Abstract
Background
The presence of pets in a home during the prenatal period and during early infancy has been associated with a lower prevalence of allergic sensitization and total IgE in middle childhood. No studies have examined the effect of pet exposure in a population-based cohort using multiple early life measures of serum total IgE.
Objective
To examine within-individual longitudinal trends in total IgE during early childhood and assess the effect of indoor prenatal pet exposure on those trends. Also, to employ a statistical method which was flexible enough to allow and account for unequally spaced study contacts and missing data.
Methods
Using the population-based Wayne County Health, Environment, Allergy and Asthma Longitudinal Study (WHEALS) birth cohort (62% African American), we analyzed 1187 infants with one to four measurements of total IgE collected from birth to 2 years of age. Effects of pet exposure on the shape and trajectory of IgE were assessed using a multilevel longitudinal model, accommodating repeated measures, missing data, and the precise time points of data collection.
Results
The best fit shape to the trajectory of IgE was non-linear, with an accelerated increase before 6 months. Total IgE was lower across the entire early life period when there was prenatal indoor pet exposure (p<0.001). This effect was statistically significantly stronger in children delivered by caesarean-section versus vaginally (p< 0.001 and p< 0.06, respectively) and in those born to non-African American (p< 0.001) versus African American mothers (p< 0.3).
Conclusion
Pet exposure and delivery mode may be markers of infant exposure to distinct microbiomes. The effect of exposures may vary by race, suggesting differential impact by ancestry.
Keywords: total IgE, cohort, longitudinal, multilevel model
Introduction
Allergic sensitization, allergies and asthma have been increasing in prevalence over the last half century among children residing in Westernized countries.1–3 One theory, the “Hygiene Hypothesis,” posits that decreased exposure to infections during early life results in an immunological shift towards an allergic phenotype.4 A refined version of this hypothesis is based on microbial ecology, suggesting that exposure to a distinct microbial community composition of home environments with certain characteristics, such as indoor pets, during pregnancy and infancy affect the developing immune system.5–7
The production of IgE in response to innocuous substances is the hallmark of allergic disease: as more IgE is produced, total IgE increases.8 Total IgE has been associated with risk for asthma in children in Westernized countries.9;10 While a number of studies have reported cross-sectional associations between early life allergic disease risk factors and total IgE, few have examined the effects of such factors on intra-individual IgE over time using repeated measures during infancy in a large population-based birth cohort. None have accommodated together two specific analytic challenges associated with birth cohort studies; the exclusion of subjects with missing data due to the difficulties of collecting blood samples from infants and the varying collection points by time since birth due to scheduling compromises, even though precise age at which a measurement is taken is critical as IgE is rapidly changing during this time period. Using an analytic approach that addresses these potential biases, we assessed the impact of exposure to pets on the trajectory of IgE development in the first two years of life in a racially diverse birth cohort based in metropolitan Detroit, Michigan.
Methods
Study population
The Wayne County Health, Environment, Allergy and Asthma Longitudinal Study (WHEALS) is a birth cohort from southeastern Michigan designed to examine the relationship of environmental factors to the subsequent development of atopic and allergic diseases in childhood. The cohort was based in the Henry Ford Health System. The population served has been shown through census data to be representative of the Detroit metropolitan area. Through an electronic medical claims database, potentially pregnant women were identified through the use of ICD-9 for visits to one of five obstetric clinics. To be eligible for the study women needed to be between 21 and 49 years of age and live in a predefined cluster of zip codes that included both urban and suburban Detroit, Michigan (see online supplement for study area map). By including not only the city of Detroit (82% African American based on 2000 Census data) but suburbs which range from 1% African American to 68% African American, we expected to recruit individuals from highly variable socioeconomic and racial backgrounds. Medical records were then reviewed to confirm pregnancy and to determine if the pregnancy had progressed beyond the first trimester (calculated by estimated due date or last menstrual period). Women in their first trimester of pregnancy qualified for the study if they remained pregnant into their second trimester and still met other study criteria. We chose the second trimester restriction to decrease the chance of miscarriage/spontaneous abortion. Women who met the above criteria were then officially enrolled in the study if they read and signed the study informed consents, indicated their intention of living in the area for at least two years, completed the pre-delivery interview and delivered at least one live birth. We recorded 5 stillborn children (not in study) and no instances of a multiple birth where one or more child died. For the 16 twin deliveries (no other multiple births in study) the first born child was designated the study child. Recruitment was completed between August 2003 and November 2007. Each follow-up contact included an interviewer administered questionnaire and was planned for 1, 6, 12 and 24 months after the child's birth. All questionnaires included questions on type of household pet(s), and for dog(s) and cat(s) more detailed information such as average time spent indoors for each pet was captured.
This research was approved by the Henry Ford Hospital Institutional Review Board.
Total IgE
The outcome of interest is the within-child pattern of total IgE development, using blood collected at birth (cord) and at approximately 6, 12 and 24-months of age. Total IgE was measured using the Pharmacia UniCAP system (Phadia, Portage, MI, USA) using the manufacturer's protocols. The high sensitivity protocol providing an assay range of 0.1 to 100 IU/mL of IgE was used for the cord and 6 month samples while the standard protocol was used for 12 and 24 month samples. The high sensitivity assay protocol was used for the earlier time points to minimize the number of samples with IgE values below the lower limit of assay calibration. One percent of all samples were reanalyzed in a different assay run on a different day enabling us to calculate an inter-assay coefficient of variation of 6.7%. One-hundred forty-seven (19.7%) of the cord blood samples and one of the 6 month samples had undetectable measures and were assigned a value of 0.05 IU/ml.
Pet exposure
A pet was defined as a cat or dog only (other types of pets were uncommon). We defined prenatal pet exposure as the mother answering yes to “Since the time you learned you were pregnant have you owned or cared for any pets for more than 1 week in your home?” We considered only those reporting pets being kept indoors at least one hour daily as exposed.
Other early life factors
Maternal race was based on self report during the enrollment interview. Women were grouped as either being African American or not; because of sample size, the non-African American group was not further subdivided. Maternal allergic history was defined as positive if she reported ever having a doctor diagnosis of hay fever or allergic rhinitis. Women were also asked to report the outcome of all prior pregnancies.
Information on maternal smoking or living with smokers and the child's exposure to tobacco smoke after delivery was also collected prior to delivery and at time of blood sampling at 6, 12 and 24 months postpartum. Delivery type (vaginal or caesarean-section) was collected from the medical record.
The number of days per week or month that the child spent 4 or more hours outside the home, defined as visiting a family, friend or babysitter's house, or child care facility, was recorded at postpartum interviews. The average number of other children and pets at such locations were also recorded and routine exposure was defined as three or more visits per week. We created four indicator variables to examine potential effects of routine external exposures: 1 or more children, pet(s), either and both.
Statistical analysis
Multilevel modeling techniques use all available data in the analyses. This means that children missing one or more IgEs did not result in their exclusion from analyses, thus addressing a common analytical challenge in asthma cohort studies/longitudinal analyses. Of the 1258 subjects enrolled, 1187 (94%) had a total IgE measure for at least 1 of the scheduled collection times (cord, 6, 12 or 24 months of age) and 825 (66%) had 2 or more IgE values. IgE was log-transformed prior to inclusion in the model due to the skew of the data.
Multilevel longitudinal models (MLM) were fit using full maximum likelihood methods and included both random and fixed effects (PROC MIXED (SAS V9.2, Release 2, Cary, NC, USA)) to assess the trajectory (rate and direction of change over time) of IgE development and between-individual change to assess the effects of early life exposures. Random effects account for the correlation within individual (repeated measures). The age of each child at each visit is included as a variable in the individual-specific component of the multilevel model to allow and account for unequal spacing of collection points, thus addressing another commonly occurring situation in many research studies where planned study contacts occur over a window of time. This study's intent was to contact every child at 6, 12, and 24 months of age, yet visits actually occurred over a range of ages. Because of the young age of the subjects and the apparent rapid change in IgE for many individuals over this time period, multilevel longitudinal models are an appropriate choice.
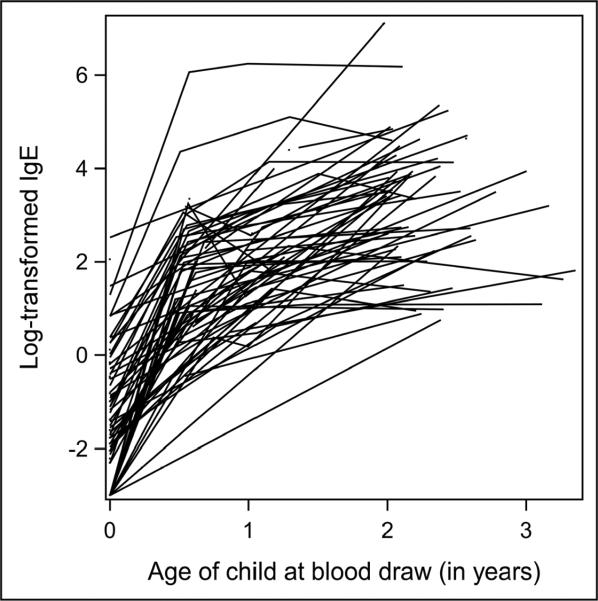
For illustrative purposes, Figure 1 is a profile plot of a 5% random selection of subjects, which indicates a fair amount of variation in the starting point (intercept), rate (slope) and shape of the trajectory (possible higher order terms). Visual inspection of all subject trajectories (each line is constructed by connecting one child's log-transformed IgE values at each age measured; single dots represent children with only one IgE measurement) showed that most were markedly nonlinear. The inspection process, via this type of graphing, can be helpful to assess if and where variability in individual growth curves might exist for the data, but is not deterministic. The six month time point appeared to be the natural choice for point where the rate (or slope) of increase changed (sometimes called `change point') but we also tested to see if using the one year time point was able to explain more of the variability (i.e. better fit) and it did not.
Figure 1.
Profile plot* of 5% randomly selected subjects *each line is a “connect-the-dots” depiction of the log-transformed IgE values, by age, for one unique child. Single dots represent children with only one IgE measurement.
A two segment linear (piecewise) model was found to have the best fit (modeling details in online supplement). Indoor pet exposure was included as a main effect and was also tested for an interaction with age of child. Indoor pet exposure was evaluated as a baseline exposure variable, as well as time-varying exposure variable (one that may change over time) since we had reports about pet exposure at each time that IgE was measured.
Effect modification for a priori selected variables was tested for by inclusion of an interaction term (p<0.10) and inspection of stratified models. Maternal race, smoke exposure during pregnancy, mode of delivery, firstborn, first pregnancy, child's gender and maternal history of allergy were tested for both effect modification and confounding of the association between indoor pets and the pattern of total IgE over early life.
Results
Cohort characteristics
The average age of the mother at child's birth was 29.5 (s.d. 5.3) and 62.2% (n=738) self-reported their race as African American. Of the 1187 subjects, 35.4% (n=420) had at least 1 indoor pet at the prenatal interview, 16.6% (n=197) had at least 1 indoor cat and 24.3% (n=289) had at least 1 indoor dog and 5.6% (n=66) had both cat(s) and dog(s). Thirty-seven (n=436) percent of the babies were delivered by c-section and 36.5% (n=433) were firstborn children. Children whose mothers reported at least 1 indoor pet at the predelivery interview were more likely to be exposed to smoke during pregnancy, be firstborn and have a mother of non-African American ethnicity (Table 1). The non-African American mothers (n=449) were 86% Caucasian, 7% Asian and 7% other.
Table 1.
Sample characteristics (N=1187)
| Indoor Pet at PD N=420 | No Indoor Pet at PD N=727 | p-value | |
|---|---|---|---|
| Mom age, mean (s.d.) | 29.7(5.1) | 29.4 (5.3) | 0.30 |
| African American race, n (%) | 186(44.3%) | 552 (72.0%) | <0.001 |
| Mom allergic history1, n (%) | 127(30.5%) | 213(27.8%) | 0.34 |
| Mother smoked, n (%) | 67(16.0%) | 72 (9.4%) | 0.001 |
| ETS during pregnancy, n (%) | 131 (31.2%) | 196(25.6%) | 0.038 |
| Delivery by c-section2, n (%) | 144(34.6%) | 292 (38.5%) | 0.19 |
| Male gender (baby), n (%) | 217(51.7%) | 375 (48.9%) | 0.36 |
| Firstborn, n (%) | 175(41.7%) | 258 (33.6%) | 0.006 |
4 with missing information
13 with missing information;
Shape of trajectory
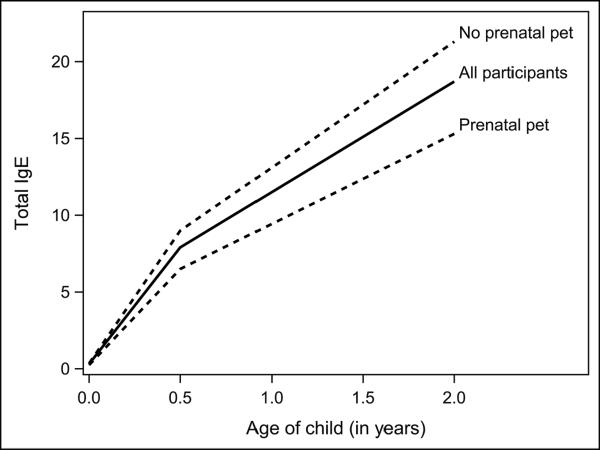
Figure 2, the solid line, shows the mean predicted piecewise model of IgE over time (within-individual change). The slope, or rate of change, between cord blood and the six month time point is significantly steeper than the slope after the six month time point (p<0.001). The average change in total IgE from cord to age 6 months estimated by the regression model is a 25.8 fold increase (95% CI=23.3 to 28.5) in the geometric mean from cord to 6 months, p<0.001. Figure 2 shows that the regression model estimates an average total IgE of 0.30 IU/ml at birth and 7.74 IU/ml at the 6 month time point, corresponding to a 25.8 fold increase (7.74/0.30). If there were no missing data and timing of study contacts did not differ among subjects, this estimate of a 25.8 fold increase (equivalent to a 2580% increase) would directly correspond to the ratio of the (cross-sectional) geometric means at birth and 6 month visit (online Table E1: geometric means of 0.31IU/ml at birth and 7.4 IU/ml at the 6 month visit is a (cross-sectional) 23.9 (7.4/0.31) fold increase). In contrast to the estimated 25.8 fold increase in the first 6 months, the estimated average fold increase in the post-6 month age period was 1.51 fold (95% CI=1.48 – 1.59) per 6 month interval (Figure 2). Thus, for example, for a child with a 6 month total IgE of 7.7 IU/ml we would expect, on average, an 11.6 IU/ml total IgE at 12 months of age.
Figure 2.
Predicted mean plot of the trajectory from two-piece random effects model
Effect of prenatal indoor pet
Overall, a constant statistically significant pet effect was found over the complete trajectory (Table 2; regression coefficient=−0.33 (s.e. 0.07), p<0.001). This means that the total IgE trajectory is an estimated and unvarying 28% lower in the group of children with prenatal pet exposure versus those who did not have prenatal pet exposure. Addition of the time-varying post-natal indoor pet exposure variable to the model was not statistically significant (p=0.50), suggesting that subsequent pet exposure did not add any additional information to the model once prenatal pet status is known. We further explored this by creating variables for loss of pet and gain of pet, and while neither was statistically significant, the numbers were small (n=83 and 55, respectively). Number of pets was not found to be significant (p=0.84). Separating the pet exposure into an indoor dog and indoor cat variables resulted in no statistically significant difference for the effect of pet type (all p≥0.5).
Table 2.
Estimated regression coefficients1 (b) and standard errors (s.e.) for the model of log-transformed IgE overtime
| Strata | Independent variable | b1 (s.e.) | Expected % decrease in total IgE with pet exposure2 | p-value |
|---|---|---|---|---|
| All | Prenatal Indoor Pet exposure | −0.33 (0.07) | 28% | <0.001 |
| Type of delivery | ||||
| Vaginal | Prenatal Indoor Pet exposure | −0.18(0.09) | 16% | 0.055 |
| C-section | Prenatal Indoor Pet exposure | −0.57(0.12) | 43% | <0.001 |
| Race | ||||
| African American | Prenatal Indoor Pet exposure | −0.11 (0.10) | 10% | 0.30 |
| Non-African American | Prenatal Indoor Pet exposure | −0.40(0.11) | 33% | <0.001 |
Due to the log-transformation of total IgE the regression coefficient can be interpreted as the average difference in log-transformed total IgE between children with prenatal indoor pet exposure vs. children without prenatal indoor pet exposure.
An easier interpretation is to use the formula (eb -1 )*100, where b is the regression coefficient.
Modification of prenatal pet effect
Male gender, maternal African American race, mode of delivery, mother's history of doctor diagnosed allergy or asthma, being firstborn or first pregnancy, and prenatal maternal or other household member smoke exposures were assessed to see whether they modified the association between pet exposure and total IgE trajectory. A statistically significant interaction was found between prenatal indoor pet exposure and mode of delivery (p=0.009). The protective effect of pet exposure on IgE trajectory was significantly greater in those delivered via caesarean section (regression coefficient = −0.57 (s.e.=0.12), p<0.001) as compared to children delivered vaginally (regression coefficient = −0.18 (0.09), borderline statistical significance of p=0.055). The regression coefficients translate to a 43% percent decrease in total IgE among children born via caesarean section as compared to a 16% overall decrease for children born vaginally. Table 3 shows that women who delivered via caesarean section tended to be older and have a firstborn female child, however adjusting for these factors did not substantially alter the findings.
Table 3.
Sample characteristics by race and mode of delivery
| Comparisons by Maternal race | Comparisons by mode of delivery | |||||
|---|---|---|---|---|---|---|
| AA mom N=738 | Non-AA N=449 | p-value | C-section N=436 | Vaginal N=738 | p-value | |
| Mom age, mean (s.d.) | 29.0 (5.3) | 30.3 (5.0) | <0.001 | 30.6 (5.3) | 28.8(5.1) | <0.001 |
| Indoor Pet, n (%) | 186(25.2%) | 234 (52.7%) | <0.001 | 144(33.0%) | 272 (36.9%) | 0.19 |
| African American race, n (%) | 284(65.1%) | 446 (60.4%) | 0.11 | |||
| Mom allergic history, n (%) | 224 (30.5%) | 116(25.9%) | 0.09 | 116(26.6%) | 219(29.8%) | 0.24 |
| Mother smoke, n (%) | 80(10.8%) | 59(13.1%) | 0.23 | 53(12.2%) | 84(11.4%) | 0.69 |
| ETS1, n(%) | 220 (29.8%) | 107(23.8%) | 0.025 | 110(25.2%) | 209 (28.3%) | 0.25 |
| Delivery by c-section, n (%) | 284 (38.9%) | 152(34.2%) | 0.11 | |||
| Male gender (baby), n (%) | 366 (49.6%) | 226 (50.3%) | 0.80 | 198(45.4%) | 389 (52.7%) | 0.016 |
| Firstborn, n (%) | 264 (35.8%) | 169(37.6%) | 0.52 | 177(40.6%) | 253 (34.3%) | 0.030 |
ETS = environmental tobacco smoke defined as an adult in household who smokes at least 1 cigarette per day inside the home
Additionally, there was evidence that maternal race was an effect modifier (interaction p-value=0.06). The protective pet effect was only statistically significant within the non African American women (associated 33% decrease; p<0.001) as compared to children of African American women (associated 10% decrease; p=0.30). African American women in this study were more likely to be younger, be exposed to environmental tobacco smoke (ETS) and less likely to have pet(s) in the household (Table 3). The remaining early life factors (male gender, firstborn or first pregnancy, mother's history of doctor diagnosed allergy or asthma and prenatal ETS) were not found to be effect modifiers (all interaction p-values > 0.45). Also, there was no strong evidence of confounding by these same early life factors (all change in effects <20%).
Early exposures outside the home
At the 6 month visit 31.8% (n=249) children had routine exposure to children outside the home and 11.4% (n=89) children had routine exposure to pets outside the home. Routine exposure to other children and/or pets, external to the home environment, did not significantly affect the trajectory of IgE post 6 months of age.
Discussion
Our study demonstrates that the trajectory of IgE in early life is affected by the presence of pets in the home during pregnancy, and the magnitude of this effect varies by the mode of delivery and self-reported race. Studies in multiple settings have evaluated the effects of both early life pet exposure11–15 and mode of delivery16;17 on the risks of allergic disease. While previous studies are not uniform, many suggest that early life pet exposures are associated with decreased risk of allergic disease18 and that delivery via caesarean-section is associated with increased risk.19 Further, it is well established that African American children and adolescents have higher IgE levels than other race groups.20
The biological mechanism explaining the effects of pets on allergy development is unknown. We hypothesize that indoor pet ownership is associated with exposure to a distinct, more broadly diverse bacterial populations in the home environment, and that this broadly diverse exposure influences early infant development of immune function in a manner that decreases the risk of development of allergy and atopic asthma. Our previous study supports this hypothesis, demonstrating that house dust from homes with dogs possessed significantly greater bacterial diversity than house dust from no-pet homes.7 Delivery by caesarean-section, which has been associated with as much as a 20% increase in the risk for allergic disease development,21 has been associated with distinct gastrointestinal microbiota deficient in Lactobaccillus and dominated by Clostridia and Escherichia species.22;23 It is possible that an enhanced household bacterial diversity provided by pets would have a greater impact on such babies. Indeed, in a Finnish study, the beneficial effects of bacterial probiotics on the risk of allergic sensitization was only apparent among children delivered by cesarean-section.24 We speculate that individuals with a predominant African ancestry and therefore an evolutionary history founded in an equatorial climate with a relatively voluminous and presumably highly diverse microbial burden may have a preponderance of genetic variants that render a differential susceptibility to the effects on IgE of microbial exposures afforded by household dogs and cats.
Few longitudinal studies of IgE have taken maximum statistical advantage of the repeated measures within children. A recent exception is a publication from the Multi-centre Allergy Study (MAS); however, their primary focus was to compare trends of total IgE to trends of allergen-specific IgE.25 While only participants with complete data over time (6% of the total sample) were analyzed in this report, and very different statistical methods were used, our results were consistent with theirs in showing a rapid increase in IgE during early childhood. Using a random effects model, the Tucson Children's Respiratory Study showed trends of general increase through childhood that were affected by breastfeeding and maternal total IgE levels, but there was only one post-natal data collection point in infancy.26 Most relevant are the results from Rothers et al. from another Tucson-based birth cohort.27 Using samples collected at 3 months and 1, 2 and 3 years of age in a cohort of 362 children and again deploying a random effects model, they reported that day care attendance by age 3 months was associated with decreased total IgE levels, but only among children whose mothers were atopic, asthmatic or both, another example of effect modification. The MLM method used in this paper differs from the random effects model in that it accounts for measures at different time points and is focused on the evaluation of within-individual change, as well as allowing the inclusion of subjects with missing data.
Examination of factors affecting early within-person total IgE trends over time is important as it may provide insight into immune mechanisms that increase the risk of developing clinical allergic disorders. In addition to the association between high total IgE and atopic dermatitis and allergic rhinitis, numerous studies have reported higher IgE among children with asthma and/or wheezing.9;10;28;29 As we follow the children in our cohort over time, we will be able to examine whether certain longitudinal patterns of total IgE are associated with increased risk of subsequent allergic disorders and asthma.
The large size of our sample and its inclusion of a high proportion of African Americans were assets of our study. A potential limitation of our study is that we used the six month time point as the `change' point. The model may have changed if a 3 or 9 month blood collection were completed. A test for a change point at the 1 year time point was not significant. In any case, this did not affect the ability of our methods of analysis to detect a dramatic increase in total IgE in the first six months of life and to analyze the effects of early life exposures on this increase. Our analysis nonetheless does not permit the conclusion that six months is necessarily the optimal time point for early life blood collection in future studies.
Additionally, no testing was done to rule out maternal contamination of the cord blood. However, it has been argued this is unnecessary since maternal IgE does not cross the placenta during gestation and the fetus is capable of producing IgE by the 11th week of gestation.30 Further, we repeated the analyses excluding the cord measurement and obtained extremely consistent results, both overall and when stratified by delivery mode and maternal race. The overall model excluding the cord measurement gave an estimated 27% decrease with indoor pet exposure as compared to the 28% decrease in the model containing cord measurements.
In summary, our data are evidence in support of the hypotheses that prenatal pet exposure affects a child's very early life immune status resulting in a lower peripheral blood total IgE trajectory. The use of MLMs and all of their advantages facilitated this within person analysis.
Supplementary Material
Key Messages
Prenatal pet exposure has a protective effect on the trajectory of total IgE in early childhood and this effect appears to be greater among babies delivered by caesarean-section and those of non-African American race.
Longitudinal studies where unequally spaced study contacts often occur, deliberate or not, can be readily modeled with existing statistical software, and this may be of particular importance in early life or any situation where rapidly occurring changes exist.
The results are consistent with the hypothesis that pet exposure and delivery mode may be markers of infant exposure to distinct microbiomes.
Acknowledgements
We would like to thank all the WHEALS study families for their past and future contributions. We also thank WHEALS research, lab and field staff; without your dedication this study would not have been possible.
Funding: This work was funded by the National Institutes of Health, USA (R01AI051598) and the Fund for Henry Ford Hospital.
Abbreviations used
- IgE
immunoglobulin E
- MLM
multilevel longitudinal model
- CI
confidence interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No author has any financial relationships with a biotechnology and/or pharmaceutical manufacturer that has an interest in the subject matter or materials discussed in the submitted manuscript.
Conflicts of interest We declare that we have no conflicts of interest.
References
- (1).Ronmark E, Bjerg A, Perzanowski M, Platts-Mills T, Lundback B. Major increase in allergic sensitization in schoolchildren from 1996 to 2006 in northern Sweden. J Allergy Clin Immunol. 2009;124:357–363. doi: 10.1016/j.jaci.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Arbes SJ, Jr., Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: Results from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–383. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- (3).McNeill G, Tagiyeva N, Aucott L, Russell G, Helms PJ. Changes in the prevalence of asthma, eczema and hay fever in pre-pubertal children: a 40-year perspective. Paediatr Perinat Epidemiol. 2009;23:506–512. doi: 10.1111/j.1365-3016.2009.01057.x. [DOI] [PubMed] [Google Scholar]
- (4).Romagnani S. The increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both? Immunol. 2004;112(3):352–363. doi: 10.1111/j.1365-2567.2004.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Bloomfield SF, Stanwell-Smith R, Crevel RW, Pickup J. Too clean, or not too clean: the hygiene hypothesis and home hygiene. Clin Exp Allergy. 2006;36:402–425. doi: 10.1111/j.1365-2222.2006.02463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Maier RM, Palmer MW, Andersen GL, Halonen MJ, Josephson KC, Maier RS, et al. Environmental determinants of and impact on childhood asthma by the bacterial community in household dust. Appl Environ Microbiol. 2010;76:2663–2667. doi: 10.1128/AEM.01665-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Fujimura KE, Johnson CC, Ownby DR, Cox MJ, Brodie EL, Havstad SL, et al. Man's best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol. 2010;126:410–2. 412. doi: 10.1016/j.jaci.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Smith PH, Ownby DR. Clinical Significance of Immunoglobulin E. Mosby; Philadelphia: 2009. pp. 845–58. [Google Scholar]
- (9).Burrows B, Martinez F, Halonen M, et al. Association of asthma with serum IgE levels and skin-test reactivity to allergens. New England Journal of Medicine. 1989;320:271–77. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- (10).Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325:1067–1071. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- (11).Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288:963–972. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- (12).Wegienka G, Johnson CC, Havstad S, Ownby DR, Zoratti EM. Indoor pet exposure and the outcomes of total IgE and sensitization at age 18 years. J Allergy Clin Immunol. 2010;126:274–9. 279. doi: 10.1016/j.jaci.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Mandhane PJ, Sears MR, Poulton R, Greene JM, Lou WY, Taylor DR, et al. Cats and dogs and the risk of atopy in childhood and adulthood. J Allergy Clin Immunol. 2009;124:745–750. doi: 10.1016/j.jaci.2009.06.038. [DOI] [PubMed] [Google Scholar]
- (14).Bufford JD, Reardon CL, Li Z, Roberg KA, Dasilva D, Eggleston PA, et al. Effects of dog ownership in early childhood on immune development and atopic diseases. Clin Exp Allergy. 2008;38:1635–1643. doi: 10.1111/j.1365-2222.2008.03018.x. [DOI] [PubMed] [Google Scholar]
- (15).Waser M, von ME, Riedler J, Nowak D, Maisch S, Carr D, et al. Exposure to pets, and the association with hay fever, asthma, and atopic sensitization in rural children. Allergy. 2005;60:177–184. doi: 10.1111/j.1398-9995.2004.00645.x. [DOI] [PubMed] [Google Scholar]
- (16).Renz-Polster H, David MR, Buist AS, Vollmer WM, O'Connor EA, Frazier EA, et al. Caesarean section delivery and the risk of allergic disorders in childhood. Clin Exp Allergy. 2005;35:1466–1472. doi: 10.1111/j.1365-2222.2005.02356.x. [DOI] [PubMed] [Google Scholar]
- (17).Pistiner M, Gold DR, Abdulkerim H, Hoffman E, Celedon JC. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J Allergy Clin Immunol. 2008;122:274–279. doi: 10.1016/j.jaci.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Bufford JD, Gern JE. Early exposure to pets: good or bad? Curr Allergy Asthma Rep. 2007;7:375–382. doi: 10.1007/s11882-007-0057-4. [DOI] [PubMed] [Google Scholar]
- (19).Bager P, Wohlfahrt J, Westergaard T. Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin Exp Allergy. 2008;38:634–642. doi: 10.1111/j.1365-2222.2008.02939.x. [DOI] [PubMed] [Google Scholar]
- (20).Visness CM, London SJ, Daniels JL, Kaufman JS, Yeatts KB, Siega-Riz AM, et al. Association of obesity with IgE levels and allergy symptoms in children and adolescents: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2009;123:1163–9. 1169. doi: 10.1016/j.jaci.2008.12.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy. 2008;38:629–633. doi: 10.1111/j.1365-2222.2007.02780.x. [DOI] [PubMed] [Google Scholar]
- (22).Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56:661–667. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kuitunen M, Kukkonen K, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, et al. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J Allergy Clin Immunol. 2009;123:335–341. doi: 10.1016/j.jaci.2008.11.019. [DOI] [PubMed] [Google Scholar]
- (25).Matricardi PM, Bockelbrink A, Gruber C, Keil T, Hamelmann E, Wahn U, et al. Longitudinal trends of total and allergen-specific IgE throughout childhood. Allergy. 2009;64:1093–1098. doi: 10.1111/j.1398-9995.2009.02055.x. [DOI] [PubMed] [Google Scholar]
- (26).Wright AL, Sherrill D, Holberg CJ, Halonen M, Martinez FD. Breastfeeding, maternal IgE, and total serum IgE in childhood. J Allergy Clin Immunol. 1999;104:589–594. doi: 10.1016/s0091-6749(99)70328-3. [DOI] [PubMed] [Google Scholar]
- (27).Rothers J, Stern DA, Spangenberg A, Lohman IC, Halonen M, Wright AL. Influence of early day-care exposure on total IgE levels through age 3 years. J Allergy Clin Immunol. 2007;120:1201–1207. doi: 10.1016/j.jaci.2007.07.036. [DOI] [PubMed] [Google Scholar]
- (28).Rotsides DZ, Goldstein IF, Canfield SM, Perzanowski M, Mellins RB, Hoepner L, et al. Asthma, allergy, and IgE levels in NYC head start children. Respir Med. 2010;104:345–355. doi: 10.1016/j.rmed.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Sherrill DL, Stein R, Halonen M, Holberg CJ, Wright A, Martinez FD. Total serum IgE and its association with asthma symptoms and allergic sensitization among children. J Allergy Clin Immunol. 1999;104:28–36. doi: 10.1016/s0091-6749(99)70110-7. [DOI] [PubMed] [Google Scholar]
- (30).Scirica CV, Gold DR, Ryan L, Abulkerim H, Celedon JC, Platts-Mills TA, et al. Predictors of cord blood IgE levels in children at risk for asthma and atopy. J Allergy Clin Immunol. 2007;119:81–88. doi: 10.1016/j.jaci.2006.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.