Abstract
Amyloid-β (Aβ) peptides are intimately involved in the inflammatory pathology of atherosclerotic vascular disease (AVD) and Alzheimer's disease (AD). Although substantial amounts of these peptides are produced in the periphery, their role and significance to vascular disease outside the brain requires further investigation.
Amyloid-β peptides present in the walls of human aorta atherosclerotic lesions as well as activated and non-activated human platelets were isolated using sequential size-exclusion columns and HPLC reverse-phase methods. The Aβ peptide isolates were quantified by ELISA and structurally analyzed using MALDI-TOF mass spectrometry procedures.
Our experiments revealed that both aorta and platelets contained Aβ peptides, predominately Aβ40. The source of the Aβ pool in aortic atherosclerosis lesions is probably the activated platelets and/or vascular wall cells expressing APP/PN2. Significant levels of Aβ42 are present in the plasma, suggesting that this reservoir makes a minor contribution to atherosclerotic plaques.
Our data reveal that although aortic atherosclerosis and AD cerebrovascular amyloidosis exhibit clearly divergent end-stage manifestations, both vascular diseases share some key pathophysiological promoting elements and pathways. Whether they happen to be deposited in vessels of the central nervous system or atherosclerotic plaques in the periphery, Aβ peptides may promote and perhaps synergize chronic inflammatory processes which culminate in the degeneration, malfunction and ultimate destruction of arterial walls.
Keywords: atherosclerosis, platelets, amyloid-beta, vascular inflammation, Alzheimer's disease, coagulation cascade
1. Introduction
Numerous studies have demonstrated that amyloid-beta (Aβ) peptides have a central role in the pathogenesis of Alzheimer's disease (AD) and recent evidence derived from epidemiologic, correlative and experimental data has strongly linked intracranial atherosclerotic vascular disease (AVD) with the pathogenesis of AD [1-3]. The Baltimore longitudinal study on aging suggests that the presence of intracranial AVD significantly increases the odds of dementia, independent of cerebral infarction [4]. Multiple established risk factors for AVD have also been recognized to be risk factors for the development of AD (reviewed in [5,6]). Clinical investigations have reported that coronary artery disease, myocardial infarction, carotid artery disease, peripheral artery disease, hypertension, diabetes mellitus, cigarette smoking and obesity are all more common in subjects with AD (reviewed in [2]). Longitudinal and twin studies have reinforced the tenet of cardiovascular failure and AVD, in the absence of stroke, as risk factors for AD in carriers of the apolipoprotein E (ApoE) ε4 allele [7]. Moreover, the association between cardiovascular disease and dementia is not explained by genetic or early life environmental factors common in both disorders [7]. Individuals with midlife hyperlipidemia have an elevated risk of developing AD, and individuals with clinically or neuropathologically diagnosed AD have higher blood cholesterol levels compared to non-demented controls [8-10]. Considerable evidence from experimental studies links cholesterol to Aβ metabolism [11,12].
Both AD and AVD evolve in an intensely inflammatory milieu [13-19] that ultimately destroys brain and vascular tissues. In AD, Aβ accumulation induces microvascular inflammation mediated by cytokines and chemokines that act on neurons, glia, endothelium, myocytes and pericytes [20-23]. Under normal and pathological conditions, the Aβ peptides play an important role in the modulation of inflammation [24,25]. In addition, pathological interactions between molecules of the coagulation cascade and Aβ have been shown to alter thrombosis and fibrinolysis thus establishing associations among atherosclerosis, thrombosis, cerebral amyloid angiopathy (CAA), brain hypoperfusion and AD [26]. The amyloidogenic 40/42 amino acid-long Aβ peptides are derived, by the proteolytic action of the β- and γ-secretases, from larger amyloid-beta precursor proteins that are 695, 751 and 770 amino acids in length (APP695, APP751 and APP770). Large amounts of Aβ peptides deposited in the brain microvascular walls result in considerable pathophysiological alterations, and the severity of cognitive decline is associated with the degree of CAA and arteriosclerosis/lipohyalinosis [27]. In particular, the capillary deposition of Aβ42 is highly correlated with AD pathology [28], blood-brain barrier (BBB) disruptions and severe alterations in brain perfusion [13,29-31].
The Aβ peptides are generated in appreciable quantities outside of the central nervous system in places such as the neuromuscular junctions of skeletal muscle fibers [32] and in the α-granules of platelets [33] that contribute to the pool of Aβ in the circulation. Other potential sources of APP/Aβ are the endothelial cells of the vasa vasorum that invade and feed the walls of the atherosclerotic lesion [34]. In platelets, the Aβ peptides are mainly derived from the APP751 and APP770, known as protease nexin-2 (PN2), which carries a Kunitz (serine) protease inhibitory (KPI) domain. The APP/PN2 molecule has important functions in the coagulation cascade as it acts as an inhibitory component of factors, IXa, Xa, XIa and tissue factor VIIa complex [35-38], thus modulating hemostasis following vascular injury by limiting thrombosis [39]. In a strain of transgenic mice (Tg-rPF4/βAPP) that over-express the APP/PN2, the thrombotic-capacity is diminished and the extent of cerebral hemorrhage is increased accordingly [39].
Activated platelets participate in the pathogenesis and evolution of atherosclerosis due to a strong contribution in the development of inflammatory responses in leukocytes, endothelial cells and vascular myocytes [40-42]. The prothrombotic interactions between platelets and vascular wall cells contribute to the establishment of the atheromatous lesions from their early stages to the final plaque rupture and thrombosis (reviewed in [43]). The elegant immunocytochemical and electron microscopic studies of De Meyer and colleagues clearly demonstrated the presence of APP and Aβ within platelets that were phagocytized by macrophages in areas of neovascularization in advanced atherosclerotic lesions [34]. The biochemical characterization of Aβ peptides presented in this study is complementary to these meticulous histological investigations. Although platelets and circulating Aβ may be a significant source of these peptides in the atherosclerotic lesions, it is possible that the Aβ peptides also originate, under pathological conditions, from the arterial tunica media myocytes and endothelial cells that also express APP [44].
In the present investigation we quantified, chromatographically isolated and characterized by mass spectrometry the Aβ peptides present in the walls of human atherosclerotic aortas. We also investigated the chemical characteristics of the Aβ peptides by mass spectrometry in freshly isolated inactivated and activated platelets as a possible source of the Aβ found in atherosclerotic lesions. In addition, we discuss the possible source of atherosclerotic Aβ as well as the potential relevance of Aβ pro-inflammatory activity to vascular pathology in the context of both AVD and AD.
2. Materials and Methods
2.1 Thoracic aortas
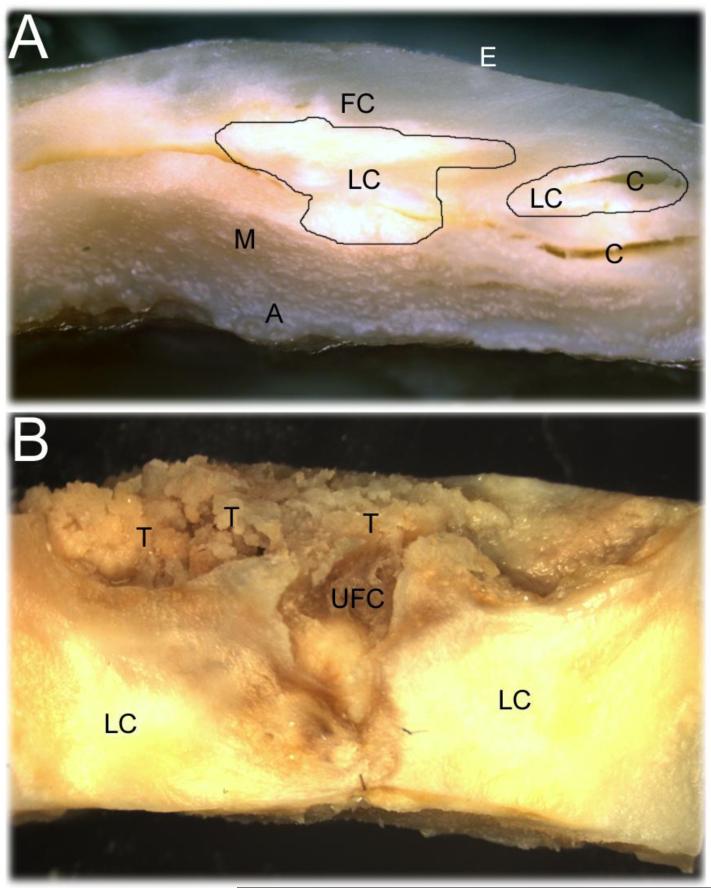
Thoracic aortic sections (2.5 g each) exhibiting severe atherosclerotic lesions were obtained in the immediate postmortem from 6 Caucasian individuals with a mean age of 84 years. There were 3 female and 3 male subjects, 4 of whom were demented with AD and one with Parkinsonian dementia. One subject, a 92 year-old male, was non-demented. The aortas exhibited severe regions of calcification complicated by ulcerations, intramural hemorrhages, rupture of fibrous caps and thrombosis as shown in Figure 1.
Figure 1.
Representative human aorta atherosclerotic lesions. A) An example of the advanced aortic atherosclerotic lesions used in this study demonstrating lipid cores with areas of calcification underlying the fibrous cap. B) Complicated atherosclerotic lesions with ulceration and rupture of the fibrous caps showing areas of thrombosis. E = endothelium; FC = fibrous cap; LC = lipid cores; C = areas of calcification; M = tunica media; A = adventitia; T = thrombosis; UFC = ulcerated (crater-like) fibrous cap.
2.2 Extraction of Aβ peptides from aortas
The aortic sections were thoroughly rinsed with cold deionized water to remove all traces of blood, and the adventitial connective tissue was detached and discarded. The intima/media of aortic sections were pooled and finely minced and ground in liquid nitrogen. Half of the pulverized tissue was placed in a beaker with 20 ml of 98% glass-distilled formic acid (GDFA), stirred overnight at 4°C and centrifuged at 250,000 × g for 1 h at 4°C. The acid-soluble phase between the floating fatty layer and the bottom pellet was collected and remaining solids were removed by gravimetric filtration using Whatman # 1 filter paper. The filtrate was centrifuged a second time and the acid-soluble fraction divided into 500 μl aliquots which were submitted to fast protein liquid chromatography (FPLC). The second half of the pulverized aortic tissue was suspended in 400 ml of 0.1 M ammonium bicarbonate, 0.01% sodium azide and stirred overnight at 4°C. The suspension was centrifuged at 30,000 × g for 1 h at 4°C. The supernatant (water-soluble fraction) was filtered through a 45 m nylon mesh, freeze-dried and lyophilized. Fifty mg aliquots of the lyophilized water-soluble fraction were then dissolved in 500 μl of 80% GDFA and submitted to FPLC.
2.3 Chromatographic separation of total aortic Aβ peptides
Both acid-soluble fractions and water-soluble fractions were separated by size-exclusion FPLC on a pre-calibrated 1 cm × 30 cm Superose 12 column (Pharmacia-Amersham-GE, Uppsala, Sweden). The column was equilibrated and developed with 80% GDFA at a flow rate of 15 ml/h at room temperature (RT) and absorbance monitored at 280 nm. The fractions containing 2-10 kDa molecules were collected and the volume reduced by vacuum centrifugation (Savant, Farmingdale, NY). To enrich the Aβ peptides and eliminate most of the flanking contaminations, 3 runs of the 2-10 kDa chromatographic eluants were pooled and re-chromatographed under the same conditions as described above.
To further purify the Aβ peptides, both the acid-soluble and water-soluble fractions obtained by size-exclusion FPLC were reduced to 100 μl and submitted to high performance liquid chromatography (HPLC, Thermo Separation Products, Waltham, MA) on a Synchropak GPC-peptide size-exclusion 7.8 cm × 90 cm column (Synchrom Inc. Lafayette, IN). The chromatographies were conducted at RT using 80% GDFA at a flow rate of 0.2 ml/min and the eluate absorbance was monitored at 280 nm. Western blots, using antibodies against Aβ40 and Aβ42, were employed to localize the Aβ peptide-containing fractions. The Aβ fraction from three column runs was pooled, the volume reduced to 500 μl and injected into a HPLC reverse-phase C8 semi-preparative 9.4 cm × 25 cm column (Zorbax 300 SB/5 μm beads; Wilmington, DE). The chromatography was developed using an elution gradient from 20% to 60% acetonitrile in water containing 0.1% trifluoroacetic acid (TFA) with a flow rate of 1.5 ml/min, at 80°C. Eluate absorbance was monitored at 214 nm. For identification of the Aβ peptide-containing fractions, the specimens were reduced in volume and submitted to Western blot analysis.
2.4 Analysis of Aβ peptides from inactivated and activated human platelets
Fresh platelets were obtained from 5 separate American Red Cross volunteer donors by platelet-pheresis and immediately processed following the procedures published by Li et al. [33,45] with modifications. A complete description of the technical details has been previously published by our group [46]. Both inactivated and thrombin-collagen activated platelets were submitted to FPLC (Superose 12), HPLC (Synchropak GPC-peptide) and reverse-phase HPLC (Zorbax 300 SB) as described above for the aortic tissue.
2.5 Western blots analysis of Aβ peptides
Samples from the HPLC, representing the total fractionation runs, were assessed for the presence of Aβ40 and Aβ42 peptides (Invitrogen, Carlsbad CA) using Western blots as described before [47].
2.6 Identification of Aβ peptides by MALDI-TOF mass spectrometry
A matrix assisted laser desorption ionization-time of flight (MALDI-TOF) Voyager DE STR mass spectrometer (Applied Biosystems, Foster City, CA) was employed to obtain data from the HPLC fractions. The applied Biosystems CalMix-2 was used as calibration mixture. The detailed protocol and composition of the calibration markers was previously published [48].
3. Results
3.1 Atherosclerotic aorta analyses
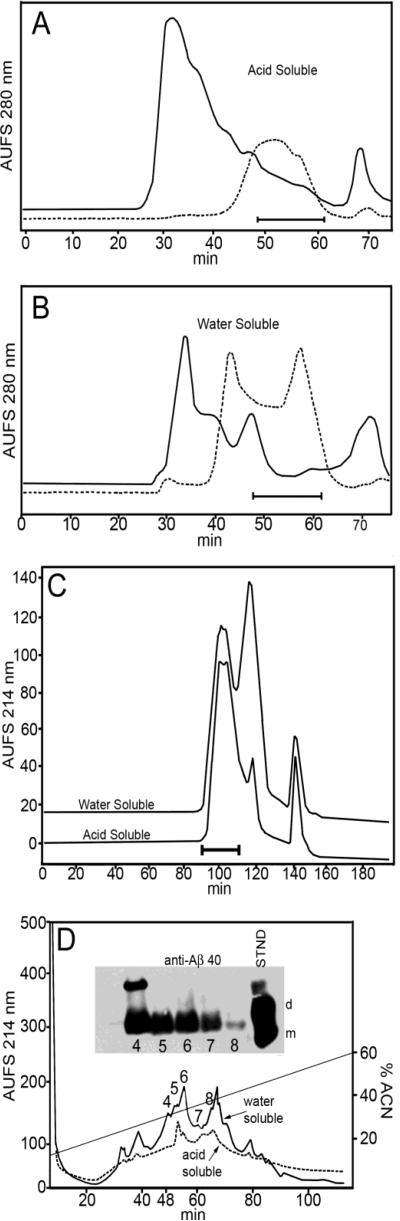
Our specific experimental goal was to establish the chemical characteristics of Aβ peptides in atherosclerotic aortic walls and in inactivated and activated platelets. Aortic vessels of elderly individuals with severe atherosclerotic lesions showed that the Aβ40 peptide was on the average 100 times more abundant than Aβ42: 75 ng/g of tissue and 0.7 ng/g of tissue, respectively [46]. MALDI-TOF mass spectrometric analysis of both the water-soluble and acid-soluble aortic fractions, that were submitted to sequential steps of FPLC and HPLC reverse-phase column separations (Figure 2A-D), revealed the presence of Aβ peptides with N-termini beginning at residues 1 through 5 and with C-termini ending at residues 39, 40, 41, 42 and 43 (Table 1). In addition, elongated Aβ peptides, extending from residues 1 to 48 (Aβ sequence numbering), was detected in peak 7 of the HPLC C3 reverse-phase column water-soluble fraction (Figure 2D). This peptide could result from γ-secretase cleavage close to APP ε-site, which corresponds in the Aβ sequence to residue 49. Western blot analyses of the reverse-phase column fractions did not show the presence of significant amounts of Aβ42 possibly due to the comparatively low values of this peptide as detected by immunoassay.
Figure 2.
Chromatographic profiles of aortic atherosclerotic wall. A) FPLC Superose 12 size-exclusion profile (solid line trace) of the whole aorta pulverized in liquid nitrogen and extracted with ammonium bicarbonate. The specimens containing the 2-10 kDa molecules (48-62 min, solid bar) were collected and re-chromatographed under the same conditions to enrich the Aβ-containing fractions (hyphened trace). B) FPLC Superose 12 size-exclusion chromatographic traces of the whole aorta pulverized in liquid nitrogen and extracted with GDFA. The continuous line represents the profile of the initial GDFA-soluble fraction. The eluants, collected from 2 of these chromatographies containing the 2-10 kDa, were pooled, concentrated and rechromatographed under the same conditions (hyphened line trace). C) Size-exclusion HPLC (GPC-Protein column) profiles of both water and GDFA fractions collected in A and B, respectively. Western blot (data not shown) demonstrated the Aβ peptides were contained in the first peak (indicated by bar). D) Reverse-phase HPLC profiles of the Aβ-containing peaks shown in C. The fractions containing the Aβ peptides were detected by Western blot (insert). Only the Aβ peptides ending at residue 40 were detected in both the water-soluble and acid-soluble fractions. The standards correspond to monomeric (m) and dimeric (d) Aβ1-40.
Table 1.
Mass spectrometric analyses of the water-soluble and formic acid-soluble aortic Aβ peptides.
| Aorta Acid Soluble | ||||
|---|---|---|---|---|
| Peak | Mass Observed | Mass Calculated | Peptide Fragment | Modification |
| 5 | 4287.89 | 4287.77 | 1-39 | 2 F |
| 5 | 4330.24 | 4330.91 | 1-40 | – |
| 5 | 4399.66 | 4400.06 | 2-42 | – |
| 5 | 4529.57 | 4529.17 | 2-43 | F |
| 6 | 4514.41 | 4515.15 | 1-42 | – |
| 6 | 3981.14 | 3981.6 | 5-41 | – |
| Aorta Water Soluble | ||||
|---|---|---|---|---|
| Peak | Mass Observed | Mass Calculated | Peptide Fragment | Modification |
| 4 | 4259.37 | 4259.77 | 1-39 | F |
| 4 | 4374.36 | 4374.91 | 1-40 | F+O |
| 4 | 4487.59 | 4488.07 | 1-41 | F+O |
| 4 | 4259.37 | 4259.82 | 2-40 | F+O |
| 5 | 4531.37 | 4531.14 | 1-42 | O |
| 5 | 4547.38 | 4547.15 | 1-42 | 2 O |
| 5 | 4643.42 | 4644.26 | 1-43 | F |
| 5 | 4243.06 | 4243.82 | 2-40 | F |
| 5 | 4516.30 | 4517.17 | 2-43 | O |
| 5 | 4243.06 | 4243.86 | 4-42 | F+O |
| 5 | 4332.14 | 4332.97 | 4-43 | 2 O |
| 6 | 4444.67 | 4444.07 | 1-41 | – |
| 6 | 4516.29 | 4515.15 | 1-42 | – |
| 6 | 4356.53 | 4356.98 | 2-41 | F |
| 6 | 4444.67 | 4444.06 | 2-42 | F+O |
| 6 | 4311.59 | 4310.98 | 3-42 | PG |
| 6 | 4356.53 | 4356.98 | 3-42 | F |
| 6 | 4356.53 | 4356.97 | 4-43 | 2 F |
| 7 | 4516.23 | 4515.15 | 1-42 | – |
| 7 | 5158.29 | 5157.96 | 1-48 | O |
The Mr deviation between the calculated and observed Aβ related peptides in all instances is less than ± 1 Dalton. Post-translational modifications: O = oxidation; F = formylation; PG = pyroglutamyl
3.2 Platelet analyses
Immunoassay analysis revealed that the inactivated platelets had an average of 84 ng/g tissue of Aβ40 and activated platelets contained an average of 57 ng/g tissue, while in both cases there were very low levels Aβ42: 1.6 ng/g tissue and 1.7 ng/g tissue, respectively [46]. The Aβ42 in the platelets, whether activated or inactivated, represents only 2-3% of the Aβ40 values, closely mimicking the values observed for the atherosclerotic aorta [46]. In agreement with these low Aβ42 values, we did not observe Aβ42 in the chromatographic fractions of the aorta (Figure 2D) or inactivated platelets by Western blotting (Figure 3A), but this peptide was found in the activated platelets (Figure 3B). Using size-exclusion FPLC and reverse-phase HPLC, the inactivated (Figure 3A) and (Figure 3B) activated platelet Aβ peptides were independently enriched and subsequently characterized by MALDI-TOF mass spectrometry (Table 2). Mass spectrometry of inactivated platelets chromatographic fractions confirmed the absence of Aβ42, while in the activated platelets, this peptide species was present, theoretically corresponding to peptide Aβ6-42 (Table 2). Intriguingly, the total Aβ value in the inactivated platelets is 30% higher than in the activated platelets [46]. A possible explanation is that aggregated Aβ becomes undetectable by the immunoassay or alternatively that upon degranulation of platelets, the Aβ was immunologically hindered by interaction with platelet-released fibrinogen [26]. Alternatively, any excess of free Aβ in the circulation will be complexed with albumin for which Aβ has a very high affinity [49-51]. Mass spectrometry showed that activated platelet Aβ peptides included species starting at residues 1, 2, 3, 4, 5 and 6, with C-termini at residues 37 through 42 (Table 2). The degraded N-terminal Aβ sequences in platelets and in atherosclerotic vessels are very similar to those observed in the brains of AD subjects [52,53].
Figure 3.
Reverse-phase HPLC profiles and Aβ Western blots of human platelets. A) Resting platelets were lysed in GDFA and the acid-soluble fraction submitted to Superose 12 FPLC and Synchropak-GPC peptide HPLC fractionation. The fractions containing the Aβ peptides were further purified by C8 reverse-phase. Fractions 11-14 contained Aβ ending in residue 40. B) Amyloid-β peptides in activated platelets (see Methods section) were chromatographically enriched as described in A. The trace shows the HPLC C8 reverse-phase elution profile. Fractions 9-12 contain the Aβ peptides ending in residue 40 (see insert). Aβ42 was also observed by Western blot in peak 8 and confirmed by mass spectrometry. In both A and B, the standards correspond to monomeric (m) and dimeric (d) Aβ1-40/42 and the diagonal line indicates the acetonitrile gradient concentration.
Table 2.
Mass spectrometric analyses of the water-soluble and formic acid-soluble activated and inactivated platelets.
| Inactivated Platelets | ||||
|---|---|---|---|---|
| Peak | Mass Observed | Mass Calculated | Peptide Fragment | Modification |
| 11 | 3915.98 | 3915.43 | 4-39 | – |
| 12 | 3943.05 | 3943.43 | 4-39 | F |
| 12 | 4030.02 | 4030.57 | 4-40 | O |
| 12 | 3882.05 | 3883.39 | 5-40 | O |
| 13 | 4043.96 | 4044.5 | 2-38 | F |
| 13 | 4043.96 | 4044.55 | 3-39 | – |
| 14 | 4028.95 | 4030.57 | 4-40 | O |
| 15 | 4070.55 | 4070.57 | 4-40 | 2 F |
| 15 | 3925.36 | 3923.39 | 5-40 | 2 F |
| Activated Platelets | ||||
|---|---|---|---|---|
| Peak | Mass Observed | Mass Calculated | Peptide Fragment | Modification |
| 8 | 4498.48 | 4499.01 | 1-41 | 2 F |
| 8 | 4070.45 | 4070.57 | 4-40 | 2 F |
| 8 | 3895.73 | 3895.39 | 5-40 | F |
| 8 | 3895.73 | 3895.44 | 6-42 | – |
| 9 | 4269.94 | 4270.76 | 2-40 | 2 F |
| 9 | 3976.77 | 3977.42 | 3-38 | O |
| 9 | 4099.60 | 4100.55 | 3-39 | 2 F |
| 9 | 4071.24 | 4070.57 | 4-40 | 2 F |
| 9 | 3883.26 | 3883.39 | 5-40 | O |
| 10 | 4016.56 | 4016.50 | 2-38 | – |
| 10 | 4072.13 | 4072.55 | 3-39 | F |
| 10 | 3883.69 | 3883.39 | 5-40 | O |
| 11 | 4072.07 | 4072.50 | 2-38 | 2 F |
| 11 | 4072.07 | 4072.55 | 3-39 | F |
| 11 | 3883.96 | 3883.39 | 5-40 | O |
| 12 | 3975.80 | 3975.44 | 2-37 | O |
| 12 | 4071.03 | 4070.57 | 4-40 | 2 F |
| 12 | 3882.80 | 3883.39 | 5-40 | O |
The Mr deviation between the calculated and observed Aβ related peptides in all instances is less than ± 1 Dalton. Post-translational modifications: O = oxidation; F = formylation.
4. Discussion
The atherosclerotic aortas contained Aβ peptides composed primarily of the Aβ40 species [46]. The Aβ peptides may have been produced at the site of the lesions by myocytes, endothelial cells [54,55] or platelets [33,34]. The preponderance of Aβ40 species in the vascular lesions may simply reflect the greater solubility and mobility of this peptide. Immunoassay studies demonstrate that significant levels of Aβ42 are found in plasma [46,49], while in the aorta this peptide species is less represented [46], suggesting that the plasma Aβ42 is not the dominant contributor to atherosclerotic plaques. Little is known about the catabolic fate of the Aβ peptides generated in skeletal muscle, a tissue composing about one third of the total body mass. The post-synaptic domain of the skeletal muscle cholinergic neuromuscular junctions contains APP and Aβ peptides [56]. The Aβ peptides may be degraded mainly in the muscle itself, but it is possible that some of the intact Aβ peptides are cleared into the circulation. The skeletal muscles contain a higher ratio of Aβ42 [57] and analogous proportions of this molecule are not observed in aortic walls. Based on the tissue ratios of Aβ40:Aβ42, the experimental observations suggest that the Aβ contributions by plasma, brain or muscle to the atherosclerotic lesions may be minimal. Measurements of Aβ40 and Aβ42 in activated and inactivated platelets revealed that Aβ40 was the dominant peptide, similar to the values observed for the atherosclerotic aorta [46]. Therefore, judging from the relative amounts of Aβ40 and Aβ42 it could be suggested that the Aβ peptides present in the atherosclerotic lesions mainly originate from activated platelets.
The α-granules of platelets are considered the major source of circulating APP/PN2 and of Aβ [33,45]. The relentless vascular wall damage that accompanies atherosclerosis and concomitant reduced ability to repair are phenomena inherent to aging-associated vascular deterioration. These events favor increased platelet activation that is mostly mediated by local secretion of tissue factor VIIa and exposure to the von Willebrand factor [58,59]. Thus, platelets carrying APP/PN-2 are abundantly available in areas of vascular injury as they participate in the coagulation cascade. In the atherosclerotic lesions, activated macrophages contain endocytic vesicles loaded with platelets and Aβ peptides [34]. A body of evidence reveals a pathological correlation between AVD, AD and CAA [3,5,13,14,60] probably mediated by sustained arterial wall injury that elicits, in all of these conditions, chronic vascular and brain inflammation. In addition to the putative platelet-atherosclerotic-linked Aβ source, the Aβ peptides in the atherosclerotic plaques may also be directly derived from the arterial walls proper, since vascular cells, like platelets, express the APP/PN2 with a KPI domain [61].
The presence of Aβ peptides in atherosclerotic plaques may synergistically increase the chronic inflammatory processes that sustain the degeneration and destruction of the arterial walls. Addition of human platelets to human and rodent macrophages in culture results in phagocytosis with induction of iNOS and foam cell formation [34]. Furthermore, in the atherosclerotic lesions, macrophage activation is triggered by, among other eliciting mechanisms, Aβ peptides that stimulate the production of iNOS and COX-2 [34]. A focal vascular inflammatory response in the vulnerable region of atheroma plaques may contribute to plaque instability and rupture [34]. A potent pro-inflammatory capacity of the Aβ peptides has been mechanistically demonstrated. The amino acid sequence of Aβ His-His-Gln-Lys, corresponding to residues 13-16, binds to heparan sulfate proteoglycans on the surface of macrophage microglia in the brain, inducing these cells to kill neurons [24,25]. In in vivo competition experiments, administration of this synthetic peptide suppressed Aβ-induced inflammation in the rat cerebral cortex [25]. Likewise, suppression of microglial synthesis of glycosaminoglycans or degradation of glycosaminoglycans also blocked activation of microglia and prevented neuronal demise [25]. We have previously shown that Aβ elicits the differentiation of monocytes into macrophages and induces the secretion of inflammation-related cytokines and chemokines [62]. Moreover, in a model of the BBB when Aβ is present on the brain side, it promotes monocyte/macrophage migration across the blood-brain barrier [62]. Vascular inflammation in AVD has traditionally been thought to be limited to large and medium-sized arteries, but recent work indicates that the systemic microvasculature is also affected [63-67] probably due to increased circulating concentrations of inflammatory mediators. In elderly humans, elevated serum C-reactive protein concentrations correlate with vascular inflammation and atherosclerosis, and with markers of brain microvascular inflammation in AD [68,69].
Hemostasis abnormalities such as increased activated factor VIIa and von Willebrand factor have been reported in AD [59]. Elevated platelet membrane fluidity [70], disturbances in protein kinase C [71] and in phospholipases C [72], and abnormal platelet activation [58] as well as increased serum levels of thrombomodulin and E-selectin [73] have also been observed in AD. In addition to the macrophage-mediated vascular inflammatory reactions, a second mechanism appears to link hemostasis with cerebrovascular amyloidosis. With advancing age the incidence of endothelial injury increases and the ability to repair decreases. This relentless emerging situation ultimately enhances APP/PN-2 production in platelets and vascular walls thereby contributing to a local reduction of hemostatic activity that favors production of cerebrovascular microhemorrhages [74,75].
5. Conclusions
Atherosclerotic vascular disease and AD cerebrovascular amyloidosis, despite their different end-stage manifestations, share pathophysiological pathways. Our studies demonstrate that the atherosclerotic lesions, common in elderly individuals, contain a heterogeneous mixture of Aβ peptides. Immunoassays suggest a preponderance of the shorter Aβ40 over Aβ42. Most likely the sources of these Aβ peptides are the cells of the vascular walls that express APP/PN2/Aβ and the platelets that participate in the atherosclerotic inflammatory and disturbed coagulation cascades inherent to chronic arterial wall degeneration. The increased and persistent synthesis of proinflammatory-related molecules by activated macrophages/microglia and activated platelets are, in part, mediated by the presence of Aβ peptides. In both AVD and AD, the Aβ peptides appear to contribute to the inflammatory reactions that ultimately culminate in vascular wall destruction.
Highlights.
Atherosclerotic aortas contained Aβ peptides primarily made of Aβ40 peptide
Activated and inactivated platelets revealed Aβ40 was the dominant peptide
Aβ peptides in atherosclerotic lesions mainly originate from activated platelets
The likely source of Aβ are vascular wall cells expressing APP/PN2/Aβ and platelets
In AVD and AD, Aβ contributes to inflammatory destruction of the vascular wall
Acknowledgements
This study was supported by: The National Institute on Aging R01 AG019795, P30 AG19610 Arizona Alzheimer's Disease Core Center, the State of Arizona Alzheimer's Disease Research Consortium, the Arizona Department of Health Services (contract 211002, Arizona Alzheimer's Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson's Disease Consortium) and the Michael J. Fox Foundation for Parkinson's Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Aging or the National Institutes of Health.
Abbreviations
- Aβ
amyloid-beta
- AD
Alzheimer's disease
- ApoE
apolipoprotein E
- APP
amyloid precursor protein
- AVD
atherosclerotic vascular disease
- BBB
blood brain barrier
- CAA
cerebral amyloid angiopathy
- FPLC
fast protein liquid chromatography
- GDFA
glass-distilled formic acid
- HPLC
high performance liquid chromatography
- KPI
Kunitz (serine) protease inhibitory
- MALDI-TOF
Matrix-assisted laser desorption/ionization time-of-flight
- PN2
protease nexin-2
- RT
room temperature
- TFA
trifluoroacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures:
The authors stipulate that they have no competing interests.
Reference List
- 1.Roher AE, Tyas SL, Maarouf CL, Daugs ID, Kokjohn TA, Emmerling MR, Garami Z, Belohlavek M, Sabbagh MN, Sue LI, Beach TG. Intracranial atherosclerosis as a contributing factor to Alzheimer's disease dementia, Alzheimers. Dement. 2011 doi: 10.1016/j.jalz.2010.08.228. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beach TG, Wilson JR, Sue LI, Newell A, Poston M, Cisneros R, Pandya Y, Esh C, Connor DJ, Sabbagh M, Walker DG, Roher AE. Circle of Willis atherosclerosis: association with Alzheimer's disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol. 2007;113:13. doi: 10.1007/s00401-006-0136-y. [DOI] [PubMed] [Google Scholar]
- 3.Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US National Alzheimer's Coordinating Center. Neurology. 2005;64:494. doi: 10.1212/01.WNL.0000150886.50187.30. [DOI] [PubMed] [Google Scholar]
- 4.Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of aging cohort. Ann. Neurol. 2010;68:231. doi: 10.1002/ana.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, Sue LI, Beach TG. Circle of Willis atherosclerosis is a risk factor for sporadic Alzheimer's disease. Arterioscler. Thromb. Vasc. Biol. 2003;23:2055. doi: 10.1161/01.ATV.0000095973.42032.44. [DOI] [PubMed] [Google Scholar]
- 6.Wolozin B, Bednar MM. Interventions for heart disease and their effects on Alzheimer's disease. Neurol. Res. 2006;28:630. doi: 10.1179/016164106X130515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksson UK, Bennet AM, Gatz M, Dickman PW, Pedersen NL. Nonstroke cardiovascular disease and risk of Alzheimer disease and dementia. Alzheimer Dis. Assoc. Disord. 2010;24:213. doi: 10.1097/WAD.0b013e3181d1b99b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann. Neurol. 1997;42:776. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 9.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322:1447. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Notkola IL, Sulkava R, Pekkanen J, Erkinjuntti T, Ehnholm C, Kivinen P, Tuomilehto J, Nissinen A. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer's disease. Neuroepidemiology. 1998;17:14. doi: 10.1159/000026149. [DOI] [PubMed] [Google Scholar]
- 11.Golde TE, Eckman CB. Cholesterol modulation as an emerging strategy for the treatment of Alzheimer's disease. Drug Discov. Today. 2001;6:1049. doi: 10.1016/s1359-6446(01)01965-1. [DOI] [PubMed] [Google Scholar]
- 12.Simons M, Keller P, Dichgans J, Schulz JB. Cholesterol and Alzheimer's disease: is there a link? Neurology. 2001;57:1089. doi: 10.1212/wnl.57.6.1089. [DOI] [PubMed] [Google Scholar]
- 13.Kalback W, Esh C, Castano EM, Rahman A, Kokjohn T, Luehrs DC, Sue L, Cisneros R, Gerber F, Richardson C, Bohrmann B, Walker DG, Beach TG, Roher AE. Atherosclerosis, vascular amyloidosis and brain hypoperfusion in the pathogenesis of sporadic Alzheimer's disease. Neurol. Res. 2004;26:525. doi: 10.1179/016164104225017668. [DOI] [PubMed] [Google Scholar]
- 14.Ferreiro JA, Ansbacher LE, Vinters HV. Stroke related to cerebral amyloid angiopathy: the significance of systemic vascular disease. J. Neurol. 1989;236:267. doi: 10.1007/BF00314454. [DOI] [PubMed] [Google Scholar]
- 15.Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, Casadei V, Grimaldi LM. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer's disease: peripheral inflammation or signals from the brain? J. Neuroimmunol. 2000;103:97. doi: 10.1016/s0165-5728(99)00226-x. [DOI] [PubMed] [Google Scholar]
- 16.Singh VK, Guthikonda P. Circulating cytokines in Alzheimer's disease. J. Psychiatr. Res. 1997;31:657. doi: 10.1016/s0022-3956(97)00023-x. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann. Neurol. 2002;52:168. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 18.Zuliani G, Ranzini M, Guerra G, Rossi L, Munari MR, Zurlo A, Volpato S, Atti AR, Ble A, Fellin R. Plasma cytokines profile in older subjects with late onset Alzheimer's disease or vascular dementia. J. Psychiatr. Res. 2007;41:686. doi: 10.1016/j.jpsychires.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 19.De Luigi A, Pizzimenti S, Quadri P, Lucca U, Tettamanti M, Fragiacomo C, De Simoni MG. Peripheral inflammatory response in Alzheimer's disease and multiinfarct dementia. Neurobiol. Dis. 2002;11:308. doi: 10.1006/nbdi.2002.0556. [DOI] [PubMed] [Google Scholar]
- 20.Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer's disease. Neurobiol. Aging. 2001;22:837. doi: 10.1016/s0197-4580(01)00276-7. [DOI] [PubMed] [Google Scholar]
- 21.Rozemuller AJ, van Gool WA, Eikelenboom P. The neuroinflammatory response in plaques and amyloid angiopathy in Alzheimer's disease: therapeutic implications. Curr. Drug Targets. CNS. Neurol. Disord. 2005;4:223. doi: 10.2174/1568007054038229. [DOI] [PubMed] [Google Scholar]
- 22.Townsend KP, Obregon D, Quadros A, Patel N, Volmar C, Paris D, Mullan M. Proinflammatory and vasoactive effects of Abeta in the cerebrovasculature. Ann. N. Y. Acad. Sci. 2002;977:65. doi: 10.1111/j.1749-6632.2002.tb04799.x. [DOI] [PubMed] [Google Scholar]
- 23.Suo Z, Tan J, Placzek A, Crawford F, Fang C, Mullan M. Alzheimer's beta-amyloid peptides induce inflammatory cascade in human vascular cells: the roles of cytokines and CD40. Brain Res. 1998;807:110. doi: 10.1016/s0006-8993(98)00780-x. [DOI] [PubMed] [Google Scholar]
- 24.Giulian D, Haverkamp LJ, Yu JH, Karshin W, Tom D, Li J, Kirkpatrick J, Kuo YM, Roher AE. Specific domains of beta-amyloid from Alzheimer plaque elicit neuron killing in human microglia. J. Neurosci. 1996;16:6021. doi: 10.1523/JNEUROSCI.16-19-06021.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giulian D, Haverkamp LJ, Yu J, Karshin W, Tom D, Li J, Kazanskaia A, Kirkpatrick J, Roher AE. The HHQK domain of beta-amyloid provides a structural basis for the immunopathology of Alzheimer's disease. J. Biol. Chem. 1998;273:29719. doi: 10.1074/jbc.273.45.29719. [DOI] [PubMed] [Google Scholar]
- 26.Cortes-Canteli M, Paul J, Norris EH, Bronstein R, Ahn HJ, Zamolodchikov D, Bhuvanendran S, Fenz KM, Strickland S. Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer's disease. Neuron. 2010;66:695. doi: 10.1016/j.neuron.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thal DR, Ghebremedhin E, Orantes M, Wiestler OD. Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J. Neuropathol. Exp. Neurol. 2003;62:1287. doi: 10.1093/jnen/62.12.1287. [DOI] [PubMed] [Google Scholar]
- 28.Attems J, Lintner F, Jellinger KA. Amyloid beta peptide 1-42 highly correlates with capillary cerebral amyloid angiopathy and Alzheimer disease pathology. Acta Neuropathol. 2004;107:283. doi: 10.1007/s00401-004-0822-6. [DOI] [PubMed] [Google Scholar]
- 29.Kalaria RN. Small vessel disease and Alzheimer's dementia: pathological considerations. Cerebrovasc. Dis. 2002;13(Suppl 2):48. doi: 10.1159/000049150. [DOI] [PubMed] [Google Scholar]
- 30.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease. Acta Neuropathol. 2009;118:103. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Princz-Kranz FL, Mueggler T, Knobloch M, Nitsch RM, Rudin M. Vascular response to acetazolamide decreases as a function of age in the arcA beta mouse model of cerebral amyloidosis. Neurobiol. Dis. 2010;40:284. doi: 10.1016/j.nbd.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Askanas V, Engel WK, Alvarez RB. Light and electron microscopic localization of beta-amyloid protein in muscle biopsies of patients with inclusion-body myositis. Am. J. Pathol. 1992;141:31. [PMC free article] [PubMed] [Google Scholar]
- 33.Li QX, Whyte S, Tanner JE, Evin G, Beyreuther K, Masters CL. Secretion of Alzheimer's disease Abeta amyloid peptide by activated human platelets. Lab Invest. 1998;78:461. [PubMed] [Google Scholar]
- 34.De Meyer GR, De Cleen DM, Cooper S, Knaapen MW, Jans DM, Martinet W, Herman AG, Bult H, Kockx MM. Platelet phagocytosis and processing of beta-amyloid precursor protein as a mechanism of macrophage activation in atherosclerosis. Circ. Res. 2002;90:1197. doi: 10.1161/01.res.0000020017.84398.61. [DOI] [PubMed] [Google Scholar]
- 35.Van Nostrand WE, Wagner SL, Farrow JS, Cunningham DD. Immunopurification and protease inhibitory properties of protease nexin-2/amyloid beta-protein precursor. J. Biol. Chem. 1990;265:9591. [PubMed] [Google Scholar]
- 36.Schmaier AH, Dahl LD, Rozemuller AJ, Roos RA, Wagner SL, Chung R, Van Nostrand WE. Protease nexin-2/amyloid beta protein precursor. A tight-binding inhibitor of coagulation factor IXa. J. Clin. Invest. 1993;92:2540. doi: 10.1172/JCI116863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahdi F, Van Nostrand WE, Schmaier AH. Protease nexin-2/amyloid beta-protein precursor inhibits factor Xa in the prothrombinase complex. J. Biol. Chem. 1995;270:23468. doi: 10.1074/jbc.270.40.23468. [DOI] [PubMed] [Google Scholar]
- 38.Mahdi F, Rehemtulla A, Van Nostrand WE, Bajaj SP, Schmaier AH. Protease nexin-2/Amyloid beta-protein precursor regulates factor VIIa and the factor VIIa-tissue factor complex. Thromb. Res. 2000;99:267. doi: 10.1016/s0049-3848(00)00245-0. [DOI] [PubMed] [Google Scholar]
- 39.Xu F, Davis J, Miao J, Previti ML, Romanov G, Ziegler K, Van Nostrand WE. Protease nexin-2/amyloid beta-protein precursor limits cerebral thrombosis. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18135. doi: 10.1073/pnas.0507798102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Totani L, Evangelista V. Platelet-leukocyte interactions in cardiovascular disease and beyond. Arterioscler. Thromb. Vasc. Biol. 2010;30:2357. doi: 10.1161/ATVBAHA.110.207480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linden MD, Jackson DE. Platelets: pleiotropic roles in atherogenesis and atherothrombosis. Int. J. Biochem. Cell Biol. 2010;42:1762. doi: 10.1016/j.biocel.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Vasina E, Heemskerk JW, Weber C, Koenen RR. Platelets and platelet-derived microparticles in vascular inflammatory disease. Inflamm. Allergy Drug Targets. 2010;9:346. doi: 10.2174/187152810793938008. [DOI] [PubMed] [Google Scholar]
- 43.Aukrust P, Halvorsen B, Ueland T, Michelsen AE, Skjelland M, Gullestad L, Yndestad A, Otterdal K. Activated platelets and atherosclerosis. Expert. Rev. Cardiovasc. Ther. 2010;8:1297. doi: 10.1586/erc.10.92. [DOI] [PubMed] [Google Scholar]
- 44.Wisniewski HM, Frackowiak J, Mazur-Kolecka B. In vitro production of beta-amyloid in smooth muscle cells isolated from amyloid angiopathy-affected vessels. Neurosci. Lett. 1995;183:120. doi: 10.1016/0304-3940(94)11129-7. [DOI] [PubMed] [Google Scholar]
- 45.Li QX, Evin G, Small DH, Multhaup G, Beyreuther K, Masters CL. Proteolytic processing of Alzheimer's disease beta A4 amyloid precursor protein in human platelets. J. Biol. Chem. 1995;270:14140. doi: 10.1074/jbc.270.23.14140. [DOI] [PubMed] [Google Scholar]
- 46.Roher AE, Esh CL, Kokjohn TA, Castano EM, Van Vickle GD, Kalback WM, Patton RL, Luehrs DC, Daugs ID, Kuo YM, Emmerling MR, Soares H, Quinn JF, Kaye J, Connor DJ, Silverberg NB, Adler CH, Seward JD, Beach TG, Sabbagh MN. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer's disease. Alzheimers. Dement. 2009;5:18. doi: 10.1016/j.jalz.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Vickle GD, Esh CL, Daugs ID, Kokjohn TA, Kalback WM, Patton RL, Luehrs DC, Walker DG, Lue LF, Beach TG, Davis J, Van Nostrand WE, Castano EM, Roher AE. Tg-SwDI transgenic mice exhibit novel alterations in AbetaPP processing, Abeta degradation, and resilient amyloid angiopathy. Am. J. Pathol. 2008;173:483. doi: 10.2353/ajpath.2008.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esh C, Patton L, Kalback W, Kokjohn TA, Lopez J, Brune D, Newell AJ, Beach T, Schenk D, Games D, Paul S, Bales K, Ghetti B, Castano EM, Roher AE. Altered APP processing in PDAPP (Val717 --> Phe) transgenic mice yields extended-length Abeta peptides. Biochemistry. 2005;44:13807. doi: 10.1021/bi051213+. [DOI] [PubMed] [Google Scholar]
- 49.Kuo YM, Kokjohn TA, Kalback W, Luehrs D, Galasko DR, Chevallier N, Koo EH, Emmerling MR, Roher AE. Amyloid-beta peptides interact with plasma proteins and erythrocytes: implications for their quantitation in plasma. Biochem. Biophys. Res. Commun. 2000;268:750. doi: 10.1006/bbrc.2000.2222. [DOI] [PubMed] [Google Scholar]
- 50.Bohrmann B, Tjernberg L, Kuner P, Poli S, Levet-Trafit B, Naslund J, Richards G, Huber W, Dobeli H, Nordstedt C. Endogenous proteins controlling amyloid beta-peptide polymerization. Possible implications for beta-amyloid formation in the central nervous system and in peripheral tissues. J. Biol. Chem. 1999;274:15990. doi: 10.1074/jbc.274.23.15990. [DOI] [PubMed] [Google Scholar]
- 51.Biere AL, Ostaszewski B, Stimson ER, Hyman BT, Maggio JE, Selkoe DJ. Amyloid beta-peptide is transported on lipoproteins and albumin in human plasma. J. Biol. Chem. 1996;271:32916. doi: 10.1074/jbc.271.51.32916. [DOI] [PubMed] [Google Scholar]
- 52.Roher AE, Lowenson JD, Clarke S, Wolkow C, Wang R, Cotter RJ, Reardon IM, Zurcher-Neely HA, Heinrikson RL, Ball MJ, Greenberg BD. Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer's disease. J. Biol. Chem. 1993;268:3072. [PubMed] [Google Scholar]
- 53.Kuo YM, Emmerling MR, Woods AS, Cotter RJ, Roher AE. Isolation, chemical characterization, and quantitation of A beta 3-pyroglutamyl peptide from neuritic plaques and vascular amyloid deposits. Biochem. Biophys. Res. Commun. 1997;237:188. doi: 10.1006/bbrc.1997.7083. [DOI] [PubMed] [Google Scholar]
- 54.Wisniewski HM, Wegiel J, Vorbrodt AW, Mazur-Kolecka B, Frackowiak J. Role of perivascular cells and myocytes in vascular amyloidosis. Ann. N. Y. Acad. Sci. 2000;903:6. doi: 10.1111/j.1749-6632.2000.tb06344.x. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi H, Yamazaki T, Kawarabayashi T, Sun X, Sakai Y, Hirai S. Localization of Alzheimer amyloid beta protein precursor and its relation to senile plaque amyloid. Gerontology. 1994;40(Suppl 2):65. doi: 10.1159/000213629. [DOI] [PubMed] [Google Scholar]
- 56.Askanas V, Engel WK, Alvarez RB. Strong immunoreactivity of beta-amyloid precursor protein, including the beta-amyloid protein sequence, at human neuromuscular junctions. Neurosci. Lett. 1992;143:96. doi: 10.1016/0304-3940(92)90241-x. [DOI] [PubMed] [Google Scholar]
- 57.Kuo YM, Kokjohn TA, Watson MD, Woods AS, Cotter RJ, Sue LI, Kalback WM, Emmerling MR, Beach TG, Roher AE. Elevated abeta42 in skeletal muscle of Alzheimer disease patients suggests peripheral alterations of AbetaPP metabolism. Am. J. Pathol. 2000;156:797. doi: 10.1016/s0002-9440(10)64947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sevush S, Jy W, Horstman LL, Mao WW, Kolodny L, Ahn YS. Platelet activation in Alzheimer disease. Arch. Neurol. 1998;55:530. doi: 10.1001/archneur.55.4.530. [DOI] [PubMed] [Google Scholar]
- 59.Mari D, Parnetti L, Coppola R, Bottasso B, Reboldi GP, Senin U, Mannucci PM. Hemostasis abnormalities in patients with vascular dementia and Alzheimer's disease. Thromb. Haemost. 1996;75:216. [PubMed] [Google Scholar]
- 60.Roher AE, Kokjohn TA, Beach TG. An association with great implications: vascular pathology and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2006;20:73. doi: 10.1097/01.wad.0000201855.39246.2d. [DOI] [PubMed] [Google Scholar]
- 61.Melchor JP, Van Nostrand WE. Fibrillar amyloid beta-protein mediates the pathologic accumulation of its secreted precursor in human cerebrovascular smooth muscle cells. J. Biol. Chem. 2000;275:9782. doi: 10.1074/jbc.275.13.9782. [DOI] [PubMed] [Google Scholar]
- 62.Fiala M, Zhang L, Gan X, Sherry B, Taub D, Graves MC, Hama S, Way D, Weinand M, Witte M, Lorton D, Kuo YM, Roher AE. Amyloid-beta induces chemokine secretion and monocyte migration across a human blood--brain barrier model. Mol. Med. 1998;4:480. [PMC free article] [PubMed] [Google Scholar]
- 63.Vogel RA. Cholesterol lowering and endothelial function. Am. J. Med. 1999;107:479. doi: 10.1016/s0002-9343(99)00261-2. [DOI] [PubMed] [Google Scholar]
- 64.John S, Jacobi J, Delles C, Schlaich MP, Alter O, Schmieder RE. Plasma soluble adhesion molecules and endothelium-dependent vasodilation in early human atherosclerosis. Clin. Sci. (Lond) 2000;98:521. [PubMed] [Google Scholar]
- 65.Stokes KY, Cooper D, Tailor A, Granger DN. Hypercholesterolemia promotes inflammation and microvascular dysfunction: role of nitric oxide and superoxide. Free Radic. Biol. Med. 2002;33:1026. doi: 10.1016/s0891-5849(02)01015-8. [DOI] [PubMed] [Google Scholar]
- 66.Fichtlscherer S, Rosenberger G, Walter DH, Breuer S, Dimmeler S, Zeiher AM. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102:1000. doi: 10.1161/01.cir.102.9.1000. [DOI] [PubMed] [Google Scholar]
- 67.Fichtlscherer S, Breuer S, Heeschen C, Dimmeler S, Zeiher AM. Interleukin-10 serum levels and systemic endothelial vasoreactivity in patients with coronary artery disease. J. Am. Coll. Cardiol. 2004;44:44. doi: 10.1016/j.jacc.2004.02.054. [DOI] [PubMed] [Google Scholar]
- 68.Uchikado H, Akiyama H, Kondo H, Ikeda K, Tsuchiya K, Kato M, Oda T, Togo T, Iseki E, Kosaka K. Activation of vascular endothelial cells and perivascular cells by systemic inflammation-an immunohistochemical study of postmortem human brain tissues. Acta Neuropathol. 2004;107:341. doi: 10.1007/s00401-003-0815-x. [DOI] [PubMed] [Google Scholar]
- 69.Genest J. C-reactive protein: risk factor, biomarker and/or therapeutic target? Can. J. Cardiol. 2010;26(Suppl A):41A. doi: 10.1016/s0828-282x(10)71061-8. [DOI] [PubMed] [Google Scholar]
- 70.Zubenko GS. Platelet membrane fluidity. Neurology. 1993;43:234. doi: 10.1212/wnl.43.1_part_1.234-a. [DOI] [PubMed] [Google Scholar]
- 71.Matsushima H, Shimohama S, Tanaka S, Taniguchi T, Hagiwara M, Hidaka H, Kimura J. Platelet protein kinase C levels in Alzheimer's disease. Neurobiol. Aging. 1994;15:671. doi: 10.1016/0197-4580(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 72.Matsushima H, Shimohama S, Fujimoto S, Takenawa T, Kimura J. Changes in platelet phospholipase C protein level and activity in Alzheimer's disease. Neurobiol. Aging. 1995;16:895. doi: 10.1016/0197-4580(95)02003-9. [DOI] [PubMed] [Google Scholar]
- 73.Borroni B, Akkawi N, Martini G, Colciaghi F, Prometti P, Rozzini L, Di LM, Lenzi GL, Romanelli G, Caimi L, Padovani A. Microvascular damage and platelet abnormalities in early Alzheimer's disease. J. Neurol. Sci. 2002;203-204:189. doi: 10.1016/s0022-510x(02)00289-7. [DOI] [PubMed] [Google Scholar]
- 74.Van Nostrand WE, Schmaier AH, Wagner SL. Potential role of protease nexin-2/amyloid beta-protein precursor as a cerebral anticoagulant. Ann. N. Y. Acad. Sci. 1992;674:243. doi: 10.1111/j.1749-6632.1992.tb27493.x. [DOI] [PubMed] [Google Scholar]
- 75.Van Nostrand WE, Schmaier AH, Farrow JS, Cunningham DD. Platelet protease nexin-2/amyloid beta-protein precursor. Possible pathologic and physiologic functions. Ann. N. Y. Acad. Sci. 1991;640:140. doi: 10.1111/j.1749-6632.1991.tb00205.x. [DOI] [PubMed] [Google Scholar]