Abstract
Animal studies have shown that life span is extended by caloric restriction (CR). This manuscript describes the design and methodology of an innovative CR intervention, which is the treatment arm of the CALERIE study. This study is a multi-center, randomized controlled trial examining the effects of two years of CR on biomarkers of longevity among non-obese (BMI ≥ 22 kg/m2 and < 28 kg/m2) adults. CALERIE is the first investigation of the effects of long-term CR on the aging process in non-obese humans. 220 healthy volunteers across 3 sites were recruited beginning in May 2007. Participants were randomized in a 2:1 ratio between the CR or Control group (i.e., ad libitum diet). An intensive intervention was designed to assist participants in adhering to the 25% CR prescription for a two-year duration. The intervention was designed to optimize the likelihood 25% CR would be achieved through a variety of nutritional and behavioral strategies, several of which are innovative methods for achieving CR. The intervention includes the following components: an intensive, “mixed” format schedule of group/individual sessions, meal provision phase with exposure to various diets, Personal Digital Assistants to monitor caloric intake, unique portion estimation training, tailored treatment using a computer tracking system, toolbox strategies and algorithms, as well as comprehensive coverage of nutrition and behavioral topics in order to assist participants in meeting their CR goal. This manuscript provides an overview of the CR intensive intervention and may be of assistance for other researchers and clinicians in designing future trials.
Keywords: Caloric restriction, randomized controlled trial, aging, intervention
1. Introduction
The purpose of the Comprehensive Assessment of the Long-term Effects of Reducing Energy Intake (CALERIE) study is to examine the effects of long-term 25% caloric restriction (CR) on non-obese humans. The study was undertaken to examine 2 years of sustained CR on: a) slowing aging as assessed by proxy indicators and b) protecting against age-related disease processes.
The distinct difference between CALERIE and previous weight loss studies is that CALERIE emphasizes adherence to a prescribed CR goal rather than to a specified degree of weight loss. When CR is maintained past the point when weight stability is established, information will be gained as to whether the effects of CR become stable or whether these effects are transitory. The distinct challenge of this study is to assist participants in achieving and maintaining the 25% CR goal for 24 months. Thus, a CR intervention was designed to assist participants with adhering to CR with no specific macronutrient composition (other than nutritional adequacy) being recommended.
1.1 Theoretical Framework for CR Intervention
A central purpose of CALERIE is to determine the similarities and differences between CR’s effects in humans compared to previous studies in laboratory animal models, which have shown an extended life span through CR [1–4]. Evidence from animal studies demonstrates that when CR is sustained, weight is relatively stable once the initial phase of weight loss has ended [5–7]. Thus, to achieve the goals of CALERIE, the period of initial weight loss must be followed by a period of relative weight stability during which adherence to the specified degree of CR is maintained. The Phase 1 study results of CALERIE demonstrated the feasibility of CR over 12 months and revealed that if CALERIE participants succeed in maintaining adherence to 25% CR it may take up to 12 months or possibly longer for the initial period of weight loss to end [8–11]. Therefore, the CALERIE Phase 2 intervention was designed to optimize the likelihood that: 1) the % CR that participants achieve will be substantial, and 2) the % CR participants achieve will be sustained at a relatively constant level over the two-year intervention.
The available literature that provided the framework for this study focused on weight loss interventions. In most long-term weight loss studies in humans, the initial period of weight loss was followed by a period, due to weight regain, body weights actually were increasing faster in the treatment than in the control group [12,13]. The CALERIE study design offers an opportunity to avoid this shortcoming.
A distinct challenge is that CALERIE participants are not obese, and some are in the “healthy” weight range (BMI 22 to 25 kg/m2). Therefore, when designing the intervention the following assumptions were made: 1) compared to obese persons, CALERIE participants may have decreased motivation for weight loss and adhering to CR on the basis of the presumed health benefits and 2) CALERIE participants’ dietary habits with regard to energy intake are likely to differ less from accepted nutritional guidelines than do those of obese persons.
1.2 Participant Recruitment and Screening
Two-hundred and twenty healthy volunteers across 3 sites (Tufts University, Pennington Biomedical Research Center, and Washington University School of Medicine) were recruited beginning in May 2007. Study participants are men within the age range of 21–50 years and women between 21–47 years who have an initial BMI ≥ 22 kg/m2 and < 28 kg/m2. Participants have been randomized in a 2:1 ratio into the CR or Control group (i.e., ad libitum diet). Potential participants were screened during a series of physical and psychological tests/interviews to identify healthy individuals who agreed to make the necessary commitments to participate in a two-year intensive CR-oriented lifestyle modification program.
1.3 Intervention Design
The CALERIE Study was designed to optimize the likelihood that a substantial degree of CR is achieved through a variety of nutritional and behavioral strategies. The conceptual framework of the CR intervention was developed from experiences during two landmark clinical trials: the Diabetes Prevention Program and the Look AHEAD Study [14–17]. These studies have successfully achieved weight loss and subsequent weight maintenance through an intensive lifestyle intervention [12, 14–17]. A very brief description of the CALERIE intervention is also mentioned in a separate manuscript outlining the overall study design, extensive methodology, and the various biomarkers being collected, which were chosen based on previous research findings [18, 19].
The primary goal of the CR intervention is to achieve and maintain a sustained reduction in caloric intake rather than a specified weight loss, with weight change being a proxy indicator of sustained CR. A two-year CR period was selected to attempt to provide for a sustained period of weight stability following weight loss. It is expected that the period of weight loss will last six to twelve months. A major emphasis of the intervention is on adherence to prescribed CR using a proactive and comprehensive plan described below for providing the participants with an array of supporting services to aid in this effort.
1.4 25% CR Intervention
The CR intervention can be conceptualized as an intensive behavioral approach [20–22] coupled with dietary modifications and daily self-monitoring of calories, designed to promote adherence to long-term CR [23–26]. All CALERIE participants are advised of the current health recommendations for physical activity of 30 minutes/day of a moderate level on a minimum of 5 days/week, but no efforts are made to change participants’ exercise habits or activity levels. In order to determine the discrete effects of CR, participants are instructed not to alter their activity habits during the course of the study. The CALERIE intervention approach was based on strategies that have been found to be effective in long-term weight management studies and in short-term studies supporting dietary composition changes for enhanced satiety and reduced hunger including the provision of meals and structured meal plans [23–26]. Within the range of recommended amounts, no specific macronutrient composition of the diet is prescribed, although participants are encouraged to incorporate concepts such as volumetrics, lower glycemic index foods, and adequate protein and fiber at meals in order to make CR adherence easier and more satisfying. In the present intervention, each participant is provided with an individualized CR prescription for 25% CR from baseline ad libitum energy intake as determined by doubly-labeled water (DLW). Group adherence to the prescribed diet is then assessed by a combination of measures of energy expenditure using the doubly labeled water technique and precise changes in body composition by duel x-ray absorptiometry (DXA) at 6, 12, 18, and 24 months [18, 27]. Six day food records are also collected at these time points [18]. However, these measurements are outcome measurements and not used to assess intervention adherence during the study; the intervention is guided by individual information recorded for each participant in a computer tracking system (CTS).
2. Key Innovative Features of the CALERIE Study
2.1 Intensive Mixed Format Schedule
Longer duration of contact with participants has been found to be associated with better adherence to interventions that promote health behavior change [26]. In order to assist participants with meeting their 25% CR goal, individual counseling sessions were chosen as the primary mode for delivering the intervention, with group counseling sessions serving as an important secondary component for the provision of information and enhancement of social support for CR adherence. A “mixed” format (i.e., individual and group sessions) was selected in order to combine the strengths of individual and group interactions. Through individual and group counseling sessions, participants are provided with information (e.g., on potential satiating effects of higher fiber intake), material aids for adherence (e.g., food scales and Personal Digital Assistants [PDAs] for self-monitoring food intake), provision of food for the first month and thereafter on an as needed basis, and incentives to enhance adherence. An overview and schedule of the individual and group counseling sessions for weeks 1–24 is provided in Table 1. A similar format was used for weeks 5–104 covering a wide variety of nutrition and behavioral topics.
Table 1.
Overview of individual and group session topics participant schedule: weeks 1–24
| WEEK | INDIVIDUAL SESSION |
INDIVIDUAL TOPIC | GROUP SESSION |
GROUP TOPIC |
|---|---|---|---|---|
| 1 | X-FP | Welcome/CR Goal/PDA Instruction | ||
| 2 | X-FP | Getting Started, Tipping the Energy Balance/ PDA Review | ||
| 3 | X-FP | Portion & Stimulus Control/PDA Review | ||
| 4 | X-FP | Managing Hunger, Satiety, and Distress Tolerance/PDA Review/Formal CR Goal | X | Portion Control |
| 5 | X | Social Support/ PDA Review | ||
| 6 | X | Hunger and Satiety | ||
| 7 | X | Problem Solving/ PDA Review | ||
| 8 | X | Putting Problem Solving Into Practice | ||
| 9 | X | How Are You Doing? Progress Review/ PDA Review | ||
| 10 | X | Goal Setting | ||
| 11 | X | Barriers & Meal Replacements / PDA Review | ||
| 12 | X | Barriers to CR | ||
| 13 | X | Eating Out with CR/ PDA Review | ||
| 14 | X | Eating Away From Home | ||
| 15 | X | Motivation/ PDA Review | ||
| 16 | X | Maintaining Motivation | ||
| 17 | X | Thoughts/ PDA Review | ||
| 18 | X | Mastering Positive Thinking | ||
| 19 | X | Social Support/ PDA Progress Review/ PDA Review | ||
| 20 | X | Enlisting Social Support | ||
| 21 | X | Food Cravings/ PDA Review | ||
| 22 | X | Urge Management of Food Cravings | ||
| 23 | X | Review of Goals/ PDA Review | ||
| 24 | X | Relapse Prevention |
FP = Food Provision to Participants X = Required Individual/Group Sessions
2.1.1 Individual Counseling Sessions
Individual counseling sessions, the cornerstone of the CALERIE intervention, provide a regular opportunity to attend to individual participant needs and an opportunity to tailor the intervention to these specific needs. The group counseling sessions are designed to complement the individual session topics and provide social support. Problems with adherence to 25% CR prescription are directly addressed in individual sessions using various pre-specified strategies, described in more detail below. The DPP and the Look AHEAD Study have used a similar approach with success [14, 15].
Each participant interacts with both a Counselor who is a behavioral expert (e.g., has a Masters or doctoral degree in psychology) and a Counselor who is an expert in nutrition (i.e., a Registered Dietitian). Both Counselors work closely with the participant to assist him/her with various aspects of CR adherence. Participants enter CALERIE and are assigned a primary Counselor who follows them in individual sessions throughout the program, beginning in week one. Individual sessions occur weekly for the first month (weeks 1–4), while the participants are developing skills in estimating their caloric intake with additional training on portion size estimation and calorie content of foods. The participant and Counselor develop a mutually-agreed-upon individual dietary/behavioral plan to achieve the targeted degree of CR within the scope of the intervention design. This plan reflects the participant’s input based on his/her preferences, needs and experience with differing types of provided meals, as well as the Counselor’s expertise concerning nutritional and behavioral strategies to achieve and maintain 25% CR.
For months 2 through 12, (weeks 5–53), individual sessions occur twice monthly, with additional biweekly phone contact. These twice-monthly individual sessions provide continued support and allow the Counselor to closely track progress. For months 13 through 24 (weeks 54–104), the participant attends monthly individual counseling sessions; additional sessions are added “as needed.” These monthly sessions reduce time demands for the participant, yet allow the Counselor to continue to track adherence to CR.
2.1.2 Group Counseling Sessions
Group sessions occur twice monthly, beginning at week four of the intervention after the participant has progressed through the initial four individual sessions. Group session content is designed to complement the information given in individual sessions as well as provide social support. Participants attend 12 group sessions at regular intervals during the first 26 weeks of the study. From weeks 27 to 104, participants attend group sessions once a month. The groups utilize open enrollment, which allows participants to begin attending group sessions as soon as they enroll in the study. This group format utilizes “modules” of group sessions that cover information that is appropriate for the participant, given the length of time that the participant has been enrolled in the study. Other studies that initially provided an intensive intervention but then held infrequent or no continuing group sessions found that participants rapidly lost momentum, regained lost weight, or increased consumption of higher-fat foods [26]. Thus, attendance at group sessions is closely monitored and recorded in the computer tracking system (CTS). Poor attendance or missed sessions trigger a rapid response by the Counselors, as described later in the paper.
2.2 Meal-Provision Phase and Exposure to Various Diets
Participants randomized to the CR group are provided with their meals for the first 27 days of the intervention and are encouraged to strictly adhere to the foods and menus provided. Participants are required to pick up the provided meals at the centers using site-specific schedules. Participants rotate through 3 different diet patterns which include a low fat (20% fat, 20% protein, 60% carbohydrate), a Mediterranean (35% fat, 15% protein, 50% carbohydrate) and a low glycemic load diet (30% fat, 30% protein, 40% carbohydrate). These varied diets were provided for educational purposes related to food selection and portion size. Three-day cycle menus are used, and each participant is on each diet type for 9 days. For participants who want to follow a vegetarian (ovo-lacto) diet, a 3-day cycle vegetarian menu (30% fat, 20% protein, 50% carbohydrate) is provided. All diets provide 14 g fiber/1000 kcal. Two-thirds of a cup (80 kcal, 19g fiber) of Fiber One® bran cereal is also provided as an optional daily addition to the menus during the meal-provision phase. Alcohol is not served by the centers and its use is discouraged during the meal-provision phase in order to maintain the 25% CR level. However, alcohol is permitted after the 4-week feeding phase for the remainder of the intervention (not more than 2 drinks per day and no more than 14 drinks per week for men and 10 drinks per week for women).
The same menu templates are used at all 3 study sites, with minor adjustments (in spices, brands) allowed to accommodate regional preferences and vendor availability. The CR prescription level for each participant is calculated from the baseline TEE results derived from DLW and was rounded up or down to the nearest hundred for the meal provision phase.
2.3 Self-Monitoring Dietary Intake and Portion Size Estimation Training
Recording food intake, as well as portion size estimation, are critical tools in helping participants achieve and maintain their 25% CR goal. The meal provision phase allows for frequent contact with the participants and during this phase, participants are required to meet with the Study Dietitian 2–3 times per week to maximize adherence to the provided food and to complete training on recording their food intake and estimating portion sizes.
On Day 1 of the intervention, participants assigned to the CR treatment arm are provided with a PDA that contains diet software and are asked to self-monitor their dietary intake throughout the two-year study. Participants complete training worksheets both on site and at home throughout the meal provision phase and work with the Study Dietitian to complete on-site training in order to become proficient with this critical behavior right at the beginning of CR. Adherence to self-monitoring is tracked in the computerized tracking system (described in Section 3.1).
To help with the accuracy of self-monitoring of dietary intake, participants are provided with food scales and measuring cups and spoons and also complete portion estimation training. Test meals using study foods are used to determine each participant’s ability to accurately estimate portions without the use of a food scale or household measuring utensil. Participants complete portion estimation training at the start of CR and during weeks 2 and 4 of the meal provision phase. The Study Dietitian determines the participant’s accuracy by comparing the reported portion size with the actual size of the foods and provides the participant with immediate feedback on his/her accuracy. Discrepancy scores are also calculated for each portion training session, and if a participant’s mean discrepancy score for a test visit is greater than +/− 30%, then the participant receives further training until accuracy criteria are met.
Counselors enter dietary self-monitoring data into the CTS, and participants graphically observe self-reported calories consumed over the two-year period. The CTS creates a graph displaying the reported dietary intake compared to each participant’s CR goal. Counselors record attendance at individual and group counseling sessions. Adherence to self-monitoring is defined as 70% complete entries during months 1–6, 50% complete entries during months 7–12 and at least 30% complete entries during months 13–24.
3. Tailoring Treatment
3.1 Computer Tracking System
Reliance on a treatment manual promotes treatment integrity among Counselors and across study sites however, the CALERIE Study also allows for individual tailoring of the intervention to meet the needs of each participant. One innovative component of the intervention is the use of a sophisticated computer tracking system (CTS) to guide delivery of individual counseling sessions. The CTS tracks changes in body weight in relation to the expected changes based on a model developed with results from CALERIE Phase 1 [28].
Information from the CTS is used by CALERIE intervention team leaders to observe adherence on a study-wide basis, for each of the three sites, and for each individual participant. Reports are generated from the CTS to describe session attendance, self-monitoring, dietary adherence, and weight loss. These reports serve as an index of adherence to the intervention and can be used by the treatment team to evaluate compliance. Aggregated data on individual participants is compiled according to site (Boston, Baton Rouge, St. Louis) for evaluation by Counselors and Investigators. The CTS tracks pre-specified strategy usage (based on Toolbox/Algorithms described below), and it records whether the strategy was effective for promoting improved adherence. The CTS also tracks monetary expenditures associated with the use of some of these strategies.
3.2 Toolbox/Algorithms
The CTS alerts Counselors if participants have sub-optimal adherence to several study-related behaviors and provides Counselors with suggested treatment strategies, in the form of toolbox options. The toolbox methodology has been used successfully in previous clinical trials [14, 15]. By systematically following the same pre-specified decision rules, it is possible to intervene quickly in order to overcome obstacles to success and improve CR adherence. This systematic approach also fosters treatment fidelity among Counselors and across sites. Additionally, the toolbox allows Counselors to tailor treatment to address personal preferences and lifestyles, as well as regional, cultural and ethnic differences among participants. At each site, approximately $150 per participant per year is allocated for utilization of toolbox options, such as provided meals or incentives such as gift certificates.
The CTS automatically determines whether participants are in or out of compliance for the following study-related behaviors: 1) adherence to CR prescription, where weight is used as a proxy, 2) session attendance, and 3) self-monitoring. Additionally, specific criteria have been developed to assist Counselors in determining if any of the following behaviors are 1interfering with adherence to the intervention: 1) poor dietary knowledge, 2) binge eating, and/or 3) emotional problems. There are two types of toolbox options that are tracked in the CTS: 1) “Open Toolbox” and 2) “Closed Toolbox.” “Open Toolbox” options are generally used first and are always available for the Counselor and participant to use in order to increase adherence. In contrast, “Closed Toolbox” options, which are not available until week 5, are used only when pre-defined adherence problems are detected by the CTS, as they typically require additional resources for implementation.
The effectiveness of selected toolbox strategies for these behaviors is evaluated by the Counselor every two to four weeks through objective criteria specific to each toolbox. By tracking this process, it is possible to empirically evaluate the efficacy of different intervention strategies as these relate to particular study-related behaviors.
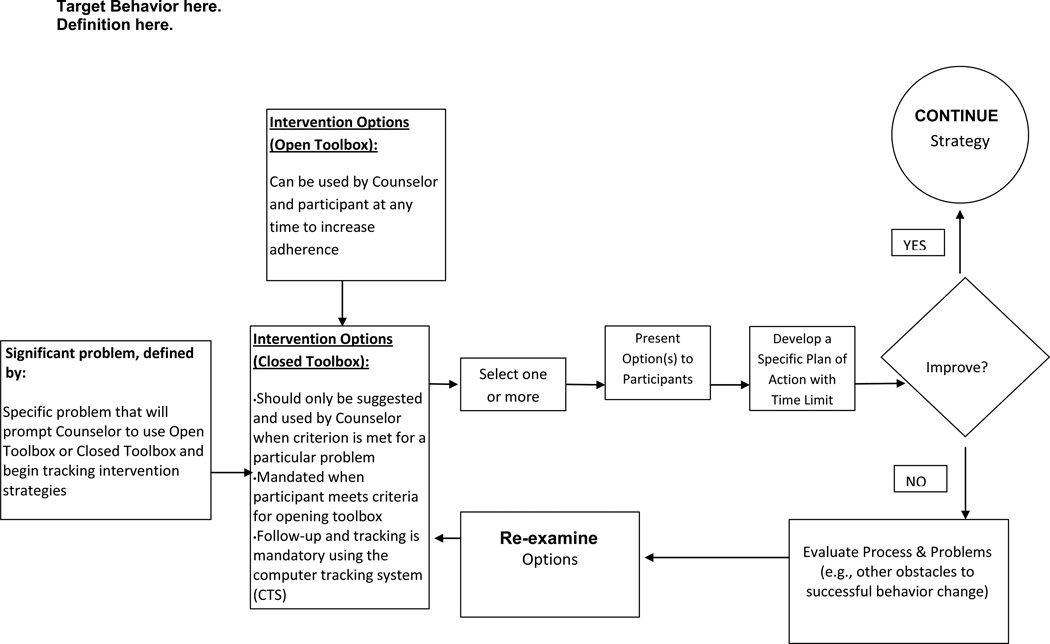
Toolbox algorithms (examples illustrated in Figures 1–2) guide the decision-making process for tailoring treatment to the unique needs of each participant. The CALERIE algorithms were specifically adapted from the Look AHEAD Study1 [14]. The algorithms operationally define specific types of adherence problems that trigger the opening the Closed Toolbox and explicitly describe the most common intervention options that should be considered to resolve the problem as quickly as possible. The General Conceptual Framework, illustrated in Figure 1, describes the basic conceptual scheme for individual tailoring during the first six months of the CALERIE intervention. This conceptual framework uses a social problem-solving approach where a problem is identified; solutions are brainstormed and selected, and then tested for a specific period of time. If the solution effectively resolves the problem, then the strategy is continued until consistent behavior change is observed. If the solution is unsuccessful after a specific time period, e.g., four weeks, the strategy is terminated and a new option for solving the problem is selected and tested for a specific period of time.
Figure 1.
General Conceptual Framework
Figure 2.
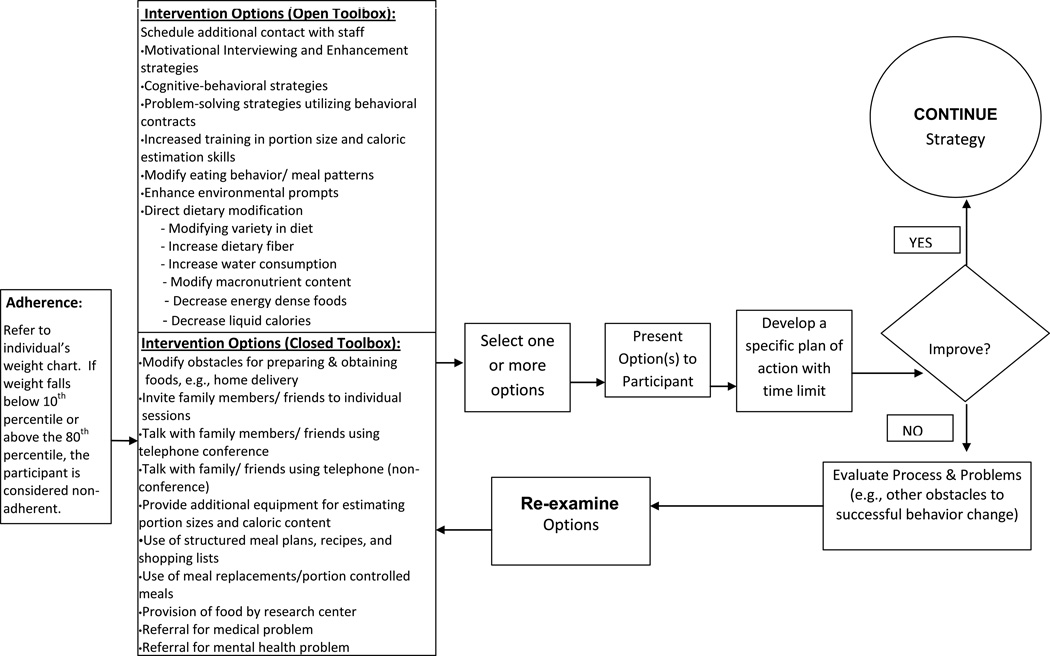
Primary Problem of Sub-Optimal Adherence to 25% Calorie Restriction
Sub-optimal adherence with CR prescription is defined by an inability to stay within appropriate parameters on an individual participant’s weight chart as represented in the CTS (e.g., if weight falls below the 10th percentile or above the 80th percentile, the participant is considered non-adherent). “Closed Toolbox” strategies should be applied when a participant’s weight is outside the adherence curve. This process should occur immediately whenever a weight measurement falls outside the adherence curve. The system shall not consider a participant to be out of adherence for this toolbox until the participant enters the 5th week of the study. “Closed Toolbox” strategies may be discontinued when the participant no longer meets criteria necessary to open the Closed Toolbox.
The Counselors track the events that open both the “Open Toolbox” and “Closed Toolbox” and record use of Toolbox options in the CTS in order to track participant progress. A coding system enables the Counselor and Investigator to track: 1) the target problem that opened the Toolbox, 2) the “Open Toolbox” and “Closed Toolbox” options that are used, 3) resolution of the problem, and 4) estimated monetary amount spent on each “Closed Toolbox” option. Using this process, it is possible to empirically evaluate the efficacy of different “clinical decisions” or at least to translate these “clinical decisions” into a set of behavioral strategies that can be objectively described. All strategies are evaluated for effectiveness every two to four weeks or at the next scheduled individual session.
In some cases, the participant may be unable to develop a treatment plan that is viable. For example, the person may refuse to initiate a portion-controlled diet or referral to a specialist. In such cases, motivational enhancement methods are employed to facilitate acceptance of the clinical recommendation. Counselors are aware that use of Toolbox options is a collaborative process between the Counselor and participant. As such, the Counselor emphasizes the role of the participant in brainstorming solutions for inadequate adherence or potential areas of difficulty.
3.3 Measuring and Tracking Changes in Body Weight
Changes in body weight were selected as a proxy indicator for CR adherence. At the start of an individual session, Counselors initially weigh participants and then enter the participant’s weight into the CTS, which graphs the participant’s predicted weight loss (based upon his/her caloric prescription) against his or her actual weight loss. This graph is used by the Counselor to determine the participant’s adherence to the intervention and is then discussed during the individual counseling session.
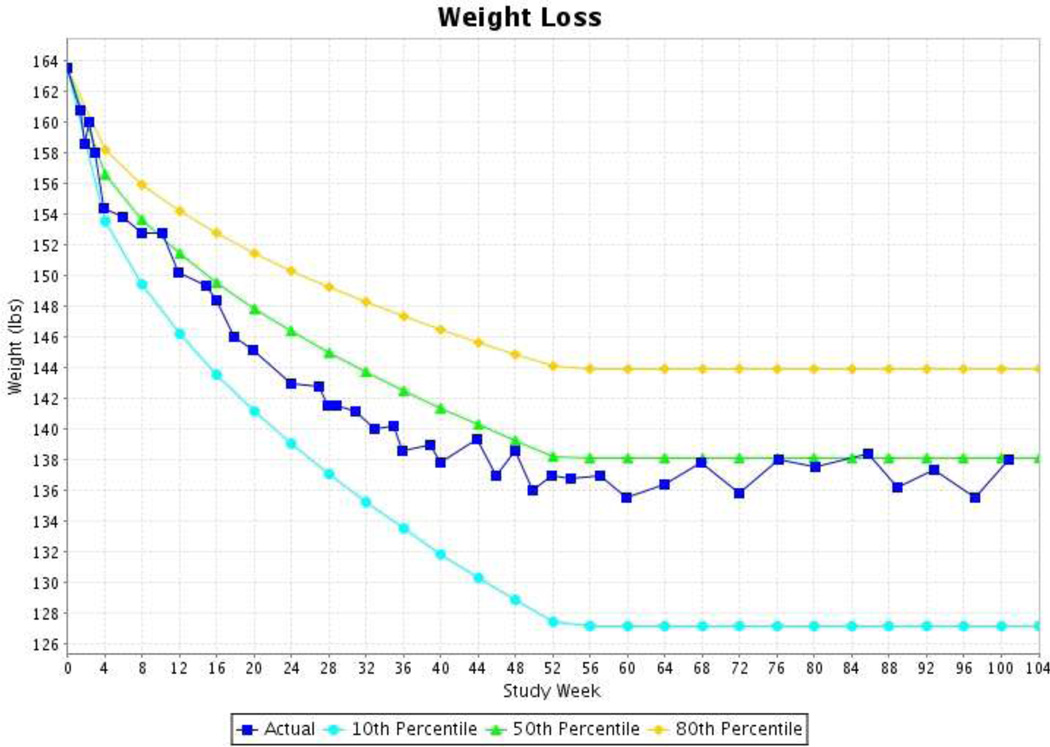
A weight loss algorithm (Figure 3), which is based on data from the CALERIE Phase I study, as a guide for expected weight change [28] with predetermined upper and lower limits. The CTS weight graph provides three lines (the 50th percentile, 80th percentile, and 10th percentile) against which the participant’s weight is plotted; this graph is viewed by the participant and Counselor at the start of each session. The upper boundary of adherence (lowest level of adherence before opening the closed toolbox) is the 80th percentile of expected weight change over time, and the lower boundary (greatest allowable weight loss) is the 10th percentile; these ranges make up what is considered the “zone of acceptable weight loss.”
FIGURE 3.
The screen shot below displays a participant who is within the expected weight loss zone the entire twenty-four months.
Decisions related to the use of Toolbox strategies are based on whether the weight changes are within the expected “zone of acceptable weight loss.” If a participant’s weight change is above the 80th percentile, the CTS alerts the Counselor that a “Closed Toolbox” option should be selected and applied to improve adherence. If the participant’s weight loss is too rapid (i.e., weight goes below the 10th percentile for maximum expected weight loss), then the CTS alerts the Counselor that the weight loss is greater than expected. In this situation, the Counselor consults with the Study Dietitian to determine if the participant is likely overly restricting their intake.
A similar approach is taken to prevent the participant’s body mass index (BMI) from falling below the 18.5 cutoff selected by CALERIE for safety purposes. In this situation, the Counselor is initially alerted when a participant’s BMI falls below 18.75 in order to intervene appropriately, by encouraging the participant to slightly increase his/her caloric intake. As long as the participant’s BMI remains above 18.5, the participant continues the intervention per the protocol. If the participant’s BMI falls below 18.5, the Counselor is alerts the Study Physician who then temporarily discontinues the participant.
3.4 Visual Analog Scales
The CTS also provides an opportunity for participants to record ratings of hunger, satiety, desire to eat, etc., and to graphically view these ratings over the two-year intervention period. Counselors ask the participant to report his or her hunger and satiety levels over the previous week by completing 100-mm visual analog scales (VAS) linked to the CTS. The system provides a report that displays reported hunger and satiety levels for each participant over time. This report is discussed with participants during individual sessions, particularly if there is a significant change in reported hunger or satiety levels over time.
4. Discussion and Conclusions
Upon completion, the CALERIE Study will be the first systematic investigation of the effects of CR on the aging process in non-obese humans. This manuscript provides an overview of the innovative study design and methods used in a CR intensive intervention. If successful, the description of the CALERIE intervention study may be of assistance for other researchers and clinicians in designing and implementing future trials.
Acknowledgments
Funding Support: This research was supported by grants 5U01AG022132-06, 5U01AG020478-06, 5U01AG020487-07 and 5U01AG020480-06 from the National Institute on Aging and K23 DK068052 (NIDDK) of the U.S. National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Williamson was the primary developer of these algorithms for the Look AHEAD Study.
REFERENCES
- 1.Spindler SR. Calorie restriction enhances the expression of key metabolic enzymes associated with protein renewal during aging. Ann N Y Acad Sci. 2001;928:296–304. doi: 10.1111/j.1749-6632.2001.tb05659.x. [DOI] [PubMed] [Google Scholar]
- 2.Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 3.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 4.Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 5.Masoro EJ, Yu BP, Bertrand HA. Action of food restriction in delaying the aging process. Proc Natl Acad Sci. 1982;79:4239–4241. doi: 10.1073/pnas.79.13.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu BP, Masoro EJ, Mc Mahan CA. Nutritional influences on aging of Fischer 344 rats: physical, metabolic and longevity characteristics. J Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- 7.Lambert J, Lamonthe JM, Zernicke RF, Auer RN, Reimer RA. Dietary restriction does not adversely affect bone geometry and mechanics in rapidly growing male Wistar rats. Pediatr Res. 2005;57:227–231. doi: 10.1203/01.PDR.0000148715.61869.4E. [DOI] [PubMed] [Google Scholar]
- 8.Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92:865–872. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer E, Rood J, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. Jama. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;84:1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 11.Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, et al. Washington University School of Medicine CALERIE Group. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci. 2006;61:943–950. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garner DM, Wooley SC. Confronting the failure of behavioral and dietary treatments for obesity. Clin Psychol Rev. 1991;11:729–780. [Google Scholar]
- 14.The Look AHEAD Research Group. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan DH, Espeland MA, Foster GD, Haffner Sm, Hubbard VS, Johnson KS, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Controlled Clinical Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 18.Rochon J, Bales CW, Ravussin E, Redman LM, Holloszy JO, Racette SB, et al. Design and conduct of the CALERIE study: Comprehensive Assessment of the Long-Term Effects of Reducing Intake of Energy. J of Gerontol A Biol Sci Med Sci. 2011;66A(1):97–108. doi: 10.1093/gerona/glq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, et al. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;811 doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- 20.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12 Suppl:151S–162S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 21.The Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williamson DA, Stewart TM. Behavior and lifestyle: approaches to treatment of obesity. J LA State Med Soc. 2005;157(Spec No 1):S50–S55. [PubMed] [Google Scholar]
- 23.Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Revs. 2001;59:129–139. doi: 10.1111/j.1753-4887.2001.tb07001.x. [DOI] [PubMed] [Google Scholar]
- 24.Jeffery RW, Wing RR, Thorson C, Burton LR, Raether C, Harvey J, et al. Strengthening behavioral interventions for weight loss: a randomized trial of food provision and monetary incentives. J Consult Clin Psychol. 1993;61:1038–1045. doi: 10.1037//0022-006x.61.6.1038. [DOI] [PubMed] [Google Scholar]
- 25.Wing RR, Jeffery RW, Burton LR, Thorson C, Nissinoff KS, Baxter JE. Food provision vs. structured meal plans in the behavioral treatment of obesity. Int J Obes Relat Metab Disord. 1996;20:56–62. [PubMed] [Google Scholar]
- 26.Perri MG, Corsica JA. Improving the maintenance of weight lost in behavioral treatment of obesity. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. New York, NY: Guilford Press; 2002. pp. 357–379. [Google Scholar]
- 27.de Jonge L, DeLany JP, Nguyen T, Howard J, Hadley EC, Redman LM, et al. Validation study of energy expenditure and intake during caloric restriction using doubly labeled water and changes in body composition. Am J Clin Nutr. 2007;85:73–79. doi: 10.1093/ajcn/85.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pieper C, Redman LM, Bapkar M, Roberts SB, Racette SB, Rochon J, et al. for the CALERIE Study Group. Development of adherence metrics for caloric restriction interventions. Clin Trials. 2011;8(2):155–164. doi: 10.1177/1740774511398369. [DOI] [PMC free article] [PubMed] [Google Scholar]