Synopsis
The concept that adrenal androgen production gradually declines with age has changed following the analysis of the longitudinal data collected in the Study of Women’s Health Across the Nation (SWAN). It is now recognized that four adrenal androgens (3-beta hydroxy-5-androsten-17-one or dehydroepiandrosterone--DHEA, its sulfate, dehydroepiandrosterone sulfate--DHEAS; androst-4-ene, 3,17-dione or androstenedione; and androst-5-ene-3-beta, 17-beta diol, also known as androstenediol or Adiol) rise during the menopausal transition in most women. Ethnic and individual differences in sex steroids are more apparent in circulating adrenal steroids than in either estradiol or cyclic ovarian steroid hormone profiles, particularly during the early and late perimenopause. Thus, adrenal steroid production may play a larger role in the occurrence of symptoms and the potential for healthier aging than previously recognized.
Keywords: menopausal transition, androgens, adrenal
Introduction
Until recently the prevailing dogma was that adrenal weak androgen production in both men and women declined after the third decade of life. In the last ten years, this concept has changed following the analysis of the longitudinal data collected in the Study of Women’s Health Across the Nation (SWAN)1. Failure to adequately attribute phenotype, symptoms and health trajectories to the observed longitudinal changes in circulating estradiol (E2) and progesterone (P) have led to investigations that focus on adrenal contributions to circulating sex steroids. Emerging data show that there are more ethnic and individual endocrine differences in mid-aged women in circulating adrenal steroids than in either estradiol or cyclic hormone profiles, particularly during the early perimenopause1,2. Thus, adrenal steroid production may play a larger role in the occurrence of symptoms and the potential for healthier aging than previously recognized.
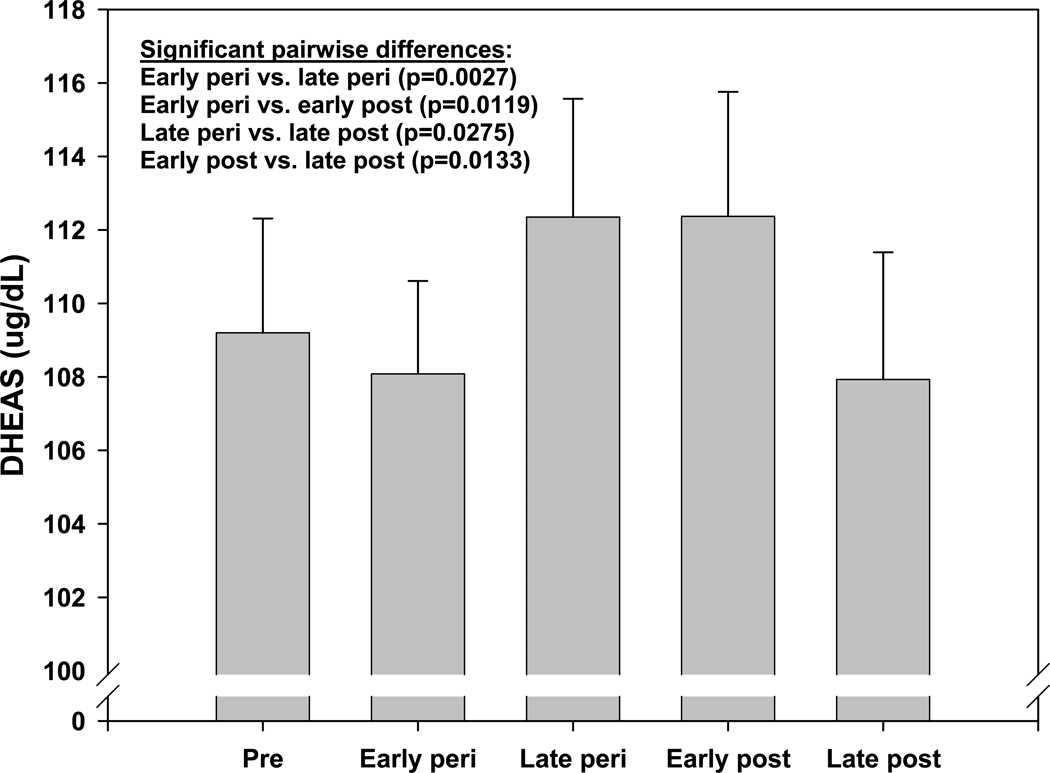
A distinct rise in circulating dehydroepiandrosterone sulfate (DHEAS) has been detected in most women during the menopausal transition. This rise was detected, however, only when the annual serum levels of DHEAS were aligned according to ovarian status, defined using the World Health Organization criteria3(Figure 1). The expected age-related, gradual decline is observed when the same data are plotted by chronological age in premenopausal women2. A similar rise in DHEAS had been observed earlier in older female laboratory macaques but has not been reported in any non-primate animal model. In women, it seems clear that most, if not all of the DHEAS rise is attributable to the adrenal and not the ovary, as a similar rise is observed in intact and ovariectomized women4. Together, these observations not only underscore the importance of longitudinal investigations such as SWAN, but also explain why this specific physiologic trait went unnoticed for decades. It also highlights the value of the nonhuman primate animal model for human reproductive endocrinology, since this steroidogenic pathway is not found in rodent adrenals and therefore would not otherwise have been investigated.
Figure 1.
Adjusted mean DHEAS (95% confidence interval) by menopause status from SWAN visits 00 – 09 (15,930 observations from 2,886 women). Reproduced from: Crawford S, Santoro N, Laughlin GA, Sowers MF, et al., Circulating Dehydroepiandrosterone Sulfate Concentrations during the Menopausal Transition. J Clin Endocrinol Metab 2009;94:2945–51. Copyright 2009, The Endocrine Society.
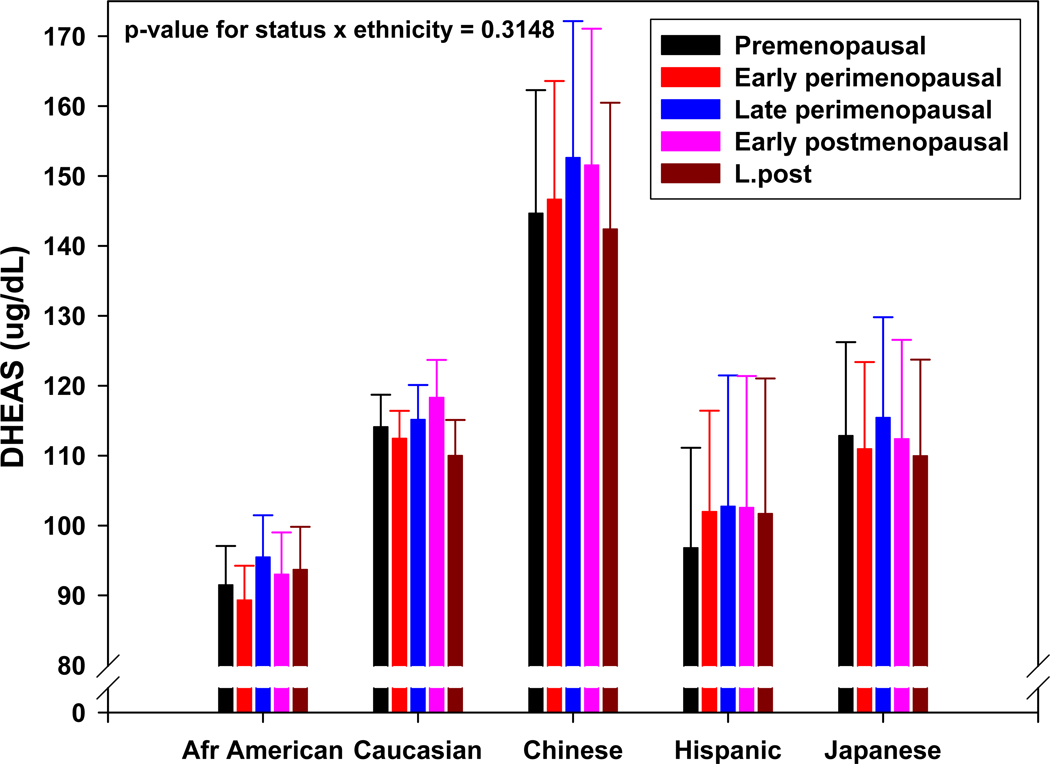
While the circulating levels of DHEAS in middle-aged women differ significantly between ethnicities at the beginning of the menopause transition (MT), the subsequent rise in DHEAS during the MT is similar, in both in the percentage of women that express it as well as the similarity in relative increase and trajectory in all five ethnicities studied in SWAN (Figure 2). The pattern of adrenal weak androgen production that emerges when the DHEAS data are aligned by ovarian status suggests several possibilities regarding control of adrenal androgen secretion in older women. First, the ethnic differences in the circulating concentration of DHEAS in adult women indicate an ethnic-specific predisposition for the regulation of the delta-5 adrenal steroidogenic pathway (Figure 3) in premenopausal women2. The between-ethnic similarities of the DHEAS trajectories, however, indicate a common physiological controlling mechanism during the MT. The time course of the rise of DHEAS, which is limited to the MT and early post menopause, suggests that changes in ovarian function are part of the controlling mechanism(s). While the finding of an increase in circulating DHEAS demonstrates a gender divergence that seems to be intimately linked to the MT, by itself it does not necessarily indicate a physiologically important event.
Figure 2.
Adjusted mean DHEAS (95% confidence interval) by menopause status within ethnicity from SWAN visits 00 – 09 (15,930 observations from 2,886 women). Reproduced from: Crawford S, Santoro N, Laughlin GA, Sowers MF, et al., Circulating Dehydroepiandrosterone Sulfate Concentrations during the Menopausal Transition. J Clin Endocrinol Metab 2009;94:2945–51. Copyright 2009, The Endocrine Society.
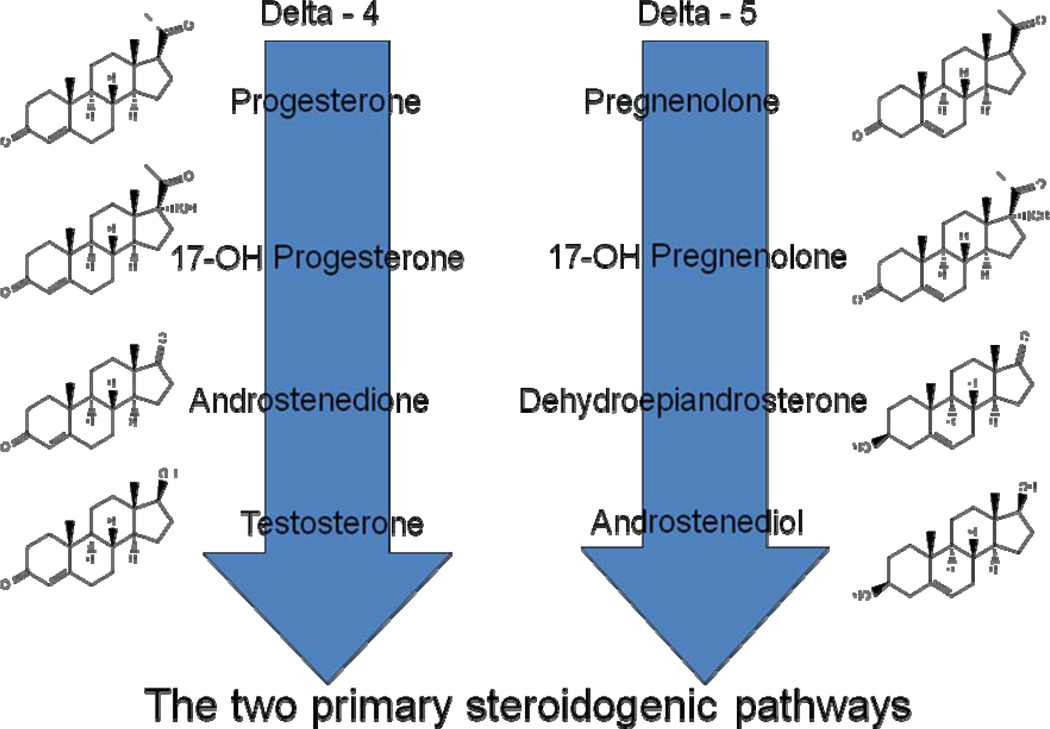
Figure 3.
The two primary adrenal steroidogenic pathways. The delta four pathway on the left has been considered to be the critical pathway giving rise to the mineralocorticoids and glucocorticoids as well as androstenedione and testosterone which can be aromatized peripherally to estrogen and estradiol, respectively. The delta five pathway on the right has been considered important mainly for the production of dehydroepiandrosterone and dehydroepiandrosterone sulfate which can be prohormones for peripheral conversion to more bioactive steroids. The steroidogenic enzyme, 3-betahydroxysteroid dehydrogenase (or delta 4/5 isomerase) converts delta-5 to delta-4 hormones. Androstenediol, because it is now recognized to circulate in relatively high concentrations, is now be considered to be important during the menopausal transition.
A great deal of attention has been focused on the delta-4 steroidogenic pathway that produces cortisol (F), androstendione (Adione) and testosterone (T; Figure 3). There are some relatively weak associations of these circulating steroids to sexual motivation, mood and the development of metabolic syndrome over the course of SWAN5,6. By comparison, the longitudinal studies of SWAN have suggested that the delta-5 steroidogenic pathway that produces dehydroepiandrosterone (DHEA), its sulfated conjugate DHEAS and androstenediol (Adiol) may play a larger role in women’s healthy aging. Specifically, two reports1,2 show that these two parallel adrenal steroidogenic pathways are controlled separately with gender-specific and ovarian-stage-specific differences in steroid production rates and trajectories for mid-aged women.
Potential Significance of the Perimenopausal Rise in Adrenal Androgens
It would be an oversimplification to conclude that the previous endocrine conundrums relating to the MT will now be explained by a new and more focused investigation of adrenal function, but these new data have provided insights. First, it has been long-speculated that DHEA provides the substrate for the many P450 c17 enzymes that can convert this relatively inert compound to more biologically active compounds (such as Adiol) and, with the help of 3-beta-hydroxysteroid dehydrogenase-isomerase, to Adione and T (see Figure 3)7,8 These products of peripheral metabolism could provide additional sex steroid activity after the loss of essential ovarian hormones at menopause. This concept is indirectly supported by the observation women with higher circulating DHEA sustain cognitive and executive function loss as the traverse the MT9. On the other hand, DHEA supplements given to mid-aged women do not provide significant measurable cognitive benefit, and this has promoted the need to investigate the ovarian-adrenal interactions of endogenously secreted hormones10. Exogenously administered DHEA, when given to women, results in circulating conversion products that are androgenic, and not primarily estrogenic11. These findings suggest that exogenously administered DHEA would not be directly or indirectly responsible for the retention of the estrogen-dependent integrity of the neural substrate and other estrogen-sensitive tissues. Within-woman changes and between-woman differences in circulating E2 are minimal during the early MT,12 while non-reproductive hormone-related changes in women progressing through the MT, such as increased incidence of the metabolic syndrome, cardiovascular disease and body composition, are dramatic13,14. These observations make it difficult to attribute between-woman differences in early perimenopausal symptoms and intermediate health outcomes to changes in circulating E2. This has led to the notion that these changes are attributable to an attendant factor, presently unidentified, that would be responsible. We find it biologically plausible that adrenal androgen dynamics during the menopausal transition are at least in part an explanatory factor.
There is now evidence that androstenediol, which has inherent androgenic and estrogenic bioactivities and is secreted in parallel with DHEAS and DHEA during the menopausal transition (Figure 4), may be such an explanatory factor. A unique aspect of androstenediol as an important, perhaps critical, endocrine component in mid-aged women lies in its ability to transduce signals through both the androgen and estrogen receptors.15–19 Thus, high circulating concentrations of androstenediol could affect the net balance of androgens and estrogens in the body, depending on the abundance or lack of abundance of specific steroid hormone receptors in target tissues. This concept is complex because, as a weaker ligand, the ability of androstenediol to transduce signals though any of these receptors depends also on the concentration of other higher-affinity ligands such as T or E2 (Figure 5).
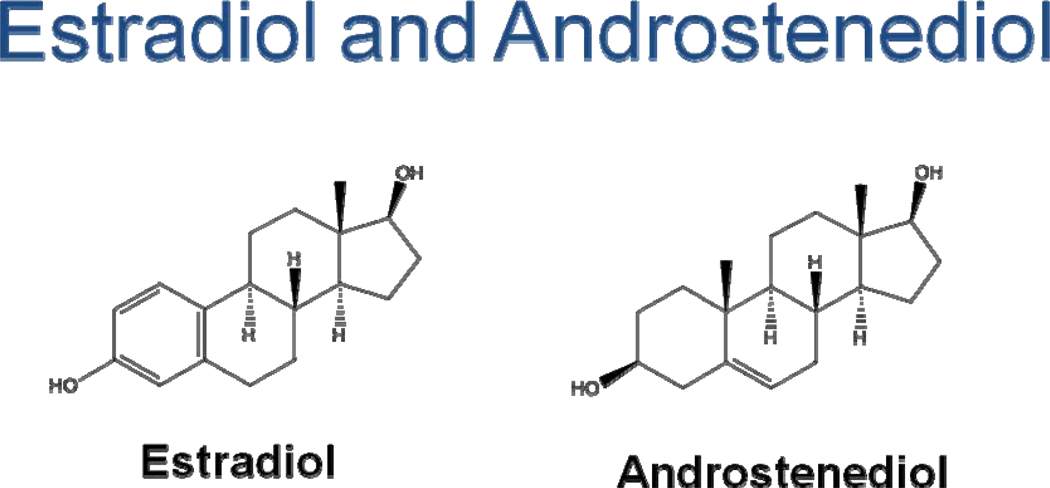
Figure 4.
Basic structures of estradiol and androstenediol. Estradiol is a classic estrogen receptor ligand while androstenediol has the classic androgen C-19 carbon structural backbone and the ability to transduce a signal through the androgen receptor. Both estradiol and androstenediol have similar 3–17 diol functional groups that most likely explain how this C-19 steroid can act as a C-18 estrogen.
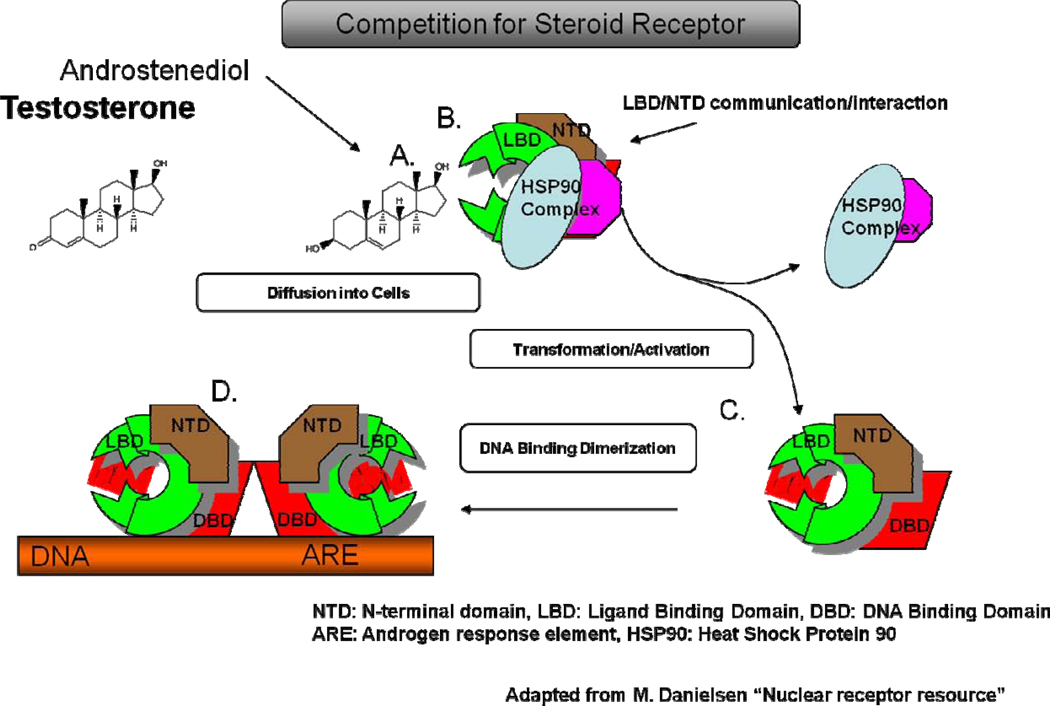
Figure 5.
Mechanism of steroid-steroid receptor interaction shown for testosterone and androsenediol. Both structures, as C-19 steroids, can compete for the androgen receptor (A.). While androstenediol has a lower binding affinity than that of testosterone, it can bind to the receptor if its concentrations are high enough. In mid-aged women androstenediol is found in concentrations that are many fold higher than testosterone at which, it can bind to the ligand binding domain (LBD) (B.). Regardless of which structure binds, the steroid-receptor complex then uncouples from the heat shock protein (HSP) to form a monimer with the ligand binding domain (LBD) exposed (C.). Two of these monimers combine to form the dimer (D.), that can then dock at the DNA androgen binding element (ARE) to initiate gene expression if the correct cofactors are also present. A similar process is true for the estrogen receptors.
The observation that there is an overall age-related decline in DHEAS, but a gender-specific rise around the time of menopause in women suggests that the delta-five steroid biosynthetic pathway in most women is influenced by subtle changes in ovarian function as women enter the MT. Evidence in support of ovarian control of adrenal androgen production is the observation that the gender- and menopause-related rise in DHEAS in women returns to a progressive decline following menopause.2 Taken together, the existing information indicates that the subtle changes in ovarian function that accompany the MT (a loss of inhibin B, no obvious change in estrogen dynamics, and a slight rise in FSH) trigger a transient increase in adrenal delta-5 steroid production that continues to and past the final menstrual period (FMP).
Potential Role of Androstenediol (Adiol)
Androstenediol, although structurally resembling an androgen, has long been recognized as a weak estrogen with a potency of 0.01 to 0.1% that of E2. Its circulating concentration in premenopausal women is less than1 nM—or about two to three times that of the average circulating concentration of E2. Because of its lower binding affinity for the estrogen receptor or other nuclear proteins that may transduce an estrogenic signal,15 this concentration is too low for androstenediol to be effective as either an androgen or estrogen16. However, the increase in DHEAS that starts in the early peri-menopause and continues into the early post-menopause, results in much higher circulating androstenediol concentrations that approach 3–4 nM--about one hundred times the average concentration of E2. At these higher circulating concentrations, protein synthesis and cell proliferation have been demonstrated in human, estrogen-sensitive cells in vitro18, and have been shown to elicit an estrogenic response in vivo in immature female rats.19 Thus, Adiol could potentially contribute to circulating estrogen action in middle-aged women. However, not all women share the robust early and late perimenopausal rise in DHEAS, DHEA and Adiol, as approximately 15% remain at or near premenopausal levels. This wide range of circulating Adiol (<1to 4 nM) therefore has the potential to explain the wide range of estrogen-related phenotypes and symptoms that are observed in perimenopausal women—data that has been difficult to reconcile with the relatively narrow range of circulating E2 levels during this same time period.
Virtually all menopausal hormone therapies (MHTs) are based on the assumption that the intervention requires the correction of low circulating estrogen concentration despite the fact that the direct measurement of low circulating estradiol prior to menopause is seldom used to justify this approach. In fact, the literature is not clear about the question of whether symptomatic women produce less estrogen in the early phases of the menopausal transition, with some reports indicating that estrogen production is not decreased in some women20–22. Clinical evidence, such as changes in estrogen-sensitive tissues, verifies that lower estrogenization is the foundation of most menopausal symptoms and is consistent with the effectiveness of E2 in ameliorating these changes. However, estrogen receptor ligands other than E2 may contribute to the clinical picture of estrogen deficiency and its attendant symptoms. The fact that there is investigative interest in such potential ligands is acknowledged in the number of failed DHEA trials that have been conducted. Furthermore, the benefits of current estrogen-based therapies seem unlikely to be totally physiologic for menopausal women, as they must be balanced against recognized risks of endometrial hyperplasia and cardiovascular diseases. In fairness to the prevailing clinical approach in applying MHT to women without substantiating low estrogen production, the day-to-day fluctuations of estrogen production in women with intact ovaries defies any practical attempt to quantify either estrogen production or circulating concentrations. However, urinary hormone excretion patterns seem to indicate that there is not a general decline in estrogen production prior to menopause in most intact women22. The effectiveness of estrogen replacement to reverse menopausal-related changes in middle-aged women does not necessarily support either a decrease in ovarian estrogen production or a decline in circulating E2. While these phenomena may be contributors in the causal pathway, they have not been shown to be singularly responsible. A clear understanding of the processes and mechanism(s) that lead to the menopause and its attendant symptoms is currently limited and there is a growing body of evidence that suggests that adrenal androgens may play an important role in the estrogen/androgen balance during the MT.
Testosterone
Circulating levels of testosterone (T) during the menopausal transition are well established in the literature and T is clearly the principal bioactive androgen in middle-aged women23. Most reports are consistent in concluding that for reproductive aged women approximately half of the bioactive androgens come from the ovary and half from the adrenal. There is less certainty regarding the ovarian contribution just before and after the FMP. Most reports indicate that the postmenopausal ovary continues to secrete androgens up to and following menopause,23,24 while a recent report using highly sensitive methods to measure circulating steroid levels indicate the ovarian contribution is less than previously accepted25. While both T and Adione gradually decline with age, both of these steroids increase concomitant with the ovarian-stage-specific increase in adrenal DHEAS2. This recent finding supports the more general, age-related decline in ovarian androgen production but also indicates an increase in adrenal derived androgens during the MT for most women.
The free androgen Index (FAI) tends to be a better predictor of many health outcomes, especially cardiovascular ones, compared to T. BMI, waist-hip ratio, waist and metabolic syndrome are more strongly correlated to FAI than T5,6,26. Predictors of obesity included an increase in free androgen index and a decrease in sex hormone-binding globulin26. Since FAI has SHBG in the denominator, and SHBG is a good predictor of CV-related outcomes, these trends make good sense. The observation that free T correlates better with hyperandrogenic conditions during the MT suggests one of two possibilities. The first is that SHBG is decreased when T is elevated, a finding that is consistent with the trend of decreasing SHBG across the MT. A second possibility is more intriguing and also consistent with observations. That explanation would be that increased SHBG ligands in the form of Adione and Adiol reduces available binding sites for T, and amplify this effect.
In two sets of longitudinal analyses, Lasley et al.2,4 found that DHEAS is negatively related to BMI, and Sowers et al27 found that DHEAS is positively related to PAI-1, tPA, and fibrinogen. Baseline analyses by Santoro et al5 did not find much of an association of DHEAS with BMI, waist-hip ratio, or waist. DHEAS was positively associated with better physical functioning, better self-reported health, less CES-D depression, and less metabolic syndrome. DHEAS wasn't significantly related to self-reported overall quality of life or sexual desire or arousal.
Based on the close association of circulating androstenediol with circulating DHEAS during the MT we would expect that effects of androstenediol on symptoms and health outcomes would also be associated with DHEAS. However, there are few longitudinal studies in which DHEAS concentrations have been analyzed across the MT. Until such analyses are reported no conclusion can be made. The rise in adrenal androgens before and immediately following menopause is quite variable and some women, perhaps 15%, reveal no increase in adrenal androgens2. Thus, a majority of women will exhibit up to a doubling of T and Adione and possibly a five-fold increase in Adiol as they traverse menopause. While this rise in T represents the major androgenic contribution, the complementary contributions of Adione and Adione to total androgencity should not be ignored. Clearly, additional investigations are required before the importance of the contribution of weak adrenal androgens to health outcomes will be completely defined. The current understanding that the adrenal participates in the estrogen:androgen balance provides more latitude in explaining androgen-related issues during the MT.
Summary
The menopause associated rise of circulating FSH12, the increase in the metabolic syndrome6,26 and other menopausal symptoms5,22 begin to occur during the early perimenopause. However, the first significant decline in circulating E2 measured on days 3–7 of the menstrual cycle does not occur until two years prior to menopause--during the late perimenopause28 and continues for two to three years beyond the FMP. This sustained, narrow range of circulating E2 concentrations occurs at the same time that mean DHEAS, DHEA and Adiol are increasing at their highest rate2. Furthermore, the between-women differences in DHEAS, DHEA and Adiol are the greatest of any hormone at that time1. More importantly, the five-fold higher circulating concentrations of Adiol in individual women, compared to the concomitantly decreasing levels of circulating E2, may compensate for the lower estrogenic bioactivity of Adiol compared to E2. This relationship indicates that women with higher circulating E2 would have little benefit from even the highest concentrations of androstenediol. In fact, it could be argued that the weaker ligand (Adiol) would attenuate to some degree the biological effects of E2. However, the higher concentration of Adiol in the presence of low circulating E2 could contribute significantly to the total circulating estrogen ligand pool in women during the menopause transition, and may have clinical significance..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose.
References
- 1.Lasley BL, Santoro N, Gold EB, et al. The relationship of circulating DHEAS, testosterone and estradiol to stages of the menopausal transition and ethnicity. J. Clin Endocrinol Metab. 2002;87:3760–3767. doi: 10.1210/jcem.87.8.8741. [DOI] [PubMed] [Google Scholar]
- 2.Crawford S, Santoro N, Laughlin GA, et al. Circulating Dehydroepiandrosterone Sulfate Concentrations during the Menopausal Transition. J Clin Endocrinol Metab. 2009;94:2945–2951. doi: 10.1210/jc.2009-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Scientific Group. Geneva, Switzerland: World Health Organization; 1996. Research on Menopause in the 1990s. (WHO Technical Report Series no. 866; 4) [PubMed] [Google Scholar]
- 4.Lasley B, Crawford S, Laughlin G, et al. Circulating Dehydroepiandrosterone Levels in Women with Bilateral Salpingo-Oophorectomy during the Menopausal Transition. Menopause. 2011;18:494–498. doi: 10.1097/gme.0b013e3181fb53fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santoro N, Torrens J, Crawford S, et al. Correlates of circulating androgens in mid-life women: The Study of Women's Health Across the Nation. J Clin Endocrinol Metab. 2005;90:4836–4845. doi: 10.1210/jc.2004-2063. [DOI] [PubMed] [Google Scholar]
- 6.Torrens JI, Sutton-Tyrrell K, Zhao X, et al. Relative androgen excess during the menopausal transition predicts incident metabolic syndrome in midlife women: Study of Women’s Health Across the Nation. Menopause. 2009;16:257–264. doi: 10.1097/gme.0b013e318185e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labrie F, Cusan L, Gomez JL, et al. Changes in serum DHEA and eleven of its metabolites during 12-month percutaneous administration of DHEA. J Steroid Biochem Mol Biol. 2008;110:1–9. doi: 10.1016/j.jsbmb.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Labrie F, Archer D, Bouchard C, et al. Intravaginal dehydroepiandrosterone (Prasterone), a physiological and highly efficient treatment of vaginal atrophy. Menopause. 2009;16:907–914. doi: 10.1097/gme.0b013e31819e8e2d. [DOI] [PubMed] [Google Scholar]
- 9.Davis SR, Shah SM, McKenzie DP, et al. Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. J Clin Endocrinol Metab. 2008;93:801–808. doi: 10.1210/jc.2007-2128. [DOI] [PubMed] [Google Scholar]
- 10.Mamas L, Mamas E. Premature ovarian failure and dehydroepiandrosterone. Fertil Steril. 2009;91:644–646. doi: 10.1016/j.fertnstert.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 11.Bird CE, Murphy J, Boroomand K, et al. Dehydroepiandrosterone: kinetics of metabolism in normal men and women. J Clin Endocrinol Metab. 1978;47:818–822. doi: 10.1210/jcem-47-4-818. [DOI] [PubMed] [Google Scholar]
- 12.Randolph JF, Jr, Sowers M, Gold EB, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88:1516–1522. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- 13.Janssen I, Powell LH, Crawford S, et al. Menopause and the metabolic syndrome: the Study of Women's Health Across the Nation. Arch Intern Med. 2008;168:1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. Sex hormone binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women's Health Across the Nation (SWAN) Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 15.Poortman J, Prenen JAC, Schwarz F, Thijssen JHH. Interaction of delta-5-androstene-3-beta,17-beta-diol with estradiol and dihydrotestosterone receptors in human myometrial and mammary cancer tissue. J Clin Endocrinol Metab. 1975;40:373–379. doi: 10.1210/jcem-40-3-373. [DOI] [PubMed] [Google Scholar]
- 16.Traish AM, Huang Y-H, Min K, et al. Binding characteristics of 3-H-delta-5-androstene-3-beta, 17-beta-diol to a nuclear protein in the rabbit vagina. Steroids. 2004;69:71–78. doi: 10.1016/j.steroids.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Adams JB, Martyn P, Lee FT, et al. Metabolism of 17 beta-estradiol and the adrenal-derived estrogen 5-androstene-3 beta,17 beta-diol (hermaphrodiol) in human mammary cell lines. Ann NY Acad Sci. 1990;595:93–105. doi: 10.1111/j.1749-6632.1990.tb34285.x. [DOI] [PubMed] [Google Scholar]
- 18.Poulan R, Labrie F. Stimulation of cell growth by C-19 steroids of adrenal origin in the ZR-75-1 human breast cancer cell line. Cancer Res. 1985;46:4933–4937. [PubMed] [Google Scholar]
- 19.Seymour-Munn K, Adams JB. Estrogenic effects of 5-androstene-3beta, 17beta diol at physiological concentrations and its possible implications in the etiology of breast cancer. Endocrinol. 1981;112:486–491. doi: 10.1210/endo-112-2-486. [DOI] [PubMed] [Google Scholar]
- 20.Santoro NS, Brown JB, Adel T, et al. Characterization of reproductive hormone dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495–1501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 21.Santoro N, Lasley B, McConnell D, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women Across the Nation (SWAN) daily hormone study. J Clin Endocrinol Metab. 2004;89:2622–2631. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 22.Skurnick JH, Weiss G, Goldsmith LT, et al. Longitudinal changes in hypothalamic and ovarian function in the perimenopausal women with anovulatory cycles: Relationship with vasomotor symptoms. Fertil Steril. 2009;91:1127–1134. doi: 10.1016/j.fertnstert.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Sowers MR, Moran FM, et al. Circulating bioactive androgens in midlife women. J Clin Endocrinol & Metab. 2006;91:4387–4394. doi: 10.1210/jc.2006-0284. [DOI] [PubMed] [Google Scholar]
- 24.Longcope C, Franz C, Morello C, et al. Steroid and gonadotropin levels in women during the peri-menopausal years. Maturitas. 1986;8:189–196. doi: 10.1016/0378-5122(86)90025-3. [DOI] [PubMed] [Google Scholar]
- 25.Labrie FMD, Martel C, Balser J. Wide distribution of the serum dehydroepiandrosterone and sex steroid levels in postmenopausal women: role of the ovary? Menopause. 2011;18:30–43. doi: 10.1097/gme.0b013e3181e195a6. [DOI] [PubMed] [Google Scholar]
- 26.Janssen I, Powell LH, Crawford S, et al. Menopause and the metabolic syndrome: the Study of Women's Health Across the Nation. Arch Intern Med. 2008;168:1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sowers MFR, Jannausch M, Randolph JF, et al. Androgens are associated with hemostatic and inflammatory factors among women at the mid-life. J Clin Endocrinol Metab. 2005;90:6064–6071. doi: 10.1210/jc.2005-0765. [DOI] [PubMed] [Google Scholar]