Abstract
Early-life seizures (ELS) are associated with long-term behavioral disorders including autism and ADHD, suggesting that frontal lobe structures may be permanently affected. We tested whether ELS produce structural alterations in the prefrontal cortex (PFC) and impair PFC-mediated function using an operant task of behavioral flexibility in rats. Adult rats that had been exposed to 75 flurothyl seizures during postnatal days 1–10 showed decreased behavioral flexibility in the task compared to controls over multiple behavioral sessions, measured as a lever preference asymmetry (p<0.001) and a decreased efficiency of attaining food rewards (p<0.05). ELS rats also showed an increased thickness of the PFC (p<0.01), primarily attributed to layer V (p<0.01) with no differences in cell density. These structural changes correlated with lever preference behavioral impairments (p<0.05). This study demonstrates that the consequences of ELS extend to the PFC, which may help explain the high prevalence of comorbid behavioral disorders following ELS.
Keywords: Behavior, Epilepsy, Flexibility, Prelimbic
Introduction
The incidence of seizures is highest during the first months to years of life. Regardless of etiology, ELS are commonly associated with later cognitive and behavioral impairments [1]. These impairments can have a substantial impact on the quality of life of patients and their families [1–8].
ELS-induced pathological alterations underlying these impairments are likely distributed among multiple neural networks that support cognitive abilities. In particular, ELS have a multitude of effects on the hippocampus and its physiology [1, 9]. Cellular, synaptic, and functional alterations in this structure often parallel memory impairments after ELS [10–12].
Abnormalities in other brain areas may help explain the behavioral disorders that are commonly associated with ELS including autism [13], attention-deficit-hyperactive-disorder or ADHD [14, 15], and obsessive compulsive disorder or OCD [16, 17]. These conditions are believed to involve alterations in frontal lobe function [18–20], implying that the neurological substrates underpinning those behaviors may be permanently altered by ELS.
The PFC is a frontal lobe structure that supports important cognitive and behavioral faculties such as working memory, decision-making, and behavioral flexibility [21]. In particular the prelimbic area of the PFC supports strategy set-shifting which is a measure of behavioral flexibility [22]. There is some evidence that ELS can affect the PFC in a rodent model. Specifically, neonatal status epilepticus can modify later dopaminergic and glutamatergic neurochemistry in the PFC, and alter behaviors related to addiction [23]. However, it is unknown whether repeated ELS affect PFC cellular architecture and behavioral functions that are typically associated with this structure.
In this study, we investigated this question by training rats that were exposed to ELS on a modified operant paradigm of behavioral flexibility [24]. We hypothesized that ELS induce structural abnormalities in the prelimbic PFC that might underlie altered behavioral flexibility later in life. We show that ELS-induced alterations include an increase in prelimbic PFC thickness and a related dysfunction in behavioral flexibility, providing a frontal lobe-based explanation for the behavioral alterations seen in children after ELS.
Methods
Animals
12 male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) from three litters were used in the study. Female rats were not included in the study in light of potential influences of fluctuating estrous cycle hormones on behavior over multiple days of testing [25]. All animal procedures were approved by the Dartmouth IACUC, under USDA and AAALAC-approved conditions in accordance with National Institutes of Health guidelines. Rats were kept with a 12-hour light/dark cycle and ad libitum access to food and water. Six of these rats (ELS group) were subjected to 75 flurothyl-induced seizures from postnatal (P) days 1–10 (7–8 seizures per day) using previously described methods from our laboratory [26]. This is an effective model of neonatal seizures [1] and does not result in lasting spontaneous seizures nor other epileptiform abnormalities in the EEG. Six control rats did not receive flurothyl treatment, but were separated from the mother for equivalent amounts of time. Animal groups thus experienced similar amounts of maternal attention and tactile stimulation, an important consideration since deficits in this could lead to decreased PFC thickness in later adulthood [27]. Following subsequent weaning from the mother at P25 rats grew to adulthood, pair-housed with a control and ELS rat in each cage. At P100 they began food-restriction to 85% of free-feeding weights before beginning the behavioral experiments approximately one week later.
Apparatus
Two operant conditioning chambers were contained in sound-attenuating cubicles and controlled with behavior software packages (Lafayette Instruments, Inc., Lafayette, IN; Med Associates Inc., St. Albans, VT). The chambers contained two retractable levers separated by a pellet dispenser on one of the walls. A stimulus light was located above each lever and a white incandescent house light bulb was above the chamber for general illumination. Food rewards were used in all tasks (45 mg Noyes food pellet; Research Diets, Incorporated, New Brunswick, NJ). Training sessions were run twice per day lasting 45 minutes each. Each rat was run at the same time each day and always in the same chamber.
Behavior
Rats were first trained to lever press with a simple fixed-ratio schedule. Both levers were continuously available, and each press produced a food reward from the pellet receptacle. After capriciously pressing one of the levers and receiving food reward, rats began methodically pressing levers. Each rat received at least 15 rewards during this initial session and pressed both levers at least once.
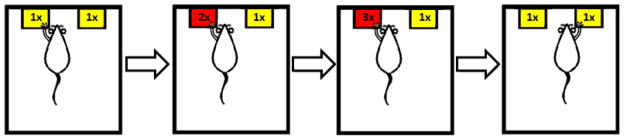
This phase was followed by four consecutive sessions of the progressive-ratio switching task (Fig. 1). In this task, the rat gained each of its first 5 rewards by simply pressing a lever a single time. The five subsequent rewards required an additional press (two presses per reward) if the rat continued on that same lever, and so on (three presses per reward for five rewards, then four presses per reward for five rewards, etc.). Switching to press the other lever reset the requirement on the original lever, while beginning to increment the requirement on the new lever after every five rewards as before. Thus it was most advantageous in this task for the rat to switch levers as often as possible. In this task, the advantage of switching levers when faced with increased work requirements would be undermined if the animal had poor behavioral flexibility (i.e. increased perseverance) [24].
Figure 1.
Behavioral flexibility task. In this progressive-ratio switching task, the rat was required to press a lever (yellow) for food an increasing number of times after each reward, creating a larger work requirement (red) as long as the rat persisted on the same lever. Upon switching to the other lever the work requirement was reset, then again increased after each reward until the rat switched again. This sequence was repeated until the 45 minute session was completed.
Histology
Following completion of the behavioral experiments, the rats were anesthetized with sodium pentobarbital, and perfused with 4% paraformaldehyde. Brain were removed, weighed, and stored in 4% paraformaldehyde for 1 week, followed by a solution of 25% sucrose in saline for another week. Brains were then frozen in dry ice and 50μm coronal sections were cut in a cryostat. These slices were mounted on glass slides, dried, and stained with thionine stain solution before being cover-slipped and stored for histological analysis. An experimenter who was blind to the treatment conditions used light microscopy and ImageJ (version 1.43; National Institutes of Health) to quantify the thickness and cell number of the separate layers of the prelimbic PFC from comparable brain slices across rats, approximately 3.0mm anterior to bregma [28, 29].
Data Analysis
All statistical analyses were performed using Stata 11 (StataCorp LP, College Station, TX), and all software for data handling was custom-written using MATLAB (The Mathworks, Inc., Natick, MA). We took a repeated measures approach to the analysis of behavioral data as each animal underwent four operant chamber sessions. Prior to carrying out the analyses we plotted a histogram of the data in order to assess which type of distribution was most appropriate to assume. Measures of behavioral flexibility in each session included total switches (i.e. the number of times the rat switched from using one lever to the other) and lever preference (the difference in presses between the right and left lever, normalized by the total number of lever presses). We assumed a Poisson distribution for count data and a gamma distribution for the lever preference and lever presses/reward data. All other data were normally distributed. Histological measures included thickness, cell count, and cell density of the prelimbic PFC (layers 2, 3, 5, and 6). We used a Students t-test to analyze these data.
Results
Behavior
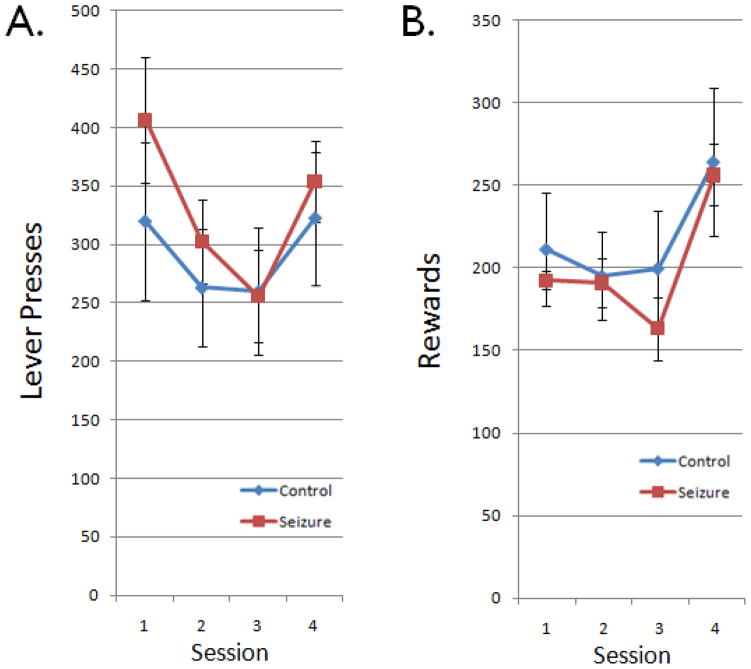
Total lever presses were analyzed to assess general activity levels (Fig. 2). Control and ELS rats did not differ in their ability to learn to press a lever for a food reward in the first session (p=0.52, Student’s t test). Further, control and ELS rats did not differ in the total number of lever presses or rewards obtained across sessions (p=0.50 and p=0.48). Thus, control and ELS rats showed similar motivations for acquisition of lever-pressing behavior and for rewards during the flexibility task.
Figure 2.
Total lever presses and rewards. Data indicate group means ± SEM for the total number of (A) lever presses and (B) rewards in a given session. Control (blue) and ELS (red) rats showed comparable numbers of lever presses and rewards during all of the sessions.
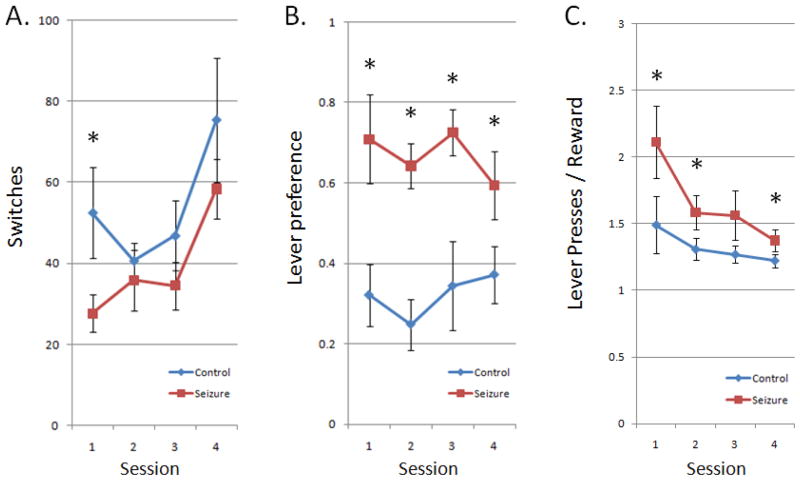
To measure behavioral flexibility, “switches” were quantified as the number of times the rat changed targets from one lever to the other per session (Fig. 3A). ELS rats had a trend to perform fewer switches per session (p=0.06) than control rats. Practice effects can be observed among both groups as increasing switches over sessions.
Figure 3.
Measures of behavioral flexibility. (A) Group means ± SEM for the total number of times the rats switched back and forth between the left and right levers. A higher value indicates more flexibility to switch between the levers. ELS rats (red) showed fewer total switches between levers during the sessions compared to controls (blue). (B) Group means ± SEM for the difference in lever presses between levers divided by total presses. A higher value indicates less flexibility to work among both levers equally. ELS rats showed much higher lever preferences than control rats in the task over all sessions. (C) Inefficiency in gaining rewards, shown as group means ± SEM for the total number of lever presses divided by total rewards. ELS rats were less efficient in gaining rewards (i.e. more lever presses per reward) than control rats over all session days. As with switches, practice effects can be seen among all animals over the consecutive sessions. Asterisks indicate statistically significant differences.
As a second measure of behavioral flexibility, lever preference was defined as the amount of activity spent on a preferred lever which indexed behavioral asymmetry and perseverance (Fig. 3B). ELS rats exhibited a higher lever preference than control rats in all sessions (p<0.001).
The work-related inefficiency of lever pressing for food reward is a practical outcome of behavioral flexibility, and was assessed by dividing the total number of lever presses by the total number of rewards in each session (Fig. 3C). There was a marked improvement across the sessions, with ELS rats averaging 2.11 presses per reward in the first session and dropping to only 1.37 by the fourth session, while controls began at 1.49 and ended at 1.22. However, as anticipated in light of the enduring deficits in lever preferences and potentially switches, ELS rats were still more inefficient than controls across all days of the task (p<0.05).
Histology
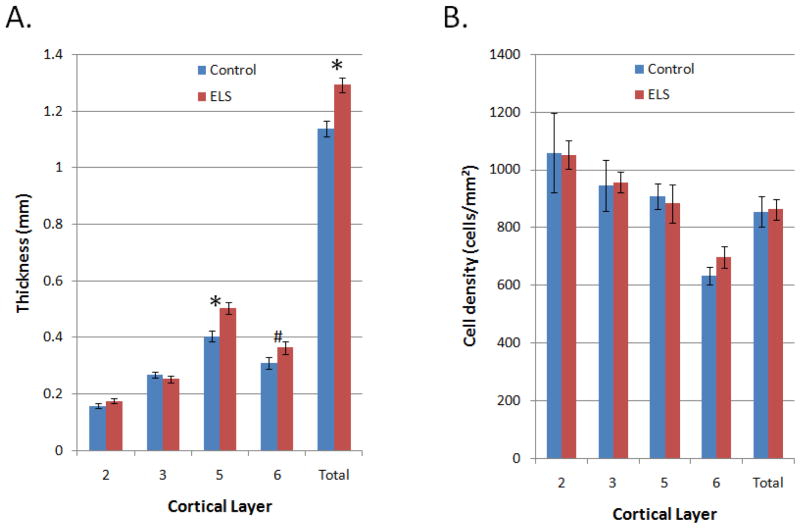
ELS rats showed increased prelimbic PFC thickness compared to control rats (p<0.01; Fig. 4), particularly due to layer V (p<0.01) and a trend in layer VI (p=0.1). Measures of cell number showed corresponding increases for ELS rats within these same layers. However, measures of cell density did not reveal group differences (p>0.05 for all). Additionally, no difference was seen between the groups in overall brain weight (p>0.05).
Figure 4.
PFC histology. Shown are group means ± SEM. (A). Compared to control rats (blue), ELS rats (red) showed thicker total PFC width (p<0.01), which was particularly prominent in the deeper layer V (p<0.01), along with a potential trend in layer VI (p=0.10). (B). No differences were seen in cell density among any of the layers.
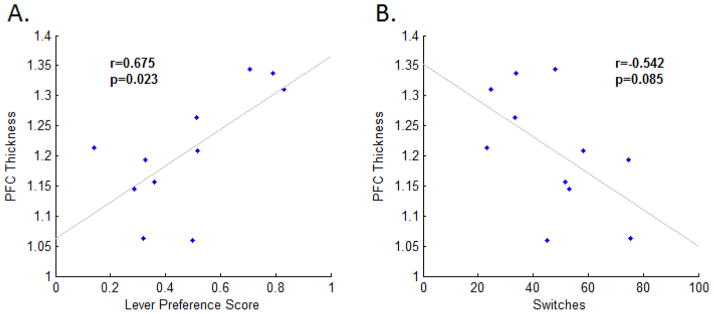
Finally, we assessed whether histological findings related to main findings of the behavioral data (Fig. 5). Behavioral measures were first averaged across the four sessions for each rat, to adjust within-animal. Lever preference and PFC thickness were significantly correlated (p<0.05, r=0.675). Similarly, there was a trend toward an inverse correlation between the number of switches and PFC thickness (p=0.085, r=−0.542).
Figure 5.
Relation of behavior and histology. PFC thicknesses were positively correlated with combined lever preference scores (A), and trended toward a negative correlation with combined switches (B), implicating an enlarged PFC as a cortical substrate of increased perseverance in this study.
Discussion
This study demonstrates that adult rats that experienced ELS have impaired behavioral flexibility, a PFC-mediated function [22]. The groups showed no differences in motivation that could account for these findings, indexed by total lever presses. Furthermore, the prelimbic PFC was thicker in these rats primarily attributed to the deeper layers, with no differences in cell density. This thickness was positively correlated with lever preference which served as a measure of behavioral flexibility (i.e. increased preference implies less flexibility). This correlation implies that ELS-induced alterations in the PFC may indeed contribute to impairments in behavioral flexibility. To our knowledge this exploratory study is the first to directly investigate PFC structure and function following repeated ELS.
A dysfunctional PFC following ELS may help explain the comorbid behavioral disorders that are relatively more common in these patients, since these disorders often involve frontal lobe dysfunction. For example, a recent report finds approximately 30% overlap between autism and childhood epilepsy [13]. Seizures are likely to contribute to autism risk, though both of these conditions can stem from a variety of etiologies [30]. The long-term alterations in excitatory and inhibitory neurotransmission that ELS engender may provide a mechanism for the co-occurrence of this disorder [9, 18, 26]. It is interesting to note that our histological findings are consistent with the observation that autism has been associated with abnormal and increased brain growth. However it is clear that we have not shown that ELS can provide a model of autism as we have not investigated two core features of this condition: socialization and language deficits. In addition, about 20% of children with epilepsy have ADHD, a four-fold increase over the general population [14]. PFC dysfunction is a likely contributor to ADHD, particularly due to its integrative role in attention and self-regulation [19]. In addition, OCD often co-occurs with refractory adult epilepsy [31] and has been associated with frontal lobe dysfunction [20].
Behavioral flexibility refers to the ability to adaptively adjust acquired responses upon a change in environmental conditions [32]. This is manifested in an operant behavior work-ratio setting by updating response strategies when reward contingencies are altered to maintain or increase efficiency (i.e. maximizing rewards while minimizing work). For example, the rats in this study were able to increase the total rewards while decreasing the total lever presses by switching between the levers more often. Taken together, all rats averaged only 1.3 presses per reward by the fourth session of the task, indicating that they were switching between the levers after almost every press and approaching the most lucrative strategy (i.e. alternation between the levers). Although control and ELS rats showed comparable levels of total lever presses, ELS rats switched levers significantly less often. This lack of switching produced a considerable burden of extra lever pressing in those sessions. Given the number of rewards obtained and lever pressing efficiency, ELS rats were pressing the levers an additional 30–120 times on average during a given session for a comparable number of rewards, which equates to a 12–42% increase in labor.
Our self-mediated progressive-ratio paradigm was unique in that the animal could reset the work ratio counter at any time by simply switching levers. Most previous studies with progressive ratio schedules use ratios that cannot be reset, and continue increasing step-wise after a specified number of presses, rewards, or time intervals. An important measure in these past studies was the “break point” [33], or the work ratio at which the animal appreciably decreases responding, or stops altogether. In these step-wise work ratio paradigms, the break point can serve as an index of motivation of the animal [34]. Our paradigm was not designed to assess motivation, but rather behavioral flexibility. This is nicely indexed by a “perseverance break point” which is the measure of inefficiency (i.e. lever presses per reward), or the animal's persistence on a lever before it resets the work requirement by pressing the other lever. Overall, the behavior data suggests that ELS animals were less likely to switch and often persevered asymmetrically on a preferred lever demonstrating impaired behavioral flexibility. Future studies of the effects of ELS on PFC function could adopt this task, which may help to address potential mechanisms of the impairments and other interplaying structures.
Our goal in this study was to elucidate the effects of ELS on the PFC. The PFC is involved in many other functions such as working memory or attention, which may also be important for performing our behavioral flexibility task. Although these tasks are heavily dependent upon PFC, the hippocampus may also play an important role. Previous research has targeted the effects of ELS on the hippocampus, noting a number of histological changes such as aberrant mossy fiber sprouting in the CA3 region [35], and reduced neurogenesis in the dentate gyrus [36]. Physiological changes can occur as well, such as alterations of glutamate and GABA receptor expression, and enhanced excitability [12, 26]. These changes parallel impairments in hippocampal function after ELS [11, 34, 37, 38], and have been extrapolated to the human condition to explain the pervasive memory and cognitive deficits seen in approximately 70% of people that experienced ELS [8]. Our results indicate that the long-term consequences of ELS extend beyond the hippocampus and also involve at least the PFC, if not more cortical or interconnected limbic structures. Further study is required to investigate whether underconnectivity between the PFC and hippocampus significantly contributes to the post-ELS phenotype observed in the current study. This would be consistent with the reported underconnectivity between brain regions in children with autism [39].
With these findings in mind, future studies could delineate the mechanism of PFC functional alterations, which are likely associated with the increased thickness of deep prelimbic PFC layers. We speculate that ELS may have enlarged this area in adulthood by altering neurodevelopmental processes, although this requires further investigation using immunohistochemical staining techniques to differentiate between neuron types, glia, and synaptic or dendritic densities.
The PFC may be a key brain area for understanding comorbidities associated with ELS that relate to behavioral disorders (e.g. ADHD, Autism, OCD). Other PFC-related functions such as attention and decision-making may be likewise disrupted and could be investigated to provide further insight into the behavioral disorders that are common to this clinical condition.
Research Highlights.
Rats exposed to the flurothyl model of early-life seizures showed impaired behavioral flexibility in later adulthood.
Early-life seizures increased the thickness and cell number of the prefrontal cortex in these rats.
Increased prefrontal cortex thickness correlated with impaired behavioral flexibility.
We suggest that behavioral disorders after early-life seizures may be related to fixed alterations in the prefrontal cortex.
Acknowledgments
This work was supported by the National Institutes of Health grants F30NS064624 (J.K.) and R01NS044295 (A.S., E.W., A.H., G.H.), and the Great Ormond Street Children’s Charity (R.S.). We thank Peter Cuadrilla for support and Pierre-Pascal Lenck-Santini for input on the manuscript.
Footnotes
Disclosures: The authors have no conflicts of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with these guidelines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holmes GL. The long-term effects of neonatal seizures. Clin Perinatol. 2009;36:901–14. vii–viii. doi: 10.1016/j.clp.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Huttenlocher PR, Hapke RJ. A follow-up study of intractable seizures in childhood. Ann Neurol. 1990;28:699–705. doi: 10.1002/ana.410280516. [DOI] [PubMed] [Google Scholar]
- 3.Glosser G, Cole LC, French JA, Saykin AJ, Sperling MR. Predictors of intellectual performance in adults with intractable temporal lobe epilepsy. J Int Neuropsychol Soc. 1997;3:252–9. [PubMed] [Google Scholar]
- 4.Bulteau C, Jambaque I, Viguier D, Kieffer V, Dellatolas G, Dulac O. Epileptic syndromes, cognitive assessment and school placement: a study of 251 children. Dev Med Child Neurol. 2000;42:319–27. doi: 10.1017/s0012162200000566. [DOI] [PubMed] [Google Scholar]
- 5.Hermann B, Seidenberg M, Bell B, Rutecki P, Sheth R, Ruggles K, Wendt G, O'Leary D, Magnotta V. The neurodevelopmental impact of childhood-onset temporal lobe epilepsy on brain structure and function. Epilepsia. 2002;43:1062–71. doi: 10.1046/j.1528-1157.2002.49901.x. [DOI] [PubMed] [Google Scholar]
- 6.Bjornaes H, Stabell K, Henriksen O, Loyning Y. The effects of refractory epilepsy on intellectual functioning in children and adults. A longitudinal study. Seizure. 2001;10:250–9. doi: 10.1053/seiz.2000.0503. [DOI] [PubMed] [Google Scholar]
- 7.Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical Neonatal Seizures are Independently Associated with Outcome in Infants at Risk for Hypoxic-Ischemic Brain Injury. J Pediatr. 2009;155:318–23. doi: 10.1016/j.jpeds.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Legido A, Clancy RR, Berman PH. Neurologic outcome after electroencephalographically proven neonatal seizures. Pediatrics. 1991;88:583–96. [PubMed] [Google Scholar]
- 9.Isaeva E, Isaev D, Savrasova A, Khazipov R, Holmes GL. Recurrent neonatal seizures result in long-term increases in neuronal network excitability in the rat neocortex. Eur J Neurosci. 2010;31:1446–55. doi: 10.1111/j.1460-9568.2010.07179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Rogalski Landrot I, Minokoshi M, Silveira DC, Cha BH, Holmes GL. Recurrent neonatal seizures: relationship of pathology to the electroencephalogram and cognition. Brain Res Dev Brain Res. 2001;129:27–38. doi: 10.1016/s0165-3806(01)00177-8. [DOI] [PubMed] [Google Scholar]
- 11.Karnam HB, Zhou JL, Huang LT, Zhao Q, Shatskikh T, Holmes GL. Early life seizures cause long-standing impairment of the hippocampal map. Exp Neurol. 2009;217:378–87. doi: 10.1016/j.expneurol.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornejo BJ, Mesches MH, Coultrap S, Browning MD, Benke TA. A single episode of neonatal seizures permanently alters glutamatergic synapses. Ann Neurol. 2007;61:411–26. doi: 10.1002/ana.21071. [DOI] [PubMed] [Google Scholar]
- 13.Brooks-Kayal A. Epilepsy and autism spectrum disorders: are there common developmental mechanisms? Brain Dev. 2010;32:731–8. doi: 10.1016/j.braindev.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann R, Goldberg-Stern H, Shuper A. Attention-deficit disorders and epilepsy in childhood: incidence, causative relations and treatment possibilities. J Child Neurol. 2009;24:727–33. doi: 10.1177/0883073808330165. [DOI] [PubMed] [Google Scholar]
- 15.Dunn DW. Neuropsychiatric aspects of epilepsy in children. Epilepsy Behav. 2003;4:101–6. doi: 10.1016/s1525-5050(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 16.Cabaleiro Goas M, Gomez-Reino y F, Carrero Martinez P, Penzol Diaz J. Obsessive syndrome of epileptic etiology in children and pre-adolescents. Arch Neurobiol (Madr) 1969;32:521–34. [PubMed] [Google Scholar]
- 17.Levin B, Duchowny M. Childhood obsessive-compulsive disorder and cingulate epilepsy. Biol Psychiatry. 1991;30:1049–55. doi: 10.1016/0006-3223(91)90124-5. [DOI] [PubMed] [Google Scholar]
- 18.Harada M, Taki MM, Nose A, Kubo H, Mori K, Nishitani H, Matsuda T. Non-Invasive Evaluation of the GABAergic/Glutamatergic System in Autistic Patients Observed by MEGA-Editing Proton MR Spectroscopy Using a Clinical 3 Tesla Instrument. J Autism Dev Disord. 2010 doi: 10.1007/s10803-010-1065-0. [DOI] [PubMed] [Google Scholar]
- 19.Sheridan MA, Hinshaw S, D'Esposito M. Efficiency of the prefrontal cortex during working memory in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:1357–66. doi: 10.1097/chi.0b013e31812eecf7. [DOI] [PubMed] [Google Scholar]
- 20.Joel D, Doljansky J, Roz N, Rehavi M. Role of the orbital cortex and of the serotonergic system in a rat model of obsessive compulsive disorder. Neuroscience. 2005;130:25–36. doi: 10.1016/j.neuroscience.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 21.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 22.Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Lin TC, Huang LT, Huang YN, Chen GS, Wang JY. Neonatal status epilepticus alters prefrontal-striatal circuitry and enhances methamphetamine-induced behavioral sensitization in adolescence. Epilepsy Behav. 2009;14:316–23. doi: 10.1016/j.yebeh.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Winter S, Dieckmann M, Schwabe K. Dopamine in the prefrontal cortex regulates rats behavioral flexibility to changing reward value. Behav Brain Res. 2009;198:206–13. doi: 10.1016/j.bbr.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 25.Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–95. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- 26.Isaeva E, Isaev D, Khazipov R, Holmes GL. Selective impairment of GABAergic synaptic transmission in the flurothyl model of neonatal seizures. Eur J Neurosci. 2006;23:1559–66. doi: 10.1111/j.1460-9568.2006.04693.x. [DOI] [PubMed] [Google Scholar]
- 27.Muhammad A, Hossain S, Pellis SM, Kolb B. Tactile stimulation during development attenuates amphetamine sensitization and structurally reorganizes prefrontal cortex and striatum in a sex-dependent manner. Behav Neurosci. 2011;125:161–74. doi: 10.1037/a0022628. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Amsterdam; Boston: Academic Press/Elsevier; 2007. [Google Scholar]
- 29.Gabbott PL, Dickie BG, Vaid RR, Headlam AJ, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: morphology and quantitative distribution. J Comp Neurol. 1997;377:465–99. doi: 10.1002/(sici)1096-9861(19970127)377:4<465::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Tuchman R, Rapin I. Epilepsy in autism. Lancet Neurol. 2002;1:352–8. doi: 10.1016/s1474-4422(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan PW. Epilepsy and obsessive-compulsive disorder. Dialogues Clin Neurosci. 2010;12:241–8. doi: 10.31887/DCNS.2010.12.2/pkaplan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghahremani DG, Monterosso J, Jentsch JD, Bilder RM, Poldrack RA. Neural components underlying behavioral flexibility in human reversal learning. Cereb Cortex. 2010;20:1843–52. doi: 10.1093/cercor/bhp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kheramin S, Body S, Herrera FM, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: implications for models of inter-temporal choice. Behav Brain Res. 2005;156:145–52. doi: 10.1016/j.bbr.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Killeen PR, Sitomer MT. Mpr. Behav Processes. 2003;62:49–64. doi: 10.1016/S0376-6357(03)00017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol. 1998;44:845–57. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- 36.McCabe BK, Silveira DC, Cilio MR, Cha BH, Liu X, Sogawa Y, Holmes GL. Reduced neurogenesis after neonatal seizures. J Neurosci. 2001;21:2094–103. doi: 10.1523/JNEUROSCI.21-06-02094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z, Yang Y, Silveira DC, Sarkisian MR, Tandon P, Huang LT, Stafstrom CE, Holmes GL. Consequences of recurrent seizures during early brain development. Neuroscience. 1999;92:1443–54. doi: 10.1016/s0306-4522(99)00064-0. [DOI] [PubMed] [Google Scholar]
- 38.Sogawa Y, Monokoshi M, Silveira DC, Cha BH, Cilio MR, McCabe BK, Liu X, Hu Y, Holmes GL. Timing of cognitive deficits following neonatal seizures: relationship to histological changes in the hippocampus. Brain Res Dev Brain Res. 2001;131:73–83. doi: 10.1016/s0165-3806(01)00265-6. [DOI] [PubMed] [Google Scholar]
- 39.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an fMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]