Abstract
As our understanding of the complexities of the various etiologies and complex genetic architecture of GnRH deficiency grows, so too does the need to apply newly-developed genetic tools in a way that: a) is meaningful to individuals and their families; b) integrates all of the phenotypic features of this syndrome into a rationale; and c) provides up-to-date diagnostic technologies in a cost-effective algorithm of genetic testing. Genetic counseling aims to accomplish these goals through ascertainment of detailed family histories, targeted comprehensive phenotypic evaluations, informed selection of genetic testing, interpretation of genetic test results, and the provision of highly specific risk assessments and psychological support to individuals diagnosed with this reproductive condition.
This chapter offers a guide to incorporating this rapidly evolving state of knowledge of the pedigree and phenotypes into the process of selecting and prioritizing genetic testing. In addition, the provision of risk assessment that accounts for nuanced genetic concepts such as variable expressivity, incomplete penetrance, and oligogenicity, all of which are emerging features of the genetics of this clinical syndrome, is considered. Beyond translating genetic information, genetic counseling should address the psychological impact of embarrassment, shame, anxiety, and guilt that are often seen among individuals with reproductive disorders.
Keywords: genetic counseling, GnRH deficiency, Kallmann syndrome
Introduction
In a field deeply impacted by the rapid changes in the diagnosis and management of genetic diseases, the dynamic practice of genetic counseling must be regularly evaluated and revised to reflect the expanding roles of genetic counselors as well as the evolving knowledge and tools used to support it. The professional organization for the field, The National Society of Genetic Counselors (NSGC), has offered a concise definition for the practice of genetic counseling as “the process of helping people understand and adapt to the medical, psychological, and familial implications of the genetic contributions to disease” (Resta et al., 2006, p. 79). This process includes providing risk assessment, educating patients regarding this risk, and assisting with management, research, and resource identification, as well as providing psychosocial counseling to facilitate informed decision making and coping.
For patients with idiopathic GnRH deficiency (IGD) with either the Kallmann Syndrome (KS) or normosmic Idiopathic Hypogonadotropic Hypogonadism (nIHH) phenotype, genetic counseling can elucidate which of the many inheritance patterns of IGD is operating in an individual’s family; delineate the full phenotypic spectrum of both the reproductive and non-reproductive phenotypes present in their family; provide a personalized recurrence risk; assist patients in deciphering complex genetic concepts relevant to their diagnosis and genetics; and navigate the growing number of options for clinical and research-based molecular testing. Additionally, genetic counseling offers a unique opportunity to identify resources needed to meet the social and emotional needs of individuals diagnosed with a highly personal reproductive condition.
The American College of Medical Genetics (ACMG) includes abnormal timing of puberty among the traits indicating a referral to a medical genetics professional (Pletcher et al., 2007). While the care of most patients with IGD is typically managed between the patient’s primary care physician and an endocrinologist, a referral to a genetics professional such as a medical geneticist or genetic counselor, should also be considered early on in the evaluation of patients with IGD. If possible, a genetics center that specializes in reproductive disorders should be considered. A genetics team is ideally suited to obtain thorough family histories and search for phenotypic clues, both reproductive and non-reproductive in nature, as well as to discuss recurrence risks, interpret test results, and address the psychosocial implications of genetic disease and testing. Specifically for patients with other, non-reproductive phenotypes, a referral to a genetics team can help clarify what evaluations may be necessary or helpful to classify an uncertain diagnosis.
The Genetics of IGD
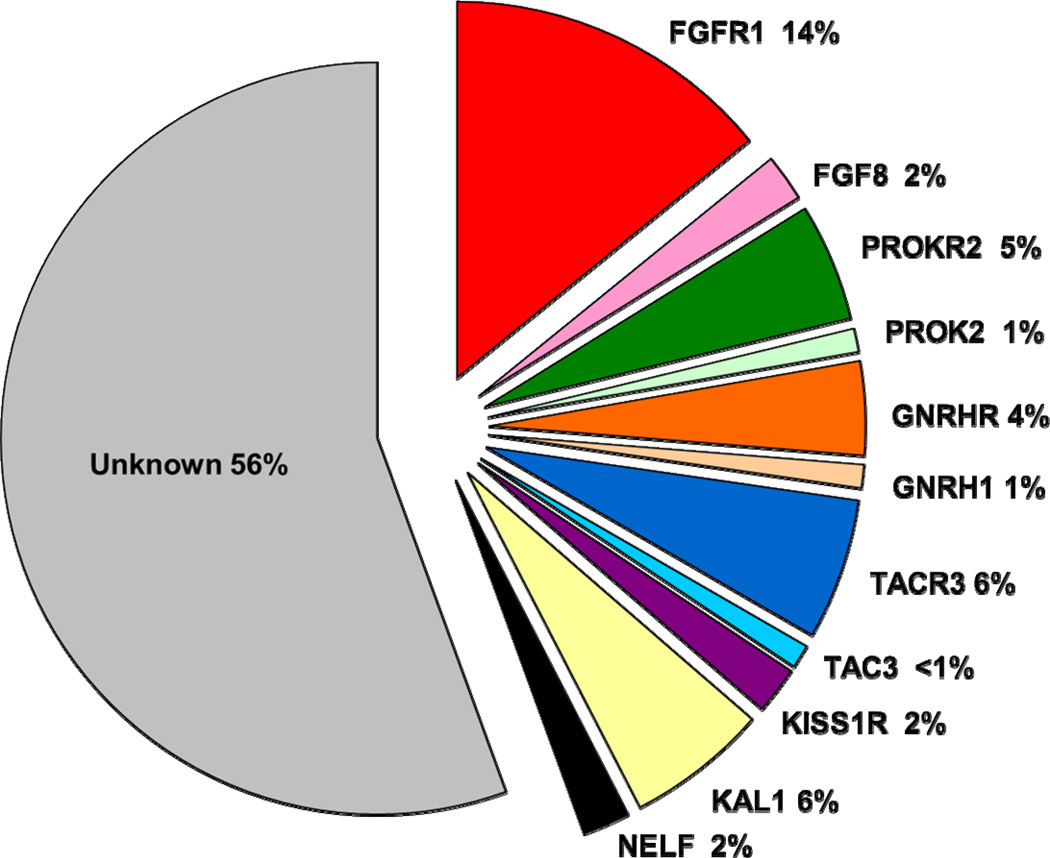
Disease-contributing mutations are currently identifiable in only about 45% of patients with IGD (Figure 1); though this percentage is rapidly increasing. However, given that for the majority of patients with IGD, there is still no discernible molecular change, obtaining a detailed, three generation family history is a critical initial step in attempting to determine the inheritance pattern and recurrence risk for patients and their family members. Key clues in the family history can also aid in the process of selecting the most appropriate genetic testing. Approximately 30% of individuals with IGD will show a familial pattern of inheritance when an accurate and detailed family history has been provided whereas the remaining cases lack an apparent family history and thus are currently considered sporadic. Familial patterns run the full Mendelian spectrum of X-linked recessive, such as that which occurs in most patients with IGD + anosmia with KAL1 mutations, and both autosomal recessive and dominant modes of inheritance (Pallais et al., 2010).
Figure 1.
Prevalence of rare sequence variants (present in less than 1% of the healthy control population) in the IGD study participants screened through the Reproductive Endocrine Unit at Massachusetts General Hospital. Rare sequence variants in known IGD genes have been detected in approximately 44% of our patient cohort with IGD, while the majority of patients with IGD have an unknown genetic etiology.
X-Linked Genes
Typical of X-linked recessive pedigrees with the KAL1 gene mutations, only males are affected and are related through unaffected females (Figure 2). However, recent data from our own group examining female carriers and/or women with GnRH deficiency and mutations in the KAL1 gene indicate that inheritance of KAL1 variant-related traits may be more complex (Shaw et al., 2011). Whether or not these clinical features of X-linked genes that occur in females with this condition represent the co-existence of mutations in other genes that often accompany Isolated GnRH Deficiency, i.e. oligogenicity (Sykiotis et al., 2010) or some degree of mosaicism of the X inactivation process currently remains unclear. Nonetheless, it is clear that some females with rare sequence variants in the KAL1 gene do have some clinical manifestions.
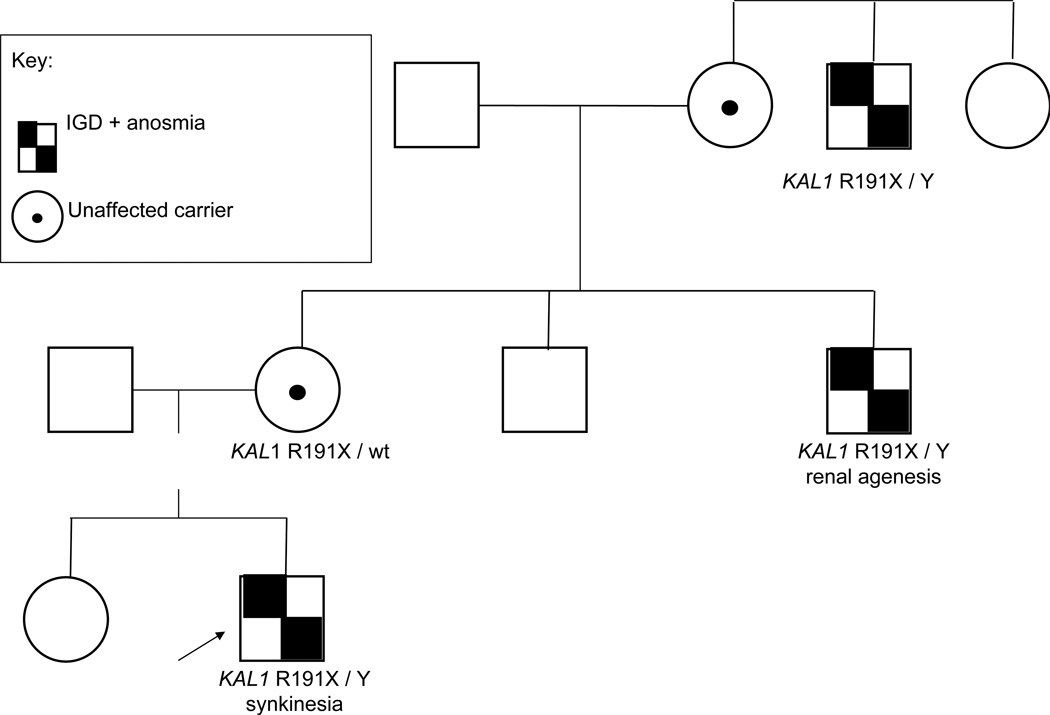
Figure 2.
A family with IGD + anosmia (Kallmann syndrome) demonstrating x-linked recessive inheritance and phenotypic features typical of KAL1. The proband, indicated by the arrow, has synkinesia in addition to his anosmic IGD, and carries a R191X mutation on the KAL1 gene on his X chromosome and a normal Y chromosome. His maternal uncle carries the same KAL1 mutation and has Kallmann syndrome with renal agenesis, and his maternal great-uncle with the mutation also has Kallmann syndrome. Typical of an x-linked recessive pedigree, no male-to-male transmission is seen and female carriers are asymptomatic.
An absolute requirement of an X-linked mode of inheritance is that there is no male-to-male transmission of the phenotype within a pedigree. In classic X-linked recessive pedigrees, carrier females have a 50% chance for their male children to be affected; in addition, 50% of their female children will be carriers of the gene. All female offspring of affected men are carriers and all male offspring are free of any genetic defect. On a molecular level, rare variants in the KAL1 gene are currently the only identified genetic contributors to X-linked recessive IGD. In addition, to date, mutations in the KAL1 gene are unique or private within families. However, some males with DAX1 variants (also X-linked recessive) can exhibit both GnRH deficiency and congenital adrenal hypoplasia. Patients with rare variants in KAL1 often show some key phenotypic traits that are clues to the presence of a KAL1 variant, including: anosmia, unilateral renal agenesis, cryptorchidism, microphallus, and/or synkinesia. Such traits can be important in selecting KAL1 gene testing as the initial diagnostic step.
Sequence variants in KAL1 are detected in 5–10% of individuals with IGD by exomic sequencing of the KAL1 gene. Using multiplex ligation-dependent probe amplification (MLPA) 7.4% of all patients with IGD were found to have deletions of the KAL1 gene. This percentage of patients in whom deletions in KAL1 were identified rose to 12% when the population was limited to anosmic males with IGD (Pederson-White et al., 2008). While KAL1 screening has been traditionally been performed as sequencing in males with IGD + anosmia, this study suggests that offering deletion/duplication studies in people with both anosmic and normosmic IGD may yield informative results. Additionally, array comparative genomic hybridization (aCGH), quantitative PCR, long-range PCR, and/or fluorescent in situ hybridization (FISH) may be used to detect deletions and duplications too large to be identified by sequencing the coding regions (Oliveira et al., 2001; Georgopoulos et al., 1997. In particular, such follow-up studies might well be appropriate where exomic sequencing failed to reveal a coding sequence mutation in a patient with IGD + anosmia.
Autosomal Recessive Genes
In autosomal recessive pedigrees, both males and females are affected in equal proportions and affected individuals typically exist in sibships with unaffected parents. Consanguinity or endogamy (a relationship between parents of the same small racial, ethnic or geographic population) can be a clue indicating possible autosomal recessive inheritance. If a patient’s IGD is determined to be autosomal recessive in its inheritance, carrier parents can then be counseled of their 25% recurrence risk for future pregnancies and the partners of affected individuals should be offered carrier testing (when available) to assess recurrence risk for their offspring accurately. Rare sequence variants known to demonstrate autosomal recessive inheritance in IGD are typically found in the following genes: GNRHR (Cerrato et al., 2006, Bedecarrats & Kaiser, 2007; de Roux et al., 1997), KISS1R (Semple et al., 2005; de Roux et al., 2003; Seminara et al., 2003), TACR3 (Gianetti et al., 2010; Guran et al., 2009; Topaloglu et al., 2009), TAC3 (Topaloglu et al., 2009), and GNRH1 (Bouligand et al., 2009; Chan et al., 2009).
Phenotypically, male patients with variants in TAC3/TACR3 (Figure 3) have two ‘signatures’ clinically - microphallus and reversal of their absent puberty in adulthood (Gianetti et al., 2010). In addition to this unusual “recovery” of their reproductive axis in adulthood, individuals with variants in the Neurokinin B (TAC3/TACR3) pathway have a normal sense of smell. Therefore, phenotypic features such as reversal of neuroendocrine profile, normosmia, and microphallus can serve as hallmarks that may call for priority testing of TAC3/TACR3 signalling system as described in Figure 6.
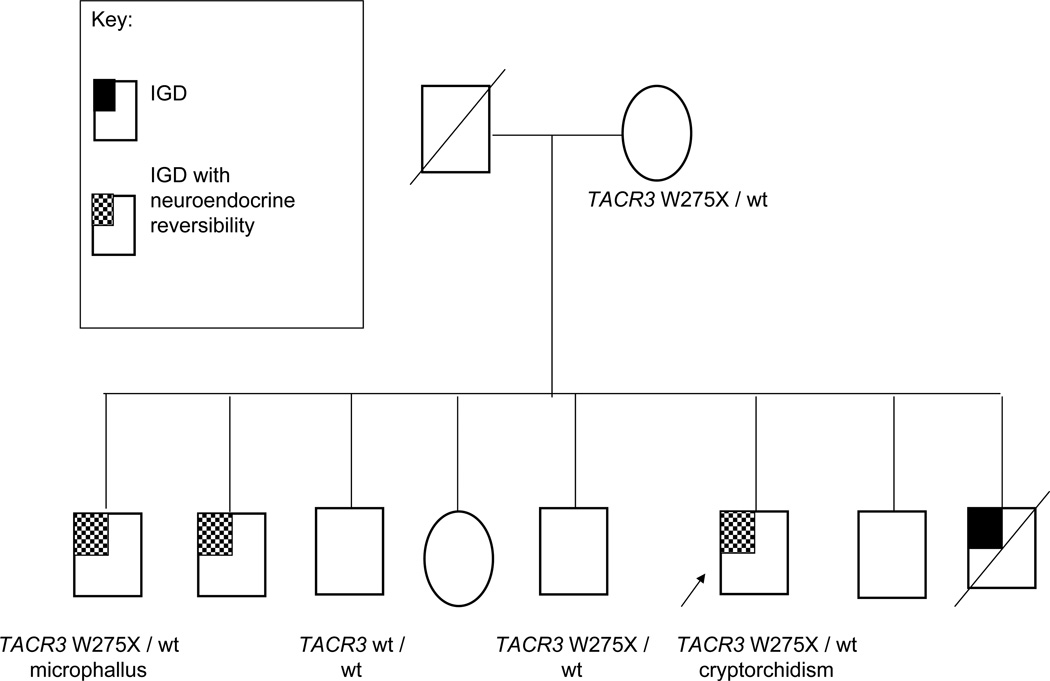
Figure 3.
A TACR3 positive family with IGD demonstrating complex autosomal recessive inheritance. Typical of an autosomal recessive pedigree, the affected family members with IGD are siblings and the mother is a carrier of a single TACR3 mutation. However, in true autosomal recessive inheritance, affected family members are expected to carry a genetic variant on each allele, while only one mutated allele has been identified in the affected members of this family. The microphallus seen in one of the brothers with IGD, as well as the neuroendocrine reversibility, can be subtle clues to suspect a TACR3 or TAC3 mutation..
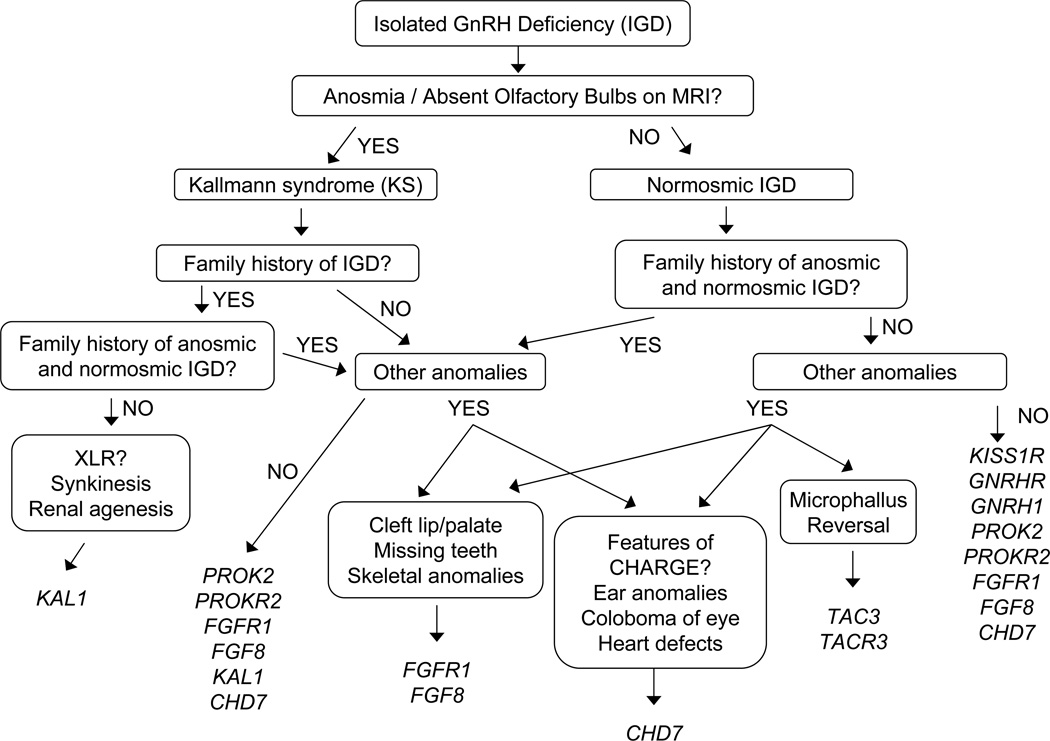
Figure 6.
A proposed algorithm for prioritizing genetic testing for IGD based on phenotypic information. Based on non-reproductive phenotypes present in patients and families with IGD, screening of patients with IGD for genetic variants can be prioritized. While clinical testing for all genes is not currently available, this tiered screening approach may be useful in saving health-care dollars as clinical testing for IGD becomes more readily available.
Typically, several of the genes demonstrating autosomal recessive inheritance (GNRH, GNRHR, KISS1, and KISS1R) have only been implicated in normosmic IGD. However, should an autosomal recessive pedigree include individuals with a congenital anosmia phenotype in their sibships with normosmic patients, i.e. ‘mixed pedigrees’, screening of PROK2/PROKR2 and the genes of the FGF signaling pathways may be prioritized.
Autosomal Dominant Genes
Autosomal dominant pedigrees typically display a “vertical” inheritance pattern in contrast to the “horizontal” pattern characteristic of autosomal recessive families. More than one generation is affected with the phenotype/trait (Figure 4). While males and females are equally affected and male-to-male transmission of the trait is usually seen, deviations from this pattern have been noted and suggest that other genes may be involved. Such digenicity (Figure 5 below) is somewhat typical of those genes in the FGF signaling pathway.
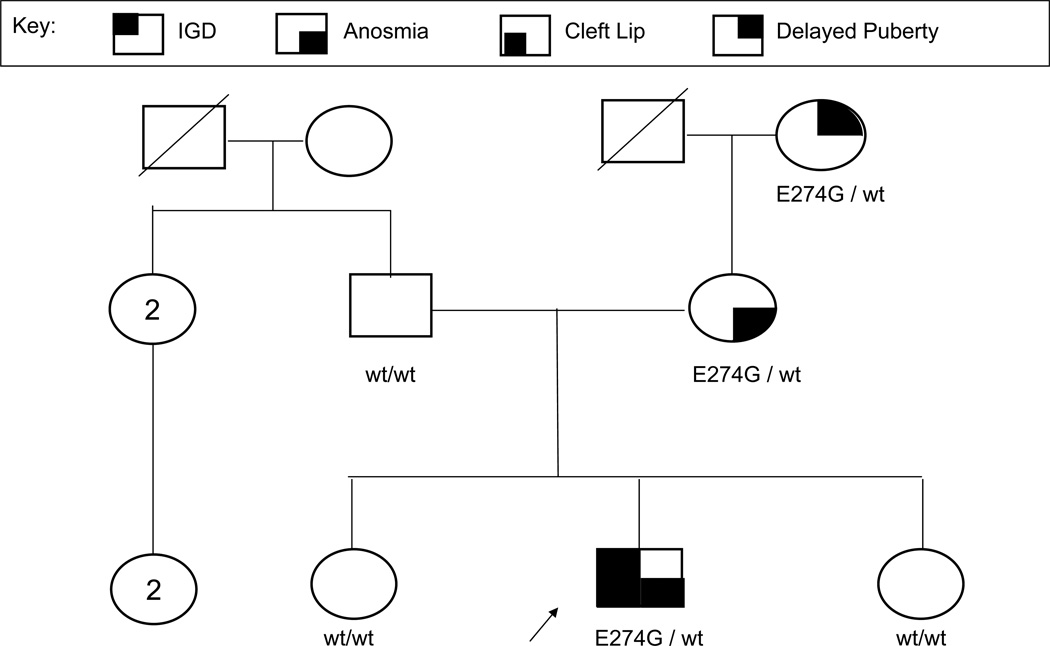
Figure 4.
An IGD pedigree showcasing autosomal dominant inheritance with variable expressivity. As is typical for an autosomal dominant inheritance pattern, several generations have an “affected” family member. Affected individuals have one wild-type FGFR1 allele and one mutated FGFR1 allele. Additionally, the concept of variable expressivity is shown by the grandmother with delayed puberty, the mother with anosmia and normal puberty, and the proband (indicated by the arrow) with IGD, anosmia, and cleft lip, though all have the same genetic change.
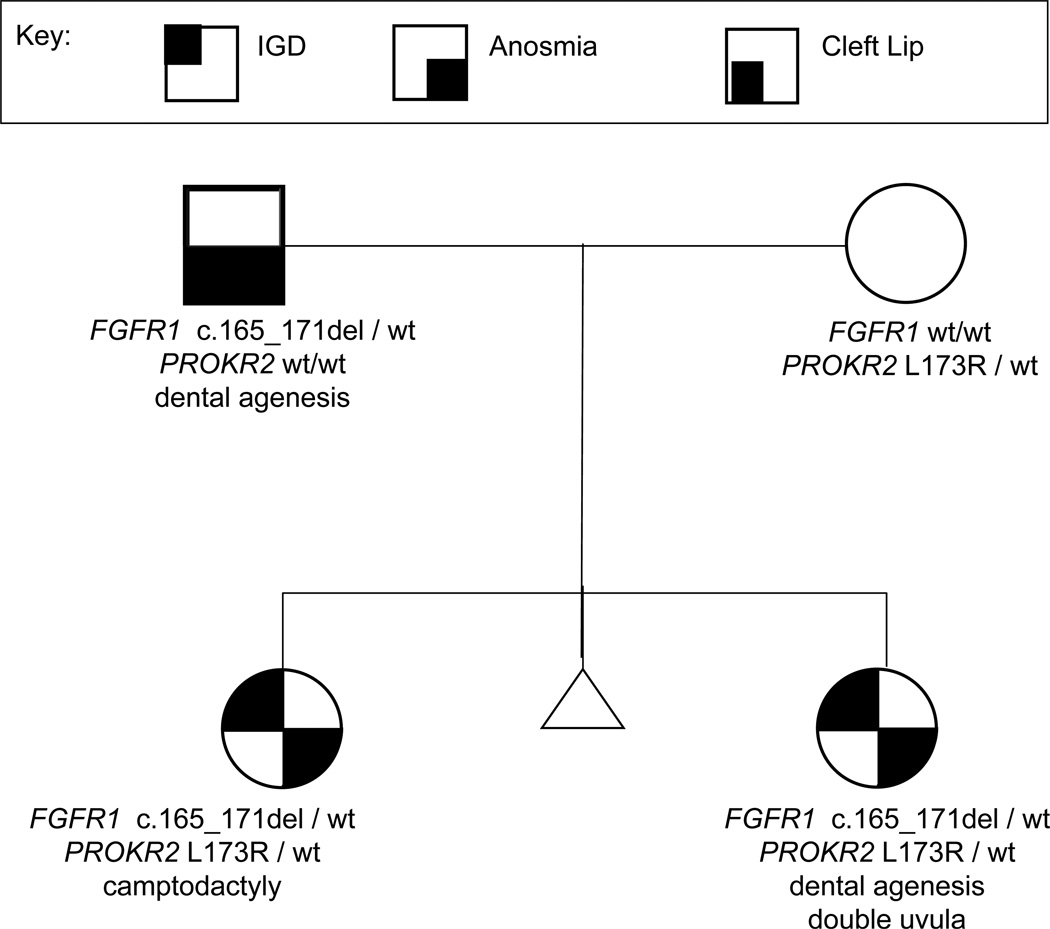
Figure 5.
An example of a family displaying digenic causes of IGD. Given the non-reproductive phenotypes present in the family (camptodactyly, dental agenesis, cleft lip), it seemed likely that the family would carry the FGFR1 variant which was identified in both daughters with Kallmann syndrome (IGD + anosmia). However, the additional presence of a PROKR2 variant helps clarify the more severe phenotype in the daughters. The PROKR2 variant likely modifies the phenotypic effects of FGFR1 leading to IGD for the daughters who have a rare sequence variant in each gene.
Curiously, affected individuals in pedigrees with mutations that are known to be autosomal recessive in mice appear to have a 50% chance of having an affected child and 50% chance of having an unaffected child. Rare variants in FGFR1, PROKR2 and PROK2 (Abreu et al., 2008; Cole et al., 2008; Dode et al., 2006; Pitteloud et al., 2007a,), CHD7 (Jongmans et al., 2009; Kim et al., 2008), and FGF8 (Falardeau et al., 2008, Trarbach et al., 2010a) have been reported to show potentially autosomal dominant inheritance in IGD. These puzzles remain to be elucidated and as more genetic variation is identified, the genetics of IGD will become more complex.
Given such multi-generational pedigrees, the non-reproductive features associated with isolated GnRH Deficiency can provide important clues as to which of these genes may be prioritized for genetic testing. For example, dental agenesis, skeletal defects of the spine or hands, or IGD with anosmia but without renal defects in a patient with a positive family history that spans more than a single generation has been reported in individuals with FGFR1 variants and somewhat distinguishes them from their KAL1-positive counterparts. Therefore, in patients with these phenotypic features, FGFR1 genetic testing is more likely to yield a positive result than other genes. Rare variants in FGFR1 have also been associated with cleft lip/palate, syndactyly, brachydactyly, and agenesis of the corpus callosum (Dode et al., 2003, Pitteloud et al., 2006, Trarbach et al., 2006). Like variants in its receptor, FGFR1, variants in FGF8 can present phenotypically as IGD +/− anosmia with midline defects such as cleft lip/palate and hypertelorism.
Families with autosomal dominant variants are also more likely to show incomplete penetrance. This means that affected individuals possessing the disease-contributing genetic variant lack the related phenotype/s. Alternatively, variable expressivity exists when differing phenotypic traits are seen among individuals (both sibs and other families) who exhibit the identical genetic variant. Figure 4 demonstrates such variable expressivity within a family. The proband, his mother, and his maternal grandmother all share the identical FGFR1 variant; however, their phenotypic traits range from isolated anosmia, to delayed puberty, to IGD with anosmia and cleft lip/palate.
Environmental and behavioral triggers as well as the influence of additional genetic variants may explain some of discrepancy between an apparent inheritance pattern seen in a family and the lack of segregation of variants within a family. The presence of rare variants in more than one gene (di- and oligogenicity) among individuals with IGD has been described by Pitteloud et al. (2007b), Sykiotis et al. (2010), and Canto et al. (2009). Figure 5 shows such digenicity within a family. The proband, his sister, and father carry the same FGFR1 frameshift variant but only the proband and his sister have IGD. However, the proband and sister also both have a mutation in PROKR2, inherited from their mother, which likely modified their disease-burden.
Genetic Testing for IGD
At the present time, the primary method for screening genes involves sequencing of coding regions. While deletion/duplication testing may be performed for these genes, such variants have only been identified in KAL1 and very rarely in FGFR1 (Trarbach et al., 2010b, Pederson-White et al., 2008). Due to the rare nature of deletions/duplications in autosomal genes, sequencing should be considered as the highest priority for testing. Furthermore, sequencing entire coding regions of these genes is necessary as most current variants tend to be private and only shared among family members.
The complexity and expense of genetic sequencing on a clinical basis currently requires that the genes selected for testing be prioritized through careful phenotyping of individual patients and collection of detailed family histories. Non-reproductive phenotypes can be quite helpful in guiding the screening priorities for an individual with IGD as described in Figure 6. For example, in a patient with a family history of Kallmann syndrome showing x-linked inheritance and/or a history of renal agenesis or synkinesia, screening of the KAL1 gene should be prioritized, while for a patient with IGD and a family history of KS and/or nIHH as well as clefting or dental agenesis, screening for FGFR1 would be expected to have a higher mutation yield. The thorough evaluation of phenotype and family history, can be a useful tool to prioritize screening and save healthcare dollars. In the near future, however, next generation sequencing promises a more rapid and affordable means to sequence multiple genes and even the entire human exomes in a clinical setting. Such technology could eliminate the need to prioritize screening as all genes could be sequenced in a single test. The implementation of this practice, however, must first thoroughly consider the possibility of identifying variants of unknown significance or otherwise unexpected and possibly undesired information (paternity, consanguinity, risk for health conditions other than IGD).
At the time of publication, clinical genetic testing conducted through a CLIA-approved laboratory for IGD is only available for KAL1, FGFR1, and CHD7, though more than a dozen genes with noted contributions to IGD have been published in peer-reviewed scientific literature are reviewed in this Special Edition. While a wider range of genetic testing for these genes is available on a research basis, expanding the menu of clinical tests accessible to patients would provide assurance of test accuracy and reliability and provide results in a timely manner. For results to have maximum benefit to patients and families, however, continued research and revision of clinical guidelines for testing and results-based management is necessary. The current list of available clinical and research-based testing, in addition to information about the laboratories conducting the testing, can be found on the National Center for Biotechnology Information’s “GeneTests” website at http://www.ncbi.nlm.nih.gov/sites/GeneTests/ (accessed March 5, 2011).
Psychosocial Aspects of IGD
The discussion of the diagnosis and potential causes of IGD with a patient or family is a sensitive subject coupled with significant psychological stress related to the essential nature of one’s sexual identity over and above the usual genetic issues. Patients often feel self-conscious when comparing their lagging sexual development to that of their peers. Particularly for adolescents, visits to their clinician to discuss their sexual maturation are particularly charged. They may feel awkward or embarrassed so healthcare providers need to be sensitive to these potential issues. Additionally, couples being seen for infertility may feel inadequate when comparing themselves to friends and family members with children. They may be looking for answers as to why this condition is happening to them and practitioners may not always have such an explanation.
Once a diagnosis of IGD has been made, there can be lingering uncertainty that may trigger ongoing emotional distress for the patient. Patients often have many questions about how they can have a child, if they will pass on the condition, or why they are the only one in their family with IGD. Even genetic testing provides limited information and may be a source of anxiety for the patient. As Baum et al. (1997) discuss, the anxiety level for the patient due to genetic testing is dependent on the individual’s testing results, coping style, perceived severity of their diagnosis, level of uncertainty, and personality. Yet over and above these general considerations, sexuality adds a unique dimension of complexity to the Genetic Counselor’s role in this disease. It also affords them a unique opportunity to be a reassuring presence in an otherwise charged series of complex discussions. Add to this the uncertainty that accompanies the fact that causative mutations still cannot be identified in a majority of patients with IGD and that even if a mutation is identified the predictive value is complicated by genetic features such as variable expressivity, reduced penetrance and oligogenicity. Additionally, if a mutation is identified, it may have implications for the patient’s entire family and the disclosure of such a diagnosis or genetic test result to family members can be an extra stressor for the patient.
Genetic counselors or other practitioners can help alleviate some of the stress by listening carefully to and validating a patient’s concerns as well as helping them brainstorm ways to share this information with their family should they desire to do so. Additionally, they can help by devoting the time to obtain a detailed family history to try to discern the inheritance pattern and remove some of the uncertainty surrounding the patient’s diagnosis. Genetic counselors can also help explain the inheritance patterns and reinforce the idea that the patient is not alone in their diagnosis to minimize feelings of isolation or embarrassment.
In addition to psychosocial matters, genetic testing in IGD can be accompanied by ethical concerns including the testing of minors, restricted diagnostic predictability of testing particularly when used in prenatal settings, and limited availability and expense of clinical genetic testing. The American Society of Human Genetics and the American College of Medical Genetics practice statement regarding genetic testing in minors (1995) encourages the clinician to work closely with the family and the patient’s other healthcare providers to determine the possible medical and psychological benefits as well as limitations and even harms of testing. Because of the often delayed diagnosis of IGD, diagnosis by genetic testing of at-risk pre-symptomatic minors offers a means to identify individuals who will benefit from increased monitoring for signs of delayed puberty and initiation of treatment before significant delays are apparent. Therefore, genetic testing for IGD can be beneficial in regards to treatment, surveillance, and early adaptation when a rare gene variant(s) with 100% penetrance and predictable expressivity is identified in an individual. Unfortunately, for the over 50% of affected individuals in whom no rare genetic variants are identified, as well as those with genetic variants with reduced penetrance, variable expressivity, or variants of unknown clinical significance, uncertainty regarding future diagnosis and prognosis can produce increased anxiety in the patient and their family members.
Thorough counseling with the patient and their parents and/or partner prior to testing is necessary to aid the family in weighing the benefits, risks, and possible uncertainty that may result from testing. Post-results counseling is also important to promote the comprehension of the result, discuss the significance of the result for patient and family members as well as any remaining uncertainty about chances for fertility and chances of passing on their condition. Throughout the process of pre- and post-test counseling, the goal must be to uphold the autonomy of the patient (in coordination with a parent when necessary), maximize the benefit, and reduce risks associated with genetic testing and diagnosis (Weil, 2000).
Summary
As research into the genetics of IGD continues, the complexity of both the genetics and the diagnosis is also growing. The continued identification of new genes, growing complexity of inheritance patterns, and expanding phenotypes are all allowing the profession to classify IGD more precisely and provide more complete information to patients about their diagnosis and recurrence risk for their family members. As the cost of screening larger numbers of genes decreases with more widely available and cheaper exomic sequencing, accurate and thorough phenotyping will become ever more important to drive the downstream analysis and identification of new candidate genes and disease classifications. Additionally, it will become increasingly important to be able to explain complex genetic concepts to patients who present to clinic as well as to explain what they should expect, both in regards to symptoms and the likelihood that their children, siblings, or other family members may also be affected. Thus, the role of the Genetic Counselor as a critical team member will only grow in the family of conditions of IGD as outlined in the Special Edition of MCE.
Acknowledgments
This research was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54 HD28138 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and R01 HD15788
References
- Abreu AP, Trarbach EB, de Castro M, Frade Costa EM, Versiani B, Matias Baptista MT, Garmes HM, Mendonca BB, Latronico AC. Loss-of-function mutations in the genes encoding prokineticin-2 or prokineticin receptor-2 cause autosomal recessive Kallmann syndrome. J. Clin. Endocrinol. Metab. 2008;93:4113–4118. doi: 10.1210/jc.2008-0958. [DOI] [PubMed] [Google Scholar]
- American Society of Human Genetics Board of Directors and the American College of Medical Genetics Board of Directors. Points to consider: Ethical, legal, and psychosocial implications of genetic testing in children and adolescents. Am. J. Hum. Genet. 1995;57:1233–1241. [PMC free article] [PubMed] [Google Scholar]
- Baum A, Friedman AL, Zakowski SG. Stress and Genetic Testing for Disease Risk. Health Psychology. 1997;16(1):8–19. doi: 10.1037//0278-6133.16.1.8. [DOI] [PubMed] [Google Scholar]
- Bédécarrats GY, Kaiser UB. Mutations in the human gonadotropin-releasing hormone receptor: insights into receptor biology and function. Semin. Reprod. Med. 2007;25:368–378. doi: 10.1055/s-2007-984743. [DOI] [PubMed] [Google Scholar]
- Bouligand J, Ghervan C, Tello JA, Brailly-Tabard S, Salenave S, Chanson P, Lombes M, Millar RP, Guiochon-Mantel A, Young J. Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation. N Engl J Med. 2009;360:2742–2748. doi: 10.1056/NEJMoa0900136. [DOI] [PubMed] [Google Scholar]
- Canto P, Munguia P, Soderlund D, Castro JJ, Mendez JP. Genetic analysis in patients with Kallmann syndrome: coexistence of mutations in prokineticin receptor 2 and KAL1. J Androl. 2009;30:41–45. doi: 10.2164/jandrol.108.005314. [DOI] [PubMed] [Google Scholar]
- Cerrato F, Shagoury J, Kralickova M, Dwyer A, Falardeau J, Ozata M, Van Vliet G, Bouloux P, Hall JE, Hayes FJ, Pitteloud N, Martin KA, Welt C, Seminara SB. Coding sequence analysis of GNRHR and GPR54 in patients with congenital and adult-onset forms of hypogonadotropic hypogonadism. Eur. J. Endocrinol. 2006;155 Suppl 1:S3–S10. doi: 10.1530/eje.1.02235. [DOI] [PubMed] [Google Scholar]
- Chan YM, de Guillebon A, Lang-Muritano M, Plummer L, Cerrato F, Tsiaras S, Gaspert A, Lavoie HB, Wu CH, Crowley WF, Amory JK, Pitteloud N, Seminara SB. GNRH1 mutations in patients with idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA. 2009;106(28):11703–11708. doi: 10.1073/pnas.0903449106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LW, Sidis Y, Zhang C, Quinton R, Plummer L, Pignatelli D, Hughes VA, Dwyer AA, Ravio T, Hayes FJ, Seminara SB, Huot C, Alos N, Speiser P, Takeshita A, Van Vliet G, Pearce S, Crowley WF, Zhou QY, Pitteloud N. Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. J. Clin. Endocrinol. Metab. 2008;93:3551–3559. doi: 10.1210/jc.2007-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Young J, Misrahi M, Genet R, Chanson P, Schaison G, Milgrom E. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N. Engl. J. Med. 1997;337:1597–1602. doi: 10.1056/NEJM199711273372205. [DOI] [PubMed] [Google Scholar]
- Dode C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler M, Lespinasse J, Lienhardt-Roussie A, Mathieu M, Moerman A, Morgan G, Murat A, Toublanc J, Wolczynski S, Delpech M, Petit C, Young J, Hardelin J. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet. 2006;2(10):e175. doi: 10.1371/journal.pgen.0020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dode C, Levilliers J, Dupont JM, De Paepe A, Le Du N, Soussi-Yanicostas N, Coimbra RS, Delmaghani S, Compain-Nouaille S, Baverel F, Pecheux C, Le Tessier D, Cruaud C, Delpech M, Speleman F, Vermeulen S, Amalfitano A, Bachelot Y, Bouchard P, Cabrol S, Carel JC, Delemarre-van de Waal H, Goulet-Salmon B, Kottler ML, Richard O, Sanchez-Franco F, Saura R, Young J, Petit C, Hardelin JP. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat. Genet. 2003;33:463–465. doi: 10.1038/ng1122. [DOI] [PubMed] [Google Scholar]
- Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, Quinton R, Na S, Hall JE, Huot C, Alois N, Pearce SH, Cole LW, Hughes V, Mohammadi M, Tsai P, Pitteloud N. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J. Clin. Invest. 2008;118:2822–2831. doi: 10.1172/JCI34538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos NA, Pralong FP, Seidman CE, Crowley WF, Jr, Vallejo M. Genetic heterogeneity evidenced by low incidence of KAL-1 gene mutations in sporadic cases of gonadtropin-releasing hormone deficiency. J Clin Endocrinol Metab. 1997;82(1):213–217. doi: 10.1210/jcem.82.1.3692. [DOI] [PubMed] [Google Scholar]
- Gianetti E, Tusset C, Noel SC, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonca BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinto R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. TAC3/TACR3 mutations reveal preferential activiation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95:2857–2867. doi: 10.1210/jc.2009-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guran T, Tolhurst G, Bereket A, Rocha N, Porter K, Turan S, Gribble FM, Kotan LD, Akcay T, Atay Z, Canan H, Serin A, O'Rahilly S, Reimann F, Semple RK, Topaloglu AK. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol Metab. 2009;94:3633–3639. doi: 10.1210/jc.2009-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongmans MC, van Ravenswaaij-Arts CM, Pitteloud N, Ogata T, Sato N, Claahsen-van der Grinten HL, van der Donk K, Seminara S, Bergman JE, Brunner HG, Crowley WF, Hoefsloot LH. CHD7 mutations in patients initially diagnosed with Kallmann syndrome--the clinical overlap with CHARGE syndrome. Clin Genet. 2009;75:65–71. doi: 10.1111/j.1399-0004.2008.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HG, Kurth I, Lan F, Meliciani I, Wenzel W, Eom SH, Kang GB, Rosenberger G, Tekin M, Ozata M, Bick DP, Sherins RJ, Walker SL, Shi Y, Gusella JF, Layman LC. Mutations in CHD7, encoding a chromatin-remodeling protein, cause idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am J Hum Genet. 2008;83:511–519. doi: 10.1016/j.ajhg.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira LM, Seminara SB, Beranova M, Hayes FJ, Valkenburgh SB, Schipani E, Costa EM, Latronico AC, Crowley WF, Vallejo M. The importance of autosomal genes in Kallmann syndrome: genotype-phenotype correlations and neuroendocrine characteristics. J. Clin. Endocrinol. Metab. 2001;86:1532–1538. doi: 10.1210/jcem.86.4.7420. [DOI] [PubMed] [Google Scholar]
- Pallais JC, Au M, Pitteloud N, Seminara S, Crowley WF. Isolated Gonadotropin-Releasing Hormone (GnRH) Deficiency Overview. In: Pagon RA, Bird TD, Dolan CR, Stephens K, editors. GeneReviews [Internet] Seattle, WA: 2010. [Google Scholar]
- Pitteloud N, Meysing A, Quinton R, Acierno JS, Dwyer AA, Plummer L, Fliers E, Boepple P, Hayes F, Seminara S, Hughes VA, Ma J, Bouloux P, Mohammadi M, Crowley WF. Mutations in fibroblast growth factor receptor 1 cause Kallmann syndrome with a wide spectrum of reproductive phenotypes. Mol Cell Endocrinol. 2006;254–255:60–69. doi: 10.1016/j.mce.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng Y, Li W, Maccoll G, Eliseenkova AV, Olsen SK, Ibrahimi OA, Hayes FJ, Boepple P, Hall JE, Bouloux P, Mohammadi M, Crowley W. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest. 2007a;117:457–463. doi: 10.1172/JCI29884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole LW, Plummer L, Jacobson-Dickman EE, Mellon PL, Zhou QY, Crowley WF. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA. 2007b;104:17447–17452. doi: 10.1073/pnas.0707173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen-White JR, Chorich LP, Bick DP, Sherins RJ, Layman LC. The prevalence of intragenic deletions in patients with idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Mol. Hum. Repro. 2008;14(6):367–370. doi: 10.1093/molehr/gan027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletcher BA, Toriello HV, Noblin SJ, Seaver LH, Driscoll DA, Bennett RL, Gross SJ. Indications for genetic referral: A guide for healthcare providers. Genetics in Medicine. 2007;9(6):385–388. doi: 10.1097/GIM.0b013e318064e70c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resta R, Biesecker BB, Bennett RL, Blum S, Hahn SE, Strecker MN, Williams JL. A new definition of Genetic Counseling: National Society of Genetic Counselors’ Task Force report. J. Genet. Couns. 2006;15(2):77–83. doi: 10.1007/s10897-005-9014-3. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Semple RK, Achermann JC, Ellery J, Farooqi IS, Karet FE, Stanhope RG. Two novel missense mutations in g protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 2005;90:1849–1855. doi: 10.1210/jc.2004-1418. [DOI] [PubMed] [Google Scholar]
- Shaw ND, Seminara SB, Welt CK, Au MG, Plummer L, Hughes VA, Dwyer AA, Martin KA, Quinton R, Meriq V, Merino PM, Gusella JF, Crowley WF, Jr, Pitteloud N, Hall JE. Expanding the phenotype and genotype of female GnRH deficiency. J. Clin. Endocrinol. Metab. 2011 Jan 5; doi: 10.1210/jc.2010-2292. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis GP, Plummer L, Hughes VA, Au M, Durrani S, Nayak-Young S, Dwyer AA, Quinton R, Hall JE, Gusella JF, Seminara SB, Crowley WF, Pitteloud N. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci USA. 2010;107:15140–15144. doi: 10.1073/pnas.1009622107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook J, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat. Genet. 2009;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trarbach EB, Abreu AP, Silveira LF, Garmes HM, Baptista MT, Teles MG, Costa EM, Mohammadi M, Pitteloud N, de Mendonca BB, Latronico AC. Nonsense mutations in FGF8 gene causing different degrees of human gonadotropin-releasing deficiency. J Clin Endocrinol Metab. 2010a;95:3491–3496. doi: 10.1210/jc.2010-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trarbach EB, Teles MG, Frade Costa EM, Abreu AP, Garmes HM, Guerre-Junior G, Matias Baptista MT, de Castro M, Mendonca BB, Latronico AC. Screening of autosomal gene deletions in patients with hypogonadotropic hypogonadism using multiplex ligation-dependent probe amplification: detection of a hemizygosis for the fibroblast growth factor receptor 1. Clinical Endocrinology. 2010b;72:371–376. doi: 10.1111/j.1365-2265.2009.03642.x. [DOI] [PubMed] [Google Scholar]
- Trarbach EB, Costa EM, Versiani B, de Castro M, Baptista MT, Garmes HM, de Mendonca BB, Latronico AC. Novel fibroblast growth factor receptor 1 mutations in patients with congenital hypogonadotropic hypogonadism with and without anosmia. J Clin Endocrinol Metab. 2006;91:4006–4012. doi: 10.1210/jc.2005-2793. [DOI] [PubMed] [Google Scholar]
- Weil J. Psychosocial Genetic Counseling. Oxford, New York: 2000. pp. 122–123. [Google Scholar]