Synopsis
Is there evidence for a perimenopausal sleep disorder? We address this question in our presentation of the SWAN “sleep story,” in which we summarize and discuss data addressing sleep quality, objective measures of sleep patterns, and sleep disorders that have been published to date by the Study of Women’s Health Across the Nation (SWAN) and the ancillary SWAN Sleep Study. In this review article, we describe what has been learned about sleep during the perimenopause. Analyses exploring racial/ethnic diversity, a hallmark of the SWAN cohort, and the role of hot flashes and mood disturbance in sleep – perimenopause associations are described. Implications for clinical practice are considered.
Keywords: aging, menopausal transition, perimenopause, psychosocial, reproductive hormones, sleep
Introduction
Is there evidence for a perimenopausal sleep disorder? Krystal et al1 raised this question in considering factors associated with sleep disruption in midlife women that could be uniquely attributed to the menopausal transition, encompassing the perimenopausal and early postmenopausal years. In recent years, many reviews addressing the topic of perimenopausal sleep have been published (e.g., 1–4).
Why the burgeoning interest? Women’s life cycles have long been defined by their reproductive capacity. Circa 1900, the average life expectancy was only about 50 years, shorter than the average age at menopause.5 Some things have not changed. The average age at menopause remains approximately 51 years. But, with improved health and sanitation conditions, medical care, and lifestyle choices, current survival trends indicate that women have a life expectancy of about 80 years.6 Thus, on average, women now live more than a third of their lifetime, almost 30 years, beyond the menopausal transition.
Recognizing this trend, attention during the past couple decades has been directed increasingly toward health problems that are either unique to or more common in women than in men. Sex/gender differences have been recognized in sleep and its disorders (such as sleep apnea and periodic limb movement disorder). Across a woman’s reproductive life span, sleep is influenced by a wide variety of intrinsic (e.g., circadian and endocrinologic) and extrinsic (e.g., psychosocial) factors. Despite the fact that sleep complaints are approximately twice as prevalent in women of all ages compared to men, most sleep research has been conducted with men while factors unique to women have been ignored.7
Perimenopause, the period encompassing the years of menstrual irregularities and the 12 months immediately following, marks the transition from reproductive to non-reproductive life. The median age of onset of the perimenopause is in the mid-to-late forties and typically lasts 2 – 10 years, with a median duration of 4 years.8
A significant proportion of women experience perimenopause as a particularly challenging period of life for preserving good sleep. To date, relatively few publications have addressed the effects of perimenopause, compared with those associated with the postmenopause, on sleep and its related physiological changes. According to the NIH State-of-the-Science Panel’s Conference Statement on the Management of Menopause-Related Symptoms,9 “(W)omen seem to have more sleep disturbances as they progress through the menopausal stages. The prevalence of sleep disturbance varies from 16% to 42% in premenopause, from 39% to 47% in perimenopause, and from 35% to 60% in postmenopause.” These estimates included surgically as well as naturally menopausal women. At that time, longitudinal data on prevalence of sleep difficulty during the menopausal transition from ongoing longitudinal studies were not yet available.10
Despite observations of increased incidence of sleep disturbances and disorders during perimenopause, the cause(s) remains unclear. Disentangling the effects of sex and aging, two important but non-modifiable factors, is not a simple matter. In a meta-analysis based on literature published between 1960 and 2003, Ohayon et al11 examined sleep across the lifespan. Men and women had similar associations between sleep measures and aging. Larger effects of age on changes in objective (polysomnographic) sleep measurements of total sleep time (TST), sleep efficiency, percentage of stage 1 sleep, and REM latency were observed in women than in men. Women also had longer TST and sleep latency, more slow wave sleep, and less stage 2 sleep than similarly aged men. However, many studies excluded middle-aged subjects, maximizing age differences in sleep variables between the young and elderly. More complex analysis of progression from young and middle-aged and from middle-aged and elderly subjects conducted in this meta-analysis showed that age progression for all sleep variables was much less marked than simple comparisons of young and elderly subjects suggested.
Woods and Mitchell12 developed a model to examine the effects of other symptoms on sleep using data from the Seattle Midlife Women’s Health Study. In addition to age, factors in their model that are likely to affect sleep symptoms include those unique to the menopausal transition, such as menopausal transition stage, vasomotor (e.g., hot flashes, night sweats), endogenous reproductive hormone levels, and exogenous hormone use. Other pertinent symptoms not specific to the transition but perhaps more relevant during this phase of women’s life cycle include depressed mood, anxiety, back aches and joint pains. Stress- and health-related and lifestyle factors also contribute to sleep disturbance.
How these factors are inter-related remains an important but unresolved question. Krystal et al1 suggested, for example, that behavioral conditioning of vasomotor-initiated insomnia, which was triggered initially by night sweats but persisted after both the night sweats and their directly associated sleep disturbances resolved, was a possible explanation for perimenopausal sleep disruption. Based on a review of population surveys, they suggested that a mood or anxiety disorder specifically associated with menopausal hormonal changes was a less likely explanation for sleep disruption during perimenopause. Dennerstein et al13 examined the influence of hormonal changes on a variety of health outcomes in the Melbourne Women’s Midlife Health Project, a prospective population-based study of Australian-born women assessed annually for nine years while traversing the menopausal transition. “Trouble sleeping” was an item from a symptom checklist measuring frequency/severity in the previous two weeks. The prevalence of this symptom increased across the transition and was not significant alleviated by hormone therapy, consistent with Krystal et al’s1 earlier observation that peri-/post-menopausal insomnia does not respond consistently to hormonal therapy, though it was indirectly related to decreasing estradiol (E2) levels, via vasomotor symptoms.13 Moreover, sleep problems negatively affected self-rated health as well as mood and well-being. Taken together, these data suggest that sleep problems can be influenced by mood as well as other health-related symptoms and behaviors during this phase of life.
On the other hand, Freeman et al,14 using data from the Penn Ovarian Aging Study, a younger population-based cohort (35–47 years and premenopausal at enrollment), also analyzed data from nine years of annual assessments. The prevalence of poor sleep (43–53% in this cohort) increased only slightly across the menopausal transition and was not significantly associated with menopausal stage, nor was this symptom associated with E2 levels. Instead, poor sleep was associated with lower levels of inhibin B, which declined during the early transition period, and with a history of depression.
Against this backdrop of these somewhat contradictory observations, we will focus this review on data from a well-characterized multi-ethnic community cohort participating in the Study of Women’s Health Across the Nation (SWAN) and the ancillary SWAN Sleep Study, and describe what we have learned about sleep thus far from the SWAN studies. Our goal is to emphasize the importance of simply asking women seen in clinical settings about their sleep as they traverse this life cycle stage because sleep problems are common and they warrant clinical attention because of the impact of sleep disturbance on quality of life and health outcomes. Where applicable, SWAN findings will be compared with research related to sleep during perimenopause available from other large epidemiological studies on the menopausal transition that have been conducted during the past 20 years.
Measuring sleep symptoms and their prevalence – early epidemiological studies of sleep and methodological considerations
Among the early published epidemiological studies on insomnia, Karacan et al15 randomly sampled 1,645 adults and reported that approximately 24% of women reported having trouble with sleeping “sometimes” and 15% reporting “often or all the time,” compared with 19% and 11%, respectively, in men. In particular, rates were higher in respondents who were female, older, African American, unpartnered (widowed, separated, or divorced), or of lower socioeconomic status. Although trouble falling asleep was the most prevalent complaint, breakdown by age showed that staying asleep and waking too early were more prominent among those 40 years and older. In a sex-by-age analysis, Bixler et al16 observed that a significantly larger number of women older than 50 years reported difficulty with falling asleep and early morning awakening. Karacan et al15 attributed their findings to age-related changes but also suggested that older individuals may “take problems to bed with them.” Thus, social and behavioral factors must be considered in addition to biological and physiological factors.
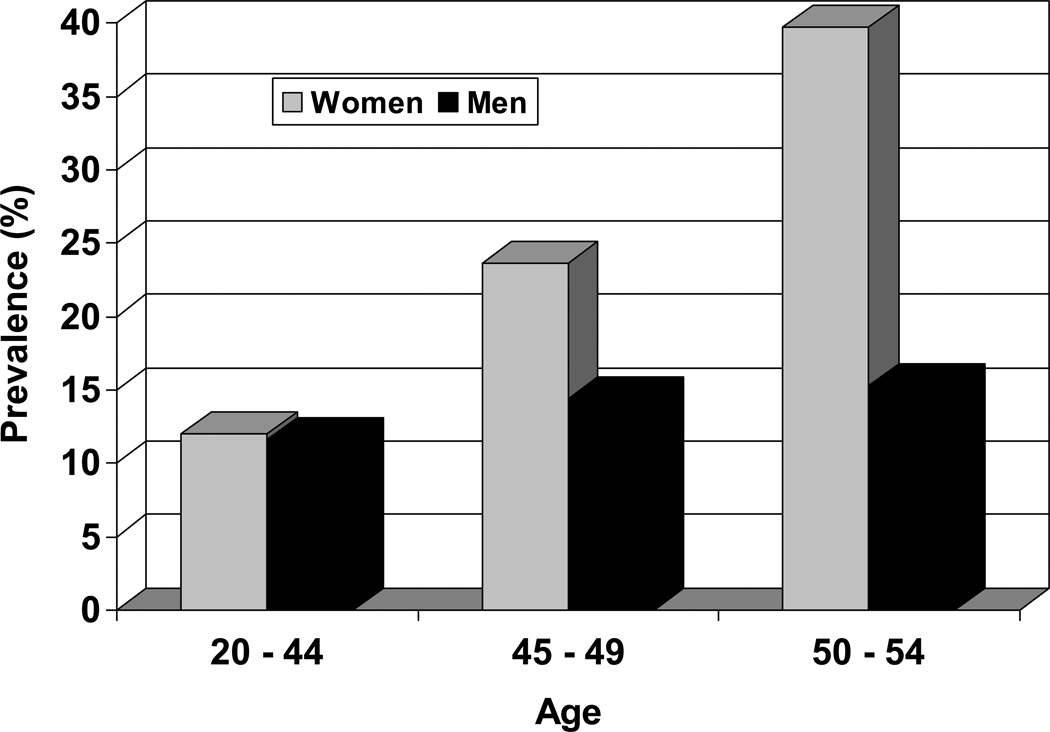
Self-reported sleep disturbances increase during midlife, with prevalences in women diverging markedly from those reported by men. Cirignotta et al17 noted that there appeared to be a critical age, around 45 years, when insomnia (defined as sleeping well without sleeping pills “rarely” or “never”) becomes a particularly frequent occurrence in women (Figure 1). Initial unadjusted estimates from the SWAN Cross-sectional Study (N = 16,065) indicated that 37.3% (N = 4,632) of 12,425 women, aged 40 – 55 years reported difficulty sleeping.18
Figure 1.
Age-related sex differences in self-reported sleep problems. Adapted using data from Cirignotta et al (1985).17
At this juncture, two points require clarification and should be kept in mind throughout our discussion. First, what is “sleep difficulty” and, second, how is it measured? In general, the definition is quite broad and diverse. Individuals report perceived sleep disturbance, their subjective experiences, rather than objectively measured sleep patterns. Ohayon19 defined four categories of “insomnia” prevalence estimates: (1) insomnia symptoms; (2) insomnia symptoms accompanied by daytime consequences (e.g., low mood, reduced function); (3) dissatisfaction with sleep quality or quantity; and (4) a diagnostic entity (i.e., insomnia diagnosis). Thus, depending on the operational criteria used, “insomnia” prevalence varied approximately 5 – 8-fold: from 30–48% if simply based on acknowledgment of a sleep problem (e.g., yes/no in the past 1–2 weeks) to 6% with the most restrictive diagnostic criteria (e.g., the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision,20 which requires symptoms plus daytime consequences such as fatigue or evidence of impaired functioning persisting for at least one month).
With regards to objective measurement of sleep, advances in our understanding of sleep and its relationship to health and other physiological functions have been achieved through use of polysomnography. Sleep laboratory studies conducted in well-controlled environments in which sleep and other physiological functions can be precisely measured have been considered the “gold standard” for recording sleep. However, this objective method “is expensive, time-consuming and necessarily limited in its capacity to generate epidemiological type data for a large population of individuals”.15
To lessen participant burden and expense in large epidemiological studies, wrist actigraphs have been used to collect objective sleep data. Actigraphy involves the use of a wristwatch-like device with a highly sensitive accelerometer to digitally record an integrated measure of motor activity. By this means, rest-activity cycles are analyzed to identify sleep-wake periods, though it must be acknowledged that “rest” does not necessarily mean “sleep.” Actigraphy has been used in to monitor sleep in epidemiological studies such as the Coronary Artery Risk Development in Young Adults (CARDIA) Study21 and the Women’s Health Initiative (WHI).22 Whereas actigraphy, compared with polysomnography, is less intrusive and does not require a technician’s presence to monitor recordings, it has a tendency to both underestimate sleep when it is significantly fragmented (eg, sleep apnea) and overestimate sleep in individuals who lie quietly immobile in bed while awake. Although it has been demonstrated that individuals with insomnia and apnea tend to underestimate how much they sleep while monitored polysomnographically, Lauderdale et al23 have observed that actigraphy tends to underestimate self-reported sleep duration.
Despite potential limitations of survey methods, such as biased reporting by insomniacs, self-report questionnaires can provide information that cannot be provided by laboratory analysis, such as daytime consequences of sleep symptoms and (dis)satisfaction with sleep quantity and/or quality. Okun et al24 used SWAN Sleep Study data to test the validity of the Insomnia Symptom Questionnaire (ISQ). This a new instrument, which was developed based on established diagnostic criteria to capture the multidimensionality of insomnia, showed high specificities (> 90%) and identified an insomnia prevalence of 9.8% in this SWAN sub-cohort of 266 pre-, peri- and post-menopausal women. This prevalence was similar to that described in the general population when insomnia was diagnosed based on symptoms and daytime consequences (9–15%)19 and among Korean pre-, peri- and post-menopausal women reporting sleep difficulties at least three times per week (14.3%).25 As Karacan et al15 concluded, “(T)he ultimate criterion as to what constitutes a good night’s sleep may be a subjective one.” Questionnaires like the ISQ can be easily implemented to describe the prevalence of insomnia symptoms in a large population.
In the SWAN Sleep Study, an ancillary study of the parent SWAN, 370 women were recruited from four of the seven sites (Chicago, IL; Detroit area, MI; Oakland, CA; Pittsburgh, PA). Participants underwent an extensive assessment of their sleep, beginning with three nights of home-monitored polysomnography during the first 3 days of the protocol, up to 35 days (one cycle) of actigraphy monitoring and sleep diaries, and questionnaires related to sleep, lifestyle and mood.26 Analyses currently are being conducted to evaluate relationships among indices of sleep duration and continuity measured by polysomnography, actigraphy and sleep diaries (Hall et al, unpublished). With these data we will be able to evaluate complex relationships between menopausal characteristics (eg, hormones, hot flashes), sleep (e.g., quantitative EEG, heart rate variability during sleep), race/ethnicity, socioeconomic status, lifestyle factors (e.g., smoking, exercise) and mood (depression, anxiety, stress), which will advance our understanding of how these measurements relate to each other in population of midlife women.26
Manber and Armitage7 described a number of other factors that complicate the study of sleep in women. These issues are particularly relevant to studying sleep during the perimenopause/menopausal transition. These include: (1) the confounding effect of menstrual phase; (2) timing of the surge of luteinizing hormone (LH), ovulation, estrogen and progesterone peaks, and menstruation, which vary from cycle to cycle as well as within and across women; (3) variability in timing of sleep measurements relative to the cycle; and (4) how many intervals in which to subdivide the menstrual cycle. In the following sections we present data from analyses of both questionnaire and polysomnogram data collected in Core SWAN and the SWAN Sleep Study that examine sleep during the perimenopause and in which we have tried to address these complicating factors that have not been accounted for in the majority of studies examining sleep disturbance in midlife women.
Results of SWAN Sleep Studies Addressing Self-Reported Sleeping Difficulties
Cross-sectional studies
From November 1995 through October 1997, a community-based survey of women’s health and menopausal symptoms was conducted at each of the seven SWAN sites (N = 16,065). Women were asked whether, over the past two weeks, they had experienced difficulty sleeping. Difficulty sleeping was reported by 37.7% of 12,603 women (78.5% of those participating in this cross-sectional survey), 40–55 years old, who completed the sleep item and whose menopausal status could be classified by bleeding criteria (ie, not taking reproductive hormones for at least 3 months) or were surgically menopausal.27
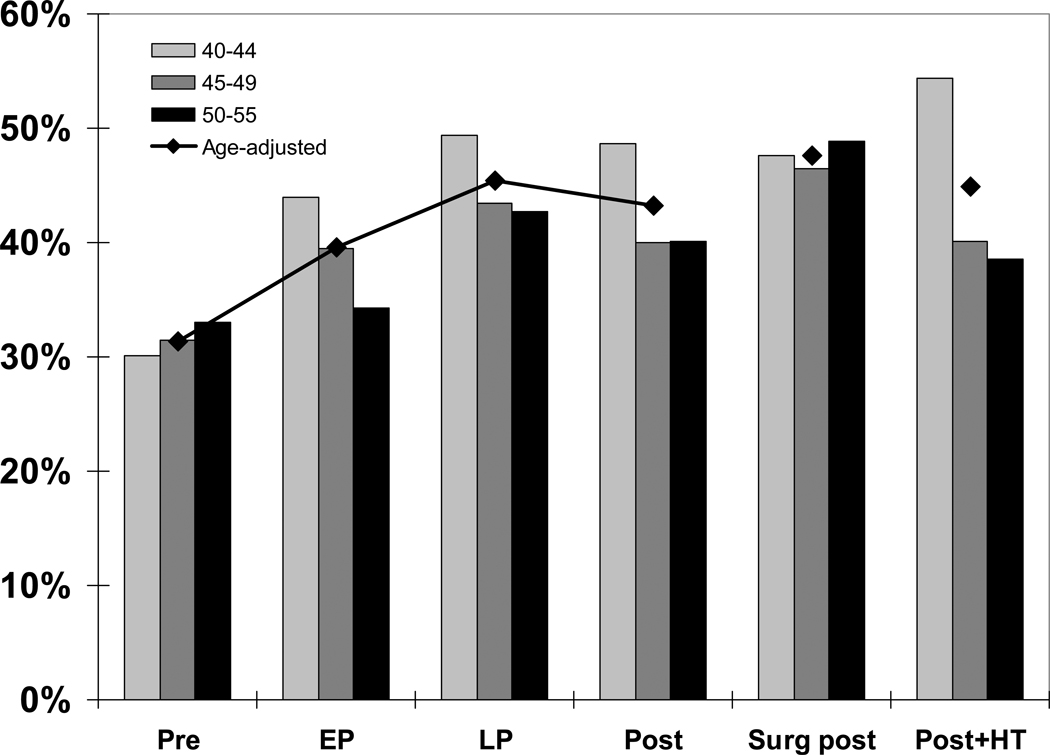
Age-adjusted prevalences as well as age differences by menopausal status are illustrated in Figure 2. Prevalences increased from pre- to late perimenopause and plateaued through postmenopause, decreasing slightly in age-adjusted analyses. Late perimenopausal and surgically menopausal groups were most likely to report difficulty sleeping. Within each group the trend was for higher rates of sleeping problems in the younger age group in all but the premenopausal (and surgically menopausal) group. This observation is remarkable in light of the relatively narrow age range examined and suggests that women beginning the menopausal transition at a younger age may experience more frequent and/or severe sleep symptoms. These cross-sectional data also suggest that age may be important but not the main determinant of the menopause – sleep relationship.
Figure 2.
Cross-sectional age-adjusted prevalence of sleep difficulty across the menopausal transition. Pre = premenopausal, EP = early perimenopausal, LP = late perimenopausal, Post = postmenopausal, Surg post = surgically postmenopausal, Post + HT = postmenopausal and using hormonal therapy (HT; this is the only group that includes HT users). Adapted using data from Kravitz et al (2003).27
A key aim of SWAN is to examine race/ethnic differences in a diverse cohort of women traversing the menopausal transition.28 Among the race/ethnic groups included in SWAN, prevalences for difficulty sleeping ranged from lowest (28.2%) in Japanese women to highest (40.3%) in Caucasian women, with Chinese (31.6%), Hispanic (38.0%) and African American (35.5%) women in between. Within each race/ethnic group, some deviance from the overall trends across menopausal transition stages may be due at least in part to relatively smaller race/ethnic sample sizes, particular among the Asian groups.
Multivariate analyses demonstrated that a number of additional variables were significantly associated with difficulty sleeping, as observed in other studies. Most notably, vasomotor and psychological (depression, anxiety) symptoms, self-perceived health, quality of life, health behaviors, and arthritis were important contributors. Results were similar when women with vasomotor symptoms were excluded. The major difference was that the magnitude of self-reported sleep difficulty within the surgically menopausal group was reduced to a level similar to that of the premenopausal group. Age was not a significant covariate in any of these analyses, perhaps related to our sample’s relatively narrow age range. Thus, these data suggest that sleep difficulties peak at late perimenopause and remain elevated in postmenopause, even in the absence of vasomotor symptoms. These results support the notion that a sizable number of women experience sleep problems during the perimenopause and that the association is not fully explained by vasomotor symptoms.
In another SWAN analysis, which focused on variables associated with vasomotor symptoms, Thurston et al29 examined correlates of “vasomotor symptom bother” beyond symptom frequency. Separate analyses were conducted, controlling for frequency of either hot flashes or night sweats as well as a number of other variables associated with bothersomeness of either of these two vasomotor symptoms. In both sets of analyses, sleep problems (defined as self-reported symptoms of falling or staying asleep or waking early at least three nights a week) had the strongest influence on bother secondary to night sweats and second strongest influence (after African American race) on bother related to hot flashes. Their findings, suggesting bidirectionality in the association between sleep disturbance and vasomotor symptoms, indicate that, in addition to being a consequence of vasomotor symptoms, sleep problems can influence the extent to which vasomotor symptoms are bothersome.
Psychosocial factors also may be important contributors to perimenopausal sleep problems. Hall et al30 showed that lower income is a correlate of perceived sleep difficulty (trouble falling asleep or waking up repeatedly or earlier than planned), or restless sleep. Financial strain, measured as “somewhat or very hard to pay for basics,” partially mediated this relationship between low income and sleep difficulty.
Relationship factors also were examined cross-sectionally. Troxel et al31 looked at marital happiness in relation to sleep disturbance (difficulty falling asleep, waking up repeatedly or early than planned, and restless sleep). Happily married women, compared with women reporting lower marital happiness, endorsed fewer sleep disturbances. This associated was found in the Caucasian group and, to a lesser extent, in the African American group, but due to small numbers, could not be examined in the other racial/ethnic (Hispanic, Chinese or Japanese) groups.
Although we should be cautious in drawing conclusions about directionality and causation due to the cross-sectional nature of these analyses, these analyses have provided us with important insights regarding sleep in perimenopausal women. The results confirmed findings from other studies that self-reported sleep disturbances increase through late perimenopause and are higher in surgically menopausal women not using hormone therapy, that there are race/ethnic differences in sleep difficulties, and that vasomotor, health, and psychosocial factors also contribute. What cross-sectional studies do not tell us is what is the effect of becoming peri- or postmenopausal on sleep. To try to answer this question, we need to look at longitudinal studies.
Longitudinal studies
SWAN longitudinal analyses have examined data collected through seven years of annual follow-up.32 Separate analyses were conducted for each of the three types of sleep disturbances: trouble falling asleep, waking up several times, and waking up earlier than planned. Sleep disturbance was considered present if it was reported as present for at least three nights weekly in the past two weeks. Three aspects of the transition were examined as predictors of sleep disturbance: transition stage by bleeding criteria, vasomotor symptoms, and reproductive hormones (follicle stimulating hormone [FSH], E2).
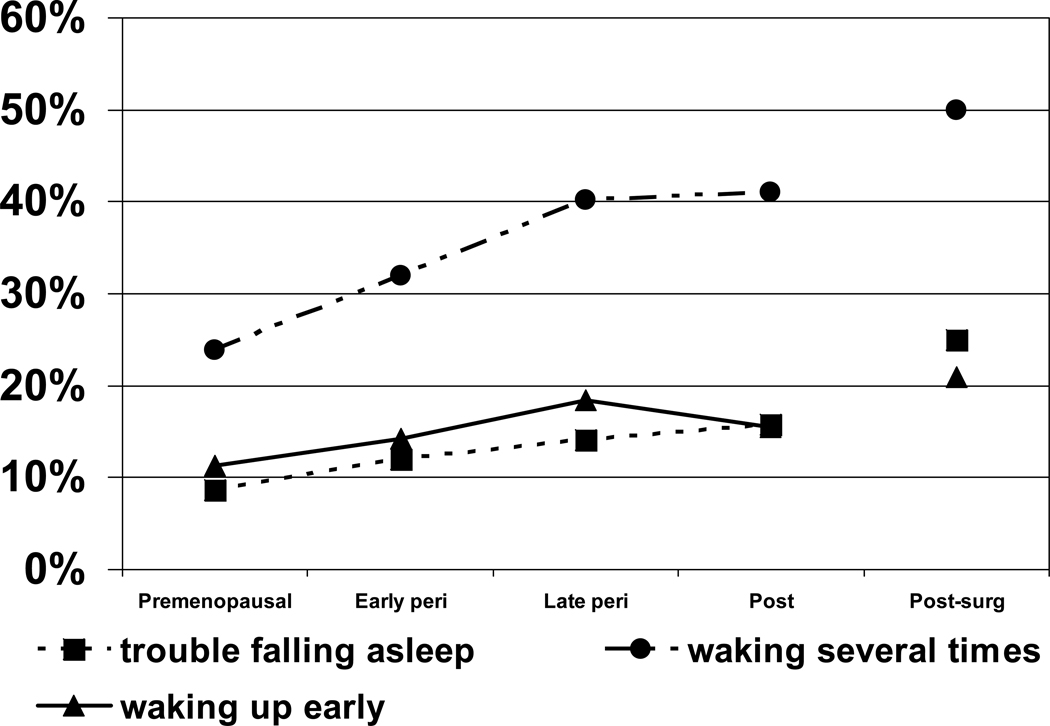
Figure 3 shows the progression of sleep problems as women traversed the transition (not all women had transitioned to post-menopausal). At baseline assessment, 30.8% of the 3,045 women included in this analysis had at least one type of sleep difficulty on 3 or more nights per week in previous 2 weeks. Waking during the night was by far the most prevalent type of sleeping problem, but trouble falling and staying asleep also increased significantly during the transition compared with remaining premenopausal. Only early morning awakening decreased during post-menopause.
Figure 3.
Prevalence of sleep difficulty across menopausal transition during longitudinal follow-up (average rates for each menopausal stage; N = 3,045). Data are reported separately for 3 types of sleep problems occurring 3 or more nights per week in the previous 2 weeks: trouble falling asleep, waking up several times during the night, and waking up earlier than planned. Adapted from Kravitz et al (2008) with permission.32
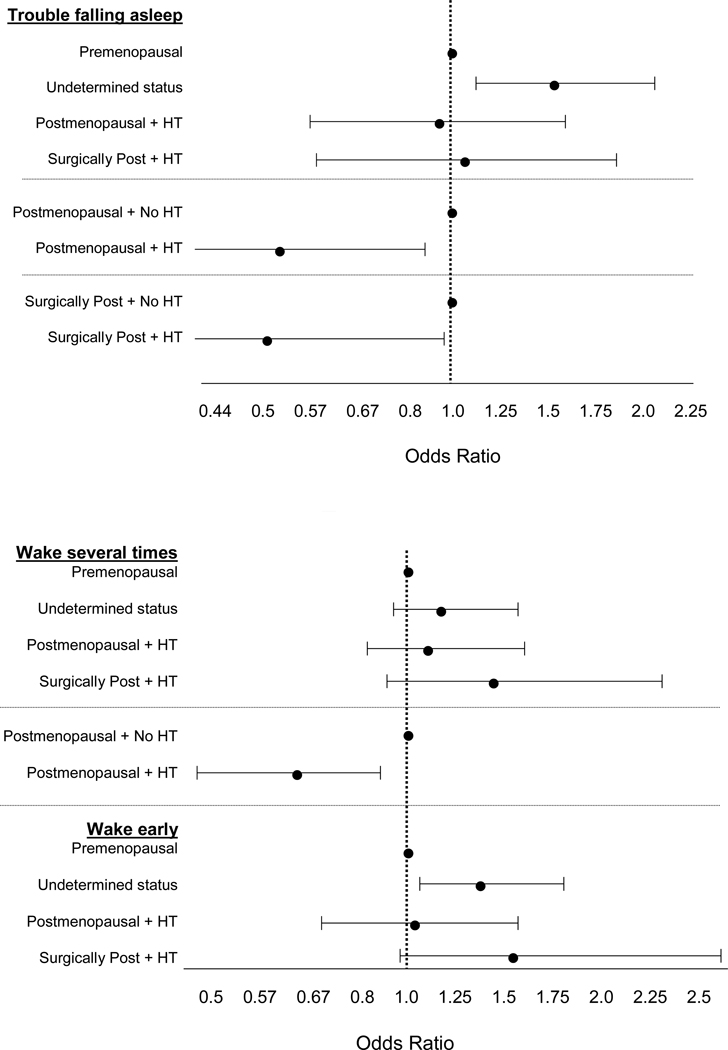
Figure 4 shows the effects of hormone therapy. Hormone therapy appeared to benefit both naturally and surgically post-menopausal women, particularly for alleviating problems falling asleep. As the figure illustrates, postmenopausal women receiving hormone therapy do not differ significantly from premenopausal women (all confidence intervals include 1). On the other hand, exogenous hormone use provided no significant benefit for pre- and perimenopausal women, compared with premenopausal women not using hormone therapy, particularly for trouble falling asleep or early morning awakening (odds ratio greater than 1 and confidence intervals do not include 1). Comparing postmenopausal women using hormones with those not using hormones indicates a clear benefit for those who were naturally post-menopausal for both falling asleep and staying asleep, but not for early morning wakenings. For surgically postmenopausal women, hormone therapy helped only for trouble falling asleep.
Figure 4.
Adjusted odds ratios for each of the three sleep difficulties in pre-/perimenopausal (“undetermined status”), naturally postmenopausal and surgically postmenopausal (“surgically post”) women using hormone therapy (+ HT) compared with premenopausal women not using HT, and naturally and surgically postmenopausal women using HT compared with their counterparts not using HT. All 3 sleep models adjusted for site, age, race/ethnicity, current HT, depressive and anxiety symptoms, sleep medications, smoking, life events, nocturia, physical symptoms, and bodily pain. Model for Trouble Falling Asleep (top figure) also adjusted for financial strain, body mass index, smoking, and education. Model for Wake Several Times (bottom figure) also adjusted for pain medications and self-reported overall health. Adapted using data from Kravitz et al (2008).32
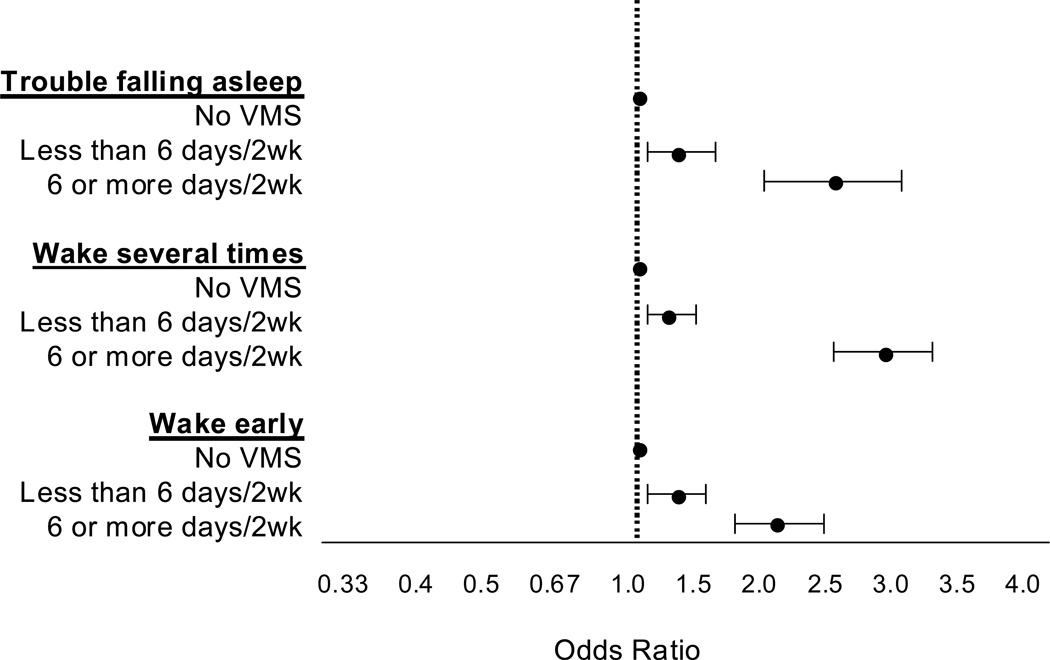
These longitudinal analyses show that vasomotor symptoms have a very clear adverse effect on sleep (Figure 5). Women experiencing these symptoms report significantly more of each of the three sleep disturbances, and do so in a dose-dependent fashion. Those experiencing symptoms on more nights per week were more likely to report a sleep disturbance than those without vasomotor symptoms, ranging from 31 – 37% more likely if vasomotor symptoms occurred for 1 – 5 days in the past two weeks to 116–193% more likely if they occurred on 6 – 14 days.
Figure 5.
Adjusted odds ratios for association of vasomotor symptoms with 3 types of sleep difficulty (trouble falling asleep, waking up several times, waking up earlier than planned) occurring 3 or more nights per week during the past 2 weeks. All models adjusted for site, age, race/ethnicity, current hormone therapy use, depressive symptoms, anxiety symptoms, sleep medication, smoking, life events, nocturia, physical symptoms, and bodily pain. Model for Trouble Falling Asleep also adjusted for difficulty paying for basics, body mass index, smoking, and education. Model for Wake Several Times also adjusted for pain medication and self-perceived overall health. Adapted using data from Kravitz et al (2008).32
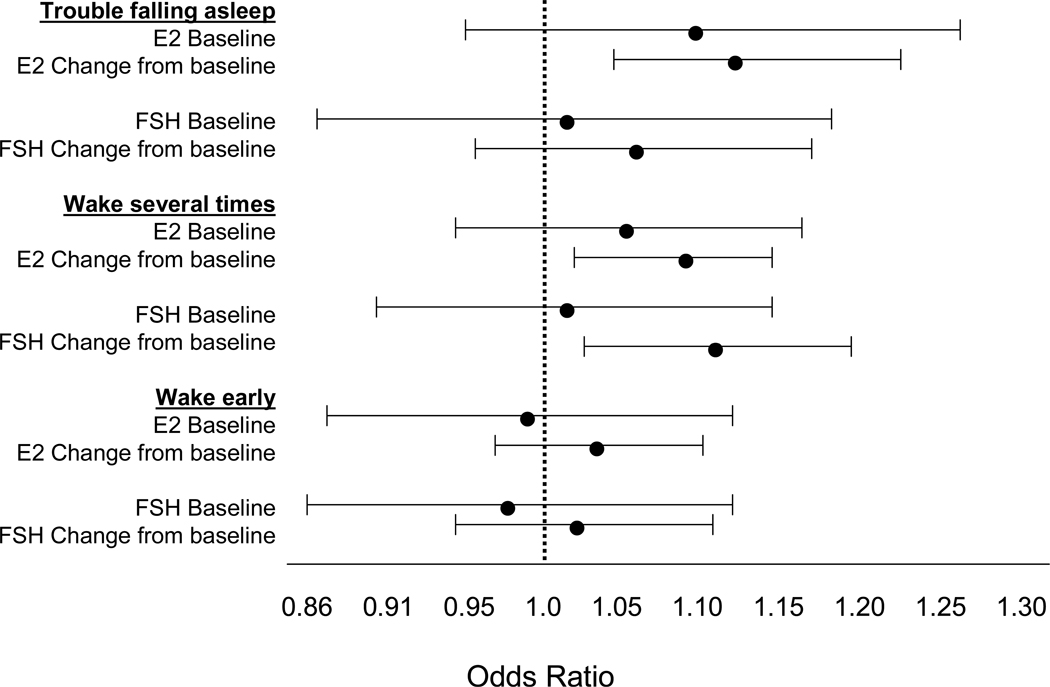
Figure 6 shows the associations between E2 and FSH, respectively, and sleep disturbances. The results indicate that only changes in hormone levels, but not baseline levels, are associated with sleep disturbances. Decrements in serum E2 levels were associated with both trouble falling and staying asleep, while increments in serum FSH levels were associated with reports of difficulty staying asleep.
Figure 6.
Adjusted odds ratios for association of 3 types of sleep difficulty (trouble falling asleep, waking up several times, waking up earlier than planned, all occurring 3 or more nights per week during the past 2 weeks) with estradiol (E2) levels (increased sleep difficulty associated with lower levels) and follicle stimulating hormone (FSH) levels (increased sleep difficulty associated with higher levels). All 3 sleep models adjusted for site, age, race/ethnicity, current hormone therapy use, blood draw in cycle day 2–5 window, depressive symptoms, anxiety symptoms, sleep medication, smoking, life events, nocturia, physical symptoms, and bodily pain. Models for Wake Several Times also adjusted for number of med conditions. Adapted using data from Kravitz et al (2008).32
Race/ethnic groups differed in both waking several times and waking earlier than planned. Caucasian women reported the most problems and Hispanic women reported the fewest. However, the impact of vasomotor symptoms on sleep differed among race/ethnic groups only for waking early, and associations between sleep difficulties and menopausal status did not vary among the race/ethnic groups.
Thus, longitudinal analyses in sleeping difficulties confirmed the findings of differences between menopausal transition stages and that, while vasomotor symptoms play an important role, they are not the only determinant of perimenopausal sleep disturbances. The associations with hormone levels was less robust in this study involving annual blood levels of estradiol and FSH. Finally, hormone therapy (alone) may not improve sleep quality for all women or at all stages of the transition.
Daily Hormone Study (DHS)
Gracia et al33 observed that, although reproductive hormone trends have been described, bleeding patterns still are used to define menopausal status because no clear cut-offs in hormone levels have distinguished women by menopause status. Differences among studies in the associations between hormone levels and sleep symptoms during the menopausal transition may be due to the complex nature of the relationship between these sex steroids and sleep as well as differences in cohorts studied and methods used.7 Single annual hormone sampling may have limited ability to provide information about the underlying hormone dynamics that occur during the menopausal transition. Thus, differences among studies in relationships between sleep symptoms and perimenopausal hormonal changes may be due to limitations of the hormone/sleep analyses with annual/infrequent sampling.
To address the association between hormone levels and self-reported trouble sleeping, we analyzed data from SWAN’s Daily Hormone Study.34 The DHS,35–37 included a subset of 848 women in SWAN who were aged 43 to 53 years and represented all SWAN race/ethnic groups. These pre- and early perimenopausal women collected their first morning urine specimen and completed a bedtime diary daily for a single menstrual cycle or 50 days (whichever came first) annually. Data for this analysis were obtained from 630 (92.6%) of the 680 women with evidence of luteal activity (ELA, i.e., women presumed to have ovulatory cycles), as determined by a substantial increase in pregnanediol glucuronide (PdG) excretion during the cycle. Urine was assayed for LH, FSH, E2 metabolites (i.e., estrone conjugates, E1c), and PdG, and sleep was measured with a single diary item, “trouble sleeping? (yes/no)”.
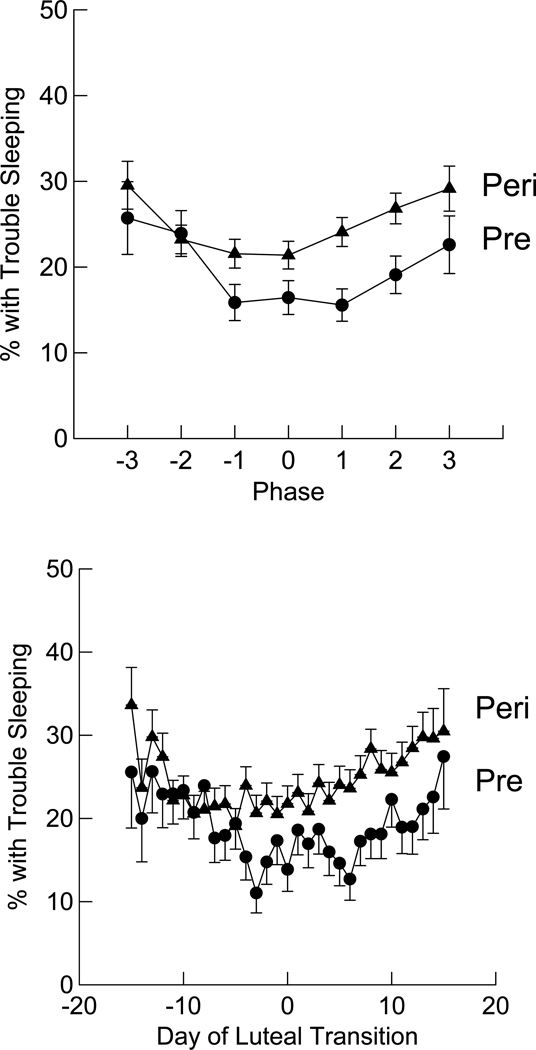
Figure 7 shows the differences in sleep outcomes by menstrual cycle phase (i.e., early, middle and late follicular or luteal, and day of luteal transition) and day-by-day. As expected, the day-by-day analysis shows more variability, and within each of the two groups significantly more women reported trouble sleeping on days at the beginning (early follicular phase) or end (late luteal phase) of their cycle, and sleep was best at mid-cycle. Across the whole cycle, early perimenopausal women were 29% more likely to report trouble sleeping compared with premenopausal group. Separate analyses were conducted for each group to determine which hormone(s) were associated with trouble sleeping. Among premenopausal women there was an 11.1% increase in the likelihood of trouble sleeping for each log-unit increment in FSH level, whereas among early perimenopausal women there was a 9.5% increment in the likelihood of trouble sleeping for each log-unit increment in PdG level. These associations between changes in reproductive hormone levels across the menstrual cycles and difficulty sleeping were observed independent of the effects of vasomotor and mood symptoms on sleep.
Figure 7.
Comparison of daily report of self-reported trouble sleeping across one menstrual cycle, by phase of cycle (top) and day-by-day (bottom), both centered on day of luteal transition. Circles represent the premenopausal group (Pre) and triangles represent early perimenopausal group (Peri). In phase of cycle figure, 0 is the day of luteal transition, −1 (days −1 to −5), −2 (days −6 to −10), −3 (days −11 to −15) correspond to late, mid, and early follicular phase, respectively, and +1 (days 1 to 5), +2 (days 6 to 10), +3 (days 11 to 15) correspond to early, mid, and late luteal phase, respectively. Bottom figure adapted from Kravitz et al (2005).34 Arch Intern Med 2005;165:2370–2376. Copyright © 2005 American Medical Association. All rights reserved.
Thus, we learned from the SWAN DHS that sleep problems are more prevalent across a menstrual cycle among perimenopausal than a similarly aged group of premenopausal women and that the prevalence of sleep difficulties varies across cycle phase, with more pre- and perimenopausal women reporting problems at the beginning and end of the cycle and fewer reporting problems at mid-cycle. Premenopausal women had more problems in association with higher FSH levels, which were higher than expected in premenopausal women in the early follicular phase, and perimenopausal women experienced more problems in concert with higher levels of the progesterone metabolite PdG in the luteal phase. Finally, the associations between hormone levels and trouble sleeping remained significant even after accounting for the contributions of mood and vasomotor symptoms to sleep symptoms.
The SWAN Sleep Study
Overview
The SWAN Sleep Study is an ancillary study conducted at four of SWAN’s seven sites (Rush University Medical Center, University of California Davis/Kaiser, University of Michigan, University of Pittsburgh). The baseline study, Sleep I used a multi-dimensional and multi-method approach to characterize (1) sleep disturbances in the perimenopause and (2) the effects of psychobiological factors on the menopause-sleep relationship in 370 women. In Sleep II, the follow-up study, 348 (94%) of the 370 Sleep I women participated in a shorter but similar protocol, as described below. Participants in the SWAN Sleep study were recruited from the Core SWAN cohort. Sleep I and II procedures were conducted during Core SWAN follow-up years 5 – 7 (2003 – 2005) and years 8 –10 (2006 –2008), respectively. Thus, the Sleep Study was enriched by up to 10 years of data on the participants’ previous health and functioning.
The Sleep Study I protocol was conducted across an entire menstrual cycle or 35 days, whichever was shorter. Study participants included 328 pre-and perimenopausal women and a small group (n=42) of postmenopausal women. Three days of in-home PSG was performed during women’s early follicular phase (if still menstruating, otherwise scheduled at the participant’s convenience), while sleep diary, actigraphy, and event monitor recordings of vasomotor symptoms were collected throughout the cycle, along with questions on sleep quality, daytime sleepiness, mood, and stress. The Sleep II protocol included the same measures but PSG sleep data were collected on only 2 nights, and actigraphy and sleep diary collections were shortened to 14 days. There were 3–4 years between the two assessments, to allow sufficient numbers of women to transition into postmenopause in order to test study aims.
Longitudinal assessment of sleep during the menopausal transition was conducted to evaluate Spielman’s38 model of the development of acute and chronic sleep disturbances and its consequences for health status. Whereas acute sleep disturbances reflect the combined effects of predisposing and precipitating factors, chronic sleep disturbances are maintained by perpetuating factors, which may develop in response to acute sleep disturbances. Moreover, these data may help to identify potential adverse effects of sleep disturbances on health status during early postmenopause as we continue annual assessments of the SWAN cohort. Thus far, data have been published from Sleep I only, and are summarized below.
Sleep I
Of the 370 women recruited, 178 were Caucasian, 134 were African American, and 58 were Chinese, and they ranged in age from 48 – 59 years. Hall et al39 examined race/ethnic differences in sleep and whether socioeconomic status measures were associated with these differences. On a measure of self-reported sleep quality, the Pittsburgh Sleep Quality Index (PSQI),40 66% of the Sleep Study cohort had scores exceeding the cut-point for clinically significant complaints (greater than 5; possible range 0 – 21; mean = 6.6, SD = 2.4). Moreover, PSQI scores were significantly higher for African Americans, but not Chinese women, than for Caucasians, indicating worse sleep quality. PSG sleep measures showed shorter sleep duration and indicators of sleep continuity demonstrated more disturbed sleep (longer sleep latency, more wakefulness after sleep onset, lower sleep efficiency) in African Americans compared with both the Caucasian and Chinese groups.
With regard to socioeconomic factors, financial strain, but not educational level, was associated with decreased sleep quality (PSQI) and lower sleep efficiency (on PSG), independent of race. Thus, race – sleep relationships did not differ according to whether a woman had earned a college or advanced degree or whether she reported financial strain, perhaps because too few of the Sleep Study women were of low socioeconomic status.39
Spectral analysis of the electroencephalogram (EEG), also known as quantitative EEG (QEEG) and completed as part of the PSG, may be a more sensitive procedure for analyzing EEG signals recorded during sleep and detecting subtle sleep EEG disturbances than traditional manual sleep stage scoring. As such, QEEG can provide additional information about sleep quality. Hall et al39 examined two QEEG sleep measures, delta and beta power. The slow wave EEG of non-rapid eye movement sleep, stages 3–4 (i.e., delta EEG), reflects deep, restful sleep, with higher delta EEG power indicating more slow wave sleep. Beta EEG power reflects cortical arousal level during sleep; higher power in the beta EEG band has been associated with psychological stress and insomnia. Consistent with the self-reported and PSG findings in this sample, beta EEG power was higher in African Americans, but not Chinese women, compared with Caucasians. Both delta EEG power and PSG measures of stage 3–4 sleep were lower in African Americans and Chinese compared with Caucasians. These sophisticated analyses of measured sleep quality support racial differences in sleep observed using both standard PSG measures and reports of poor sleep quality.
Clinical disorders of sleep were also examined in Sleep I. Clinically significant levels of sleep apnea and periodic leg movements (PLMs) were observed in a substantial proportion the sample; 20% had apnea + hypopnea values above 15, and 8% had periodic leg movements with arousal (PLMAr) values above 10.39 The mean apnea-hypopnea index (AHI) was 10.4 and mean PLMAr index was 3.9. In contrast, the estimated prevalence of AHI greater than 15 in similarly aged middle-aged women (50 – 60 years) in the Wisconsin Sleep Cohort was 4.0%.41 Young et al42 reported that menopausal status was associated with AHI level, which increased across the menopausal transition, after controlling for age, body mass index (BMI), and lifestyle factors. The mean BMI at baseline of Young et al’s cohort was 30.1 mg/kg2,41 similar to that reported by Hall et al,39 (29.96 mg/kg2). Thus, an explanation for the difference in AHI between the SWAN Sleep Study and the Wisconsin Sleep Cohort Study, which were conducted approximately a decade apart, is not immediately clear. Possible contributing factors could include differences in sleep recording and apnea scoring procedures as well as the fact that the SWAN Sleep Study was conducted in-home while the Wisconsin Sleep Cohort Study used an in-laboratory protocol. Whereas sleep apnea43 and PLMs44 can affect sleep duration and continuity measures in peri- and postmenopausal women, we found no racial differences in either of these sleep pathologies in our sample, suggesting that sleep disorders are unlikely to explain racial differences seen on sleep EEG analyses.
Analyses of SWAN Sleep Study data have shown the association of sleep disorders with other health conditions. These data show that indices of sleep disordered breathing (including sleep apnea, oxygen desaturation event frequency and percent of total sleep time with oxygen saturation levels of 90% or less), may be risk factors for cardiometabolic disorders in perimenopausal women. Matthews et al45 examined associations between inflammation and pro-coagulation biomarkers (C-reactive protein (CRP), fibrinogen, factor VIIc, and plasminogen activator inhibitor (PAI)-1) and measures of PSG sleep and sleep disordered breathing. Regression analyses revealed that each of these four inflammatory and coagulation indices were associated with indicators of sleep disordered breathing after adjusting for a variety of covariates, including BMI (41% of the sample were categorized as obese, i.e., BMI 30 or greater). In adjusted models, AHI and oxygen desaturation indices (desaturation event frequency and percent of total sleep with oxygen saturation 90% or less) were significantly associated with all biomarkers except factor VIIc (only the association with desaturation frequency was significant). These observations suggest that inflammation and pro-coagulation processes may be an important pathway connecting sleep disordered breathing and cardiometabolic disorders in women. Analyses exploring racial variation showed that African Americans with elevated inflammatory markers had shorter sleep duration and lower sleep efficiency.
Of particular importance for clinicians is that the cross-sectional associations of sleep with race/ethnicity, financial strain, and marital happiness, and of sleep disordered breathing indices with inflammatory and coagulation indices were obtained in community samples of midlife women, rather than women referred because of sleep problems. In addition, the study findings were based on in-home sleep assessments and were adjusted for potential confounders of the association. Whereas inflammation and coagulation may provide a pathway that connects sleep characteristics with risk for cardiometabolic disorders, longitudinal assessment of the impact of the menopause transition, reproductive hormones, vasomotor symptoms, and other factors on sleep, and of the associations between sleep disorders and sleep patterns with inflammation and other cardiometabolic risk factors, is needed to more clearly delineate the temporal relationship of sleep with inflammation and other menopause-related and health outcomes. Longitudinal analyses involving Sleep II data and continued follow-up of the cohort are underway.
While longitudinal analyses involving changes in sleep and other factors between the Sleep I and Sleep II studies are not yet available, several analyses of Sleep I data have been conducted that examine the associations between sleep and subject characteristics collected as part of the overall SWAN study during the years preceding and following the Sleep I study. One example of such an analysis examines the longitudinal changes in the sex steroid hormones and gonadotropins in relation to measures of sleep duration, continuity, and architecture. The SWAN Sleep Study benefits from the standardized collection of hormone data obtained in the years prior to and after the sleep studies, which allow for changes in gonadotropins to be examined in relationship to sleep parameters.
Sowers et al46 examined associations between objectively and subjectively measured sleep characteristics and endogenous levels of serum FSH, E2, or testosterone (T), or changes in these levels, over the preceding 5- to 7-year time period. A number of interesting sleep – perimenopause relationships were revealed. In adjusted analyses, a greater rate of increase in FSH from the baseline value to the time of the Sleep Study was associated with significantly longer sleep duration and higher delta (slow wave) sleep percent on the PSG, and a perception of worse sleep quality. On the other hand, women who had a slower rate of FSH change had significantly lower sleep efficiency. However, the FSH cutpoint of 40 mIU/mL, which is commonly used to denote the transition to postmenopause, was not associated with the sleep measures being evaluated.
In contrast to associations observed between changes in FSH and sleep, sleep measures did not reflect changing levels of E2 preceding the sleep studies, although a higher baseline E2 was associated with a slightly poorer sleep quality 7 years later. Relatively limited change in T occurred across the visits preceding the Sleep Study. However, the E2/T ratio, which may reflect the increasing androgenemia associated with progression of the transition, was reduced progressively across the menopausal transition stages. A lower E2/T ratio preceding the Sleep Study was significantly associated with less time awake after sleep onset, indicating better sleep consolidation. Notably, menopausal status (based on variability in menstrual bleeding) was not associated with any sleep measure when any of the serum hormones were in the analytic model, indicating that the hormone levels may be a better predictor of sleep than menopausal stage per se. Similarly, Young et al43 observed that PSG-measured sleep was not worse in perimenopausal or postmenopausal women, compared with premenopausal women, although peri- and postmenopausal women reported experiencing more sleep dissatisfaction.
Our results suggest that measures of a more rapid rate of progress through the menopause transition, as indicated by the trajectory of FSH change (i.e., rate of change over time), together with objective measures of sleep may be clinically useful for validating sleep complaints in perimenopausal women. However, the cost-benefit of using PSG for this purpose remains to be evaluated.
Sleep also has a social context. Extending previous work with the SWAN cohort, showing that marital happiness was associated with higher sleep quality in women,31 Troxel et al47 explored whether the marital or cohabiting relationship, current and/or past, is associated with sleep, as measured both subjectively (PSQI) and objectively (PSG and actigraphy). Analyses of women’s relationship histories over the 6–8 years prior to the sleep study showed advantages in sleep for women who were consistently partnered versus women who were unpartnered throughout this interval, or those who had lost or gained a partner over that time course.
For those who gained a partner, there were discrepancies in the effects of sleeping with a partner on subjective versus objective sleep measures. Whereas women who had gained a partner were similar to consistently married women in terms of subjective sleep quality and PSG measures of sleep continuity and architecture, their differences, and the discrepancies in subjective versus objective sleep parameters in regards to the effects of sleeping with a partner, suggest that although the presence of a bed partner can negatively affect objective measures of sleep, participants prefer to sleep with the partner despite objective costs vis-à-vis sleep.
These findings make unique contributions toward our understanding of how a positive relationship transition, the gain of a new partner, is associated with better sleep in midlife women. Thus, asking questions regarding relationships need to be part of a sleep history in women, particularly during this period of social as well as biological transition.
Summary/Conclusions
Converging evidence supports the existence of perimenopausal sleep disturbances, rather than a single specific disorder, as distinct phenomena that exist independent from other factors that are common in this population and likely to influence sleep (i.e., vasomotor symptoms, mood disturbance, sleep apnea). Moreover, the perimenopausal transition is a plausible determinant of worsening sleep in mid-life women. In addition to the hormone changes underlying the perimenopause, a diverse variety of factors, both directly related (e.g., vasomotor symptoms) and unrelated to the transition (e.g., age-related sleep changes, sleep apnea, mood disturbance, relationship and co-sleeping habits), contribute to sleep disturbances in the menopausal transition.12–14,32 SWAN data show that the menopausal transition is related to self-reported difficulty sleeping, independent of age,27 indicating that age is not the primary determinant of the perimenopause – sleep relationship. As another source of sleeping difficulty in perimenopausal women, SWAN and other studies have shown that vasomotor symptoms are strongly associated with poor sleep quality.2,27 However, subgroup analyses restricted to women without vasomotor symptoms continue to find an association between the perimenopause and poor sleep quality, albeit a weaker association, providing evidence that the perimenopause – sleep relationship is not entirely explained by vasomotor symptoms.27 We have also found,32 as shown in Figure 4, that exogenous sex steroid hormones (alone) may be an insufficient intervention for improving sleep quality, except in naturally and surgically postmenopausal women.
Although the perimenopause is clearly associated with sleep dissatisfaction, objective measures of sleep patterns and sleep disorders must also be analyzed to characterize the relations among sleep symptoms, vasomotor symptoms, physical and emotional health consequences, aging and menopause. Ongoing SWAN Sleep Study analyses will address these issues to more fully distinguish whether the perimenopause – sleep relationship exists independent of age, vasomotor, mood and other health conditions.
Sleep disturbances are associated with increased health care costs and many negative health outcomes, such as decreased quality of life, poor work performance, and mood and anxiety symptoms. Sleep apnea prevalence, which seems to be higher in SWAN participants than in other epidemiological studies that include community middle-aged women not selected for any particular health problems, increases in middle-aged women and may affect cardiovascular disease risk.
Characterizing sleep and its psychobiological and psychosocial correlates is vital for identifying factors that may ease menopause-related sleep disturbances and their impact on health status in midlife women of differing racial and ethnic groups. The menopausal transition is an important time for change in risk for sleep disturbances and their associated health consequences. The SWAN Sleep Study provides a unique opportunity to collect longitudinal data on multiple high-quality objective and subjective measures of sleep in a multi-ethnic cohort of midlife women. Sleep I has provided baseline data on menopausal-related sleep disturbances, and these measures have been evaluated in conjunction with longitudinal measures drawn from the Core SWAN study. Anticipated results from Sleep II will provide further insights into the course of sleep across the menopause transition.
Acknowledgment
Funding for the SWAN Sleep Study is from the National Institute on Aging (Grants AG019360, AG019361, AG019362, AG019363). Support for this manuscript was also provided in part by National Institute of Mental Health (5R01MH082922; H. Joffe, PI). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. The authors also acknowledge the graphics assistance of Dr. Imke Janssen in preparing Figure 7.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Kravitz has nothing to disclose. Dr. Joffe discloses that over the past 12 months she has served as a co-investigator on clinical trials supported by Bayer HealthCare Pharmaceuticals, Forest Laboratories, and GlaxoSmithKline, and served as a consultant to Sunovion.
References
- 1.Krystal AD, Edinger J, Wohlgemuth, et al. Sleep in peri-menopausal and post-menopausal women. Sleep Med Rev. 1998;2:243–253. doi: 10.1016/s1087-0792(98)90011-9. [DOI] [PubMed] [Google Scholar]
- 2.Joffe H, Massler A, Sharkey KM. Evaluation and management of sleep disturbance during the menopausal transition. Semin Reprod Med. 2010;28:404–421. doi: 10.1055/s-0030-1262900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moline ML, Broch L, Zak R, et al. Sleep in women across the life cycle from adulthood through menopause. Sleep Med Rev. 2003;7:155–177. doi: 10.1053/smrv.2001.0228. [DOI] [PubMed] [Google Scholar]
- 4.Parry BL, Martinez LF, Maurer EL, et al. Sleep, rhythms and women’s mood. Part II. Menopause. Sleep Med Rev. 2006;10:197–208. doi: 10.1016/j.smrv.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Soules MR, Bremner WJ. The menopause and climacteric: Endocrinologic basis and associated symptomatology. J Am Geriatr Soc. 1982;30:547–561. doi: 10.1111/j.1532-5415.1982.tb05661.x. [DOI] [PubMed] [Google Scholar]
- 6.Kochanek KD, Xu J, Murphy SL, et al. Deaths: Preliminary data for 2009. National Vital Statistics Reports. 2011;59:1–68. [PubMed] [Google Scholar]
- 7.Manber R, Armitage R. Sex, steroids, and sleep: a review. Sleep. 1999;22:540–555. [published correction appears in Sleep 2000;23:145-9] [PubMed] [Google Scholar]
- 8.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14:103–115. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]
- 9.NIH State-of-the-Science Panel. National Institutes of Health State-of-the-Science conference statement: management of menopause-related symptoms. Ann Intern Med. 2005;142:1003–1013. [PubMed] [Google Scholar]
- 10.Woods NF, Mitchell ES. Symptoms during the perimenopause: prevalence, severity, trajectory, and significance in women’s lives. Am J Med. 2005;118 Suppl 12B:14S–24S. doi: 10.1016/j.amjmed.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 11.Ohayon MM, Carskadon MA, Guilleminualt C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 12.Woods NF, Mitchell ES. Sleep symptoms during the menopausal transition and early postmenopause: Observations from the Seattle Midlife Women’s Health Study. Sleep. 2010;33:539–549. doi: 10.1093/sleep/33.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dennerstein L, Lehert P, Guthrie JR, et al. Modeling women’s health during the menopausal transition: A longitudinal analysis. Menopause. 2007;14:53–62. doi: 10.1097/01.gme.0000229574.67376.ba. [DOI] [PubMed] [Google Scholar]
- 14.Freeman EW, Sammel MD, Lin H, et al. Symptoms Associated With Menopausal Transition and Reproductive Hormones in Midlife Women. Obstet Gynecol. 2007;110:230–240. doi: 10.1097/01.AOG.0000270153.59102.40. [DOI] [PubMed] [Google Scholar]
- 15.Karacan I, Thornby JI, Anch M, et al. Prevalence of sleep disturbance in a primarily urban Florida county. Soc Sci Med. 1976;10:239–244. doi: 10.1016/0037-7856(76)90006-8. [DOI] [PubMed] [Google Scholar]
- 16.Bixler EO, Kales A, Soldatos CR, et al. Prevalence of sleep disor-ders in the Los Angeles metropolitan area. Am J Psychiatry. 1979;136:1257–1262. doi: 10.1176/ajp.136.10.1257. [DOI] [PubMed] [Google Scholar]
- 17.Cirignotta F, Mondini S, Zucconi M, et al. Insomnia: an epidemiological survey. Clin Neuropharmacol. 1985;8 suppl 1:S49–S54. doi: 10.1097/00002826-198508001-00007. [DOI] [PubMed] [Google Scholar]
- 18.Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. Am J Epidemiology. 2000;152:463–473. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 19.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 21.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: The CARDIA Study. Am J Epidemiology. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 22.Kripke DF, Langer RD, Elliott JA. Mortality related to actigraphic long and short sleep. Sleep Medicine. 2010;12:28–33. doi: 10.1016/j.sleep.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauderdale DS, Knutson KL, Yan LL, et al. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okun ML, Kravitz HM, Sowers MF, et al. Psychometric evaluation of the Insomnia Symptom Questionnaire: A self-report measure to identify chronic insomnia. J Clin Sleep Med. 2009;5:41–51. [PMC free article] [PubMed] [Google Scholar]
- 25.Shin C, Lee S, Lee T, et al. Prevalence of insomnia and its relationship to menopausal status in middle-aged Korean women. Psychiatry Clin Neurosci. 2005;59:395–402. doi: 10.1111/j.1440-1819.2005.01391.x. [DOI] [PubMed] [Google Scholar]
- 26.Hall M, Kravitz HM, Gold E, et al. Sleep during the menopausal transition in a multi-ethnic cohort: Feasibility and preliminary results. Sleep. 2005;28 Abstract Supplement:A119. [Abstract 0350] [Google Scholar]
- 27.Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 28.Sowers MF, Crawford SL, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Marcus R, Kelsey J, editors. Menopause: biology and pathobiology. Orlando, Fla: Academic Press Inc; 2000. pp. 175–188. [Google Scholar]
- 29.Thurston RC, Bromberger JT, Joffe H, et al. Beyond frequency: Who is most bothered by vasomotor symptoms? Menopause. 2008;15:841–847. doi: 10.1097/gme.0b013e318168f09b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall M, Bromberger J, Matthews K. Socioeconomic status as a correlate of sleep in African-American and Caucasian women. Ann N Y Acad Sci. 1999;896:427–430. doi: 10.1111/j.1749-6632.1999.tb08161.x. [DOI] [PubMed] [Google Scholar]
- 31.Troxel WM, Buysse DJ, Hall M, et al. Marital happiness and sleep disturbances in a multi-ethnic sample of middle-aged women. Behav Sleep Med. 2009;7:2–19. doi: 10.1080/15402000802577736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31:979–990. [PMC free article] [PubMed] [Google Scholar]
- 33.Gracia CR, Sammel MD, Freeman EW, et al. Defining menopause status: Creation of a new definition to identify the early changes of the menopausal transition. Menopause. 2005;12:128–135. doi: 10.1097/00042192-200512020-00005. [DOI] [PubMed] [Google Scholar]
- 34.Kravitz HM, Janssen I, Santoro N, et al. Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch Intern Med. 2005;165:2370–2376. doi: 10.1001/archinte.165.20.2370. [DOI] [PubMed] [Google Scholar]
- 35.Santoro N, Crawford SL, Allsworth JE, et al. Assessing menstrual cycles with urinary hormone assays. Am J Physiol Endocrinol Metab. 2003;284:E521–E530. doi: 10.1152/ajpendo.00381.2002. [DOI] [PubMed] [Google Scholar]
- 36.Santoro N, Lasley BL, McConnell D, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: the Study of Women’s Health Across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab. 2004;89:2622–2631. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 37.Weiss G, Skurnick JH, Goldsmith LT, et al. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004;292:2991–2996. doi: 10.1001/jama.292.24.2991. [published correction appears in JAMA. 2005;293:163] [DOI] [PubMed] [Google Scholar]
- 38.Spielman AJ. Assessment of Insomnia. Clin Psychol Rev. 1986;6:11–25. [Google Scholar]
- 39.Hall MH, Matthews KA, Kravitz HM, et al. Race and financial strain are independent correlates of sleep in mid-life women: The SWAN Sleep Study. Sleep. 2009;32:73–82. [PMC free article] [PubMed] [Google Scholar]
- 40.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–2I3. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 41.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 42.Young T, Finn L, Austin D, et al. Menopausal status and sleep disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167:1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 43.Young T, Rabago D, Zgierska A, et al. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–672. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
- 44.Polo-Kantola P, Rauhala E, Erkkola R, et al. Estrogen replacement therapy and nocturnal periodic limb movements: A randomized controlled trial. Obstet Gynecol. 2001;97:548–554. doi: 10.1016/s0029-7844(00)01191-1. [DOI] [PubMed] [Google Scholar]
- 45.Matthews KM, Zheng H, Kravitz HM, et al. Are Inflammatory and Coagulation Biomarkers Related to Sleep Characteristics in Mid-Life Women?: Study of Women’s Health Across the Nation Sleep Study. Sleep. 2010;33:1649–1655. doi: 10.1093/sleep/33.12.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sowers MF, Zheng H, Kravitz HM, et al. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31:1339–1349. [PMC free article] [PubMed] [Google Scholar]
- 47.Troxel WM, Buysse DJ, Matthews KA, et al. Marital/cohabitation status and history in relation to sleep in midlife women. Sleep. 2010;33:973–981. doi: 10.1093/sleep/33.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]