Abstract
Background and Purpose
We compared the associations of self-reported atrial fibrillation (SR-AF) and electrocardiogram-detected AF (ECG-AF) with incident stroke in the REGARDS study.
Methods
27,109 participants aged ≥45 years without prior stroke were included in this analysis. Stroke cases were identified and adjudicated during an average of 4.4 years of follow-up. Cox proportional hazards analysis was used to calculate hazard ratios of SR-AF, ECG-AF, and AF detected by either method with incident stroke. We also examined the predictive ability of the Framingham Stroke Risk Score (FSRS) where the component AF was defined by different methods.
Results
After adjustment for components of the FSRS, SR-AF, ECG-AF, and AF by either method were predictive of incident stroke [HR (95% CI): 1.41 (1.05,1.88), 1.90 (1.10,3.27), 1.53 and (1.16,2.01), respectively]. When self-report, ECG or either method, separately, were considered as the method of AF ascertainment in the FSRS, the Hazard ratios per 1% increase in the FSRS were identical across AF ascertainment methods [1.04 (1.03,1.04); 1.04 (1.04,1.05); 1.04 (1.03,1.04) respectively].
Conclusions
SR-AF is a strong predictor of stroke that can be used interchangeably or in combination with ECG-AF in stroke risk prediction models.
Keywords: Atrial fibrillation, self-report, Electrocardiogram
Introduction
Atrial fibrillation (AF) is a major risk factor for stroke (1). Electrocardiogram (ECG-AF) and self-reported history of a physician diagnosis of atrial fibrillation (SR-AF) are the most commonly used methods for detecting this serious arrhythmia in population studies (2). Nevertheless, the usefulness of SR-AF data is often called into question because of the possibility of unreliability.
Several studies attempted to assess the validity of self-reported cardiovascular diseases by comparing self-reports with medical records (3). The challenge in applying this validation approach to AF is its paroxysmal (intermittent) nature in 40% of cases (4). Since the clinical significance of detecting AF relates mainly to identifying those at increased stroke risk, demonstrating that SR-AF is an equivalent stroke risk factor to ECG-AF would provide validation of self-reported data.
Methods
The goals and design of the REGARDS study, a US national longitudinal study, have been published (5). REGARDS was designed to investigate causes of regional and racial disparities in stroke mortality, oversampling blacks and residents of the southeastern “stroke belt region”. Individuals were recruited from a commercially available list of residents using mail and telephone contact with a 49% cooperation rate. Demographic information and medical history were obtained by telephone interview. All methods were approved by institutional human subjects review boards. A brief physical examination was conducted 3-4 weeks after the telephone interview, including standardized measurements of risk factors, collection of blood and urine and recording of resting ECG. Participants are followed every 6 months by telephone for possible stroke outcomes.
Of 30,239 REGARDS participants enrolled between 2003-2007, 29,648 had follow-up data. Of these, we excluded 1,886 participants reporting prior stroke, 331 not answering questions on SR-AF, and 322 without an ECG.
AF ascertainment
Information on AF ascertainment was previously published (6, 7). Briefly, SR-AF was defined as a positive response to the question, “Has a physician or a health professional ever told you that you had atrial fibrillation?” ECG-AF was identified from the baseline ECGs which were centrally read by electrocardiographers blinded to clinical data.
Stroke events
Details on stroke events adjudication has been previously published (8). In summary, reports of possible stroke during follow-up generated a request for retrieval of medical records that were centrally adjudicated by physicians. Stroke events were defined following the World Health Organization (WHO) definition (9). Events not meeting the WHO definition but with symptoms lasting <24 hours with neuro-imaging consistent with acute ischemia or hemorrhage were classified as “clinical strokes”. This analysis included WHO-defined and clinical ischemic stroke cases.
Statistical Analysis
Cox proportional hazards analysis was used to examine the hazard ratios (HR) and 95% confidence interval (95% CI) for incident stroke associated with SR-AF, ECG-AF and AF by either method, separately. Models were adjusted for age, sex, and race (demographic models), then further adjusted for components of the Framingham Stroke Risk Score (FSRS; Framingham models) which included use of antihypertensive drugs, systolic blood pressure, current smoking, diabetes, left ventricular hypertrophy (LVH), and prior heart disease (10). In additional models, we examined effects of including socio-economic status (SES) and warfarin use, to the Framingham models. We examined the effect of the method of AF ascertainment on the predictive ability of the FSRS. To reduce potential biases due to missing data and to improve precision, we applied multiple imputation techniques to classify potential stroke events still in process and for records where medical records could not be retrieved (approximately 10% each), details are available elsewhere (11).
Results
There were 27,109 participants (45% males, 40% African Americans) included in this analysis. AF was detected by self-report in 2084 participants, by ECG in 360 and by both methods in 222. Table 1 shows participant characteristics by the method of AF ascertainment.
Table 1. Characteristics of the study population*.
| Self-reported atrial fibrillation | ECG- detected atrial fibrillation | ||||
|---|---|---|---|---|---|
| Absent N = 25,025 |
Present N= 2084 |
Absent N= 26,749 |
Present N= 360 |
||
| Age (year) | 64.5 ± 9.3 | 67.0 ± 9.6 | 64.5 ± 9.3 | 73.6 ± 8.4 | |
| Categorical Age | <65 | 52.3 | 42.5 | 52.0 | 14.4 |
| 65-74 | 32.0 | 32.8 | 32.0 | 36.7 | |
| 75+ | 15.8 | 24.7 | 16.0 | 48.9 | |
| Black race (%) | 40.6 | 35.4 | 40.5 | 19.7 | |
| Male sex (%) | 44.7 | 44.2 | 44.3 | 71.1 | |
| Systolic blood pressure (mmHg) | 127.3 ± 16.5 | 127.8 ± 17.1 | 127.3 ± 16.5 | 125.6 ± 16.1 | |
| Use of antihypertensive drugs (%) | 48.8 | 62.0 | 49.8 | 51.9 | |
| Current smoking (%) | 14.2 | 13.2 | 14.2 | 7.8 | |
| Diabetes (%) | 20.4 | 24.8 | 20.7 | 26.9 | |
| Left vent hypertrophy (%) | 9.5 | 10.3 | 9.6 | 5.6 | |
| History of heart disease (%) | 20.0 | 39.1 | 21.3 | 40.3 | |
| Warfarin use (%) | 1.7 | 19.3 | 2.2 | 66.7 | |
Continuous variable expressed as mean ± standard deviation
During a mean follow-up of 4.4 years, 378 strokes occurred (2,084 with baseline SR-AF, 360 with ECG-AF and 2,222 with AF by either method). Figure 1 shows the stroke-free survival curves by AF detection.
Figure 1.
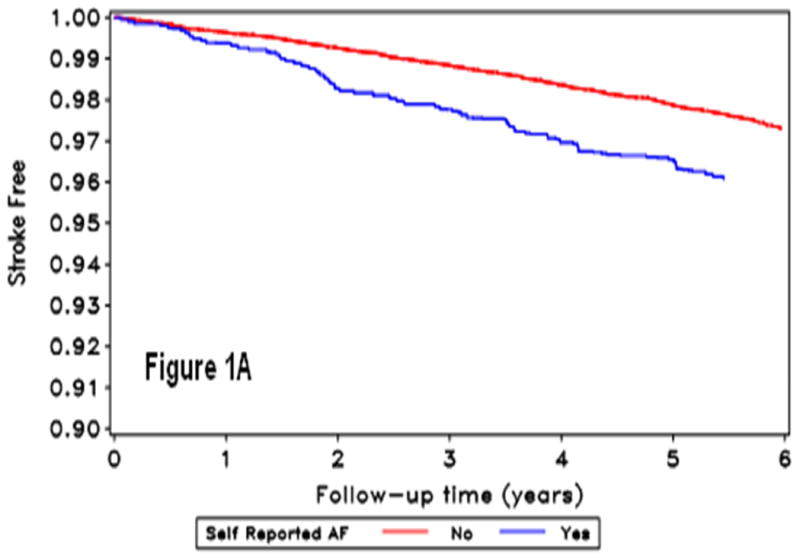
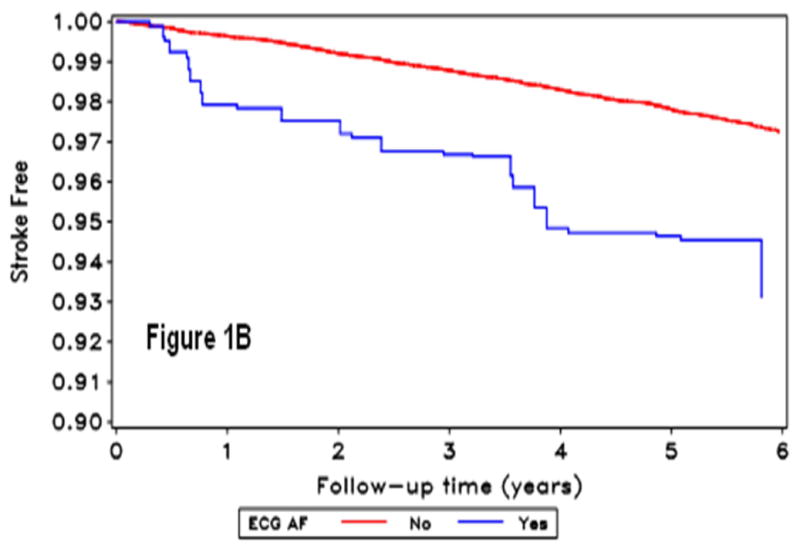
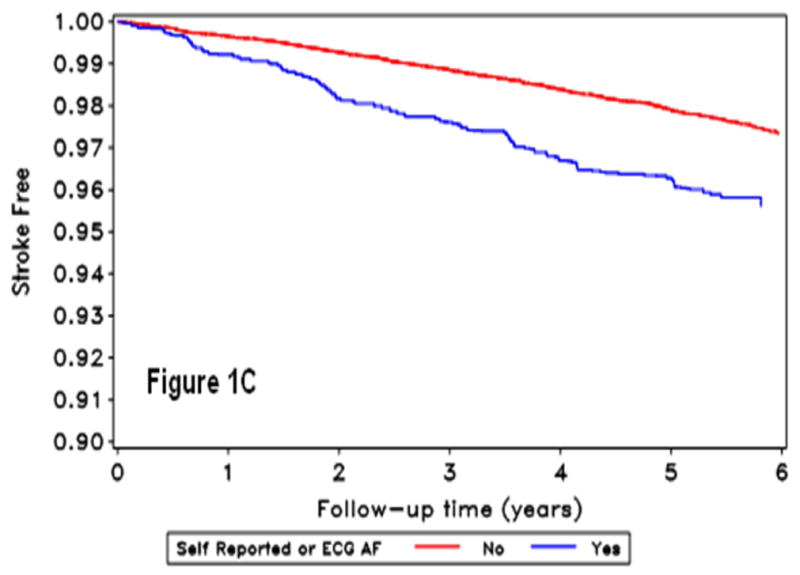
Kaplan Meir curves for incident stroke by self-reported-AF (A), ECG detected-AF (B), and AF by either self-report or ECG (C). The horizontal axis shows follow-up time in years, and the vertical axis the proportion of the population stroke-free for those without (red line) and with (blue line) atrial fibrillation condition.
In Table 2, SR-AF, ECG-AF and AF by either method were predictive of stroke in all models, with a slightly higher HR for ECG-AF. Adding SES to the Framingham models did not substantially change the HR associated with SR-AF [HR (95% CI): 1.41 (1.05,1.88), p=0.022] or ECG-AF [1.88 (1.09,3.25), p=0.023] compared to the Framingham models in Table 2. On the other hand, adding warfarin use to the Framingham model attenuated the HRs for incident stroke associated with both SR-AF [(1.30 (0.95,1.77), p=0.10] and ECG-AF [1.52 (0.81,2.86), p=0.19].
Table 2. Association between atrial fibrillation identified using different methods and incident stroke.
| Demographic model * HR (95% CI) |
p-value | Framingham model † HR (95% CI) |
p-value | |
|---|---|---|---|---|
| Self-reported atrial fibrillation | 1.55 (1.17, 2.04) | 0.002 | 1.41 (1.05,1.88) | 0.020 |
| ECG-detected atrial fibrillation | 1.78 (1.03, 3.09) | 0.039 | 1.90 (1.10, 3.27) | 0.022 |
| Atrial fibrillation by either self report or ECG | 1.66 (1.27, 2.17); | <0.001 | 1.53 (1.16, 2.01) | 0.003 |
Age, race and sex
Demographic model + use of antihypertensive medications, systolic blood pressure, current smoking, diabetes, left ventricular hypertrophy, and prior heart disease.
When self-report, ECG or either method, separately, were considered as the method of AF ascertainment in the FSRS, the Hazard ratios per 1% increase in the FSRS were identical for each AF ascertainment method [1.04 (1.03,1.04); 1.04 (1.04,1.05); 1.04 (1.03,1.04) respectively; p<0.001 for all]. There were no meaningful differences in observed associations when we stratified the analyses by race or sex (data not shown).
Discussion
In this analysis, SR-AF was a strong independent risk factor for stroke. We also showed that SR-AF can be used interchangeably with ECG-AF for stroke risk prediction using the FSRS. These findings suggest that important predictive information can be derived from a simple AF ascertainment method: self-report of a previous physician diagnosis. Adjustment for warfarin use in this analysis attenuated the risk of stroke associated with SR-AF and with ECG-AF. This means that SR-AF may be not only an independent risk factor for stroke, but also a modifiable risk factor if treated in a manner similar to the ECG- AF. However, the risk-benefit of routine use of warfarin in patients with SR-AF without ECG evidence needs to be evaluated in future studies.
Based on our findings, we propose that “SR-AF and/or ECG-AF” would be a good choice if both methods are available. Compared to ECG or self-report alone, using a classification as “either” was a good predictor in this study in terms of hazard ratio and significance/precision (p-value and confidence interval). However, in case of unavailable ECG data, SR-AF could be a useful predictor that provides important information.
So, why is SR-AF a strong risk factor for stroke despite the possibility of misclassification due to inaccuracy of reporting past medical history? An explanation can be derived from the possible reasons why an individual may wrongfully report having AF. It is possible that positive reports for AF could be triggered by past history of other arrhythmias or heart disease that are recollected as AF. Since history of heart disease and arrhythmias are risk factors for stroke (12), it is not surprising that SR-AF carries prognostic significance, even if not true in all cases. Further, results from the Cardiovascular Health Study (CHS) showed that SR-AF have similar associations with risk factors and medication use as ECG-AF (13), so it is plausible that SR-AF could have a similar prognostic significance as ECG.
Our study has some limitations. We validated SR-AF in terms of prognostic significance not diagnostic accuracy. This enabled us to comment on the usefulness of SR-AF as a predictor for stroke. We could not confirm the appropriateness of SR-AF to estimate prevalence of AF. Nevertheless, we previously reported that combining data from self-report and ECG provided more logical estimates for the racial distribution of AF (7) that partially explain the paradox of high stroke and low reported prevalence of AF in blacks (2, 14). Another limitation of our study is that we examined the usefulness of SR-AF in the context of the FSRS, which was developed in whites not in blacks. However, we stratified the results by race and examined interactions, and there were no meaningful black-white differences. Finally, since REGARDS population was collected from commercial listing which means that more affluent might have been over represented, and given the relatively low cooperation rate, the generalizability of our findings might have been affected. Despite these limitations, our study answered a number of questions related to the validity of SR-AF using a novel validation approach in a well-defined large biracial cohort.
In conclusion, SR-AF is a strong predictor for stroke which can be used interchangeably or in combination with ECG-AF in stroke risk prediction models. Findings support using self-report as a method for AF ascertainment.
Acknowledgments
The authors thank the investigators, staff, and participants of the REGARDS study for their valuable contributions. A full list of REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Source of Funding: This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services.
Footnotes
Disclosures: None
References
- 1.Wolf PA, Mitchell JB, Baker CS, Kannel WB, D'Agostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med. 1998;158:229–234. doi: 10.1001/archinte.158.3.229. [DOI] [PubMed] [Google Scholar]
- 2.Soliman EZ, Alonso A, Goff DC., Jr Atrial fibrillation and ethnicity: the known, the unknown and the paradox. Future Cardiol. 2009;5:547–556. doi: 10.2217/fca.09.49. [DOI] [PubMed] [Google Scholar]
- 3.Bowlin SJ, Morrill BD, Nafziger AN, Jenkins PL, Lewis C, Pearson TA. Validity of cardiovascular disease risk factors assessed by telephone survey: the Behavioral Risk Factor Survey. J Clin Epidemiol. 1993;46:561–571. doi: 10.1016/0895-4356(93)90129-o. [DOI] [PubMed] [Google Scholar]
- 4.Aboaf AP, Wolf PS. Paroxysmal atrial fibrillation. A common but neglected entity. Arch Intern Med. 1996;156:362–367. [PubMed] [Google Scholar]
- 5.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, et al. The REasons for Geographic and Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 6.Meschia JF, Merrill P, Soliman EZ, Howard VJ, Barrett KM, Zakai NA, et al. Racial disparities in awareness and treatment of atrial fibrillation: The REGARDS study. Stroke. 2010;41:581–781. doi: 10.1161/STROKEAHA.109.573907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prineas RJ, Soliman EZ, Howard G, Howard VJ, Cushman M, Zhang ZM, et al. The sensitivity of the method used to detect atrial fibrillation in population studies affects group-specific prevalence estimates: ethnic and regional distribution of atrial fibrillation in the REGARDS study. J Epidemiol. 2009;19:177–181. doi: 10.2188/jea.JE20081032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, et al. The contribution of disparities in stroke incidence to the disparities in stroke mortality: The REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Ann Neurol. 2011 doi: 10.1002/ana.22385. Epub ahead of print. [DOI] [Google Scholar]
- 9.Recommendations on stroke prevention, diagnosis and therapy. Report of the WHO Task Force on stroke and other cerebrovascular disorders. Stroke. 1989;20:1407–1431. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 10.D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 11.Howard G, McClure LA, Moy CS, Safford MM, Cushman M, Judd SE, et al. Imputation of events for “incomplete adjudication” in observational epidemiologic studies. Am J Epidemiol. 2011 in press. [Google Scholar]
- 12.Engstrom G, Hedblad B, Juul-Moller S, Tyden P, Janzon L. Cardiac arrhythmias and stroke: increased risk in men with high frequency of atrial ectopic beats. Stroke. 2000;31:2925–2929. doi: 10.1161/01.str.31.12.2925. [DOI] [PubMed] [Google Scholar]
- 13.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al. Incidence and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 14.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the ARIC study. Stroke. 2009;40:1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]


