Abstract
Background
Studies have demonstrated that IgE-binding cross-reactive epitopes between shrimp, cockroach and house dust mite tropomyosins can account for the presence of detectable IgE to shrimp in people who have cockroach and dust mite allergies.
Objective
We investigated the correlation between IgE-mediated sensitization to shrimp, cockroach, and dust mite in relation to allergen exposure in inner-city children.
Methods
Five hundred and four serum samples from the National Cooperative Inner City Asthma Study (NCICAS) were evaluated for specific IgE to shrimp and the results were compared to specific IgE to cockroach (Blattella germanica) and dust mite (Dermatophagoides farinae). Associations between IgE sensitization to these allergens and environmental exposures were determined.
Results
There was a strong positive correlation between shrimp, cockroach, and dust mite IgE levels. High exposure to cockroach (Bla g) in the home, particularly in the bedroom and television room, was significantly correlated with higher shrimp and cockroach IgE levels. In contrast, high exposure to dust mite in the home was highly correlated with IgE to D.farinae, but not with shrimp IgE levels. There is a synergistic relationship between cockroach IgE and exposure in predicting shrimp IgE levels.
Conclusions
For children with evidence of IgE-mediated sensitization to cockroach and shrimp, having high exposure to cockroach in the home can contribute to higher shrimp IgE levels, which may not correlate with clinical reactivity. Further patient evaluations with clinical histories of shrimp exposure and reactions as well as oral food challenges would have to be performed to confirm these findings.
Keywords: cockroach, dust mite, shrimp, tropomyosin, cross-reactivity
Introduction
Food allergy affects nearly 4% of the US population [1], and a recent study by Liu et al. [2] suggests that black male children have the highest rates of food allergy. Over 8,000 participants in the NHANES 2005–2006 had serum-specific IgE measured to egg, milk, peanut, and shrimp. The prevalence of sensitization to any of these foods was 16.8% and was significantly higher in children (23.2%), males (20.4%), non-Hispanic blacks (27.0%) and persons of lower income (19.0%). The largest disparity between estimated clinical allergy rates between races/ethnicities, high versus low poverty income ratio, and high versus low household education level was seen for shrimp allergy.
In the same study [2], the authors found that subjects with asthma had increased risk of all measures of food sensitization, adding further support for the association between food allergy and asthma [3–5]. However, it is well known that IgE-binding cross-reactive epitopes between food allergens and environmental allergens may contribute to elevated IgE levels [6–8]. In particular, house dust mite and cockroach tropomyosins can account for the presence of detectable IgE to shrimp, even in unexposed individuals [9].
Since inner city children tend to have high rates of exposure to environmental allergens, we hypothesized that sensitization to cockroach and dust mite might correlate with sensitization to shrimp. This could be an important factor to consider when assessing prevalence of food allergies based only on serologic tests. If environmental allergen exposure does have an influence on food-specific IgE levels, this may have a significant impact on management of food allergies, particularly when deciding whether to perform oral food challenges.
Methods
Study population
NCICAS included 1528 children 4–9 years of age with asthma who were recruited from emergency departments and clinics in inner city areas in the United States (Bronx, New York; East Harlem, New York; St. Louis; Washington, D.C.; Baltimore; Chicago; Cleveland; and Detroit) [10,11]. The study was approved by the institutional review board at each site, and written informed consent was obtained from the parents or legal guardians of participants. At enrollment, participants were invited to provide a voluntary blood sample with the understanding that it would be stored for future analyses of markers of atopy (i.e. allergen-specific IgE). Five hundred four of the children had serum samples available for IgE analysis.
Home visits were performed in a subset of participants and allergen levels for Dermatophagoides farinae (Der f 1), D. pteronyssinus (Der p 1), and Blattella germanica (Bla g 1) were measured from settled dust in 198 homes of participants who also had serum available [12].
Assays
The serum samples were evaluated for specific IgE (ImmunoCAP® system; Phadia; Uppsala, Sweden) to shrimp (boiled, frozen Atlantic shrimp and raw, frozen prawns from the Indo-West-Pacific). A specific IgE (SIgE) level ≥ 0.35 kUA/L indicated sensitization. From a previous study, specific IgE levels (ImmunoCAP® system; Phadia; Uppsala, Sweden) to w hole body cockroach (Blattella germanica) and whole body culture of Dermatophagoides farinae were available.
Statistical analysis
We used χ2- statistics to test differences in the prevalence of shrimp-specific IgE levels by population characteristics. Pearson correlations were used to assess the association between serum specific IgE to shrimp, cockroach (Blattella germanica), dust mite (Dermatophagoides farinae) and home allergen exposure levels. Effect modification of the relationship of home exposures to shrimp-specific IgE levels stratified by sensitivity to other allergens were tested with an interaction term. Allergen-specific serum IgEs and allergen exposure levels were logarithmically transformed (base 10) for the statistical analysis because of skewed distributions.
The nationally representative NHANES 2005–2006 data were analyzed using the sampling weights and survey design variables to account for the complex sample design. All analyses were conducted using the R system for statistical computing (version 2.12.1). Statistical significance was established a priori at 0.05.
Results
Overall, 32.7% of the children in the NCICAS study had a positive shrimp-specific IgE result. Positive shrimp-specific IgE was associated with age and race, but not sex or caretaker’s education level (Table 1).
Table 1.
Demographic characteristics
| Subject Characteristics | N | % Shrimp Positive (SE) |
Chi-square p-value |
|---|---|---|---|
| Overall | 504 | 32.7 (2.1) | |
| Age | |||
| 4–6 | 285 | 28.8 (2.7) | 0.03 |
| 7–9 | 218 | 38.1 (3.3) | |
| Sex | |||
| Male | 319 | 33.5 (2.6) | 0.64 |
| Female | 184 | 31.5 (3.4) | |
| Race-ethnicity | |||
| Hispanic | 58 | 53.4 (6.6) | 0.001 |
| Black | 410 | 30.5 (2.3) | |
| Other | 26 | 19.2 (7.8) | |
| Education (mother) | |||
| < 12th grade | 171 | 32.2 (3.6) | 0.64 |
| 12th grade | 193 | 30.6 (3.3) | |
| > 12th grade | 132 | 35.6 (4.2) | |
| Cockroach Specific IgE | |||
| Not detectable | 280 | 3.6 (1.1) | <0.001 |
| Detectable | 224 | 69.2 (3.1) |
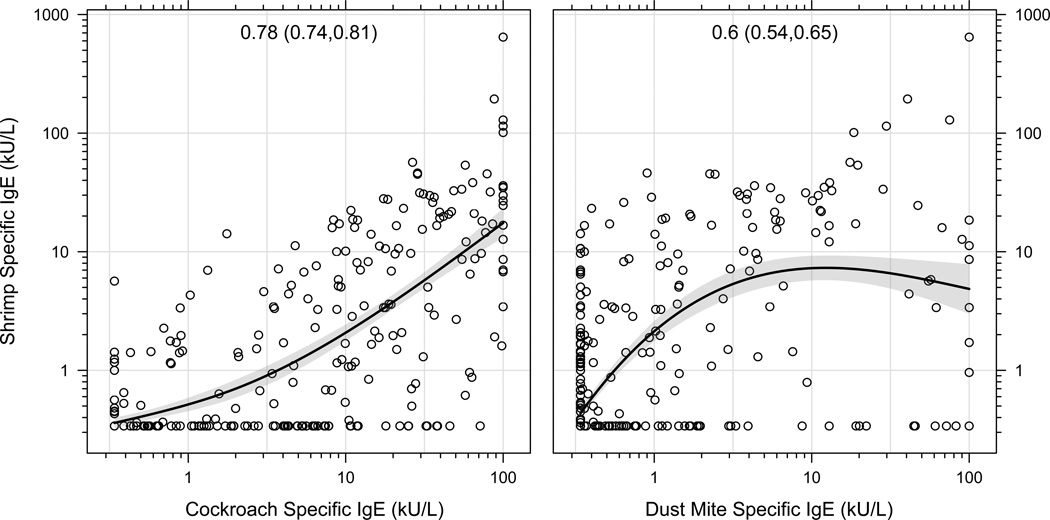
Shrimp IgE and cockroach and dust mite IgE levels were highly correlated (Figure 1). High exposure to cockroach (Bla g 1) in the home, particularly in the bedroom and television room, was significantly correlated with higher shrimp and cockroach IgE levels. In contrast, high exposure to dust mite (Der f 1) in the home was significantly correlated with IgE to D.farinae, but not with shrimp IgE levels (Table 2).
Figure 1.
Shrimp IgE and cockroach and dust mite IgE levels were highly correlated
Table 2.
Correlation between allergen lev els in settled dust and specific IgE levels
| Cockroach sIgE |
Dust mite (Dermatophagoides farinae) sIgE |
Shrimp sIgE |
|||||
|---|---|---|---|---|---|---|---|
| Exposure | Corr | P | Corr | P | Corr | P | |
| Bla g 1 | Bedroom | 0.32 | <0.001 | 0.14 | <0.10 | 0.27 | <0.001 |
| TV room | 0.26 | <0.001 | 0.13 | <0.10 | 0.20 | <0.01 | |
| Kitchen | 0.20 | <0.01 | 0.05 | 0.47 | 0.08 | 0.25 | |
| Der f 1 | Bedroom | −0.03 | 0.65 | 0.15 | <0.05 | 0.01 | 0.93 |
| TV room | −0.06 | 0.41 | 0.25 | <0.001 | 0.04 | 0.60 | |
| Kitchen | −0.04 | 0.56 | 0.02 | 0.76 | −0.02 | 0.83 | |
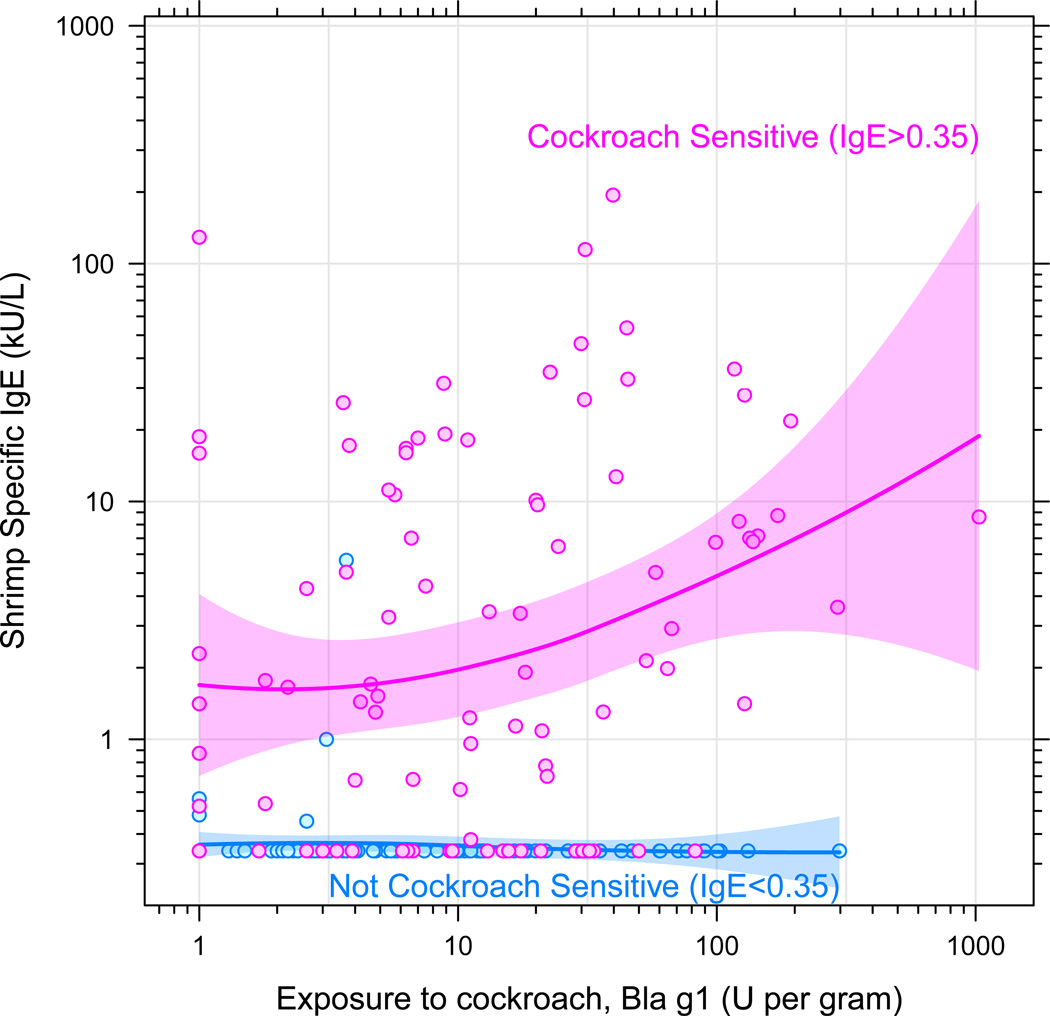
When examining the roles of sensitization and exposure to cockroach on shrimp IgE levels, a synergistic relationship is found between cockroach IgE and exposure in predicting shrimp IgE (interaction p-value = 0.01). For those with sensitization to cockroach, there is a strong correlation between exposure to cockroach in the home and shrimp IgE level, indicating that exposure may be an important contributor to elevations in shrimp IgE in some individuals (Figure 2).
Figure 2.
Shrimp-specific IgE was correlated with exposure to cockroach, but only among children IgE positive to cockroach
Discussion
Results from the NHANES data indicate that young males of lower socio-economic status are at highest risk for food allergies [2]. Prevalence of shrimp sensitization demonstrated the greatest disparity between race, poverty income ratio and education. This raises the question of how these demographic categorizations are associated with shrimp sensitization. One possibility is environmental exposures, as it is well known that cockroach allergen exposure is a big factor for inner city asthmatics. Therefore, we sought to investigate the role of environmental exposure to allergens in relation to shrimp sensitization.
Nationally representative data from NHANES 2005–2006 demonstrate similar associations to NCICAS between high shrimp sensitization in non-hispanic blacks of lower socio-economic status (Table 3). It is well-known that cockroach, dust mite and shrimp tropomyosins share high sequence identity (~80%) [8] and with the additional exposure data that was collected in thi s population, we not only found a high correlation between both cockroach IgE and shrimp IgE, but a high correlation between exposure to cockroach and shrimp IgE as well. In contrast, we did not find a correlation between dust mite exposure and shrimp IgE.
Table 3.
Percent (SE) detectable shrimp- specific serum IgE in National U.S. Data: NHANES 2005–2006, Ages 6–19 years
| Subject Characteristics | All Children Ages 6–19 |
Children w/ Asthma Ages 6–19 |
|||
|---|---|---|---|---|---|
| N | % (SE) Shrimp Positive |
N | % (SE) Shrimp Positive |
||
| Overall | 3433 | 6.1 (0.73) | 360 | 9.9 (2.49) | |
| Age | <0.01 | 0.15 | |||
| 6–11 | 1145 | 3.3 (0.65) | 120 | 9.8 (3.78) | |
| 12–15 | 1128 | 7.2 (1.09) | 121 | 5.7 (2.01) | |
| 16–19 | 1160 | 8.7 (1.46) | 119 | 15.0 (4.54) | |
| Sex | 0.65 | 0.59 | |||
| Male | 1697 | 6.3 (0.91) | 197 | 8.9 (3.24) | |
| Female | 1736 | 5.9 (0.86) | 163 | 11.2 (3.29) | |
| Race-ethnicity | <0.001 | <0.01 | |||
| Non-Hispanic white | 882 | 4.2 (0.79) | 102 | 6.5 (3.11) | |
| Non-Hispanic black | 1124 | 12.5 (1.57) | 143 | 26.5 (4.78) | |
| Mexican American | 1130 | 7.8 (1.27) | 87 | 11.5 (3.57) | |
| Other | 297 | 6.3 (2.23) | 28 | 4.4 (4.21) | |
| Education (family referent) | <0.01 | 0.09 | |||
| < 12th grade | 1030 | 10.1 (1.13) | 94 | 20.8 (4.51) | |
| 12th grade | 790 | 5.4 (0.98) | 82 | 8.9 (3.23) | |
| > 12th grade | 1450 | 5.1 (0.98) | 169 | 8.3 (3.53) | |
| Missing/unknown | 163 | 6.2 (3.22) | 15 | 1.4 (1.42) | |
| Poverty Index Quartiles | <0.01 | 0.37 | |||
| 1st [0 – 1.59] | 1209 | 8.9 (0.78) | 121 | 12.8 (2.71) | |
| 2nd [1.60 – 3.05] | 901 | 5.1 (1.09) | 97 | 7.7 (3.11) | |
| 3rd [3.06 – 4.96] | 624 | 4.2 (0.90) | 64 | 11.5 (6.40) | |
| 4th [4.97 – 5] | 537 | 6.3 (1.81) | 68 | 7.8 (4.06) | |
| Missing/unknown | 162 | 4.5 (1.45) | 10 | 15.2 (13.99) | |
| > 1 non-food positive specific IgE | <0.001 | <0.001 | |||
| No | 1427 | 0.2 (0.15) | 92 | 0 (0) | |
| Yes | 1449 | 13.5 (1.44) | 213 | 15.6 (3.45) | |
| Missing/unknown | 557 | 0 (0) | 55 | 0 (0) | |
| Cockroach Specific IgE | <0.001 | <0.001 | |||
| Not detectable | 2456 | 1.9 (0.33) | 227 | 3.6 (1.79) | |
| Detectable | 448 | 42.1 (3.68) | 81 | 39.9 (7.06) | |
These findings provide a potential explanation for the higher observed rates of sensitization to shrimp in black male children from the inner city. Shrimp IgE levels may need to be interpreted in the context of environmental exposures for this particular population. Studies have reported different predictive values for food-specific IgE levels depending on the study population, which likely is a reflection of diet, demographics (i.e. age), and disease states (i.e. presence or absence of atopic dermatitis) [13–17]. The results of this study suggest that there may be racial or socio-economic differences for food-specific IgE cutoff levels as well. Further studies using oral food challenges to confirm clinical reactivity will be necessary to further explore this possibility.
Acknowledgments
Funding Source: The National Cooperative Inner-City Asthma Study was supported by grants UOI A1-30751, A1-30752, A1-30756, A1-30772, A1-30773-01, A1-30777, A1-30779, A1-30780, N01-A1-15105 from the National Institutes of Allergy and Infectious Disease (National Institutes of Health, Bethesda, MD).
The project described was supported in part by Grant Number M01-RR-00071 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Julie Wang, M.D. is funded in part by a grant from the National Institutes of Health/National Institute of Allergy and Infectious Diseases; K23 AI083883
Abbreviations
- NCICAS
National Cooperative Inner City Asthma Study
- IgE
Immunoglobulin E
- Bla g 1
Blattella germanica 1
- Der f 1
Dermatophagoides farinae 1
- SIgE
Specific IgE
- SIgG
Specific IgG
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Implications: Shrimp IgE is correlated with cockroach IgE and exposure to cockroach allergen in the home, suggesting that shrimp IgE may need to be interpreted in the context of environmental exposures.
References
- 1.Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009 Dec;124(6):1549–1555. doi: 10.1542/peds.2009-1210. [DOI] [PubMed] [Google Scholar]
- 2.Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2010;126:798–806. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Visness CM, Sampson HA. Food allergen sensitization in inner-city children with asthma. J Allergy Clin Immunol. 2005;115:1076–1080. doi: 10.1016/j.jaci.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Simpson AB, Glutting J, Yousef E. Food allergy and asthma morbidity in children. Pediatr Pulmonol. 2007;42:489–495. doi: 10.1002/ppul.20605. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder A, Kumar R, Pongracic JA, et al. Food allergy is associated with an increased risk of asthma. Clin Exp Allergy. 2009;39:261–270. doi: 10.1111/j.1365-2222.2008.03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mittag D, Vieths S, Vogel L, Becker WM, Rihs HP, Helbling A, Wüthrich B, Ballmer-Weber BK. Soybean allergy in patients allergic to birch pollen: clinical investigation and molecular characterization of allergens. J Allergy Clin Immunol. 2004 Jan;113(1):148–154. doi: 10.1016/j.jaci.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Mittag D, Akkerdaas J, Ballmer-Weber BK, Vogel L, Wensing M, Becker WM, Koppelman SJ, Knulst AC, Helbling A, Hefle SL, Van Ree R, Vieths S. Ara h 8, a Bet v 1-homologous allergen from peanut, is a major allergen in patients with combined birch pollen and peanut allergy. J Allergy Clin Immunol. 2004 Dec;114(6):1410–1417. doi: 10.1016/j.jaci.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Ayuso R, Resse G, Leong-Kee S, et al. Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int Arch Allergy Immunol. 2002;129:38–48. doi: 10.1159/000065172. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes J, Reshef A, Patton L, et al. Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed Orthodox Jews. Clin Exp Allergy. 2003;33:956–961. doi: 10.1046/j.1365-2222.2003.01722.x. [DOI] [PubMed] [Google Scholar]
- 10.Kattan M, Mitchell H, Eggleston P, Gergen P, Crain E, Redline S, et al. Characteristics of inner-city children with asthma: the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997;24:253–262. doi: 10.1002/(sici)1099-0496(199710)24:4<253::aid-ppul4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell H, Senturia Y, Gergen P, Baker D, Joseph C, McNiff-Mortimer K, et al. Design and methods of the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997;24:237–252. doi: 10.1002/(sici)1099-0496(199710)24:4<237::aid-ppul3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 13.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 14.Perry TT, Matsui EC, Kay Conover-Walker M, Wood RA. The relationship of allergen-specific IgE levels and oral food challenge outcome. J Allergy Clin Immunol. 2004;114:144–149. doi: 10.1016/j.jaci.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Ara C, Boyano-Martinez T, Diaz-Pena JM, Martin-Munoz F, Reche-Frutos M, Martin-Esteban M. Specific IgE levels in the diagnosis of immediate hypersensitivity to cows' milk protein in the infant. J Allergy Clin Immunol. 2001;107:185–190. doi: 10.1067/mai.2001.111592. [DOI] [PubMed] [Google Scholar]
- 16.Boyano Martinez T, Garcia-Ara C, Diaz-Pena JM, Munoz FM, Garcia Sanchez G, Esteban MM. Validity of specific IgE antibodies in children with egg allergy. Clin Exp Allergy. 2001;31:1464–1469. doi: 10.1046/j.1365-2222.2001.01175.x. [DOI] [PubMed] [Google Scholar]
- 17.Hill DJ, Heine RG, Hosking CS. The diagnostic value of skin prick testing in children with food allergy. Pediatr Allergy Immunol. 2004;15:435–441. doi: 10.1111/j.1399-3038.2004.00188.x. [DOI] [PubMed] [Google Scholar]