF1 stimulates the DAX1 promoter (Yu, Ito and Jameson, 1998). Although DAX1 typically represses SF1-mediated transcription, low doses of DAX1 can activate certain SF1-regulated promoters, such as CYP11A1 and CYP11B1 (Verrijn Stuart et al., 2007). Perhaps more important from a physiologic perspective, both SF1 and DAX1 play important, and incompletely understood, roles in development. Current models suggest that DAX1 may favor a pluripotential state whereas SF1 directs cell differentiation (Kim, Barlaskar, Heaton et al., 2009).
In addition to SF1, DAX1 acts as a repressor of other nuclear receptors including the estrogen receptor (ER or NR3A1-2), the progesterone receptor (PR or NR3C3), the androgen receptor (AR or NR3C4), and liver receptor homologue-1 (LRH-1 or NR5A2) (Suzuki et al., 2003, Zhang, Thomsen, Johansson et al., 2000, Holter, Kotaja, Makela et al., 2002, Agoulnik, Krause, Bingman et al., 2003). DAX1 antagonizes cooperation between different transcription factors like SF1 and Wilms Tumor 1 (WT1) in the regulation of the Mullerian inhibiting substance (MIS) gene (Nachtigal, Hirokawa, Enyeart-VanHouten et al., 1998). DAX1 also inhibits SF1/EGR1 stimulation of LH3 gene expression (Dorn, Ou, Svaren et al., 1999) and SF1/GATA6 regulation of androgen biosynthesis genes (Jimenez, Saner, Mayhew et al., 2003).
In cells coexpressing DAX1 and SF1, DAX1 interacts with SF1 through its LXXLL motifs and translocates to the nucleus (Kawajiri et al., 2003). DAX1 mutations in AHC result in aberrant localization to the cytoplasm (Lehmann et al., 2002). Subcellular localization of DAX1 also plays an important role in DAX1 function in various cell types, including ES cells (Clipsham et al., 2004). DAX1 has been proposed to play a novel role as an RNA binding protein in the cell cytoplasm (Lalli, Ohe, Hindelang et al., 2000).
DAX1 Function and AHC
AHC patients fail to develop the adult zone of the adrenal gland, emphasizing the role of DAX1 in development, as well as a regulator of steroidogenesis. DAX1 is expressed in the adrenal cortex throughout development, although its precise role in adrenal morphogenesis is not fully understood. In Dax1-deficient mice, the X-zone fails to regress in male mice at puberty (Yu et al., 1998), suggesting that Dax1 may play an important role in fetal adrenal regression as opposed to directing zonation of the adult adrenal gland. The function of DAX1 in the development of the adrenal gland may be related to its role in maintaining pluripotency (Niakan et al., 2006). In the adult, expression of Dax1 and Sf1 is layer-specific (Kim et al., 2009). Neither Dax1 nor Sf1 are expressed in the outermost layer, the capsule. Both Dax1 and Sf1 are expressed in the subcapsular cortex whereas the mature steroidogenic layers of the cortex express only Sf1. It is plausible that DAX1 maintains a progenitor pool whereas SF1 directs various stages of cell differentiation (Kim et al., 2009). This layer-specific expression pattern suggests that Sf1 and Dax1 expression, along with paracrine and endocrine signals, control the differentiation and development of the adrenal cortex by maintaining a pool of stem or progenitor cells (Kim et al., 2009).
As noted above, the development of HHG in AHC is thought to reflect a combined defect of hypothalamic and pituitary function (Habiby et al., 1996). Dax1 is expressed in the VMH, Rathke’s pouch, and in the pituitary (Ikeda et al., 2001). These observations again raise the possibility that DAX1 plays a role in development of the pituitary and hypothalamus.
Conclusions
The majority of X-linked AHC patients present with adrenal insufficiency during the first few months of life. It is estimated that more than 50% of boys with idiopathic primary adrenal insufficiency have mutations in DAX1 (Lin 2006). As our understanding of the disease has evolved, improved treatments have extended patient life expectancy. Patients with HHG secondary to AHC have low serum gonadotropins. It appears that both the hypothalamus and pituitary are affected by mutations in DAX1; however, the mechanism of DAX1 action in these tissues remains unclear.
Although the mouse models of Dax1 have provided a wealth of information about the developmental expression pattern of Dax1 and the molecular mechanism of Dax1 action, these models do not faithfully replicate the AHC and HHG phenotypes seen in humans. Mouse studies have shown that Dax1 is expressed not solely in the adult steroidogenic tissues but also in the early embryo and in the developing gonad, adrenal, hypothalamus, and pituitary. Increasing evidence supports a model in which DAX1 functions in a network of other transcription factors to regulate the transition from pluripotency (DAX1 high) to differentiation (DAX1 low). Future questions will focus on the developmental roles of DAX1, its target genes, and its interplay with SF1 and other transcription factors. From a clinical perspective, the main challenges continue to be early diagnosis and treatment of affected infants, distinguishing AHC from other causes of neonatal and childhood adrenal insufficiency, and the hormonal management of puberty and fertility.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Sikl H. Addison’s disease due to congenital hypoplasia of the adrenals in an infant aged 33 days. J Pathol Bacteriol. 1948;60:323. doi: 10.1002/path.1700600220. [DOI] [PubMed] [Google Scholar]
- Mitchell RG, Rhaney K. Congenital adrenal hypoplasia in siblings. Lancet. 1959;1:488–92. doi: 10.1016/s0140-6736(59)91020-7. [DOI] [PubMed] [Google Scholar]
- Dacou C, Di George AM. Evidence for an X-linked form of congenital adrenal hypoplasia (abstract 89). Program of the thirty-eighth annual meeting of the soiety for pediatric research. Atlantic City; New Jersey. 1968. [Google Scholar]
- Uttley WS. Familial congenital adrenal hypoplasia. Arch Dis Child. 1968;43:724–30. doi: 10.1136/adc.43.232.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KE, Bille T, Jacobsen BB, Iversen T. X-linked congenital adrenal hypoplasia. A study of five generations of a Greenlandic Family. Acta Paediatr Scand. 1982;71:947–51. doi: 10.1111/j.1651-2227.1982.tb09554.x. [DOI] [PubMed] [Google Scholar]
- Kelch RP, Virdis R, Rapaport R, Greig F, Levine LS. Congenital adrenal hypoplasia. Pediatric Adolescent Endocrinology. 1984;13:156–161. [Google Scholar]
- Yu RN, Ito M, Saunders TL, Camper SA, Jameson JL. Role of Ahch in gonadal development and gametogenesis. Nat Genet. 1998;20:353–7. doi: 10.1038/3822. [DOI] [PubMed] [Google Scholar]
- Reutens AT, Achermann JC, Ito M, Gu WX, Habiby RL, Donohoue PA, Pang S, Hindmarsh PC, Jameson JL. Clinical and functional effects of mutations in the DAX-1 gene in patients with adrenal hypoplasia congenita. J Clin Endocrinol Metab. 1999;84:504–11. doi: 10.1210/jcem.84.2.5468. [DOI] [PubMed] [Google Scholar]
- Lin L, Gu WX, Ozisik G, To WS, Owen CJ, Jameson JL, Achermann JC. Analysis of DAX1 (NR0B1) and steroidogenic factor-1 (NR5A1) in children and adults with primary adrenal failure: ten years’ experience. J Clin Endocrinol Metab. 2006;91:3048–54. doi: 10.1210/jc.2006-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerenyi N. Congenital adrenal hypoplasia. Report of a case with extreme adrenal hypoplasia and neurohypophyseal aplasia, drawing attention to certain aspects of etiology and classification. Arch Pathol. 1961;71:336–43. [PubMed] [Google Scholar]
- Mamelle JC, David M, Riou D, Gilly J, Trouillas J, Dutruge J, Gilly R. [Congenital adrenal hypoplasia of cytomegalic type. Recessive, sex-linked form. Apropos of 3 cases] Arch Fr Pediatr. 1975;32:139–59. [PubMed] [Google Scholar]
- Achermann JC, Silverman BL, Habiby RL, Jameson JL. Presymptomatic diagnosis of X-linked adrenal hypoplasia congenita by analysis of DAX1. J Pediatr. 2000;137:878–81. doi: 10.1067/mpd.2000.108567. [DOI] [PubMed] [Google Scholar]
- Prader A, Zachmann M, Illig R. Luteinizing hormone deficiency in hereditary congenital adrenal hypoplasia. J Pediatr. 1975;86:421–2. doi: 10.1016/s0022-3476(75)80978-4. [DOI] [PubMed] [Google Scholar]
- Golden MP, Lippe BM, Kaplan SA. Congenital adrenal hypoplasia and hypogonadotropic hypogonadism. Am J Dis Child. 1977;131:1117–8. doi: 10.1001/archpedi.1977.02120230063010. [DOI] [PubMed] [Google Scholar]
- Zachmann M, Illig R, Prader A. Gonadotropin deficiency and cryptorchidism in three prepubertal brothers with congenital adrenal hypoplasia. J Pediatr. 1980;97:255–7. doi: 10.1016/s0022-3476(80)80486-0. [DOI] [PubMed] [Google Scholar]
- Kelly WF, Joplin GF, Pearson GW. Gonadotrophin deficiency and adrenocortical insufficiency in children: a new syndrome. Br Med J. 1977;2:98. doi: 10.1136/bmj.2.6079.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond J, Howard NJ, Brookwell R, Purvis-Smith S, Wilcken B, Hoogenraad N. Proposed assignment of loci for X-linked adrenal hypoplasia and glycerol kinase genes. Lancet. 1985;1:54. doi: 10.1016/s0140-6736(85)91009-8. [DOI] [PubMed] [Google Scholar]
- Bartley JA, Patil S, Davenport S, Goldstein D, Pickens J. Duchenne muscular dystrophy, glycerol kinase deficiency, and adrenal insufficiency associated with Xp21 interstitial deletion. J Pediatr. 1986;108:189–92. doi: 10.1016/s0022-3476(86)80980-5. [DOI] [PubMed] [Google Scholar]
- Francke U, Harper JF, Darras BT, Cowan JM, McCabe ER, Kohlschutter A, Seltzer WK, Saito F, Goto J, Harpey JP, et al. Congenital adrenal hypoplasia, myopathy, and glycerol kinase deficiency: molecular genetic evidence for deletions. Am J Hum Genet. 1987;40:212–27. [PMC free article] [PubMed] [Google Scholar]
- Mlandel JL, Willard HF, Nussbaum RL, Romeo G, Puck JMl, Davies KE. Report of the committee on the genetic constitution of the X chromosome. Cytogenet Cell Genet. 1989;51:384–437. doi: 10.1159/000132801. [DOI] [PubMed] [Google Scholar]
- Goonewardena P, Dahl N, Ritzen Ml, van Ommen GJ, Pettersson U. Molecular Xp deletion in a male: suggestion of a locus for hypogonadotropic hypogonadism distal to the glycerol kinase and adrenal hypoplasia loci. Clin Genet. 1989;35:5–12. doi: 10.1111/j.1399-0004.1989.tb02899.x. [DOI] [PubMed] [Google Scholar]
- Worley KC, Ellison KA, Zhang YH, Wang DF, Mason J, Roth EJ, Adams V, Fogt DD, Zhu XM, Towbin JA, et al. Yeast artificial chromosome cloning in the glycerol kinase and adrenal hypoplasia congenita region of Xp21. Genomics. 1993;16:407–16. doi: 10.1006/geno.1993.1204. [DOI] [PubMed] [Google Scholar]
- Muscatelli F, Strom TM, Walker AP, Zanaria E, Recan D, Meindl A, Bardoni B, Guioli S, Zehetner G, Rabl W, et al. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature. 1994;372:672–6. doi: 10.1038/372672a0. [DOI] [PubMed] [Google Scholar]
- Zanaria E, Muscatelli F, Bardoni B, Strom TM, Guioli S, Guo W, Lalli E, Moser C, Walker AP, McCabe ER, et al. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature. 1994;372:635–41. doi: 10.1038/372635a0. [DOI] [PubMed] [Google Scholar]
- Guo W, Mason JS, Stone CG, Jr, Morgan SA, Madu SI, Baldini A, Lindsay EA, Biesecker LG, Copeland KC, Horlick MN, et al. Diagnosis of X-linked adrenal hypoplasia congenita by mutation analysis of the DAX1 gene. JAMA. 1995;274:324–30. [PubMed] [Google Scholar]
- Takahashi T, Shoji Y, Haraguchi N, Takahashi I, Takada G. Active hypothalamic-pituitary-gonadal axis in an infant with X-linked adrenal hypoplasia congenita. J Pediatr. 1997;130:485–8. doi: 10.1016/s0022-3476(97)70217-8. [DOI] [PubMed] [Google Scholar]
- Meloni A, Cao A, Rosatelli MC. New frameshift mutation in the DAX-1 gene in a patient with X-linked adrenal hypoplasia and hypogonadotropic hypogonadism. Hum Mutat. 1996;8:183–4. doi: 10.1002/(SICI)1098-1004(1996)8:2<183::AID-HUMU12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Nakae J, Abe S, Tajima T, Shinohara N, Murashita M, Igarashi Y, Kusuda S, Suzuki J, Fujieda K. Three novel mutations and a de novo deletion mutation of the DAX-1 gene in patients with X-linked adrenal hypoplasia congenita. J Clin Endocrinol Metab. 1997;82:3835–41. doi: 10.1210/jcem.82.11.4342. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Blichfeldt S, Muller J. X-linked adrenal hypoplasia in a large Greenlandic family. Detection of a missense mutation (N4401) in the DAX-1 gene; implication for genetic counselling and carrier diagnosis. Hum Genet. 1997;99:83–7. doi: 10.1007/s004390050316. [DOI] [PubMed] [Google Scholar]
- Peter M, Viemann M, Partsch CJ, Sippell WG. Congenital adrenal hypoplasia: clinical spectrum, experience with hormonal diagnosis, and report on new point mutations of the DAX-1 gene. J Clin Endocrinol Metab. 1998;83:2666–74. doi: 10.1210/jcem.83.8.5027. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Achermann JC, Genel M, Jameson JL, Crowley WF., Jr X-linked adrenal hypoplasia congenita: a mutation in DAX1 expands the phenotypic spectrum in males and females. J Clin Endocrinol Metab. 1999;84:4501–9. doi: 10.1210/jcem.84.12.6172. [DOI] [PubMed] [Google Scholar]
- Bassett JH, O’Halloran DJ, Williams GR, Beardwell CG, Shalet SM, Thakker RV. Novel DAX1 mutations in X-linked adrenal hypoplasia congenita and hypogonadotrophic hypogonadism. Clin Endocrinol (Oxf) 1999;50:69–75. doi: 10.1046/j.1365-2265.1999.00601.x. [DOI] [PubMed] [Google Scholar]
- Caron P, Imbeaud S, Bennet A, Plantavid M, Camerino G, Rochiccioli P. Combined hypothalamic-pituitary-gonadal defect in a hypogonadic man with a novel mutation in the DAX-1 gene. J Clin Endocrinol Metab. 1999;84:3563–9. doi: 10.1210/jcem.84.10.6030. [DOI] [PubMed] [Google Scholar]
- Merke DP, Tajima T, Baron J, Cutler GB., Jr Hypogonadotropic hypogonadism in a female caused by an X-linked recessive mutation in the DAX1 gene. N Engl J Med. 1999;340:1248–52. doi: 10.1056/NEJM199904223401605. [DOI] [PubMed] [Google Scholar]
- Tabarin A, Achermann JC, Recan D, Bex V, Bertagna X, Christin-Maitre S, Ito M, Jameson JL, Bouchard P. A novel mutation in DAX1 causes delayed-onset adrenal insufficiency and incomplete hypogonadotropic hypogonadism. J Clin Invest. 2000;105:321–8. doi: 10.1172/JCI7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenice S, Latronico AC, Brito VN, Arnhold IJ, Kok F, Mendonca BB. Adrenocorticotropin-dependent precocious puberty of testicular origin in a boy with X-linked adrenal hypoplasia congenita due to a novel mutation in the DAX1 gene. J Clin Endocrinol Metab. 2001;86:4068–71. doi: 10.1210/jcem.86.9.7816. [DOI] [PubMed] [Google Scholar]
- Wiltshire E, Couper J, Rodda C, Jameson JL, Achermann JC. Variable presentation of X-linked adrenal hypoplasia congenita. J Pediatr Endocrinol Metab. 2001;14:1093–6. doi: 10.1515/jpem-2001-0804. [DOI] [PubMed] [Google Scholar]
- Sekiguchi Y, Hara Y, Matsuoka H, Hayashi Y, Katsumata N, Hirata Y. Sibling cases of Addison’s disease caused by DAX-1 gene mutations. Intern Med. 2007;46:35–9. doi: 10.2169/internalmedicine.46.6082. [DOI] [PubMed] [Google Scholar]
- Bernard P, Ludbrook L, Queipo G, Dinulos MB, Kletter GB, Zhang YH, Phelan JK, McCabe ER, Harley VR, Vilain E. A familial missense mutation in the hinge region of DAX1 associated with late-onset AHC in a prepubertal female. Mol Genet Metab. 2006;88:272–9. doi: 10.1016/j.ymgme.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Verrijn Stuart AA, Ozisik G, de Vroede MA, Giltay JC, Sinke RJ, Peterson TJ, Harris RM, Weiss J, Jameson JL. An amino-terminal DAX1 (NROB1) missense mutation associated with isolated mineralocorticoid deficiency. J Clin Endocrinol Metab. 2007;92:755–61. doi: 10.1210/jc.2005-2429. [DOI] [PubMed] [Google Scholar]
- Landau Z, Hanukoglu A, Sack J, Goldstein N, Weintrob N, Eliakim A, Gillis D, Sagi M, Shomrat R, Kosinovsky EB, Anikster Y. Clinical and genetic heterogeneity of congenital adrenal hypoplasia due to NR0B1gene mutations. Clin Endocrinol (Oxf) 2010;72:448–54. doi: 10.1111/j.1365-2265.2009.03652.x. [DOI] [PubMed] [Google Scholar]
- Li N, Liu R, Zhang H, Yang J, Sun S, Zhang M, Liu Y, Lu Y, Wang W, Mu Y, Ning G, Li X. Seven novel DAX1 mutations with loss of function identified in Chinese patients with congenital adrenal hypoplasia. J Clin Endocrinol Metab. 2010;95:E104–11. doi: 10.1210/jc.2009-2408. [DOI] [PubMed] [Google Scholar]
- Sykiotis GP, Hoang XH, Avbelj M, Hayes FJ, Thambundit A, Dwyer A, Au M, Plummer L, Crowley WF, Jr, Pitteloud N. Congenital idiopathic hypogonadotropic hypogonadism: evidence of defects in the hypothalamus, pituitary, and testes. J Clin Endocrinol Metab. 2010;95:3019–27. doi: 10.1210/jc.2009-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe S, Nakae J, Yasoshima K, Tajima T, Shinohara N, Murashita M, Satoh K, Koike A, Takahashi Y, Fujieda K. Novel missense mutation (Leu466Arg) of the DAX1 gene in a patient with X-linked congenital adrenal hypoplasia. Am J Med Genet. 1999;84:87–9. [PubMed] [Google Scholar]
- Achermann JC, Meeks JJ, Jameson JL. Phenotypic spectrum of mutations in DAX-1 and SF-1. Mol Cell Endocrinol. 2001;185:17–25. doi: 10.1016/s0303-7207(01)00619-0. [DOI] [PubMed] [Google Scholar]
- Ahmad I, Paterson WF, Lin L, Adlard P, Duncan P, Tolmie J, Achermann JC, Donaldson MD. A novel missense mutation in DAX-1 with an unusual presentation of X-linked adrenal hypoplasia congenita. Horm Res. 2007;68:32–7. doi: 10.1159/000099835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argente J, Ozisik G, Pozo J, Teresa Munoz M, Soriano-Guillen L, Larry Jameson J. A novel single base deletion at codon 434 (1301delT) of the DAX1 gene associated with prepubertal testis enlargement. Mol Genet Metab. 2003;78:79–81. doi: 10.1016/s1096-7192(02)00198-1. [DOI] [PubMed] [Google Scholar]
- Balsamo A, Antelli A, Baldazzi L, Baronio F, Lazareva D, Cassio A, Cicognani A. A new DAX1 gene mutation associated with congenital adrenal hypoplasia and hypogonadotropic hypogonadism. Am J Med Genet A. 2005;135:292–6. doi: 10.1002/ajmg.a.30689. [DOI] [PubMed] [Google Scholar]
- Brown P, Scobie GA, Townsend J, Bayne RA, Seckl JR, Saunders PT, Anderson RA. Identification of a novel missense mutation that is as damaging to DAX-1 repressor function as a nonsense mutation. J Clin Endocrinol Metab. 2003;88:1341–9. doi: 10.1210/jc.2002-021560. [DOI] [PubMed] [Google Scholar]
- Calliari LE, Longui CA, Rocha MN, Faria CD, Kochi C, Melo MR, Melo MB, Monte O. A novel mutation in DAX1 gene causing different phenotypes in three siblings with adrenal hypoplasia congenita. Genet Mol Res. 2007;6:277–83. [PubMed] [Google Scholar]
- Frapsauce C, Ravel C, Legendre M, Sibony M, Mandelbaum J, Donadille B, Achermann JC, Siffroi JP, Christin-Maitre S. Birth after TESE-ICSI in a man with hypogonadotropic hypogonadism and congenital adrenal hypoplasia linked to a DAX-1 (NR0B1) mutation. Hum Reprod. 2011;26:724–8. doi: 10.1093/humrep/deq372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Malpartida K, Gomez-Balaguer M, Sola-Izquierdo E, Fuentes-Pardilla MJ, Jover-Fernandez A, Sanz-Ruiz I, Hernandez-Mijares A. A novel mutation in DAX1 (NR0B1) causing X-linked adrenal hypoplasia congenita: clinical, hormonal and genetic analysis. Endocrine. 2009;36:275–80. doi: 10.1007/s12020-009-9232-9. [DOI] [PubMed] [Google Scholar]
- Guo W, Burris TP, Zhang YH, Huang BL, Mason J, Copeland KC, Kupfer SR, Pagon RA, McCabe ER. Genomic sequence of the DAX1 gene: an orphan nuclear receptor responsible for X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1996;81:2481–6. doi: 10.1210/jcem.81.7.8675564. [DOI] [PubMed] [Google Scholar]
- Habiby RL, Boepple P, Nachtigall L, Sluss PM, Crowley WF, Jr, Jameson JL. Adrenal hypoplasia congenita with hypogonadotropic hypogonadism: evidence that DAX-1 mutations lead to combined hypothalmic and pituitary defects in gonadotropin production. J Clin Invest. 1996;98:1055–62. doi: 10.1172/JCI118866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi K, Arikawa M, Yasunaga S, Kakuma T, Fukagawa K, Yanase T, Nawata H, Sakata T. Novel mutation of the DAX1 gene in a patient with X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Am J Med Genet. 1998;76:62–6. doi: 10.1002/(sici)1096-8628(19980226)76:1<62::aid-ajmg11>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Kinoshita E, Yoshimoto M, Motomura K, Kawaguchi T, Mori R, Baba T, Nishij’o K, Hasegawa T, Momoi T, Yorihuji T. DAX-1 gene mutations and deletions in Japanese patients with adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Horm Res. 1997;48:29–34. doi: 10.1159/000185364. [DOI] [PubMed] [Google Scholar]
- Krone N, Riepe FG, Dorr HG, Morlot M, Rudorff KH, Drop SL, Weigel J, Pura M, Kreze A, Boronat M, de Luca F, Tiulpakov A, Partsch CJ, Peter M, Sippell WG. Thirteen novel mutations in the NR0B1 (DAX1) gene as cause of adrenal hypoplasia congenita. Hum Mutat. 2005;25:502–3. doi: 10.1002/humu.9331. [DOI] [PubMed] [Google Scholar]
- Lam CW, Cheng AW, Poon WT, Yuen YP, Huen KF. Novel mutation, c.1234delA, in the DAX1 gene in congenital adrenal hypoplasia. Clin Chim Acta. 2006;374:151–2. doi: 10.1016/j.cca.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Mantovani G, Ozisik G, Achermann JC, Romoli R, Borretta G, Persani L, Spada A, Jameson JL, Beck-Peccoz P. Hypogonadotropic hypogonadism as a presenting feature of late-onset X-linked adrenal hypoplasia congenita. J Clin Endocrinol Metab. 2002;87:44–8. doi: 10.1210/jcem.87.1.8163. [DOI] [PubMed] [Google Scholar]
- Mericq V, Ciaccio M, Marino R, Lamoglia JJ, Viterbo G, Rivarola MA, Belgorosky A. A new DAX-1 mutation in a family with a case of neonatal adrenal insufficiency and a sibling with adrenal hypoplasia and sudden death at 3 years of age. J Pediatr Endocrinol Metab. 2007;20:1039–43. doi: 10.1515/jpem.2007.20.9.1039. [DOI] [PubMed] [Google Scholar]
- Nakae J, Tajima T, Kusuda S, Kohda N, Okabe T, Shinohara N, Kato M, Murashita M, Mukai T, Imanaka K, Fujieda K. Truncation at the C-terminus of the DAX-1 protein impairs its biological actions in patients with X-linked adrenal hypoplasia congenita. J Clin Endocrinol Metab. 1996;81:3680–5. doi: 10.1210/jcem.81.10.8855822. [DOI] [PubMed] [Google Scholar]
- Ozisik G, Mantovani G, Achermann JC, Persani L, Spada A, Weiss J, Beck-Peccoz P, Jameson JL. An alternate translation initiation site circumvents an amino-terminal DAX1 nonsense mutation leading to a mild form of X-linked adrenal hypoplasia congenita. J Clin Endocrinol Metab. 2003;88:417–23. doi: 10.1210/jc.2002-021034. [DOI] [PubMed] [Google Scholar]
- Salvi R, Gomez F, Fiaux M, Schorderet D, Jameson JL, Achermann JC, Gaillard RC, Pralong FP. Progressive onset of adrenal insufficiency and hypogonadism of pituitary origin caused by a complex genetic rearrangement within DAX-1. J Clin Endocrinol Metab. 2002;87:4094–100. doi: 10.1210/jc.2001-011930. [DOI] [PubMed] [Google Scholar]
- Tsai WY, Tung YC. Novel deletion mutations of the DAX1 (NR0B1) gene in two Taiwanese families with X-linked adrenal hypoplasia congenita. J Pediatr Endocrinol Metab. 2005;18:991–7. doi: 10.1515/jpem.2005.18.10.991. [DOI] [PubMed] [Google Scholar]
- Wang J, Killinger DW, Hegele RA. A microdeletion within DAX-1 in X-linked adrenal hypoplasia congenita and hypogonadotrophic hypogonadism. J Investig Med. 1999;47:232–5. [PubMed] [Google Scholar]
- Wu CM, Zhang HB, Zhou Q, Wan L, Jin J, Ni L, Pan YJ, Wu XY, Ruan LY. Two novel DAX1 gene mutations in Chinese patients with X-linked adrenal hypoplasia congenita: clinical, hormonal and genetic analysis. J Endocrinol Invest. 2011 doi: 10.3275/7484. [DOI] [PubMed] [Google Scholar]
- Yanase T, Takayanagi R, Oba K, Nishi Y, Ohe K, Nawata H. New mutations of DAX-1 genes in two Japanese patients with X-linked congenital adrenal hypoplasia and hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1996;81:530–5. doi: 10.1210/jcem.81.2.8636263. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Guo W, Wagner RL, Huang BL, McCabe L, Vilain E, Burris TP, Anyane-Yeboa K, Burghes AH, Chitayat D, Chudley AE, Genel M, Gertner JM, Klingensmith GJ, Levine SN, Nakamoto J, New MI, Pagon RA, Pappas JG, Quigley CA, Rosenthal IM, Baxter JD, Fletterick RJ, McCabe ER. DAX1 mutations map to putative structural domains in a deduced three-dimensional model. Am J Hum Genet. 1998;62:855–64. doi: 10.1086/301782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Huang BL, Anyane-Yeboa K, Carvalho JA, Clemons RD, Cole T, De Figueiredo BC, Lubinsky M, Metzger DL, Quadrelli R, Repaske DR, Reyno S, Seaver LH, Vaglio A, Van Vliet G, McCabe LL, McCabe ER, Phelan JK. Nine novel mutations in NR0B1 (DAX1) causing adrenal hypoplasia congenita. Hum Mutat. 2001;18:547. doi: 10.1002/humu.1236. [DOI] [PubMed] [Google Scholar]
- Franzese A, Brunetti-Pierri N, Spagnuolo MI, Spadaro R, Giugliano M, Mukai T, Valerio G. Inappropriate tall stature and renal ectopy in a male patient with X-linked congenital adrenal hypoplasia due to a novel missense mutation in the DAX-1 gene. Am J Med Genet A. 2005;135:72–4. doi: 10.1002/ajmg.a.30670. [DOI] [PubMed] [Google Scholar]
- Laissue P, Copelli S, Bergada I, Bergada C, Barrio G, Karaboga S, Wurtz JM, Fellous M, Lalli E, Veitia RA. Partial defects in transcriptional activity of two novel DAX-1 mutations in childhood-onset adrenal hypoplasia congenita. Clin Endocrinol (Oxf) 2006;65:681–6. doi: 10.1111/j.1365-2265.2006.02649.x. [DOI] [PubMed] [Google Scholar]
- Okuhara K, Abe S, Kondo T, Fujita K, Koda N, Mochizuki H, Fujieda K, Tajima T. Four Japanese patients with adrenal hypoplasia congenita and hypogonadotropic hypogonadism caused by DAX-1 gene mutations: mutant DAX-1 failed to repress steroidogenic acute regulatory protein (StAR) and luteinizing hormone beta-subunit gene promoter activity. Endocr J. 2008;55:97–103. doi: 10.1507/endocrj.k07e-008. [DOI] [PubMed] [Google Scholar]
- Ito M, Yu R, Jameson JL. DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol Cell Biol. 1997;17:1476–83. doi: 10.1128/mcb.17.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann SG, Wurtz JM, Renaud JP, Sassone-Corsi P, Lalli E. Structure-function analysis reveals the molecular determinants of the impaired biological function of DAX-1 mutants in AHC patients. Hum Mol Genet. 2003;12:1063–72. doi: 10.1093/hmg/ddg108. [DOI] [PubMed] [Google Scholar]
- Lehmann SG, Lalli E, Sassone-Corsi P. X-linked adrenal hypoplasia congenita is caused by abnormal nuclear localization of the DAX-1 protein. Proc Natl Acad Sci USA. 2002;99:8225–30. doi: 10.1073/pnas.122044099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawajiri K, Ikuta T, Suzuki T, Kusaka M, Muramatsu M, Fujieda K, Tachibana M, Morohashi K. Role of the LXXLL-motif and activation function 2 domain in subcellular localization of Dax-1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1) Mol Endocrinol. 2003;17:994–1004. doi: 10.1210/me.2002-0360. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kasahara M, Yoshioka H, Morohashi K, Umesono K. LXXLL-related motifs in Dax-1 have target specificity for the orphan nuclear receptors Ad4BP/SF-1 and LRH-1. Mol Cell Biol. 2003;23:238–49. doi: 10.1128/MCB.23.1.238-249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh MG, Boyes L, Kingston H, Collins R, Besley GT, Padmakumar B, Ismayl O, Hughes I, Hall CM, Hellerud C, Achermann JC, Clayton PE. Skewed X inactivation is associated with phenotype in a female with adrenal hypoplasia congenita. J Med Genet. 2008;45:e1. doi: 10.1136/jmg.2007.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse K, Sippell WG, Schnakenburg KV. Hypogonadism in congenital adrenal hypoplasia: evidence for a hypothalamic origin. J Clin Endocrinol Metab. 1984;58:12–7. doi: 10.1210/jcem-58-1-12. [DOI] [PubMed] [Google Scholar]
- Jeffs B, Meeks JJ, Ito M, Martinson FA, Matzuk MM, Jameson JL, Russell LD. Blockage of the rete testis and efferent ductules by ectopic Sertoli and Leydig cells causes infertility in Dax1-deficient male mice. Endocrinology. 2001;142:4486–95. doi: 10.1210/endo.142.10.8447. [DOI] [PubMed] [Google Scholar]
- Meeks JJ, Weiss J, Jameson JL. Dax1 is required for testis determination. Nat Genet. 2003;34:32–3. doi: 10.1038/ng1141. [DOI] [PubMed] [Google Scholar]
- Dipple KM, McCabe ER. Phenotypes of patients with “simple” Mendelian disorders are complex traits: thresholds, modifiers, and systems dynamics. Am J Hum Genet. 2000;66:1729–35. doi: 10.1086/302938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriver CR, Waters PJ. Monogenic traits are not simple: lessons from phenylketonuria. Trends Genet. 1999;15:267–72. doi: 10.1016/s0168-9525(99)01761-8. [DOI] [PubMed] [Google Scholar]
- Battista MC, Otis M, Cote M, Laforest A, Peter M, Lalli E, Gallo-Payet N. Extracellular matrix and hormones modulate DAX-1 localization in the human fetal adrenal gland. J Clin Endocrinol Metab. 2005;90:5426–31. doi: 10.1210/jc.2005-0666. [DOI] [PubMed] [Google Scholar]
- Hay ID, Smail PJ, Forsyth CC. Familial cytomegalic adrenocortical hypoplasia: an X-linked syndrome of pubertal failure. Arch Dis Child. 1981;56:715–21. doi: 10.1136/adc.56.9.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D, Cohen HN, Beastall GH, Hay ID, Thomson JA. Contrasting effects of subcutaneous pulsatile GnRH therapy in congenital adrenal hypoplasia and Kallmann’s syndrome. Clin Endocrinol (Oxf) 1984;21:597–603. doi: 10.1111/j.1365-2265.1984.tb01401.x. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Kaji M, Momoi T, Mikawa H, Shigematsu Y, Sudo M. Failure to induce puberty in a man with X-linked congenital adrenal hypoplasia and hypogonadotropic hypogonadism by pulsatile administration of low-dose gonadotropin-releasing hormone. Acta Endocrinol (Copenh) 1987;114:153–60. doi: 10.1530/acta.0.1140153. [DOI] [PubMed] [Google Scholar]
- Bovet P, Reymond MJ, Rey F, Gomez F. Lack of gonadotropic response to pulsatile gonadotropin-releasing hormone in isolated hypogonadotropic hypogonadism associated to congenital adrenal hypoplasia. J Endocrinol Invest. 1988;11:201–4. doi: 10.1007/BF03350135. [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Thambundit A, Dwyer AA, Falardeau JL, Plummer L, Caronia LM, Hayes FJ, Lee H, Boepple PA, Crowley WF., Jr Role of seminiferous tubular development in determining the FSH versus LH responsiveness to GnRH in early sexual maturation. Neuroendocrinology. 2009;90:260–8. doi: 10.1159/000245383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletter GB, Gorski JL, Kelch RP. Congenital adrenal hypoplasia and isolated gonadotropin deficiency. Trends in Endocrinology and Metabolism. 1991;2:123–128. [Google Scholar]
- Partsch CJ, Sippell WG. Hypothalamic hypogonadism in congenital adrenal hypoplasia. Horm Metab Res. 1989;21:623–5. doi: 10.1055/s-2007-1009303. [DOI] [PubMed] [Google Scholar]
- Goto M, Katsumata N. X-linked adrenal hypoplasia congenita caused by a novel intronic mutation of the DAX-1 gene. Horm Res. 2009;71:120–4. doi: 10.1159/000183901. [DOI] [PubMed] [Google Scholar]
- Smyk M, Berg JS, Pursley A, Curtis FK, Fernandez BA, Bien-Willner GA, Lupski JR, Cheung SW, Stankiewicz P. Male-to-female sex reversal associated with an approximately 250 kb deletion upstream of NR0B1 (DAX1) Hum Genet. 2007;122:63–70. doi: 10.1007/s00439-007-0373-8. [DOI] [PubMed] [Google Scholar]
- Tee MK, Lin D, Sugawara T, Holt JA, Guiguen Y, Buckingham B, Strauss JF, 3rd, Miller WL. T-->A transversion 11 bp from a splice acceptor site in the human gene for steroidogenic acute regulatory protein causes congenital lipoid adrenal hyperplasia. Hum Mol Genet. 1995;4:2299–305. doi: 10.1093/hmg/4.12.2299. [DOI] [PubMed] [Google Scholar]
- Sills IN, Voorhess ML, MacGillivray MH, Peterson RE. Prolonged survival without therapy in congenital adrenal hypoplasia. Am J Dis Child. 1983;137:1186–8. doi: 10.1001/archpedi.1983.02140380046015. [DOI] [PubMed] [Google Scholar]
- Burris TP, Guo W, McCabe ER. The gene responsible for adrenal hypoplasia congenita, DAX-1, encodes a nuclear hormone receptor that defines a new class within the superfamily. Recent Prog Horm Res. 1996;51:241–59. discussion 259–60. [PubMed] [Google Scholar]
- Iyer AK, McCabe ER. Molecular mechanisms of DAX1 action. Mol Genet Metab. 2004;83:60–73. doi: 10.1016/j.ymgme.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Swain A, Zanaria E, Hacker A, Lovell-Badge R, Camerino G. Mouse Dax1 expression is consistent with a role in sex determination as well as in adrenal and hypothalamus function. Nat Genet. 1996;12:404–9. doi: 10.1038/ng0496-404. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Takeda Y, Shikayama T, Mukai T, Hisano S, Morohashi KI. Comparative localization of Dax-1 and Ad4BP/SF-1 during development of the hypothalamic-pituitary-gonadal axis suggests their closely related and distinct functions. Dev Dyn. 2001;220:363–76. doi: 10.1002/dvdy.1116. [DOI] [PubMed] [Google Scholar]
- Bae DS, Schaefer ML, Partan BW, Muglia L. Characterization of the mouse DAX-1 gene reveals evolutionary conservation of a unique amino-terminal motif and widespread expression in mouse tissue. Endocrinology. 1996;137:3921–7. doi: 10.1210/endo.137.9.8756567. [DOI] [PubMed] [Google Scholar]
- Swain A, Narvaez V, Burgoyne P, Camerino G, Lovell-Badge R. Dax1 antagonizes Sry action in mammalian sex determination. Nature. 1998;391:761–7. doi: 10.1038/35799. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Washburn LL, Schork NJ, Lee BK, Shown EP, Xu X, Dredge RD, Pringle MJ, Page DC. Sex-determining genes on mouse autosomes identified by linkage analysis of C57BL/6J-YPOS sex reversal. Nat Genet. 1996;14:206–9. doi: 10.1038/ng1096-206. [DOI] [PubMed] [Google Scholar]
- Clipsham R, Niakan K, McCabe ER. Nr0b1 and its network partners are expressed early in murine embryos prior to steroidogenic axis organogenesis. Gene Expr Patterns. 2004;4:3–14. doi: 10.1016/j.modgep.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Niakan KK, Davis EC, Clipsham RC, Jiang M, Dehart DB, Sulik KK, McCabe ER. Novel role for the orphan nuclear receptor Dax1 in embryogenesis, different from steroidogenesis. Mol Genet Metab. 2006;88:261–71. doi: 10.1016/j.ymgme.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Kelly VR, Hammer GD. LRH-1 and Nanog regulate Dax1 transcription in mouse embryonic stem cells. Mol Cell Endocrinol. 2010 doi: 10.1016/j.mce.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly VR, Xu B, Kuick R, Koenig RJ, Hammer GD. Dax1 Up-Regulates Oct4 Expression in Mouse Embryonic Stem Cells via LRH-1 and SRA. Mol Endocrinol. 2010 doi: 10.1210/me.2010-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–61. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Aragoncillo E, Carrillo J, Lalli E, Agra N, Gomez-Lopez G, Pestana A, Alonso J. DAX1, a direct target of EWS/FLI1 oncoprotein, is a principal regulator of cell-cycle progression in Ewing’s tumor cells. Oncogene. 2008;27:6034–43. doi: 10.1038/onc.2008.203. [DOI] [PubMed] [Google Scholar]
- Meeks JJ, Crawford SE, Russell TA, Morohashi K, Weiss J, Jameson JL. Dax1 regulates testis cord organization during gonadal differentiation. Development. 2003;130:1029–36. doi: 10.1242/dev.00316. [DOI] [PubMed] [Google Scholar]
- Bouma GJ, Albrecht KH, Washburn LL, Recknagel AK, Churchill GA, Eicher EM. Gonadal sex reversal in mutant Dax1 XY mice: a failure to upregulate Sox9 in pre-Sertoli cells. Development. 2005;132:3045–54. doi: 10.1242/dev.01890. [DOI] [PubMed] [Google Scholar]
- Park SY, Lee EJ, Emge D, Jahn CL, Jameson JL. A phenotypic spectrum of sexual development in Dax1 (Nr0b1)-deficient mice: consequence of the C57BL/6J strain on sex determination. Biol Reprod. 2008;79:1038–45. doi: 10.1095/biolreprod.108.069492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuschlein F, Keegan CE, Bavers DL, Mutch C, Hutz JE, Shah S, Ulrich-Lai YM, Engeland WC, Jeffs B, Jameson JL, Hammer GD. SF-1, DAX-1, and acd: molecular determinants of adrenocortical growth and steroidogenesis. Endocr Res. 2002;28:597–607. doi: 10.1081/erc-120016972. [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–90. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Swain A, Weber TJ, Hentges KE, Zanaria E, Lalli E, Tamai KT, Sassone-Corsi P, Lovell-Badge R, Camerino G, Parker KL. Steroidogenic factor 1 and Dax-1 colocalize in multiple cell lineages: potential links in endocrine development. Mol Endocrinol. 1996;10:1261–72. doi: 10.1210/mend.10.10.9121493. [DOI] [PubMed] [Google Scholar]
- Lonard DM, Lanz RB, O’Malley BW. Nuclear receptor coregulators and human disease. Endocr Rev. 2007;28:575–87. doi: 10.1210/er.2007-0012. [DOI] [PubMed] [Google Scholar]
- Clipsham R, McCabe ER. DAX1 and its network partners: exploring complexity in development. Mol Genet Metab. 2003;80:81–120. doi: 10.1016/j.ymgme.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Crawford PA, Dorn C, Sadovsky Y, Milbrandt J. Nuclear receptor DAX-1 recruits nuclear receptor corepressor N-CoR to steroidogenic factor 1. Mol Cell Biol. 1998;18:2949–56. doi: 10.1128/mcb.18.5.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altincicek B, Tenbaum SP, Dressel U, Thormeyer D, Renkawitz R, Baniahmad A. Interaction of the corepressor Alien with DAX-1 is abrogated by mutations of DAX-1 involved in adrenal hypoplasia congenita. J Biol Chem. 2000;275:7662–7. doi: 10.1074/jbc.275.11.7662. [DOI] [PubMed] [Google Scholar]
- Yu RN, Ito M, Jameson JL. The murine Dax-1 promoter is stimulated by SF-1 (steroidogenic factor-1) and inhibited by COUP-TF (chicken ovalbumin upstream promoter-transcription factor) via a composite nuclear receptor-regulatory element. Mol Endocrinol. 1998;12:1010–22. doi: 10.1210/mend.12.7.0131. [DOI] [PubMed] [Google Scholar]
- Kim AC, Barlaskar FM, Heaton JH, Else T, Kelly VR, Krill KT, Scheys JO, Simon DP, Trovato A, Yang WH, Hammer GD. In search of adrenocortical stem and progenitor cells. Endocr Rev. 2009;30:241–63. doi: 10.1210/er.2008-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Thomsen JS, Johansson L, Gustafsson JA, Treuter E. DAX-1 functions as an LXXLL-containing corepressor for activated estrogen receptors. J Biol Chem. 2000;275:39855–9. doi: 10.1074/jbc.C000567200. [DOI] [PubMed] [Google Scholar]
- Holter E, Kotaja N, Makela S, Strauss L, Kietz S, Janne OA, Gustafsson JA, Palvimo JJ, Treuter E. Inhibition of androgen receptor (AR) function by the reproductive orphan nuclear receptor DAX-1. Mol Endocrinol. 2002;16:515–28. doi: 10.1210/mend.16.3.0804. [DOI] [PubMed] [Google Scholar]
- Agoulnik IU, Krause WC, Bingman WE, 3rd, Rahman HT, Amrikachi M, Ayala GE, Weigel NL. Repressors of androgen and progesterone receptor action. J Biol Chem. 2003;278:31136–48. doi: 10.1074/jbc.M305153200. [DOI] [PubMed] [Google Scholar]
- Nachtigal MW, Hirokawa Y, Enyeart-VanHouten DL, Flanagan JN, Hammer GD, Ingraham HA. Wilms’ tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell. 1998;93:445–54. doi: 10.1016/s0092-8674(00)81172-1. [DOI] [PubMed] [Google Scholar]
- Dorn C, Ou Q, Svaren J, Crawford PA, Sadovsky Y. Activation of luteinizing hormone beta gene by gonadotropin-releasing hormone requires the synergy of early growth response-1 and steroidogenic factor-1. J Biol Chem. 1999;274:13870–6. doi: 10.1074/jbc.274.20.13870. [DOI] [PubMed] [Google Scholar]
- Jimenez P, Saner K, Mayhew B, Rainey WE. GATA-6 is expressed in the human adrenal and regulates transcription of genes required for adrenal androgen biosynthesis. Endocrinology. 2003;144:4285–8. doi: 10.1210/en.2003-0472. [DOI] [PubMed] [Google Scholar]
- Lalli E, Ohe K, Hindelang C, Sassone-Corsi P. Orphan receptor DAX-1 is a shuttling RNA binding protein associated with polyribosomes via mRNA. Mol Cell Biol. 2000;20:4910–21. doi: 10.1128/mcb.20.13.4910-4921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.