Abstract
Objective
Adult major depressive disorder (MDD) is associated with reduced cortico-limbic functional connectivity thought to indicate decreased top-down control of emotion. However, it is unclear whether such connectivity alterations are also present in early childhood onset MDD.
Method
Fifty-one children ages 7–11 years, prospectively studied since preschool age, completed resting state fMRI and were assigned to four groups: 1) C-MDD (N=13) personal history of early childhood onset MDD; 2) M-MDD (N=11) a maternal history of affective disorders; 3) CM-MDD (N=13) both maternal and early childhood onset MDD or 4) CON (N=14) without either a personal or maternal history. We used seed-based resting state functional connectivity (rsfcMRI) analysis in an independent sample of adults to identify networks showing both positive (e.g., limbic regions) and negative (e.g., dorsal frontal/parietal regions) connectivity with the amygdala. These regions were then used in ROI based analyses of our child sample.
Results
We found a significant interaction between maternal affective disorder history and the child's MDD history for both positive and negative rsfcMRI networks. Specifically, when copared to CON, we found reduced connectivity between the amygdala and the “Negative Network” in children with C-MDD, M-MDD and CM-MDD. Children with either C-MDD or a maternal history of MDD (but not CM-MDD) displayed reduced connectivity between the amygdala and the “Positive Network”.
Conclusions
Our finding of an attenuated relationship between the amygdala, a region affected in MDD and involved in emotion processing, and cognitive control regions is consistent with a hypothesis of altered regulation of emotional processing in C-MDD suggesting developmental continuity of this alteration into early childhood.
Keywords: Functional Connectivity, Depression, Amygdala, fMRI, Childhood-Onset
Introduction
There is growing empirical evidence suggesting that clincially signficant episodes of Major Depressive Disorder (MDD) can be identified and reliably diagnosed in preschool and early school aged children when age-adjusted DSM criteria are implemented.1–5 However, little is known about the neural correlates of the illness in this age group. Understanding the neural correlates in the earliest known forms of MDD will provide clues necessary to characterize the neurodevelopmental pathophysiology of this disorder. The adult and adolescent depression literatures point to behavioral and neural problems with emotion regulation, or the ability to effectively and adaptively control negative thoughts, feelings and moods.2, 6, 7 Further, fMRI studies highlight the importance of a network of regions for emotion regulation, including the amygdala and various regions of the prefrontal cortex,8–10 including the anterior cingulate, dorsolateral prefrontal cortex, ventral lateral prefrontal cortex, and the medial prefrontal cortex.6, 11–15 Based on the evidence that problems with emotion regulation are an important component of MDD and that emotion regulation involves a host of regions affected in adult and adolescent MDD, we utilized a resting state functional connectivity approach to investigate amygdala connectivity with prefrontal cortex and other cortical regions, as well as with other limbic regions, in children (ages 7–11) propsectively studied since the preschool period and known to have early onset MDD and/or increased risk for MDD on the basis of family history of the disorder.6, 11–15
MDD has been well validated and widely recognized in children for decades. Preschool-onset MDD has been shown more recently to share core clinical characteristics with adolescent and adult-onset MDD, as well as significant homotypic continuity with later childhood forms of MDD, suggesting it is an early onset form of the well known lifespan disorder.5, 16 Problems with emotion regulation are well established in both childhood and adult MDD; both age groups have difficulty generating and maintaining/recognizing appropriate positive emotions and also experience an inordinate amount of intense negative emotions including sadness, guilt and feelings of worthlessness.17 The importance of understanding and successfully treating MDD in childhood is underscored by findings that children and adolescents with greater MDD episode duration show greater risk of relapse.18–21 It is important to understand the specific neural systems affected in pediatric MDD not only because childhood onset of the disease is linked to a poorer lifetime prognosis, but also because these children are affected during critical years for cognitive, social and neural development and currently effective treatment options are limited.22
The Amygdala and Emotion Regulation in MDD
Task-based fMRI studies investigating emotion processing in depressed samples report increased responses to negative emotional cues (e.g., faces, pictures, words) in limbic regions, including the amygdala, along with altered activity in cognitive control regions during emotional distraction and regulation.12, 23–37 This pattern of hyperactivity of limbic regions, including the amygdala, while viewing emotional faces has received mixed support in the adolescent MDD literature.38–40 Interestingly, adolescents with MDD also display reduced activation in dorsal prefrontal and cingulate cortex during cognitive control tasks.41 There is also evidence for altered pregenual cingulate activity during an emotional stroop and increased amygdala response to fearful face stimuli in school-aged children at high risk for MDD.42 Many of these regions affected in MDD are implicated in emotion regulation processes in healthy populations.
Specifically, regions such as the dorsolateral prefrontal cortex and the medial prefrontal cortex are thought to modulate activity of regions involved in emotionality, such as the amygdala8. During successful emotion regulation, both dorsal and medial prefrontal regions display increased activity, along with a decrease in amygdala activity. Moreover, stronger connectivity between these regions is associated with a greater reduction in negative affect after attempts to regulate emotion.43 This evidence, along with the amygdala's central role in assigning affective salience to stimuli and involvement in emotional memory, attention, and fear conditioning, suggests it may be a critical node in the pathway through which regions involved in control influence the activation of emotion regulatory processes.44–46
Investigating the functional relationship (i.e. functional connectivity) between the amygdala and other emotion regulation regions may help elucidate the dynamics of regulation and inform our understanding of brain system alterations that contribute to maladaptive emotion regulation processees associated with MDD. Work in healthy adults has shown that the timing of, and relationship between activity in the amygdala and regions of the prefrontal cortex (dorsolateral, dorsomedial and orbital) may vary depending on the efficacy of emotion regulation and type of regulation strategy employed.9, 43, 47 Thus, even in the absence of utilization of an explicit emotion regulation task in the scanner, one can make inferences about the regions involved in emotion regulation using resting state functional connectivity, as explained below.
Resting State Functional Connectivity of the Amygdala
As mentioned above, research has found the amygdala to be intricately linked with both cortical and subcortical regions during tasks involving preattentive and purposeful emotion regulation and recognition. Interestingly, a similar pattern of relationships has also been found using resting state functional connectivity MRI. Data collected during resting state functional connectivity measures spontaneous low-frequency blood oxygen level-dependent (BOLD) activity while an individual is `at rest' (i.e., lying quietly with eyes open or closed) and has been used to demonstrate `functional connectivity' (i.e., similar patterns of fluctuation in the BOLD signal) among brain regions.48 Importantly, research using resting state functional connectivity has demonstrated that groups of brain regions that often activate (or deactivate) at the same time in task settings also exhibit functional connectivity at rest, suggesting a history of coactivation that can be used to examine functional relationships within the brain. A growing body of literature using this technique has identified functional brain systems related to basic sensory functions such as vision and somatosensory as well as networks of regions related to task control, attention and default mode activity. As such, resting state functional connectivity is a useful tool for examining the participation of a given brain region within a larger neural system as well as identifying the potential influence of early occurring psychopathology on these functional relationships.
Stein and colleagues used path analysis to examine a network of regions displaying functional connectivity with the amygdala, including cortical structures such as the cingulate, prefrontal, and insular cortices along with the hippocampus and parahippocampal gyrus.49 Recently, Roy et al. investigated amygdala connectivity using seed-based resting state functional connectivity techniques in healthy adults.50 Many regions involved in the generation, regulation, and/or memory of emotions demonstrated negative or positive resting state functional connectivity relationships with the amygdala. More specifically, regions typically involved in control processes (frontal/parietal regions) displayed a negative functional relationship with the amygdala, which was interpreted as a potential method of top-down regulation of amygdala activity by these control regions. Conversely limbic regions such as the hippocampus displayed a positive functional relationship with the amygdala.
Amygdala Functional Connectivity in MDD
Studies investigating functional connectivity of the amygdala in adult MDD have revealed some important differences between patient and control populations. Chen et al., found reduced connectivity among individuals with MDD between the amygdala and both control and limbic regions that reversed following SSRI treatment.51 In a series of studies Anand and colleagues found decreased cortico-limbic connectivity in individuals with MDD both at rest and while viewing emotional pictures.52–54 Further studies indicated that treatment with an SSRI served to increase cortico-limbic connectivity. There is also evidence that amygdala-prefrontal cortex connectivity varies along with Monoamine oxidase A genotype, such that depressed individuals and people with the Monoamine oxidase A-H variant show decreased functional connectivity.55 Matthews et al., found reduced amygdala to supragenual cingulate connectivity in MDD patients, with greater reductions in connectivity related to greater symptom severity.56 Lui et al., found reduced resting state functional connectivity within a network of regions involved in emotion regulation and interestingly while the refractory MDD group showed this decrease compared to controls, a nonrefractory MDD group showed even further reduction in thalamo-cortical functional connectivity.57 Cullen and colleagues found reduced fractional anisotropy between the amygdala and right subgenual ACC in adolescentswith MDD, using diffusion tensor imaging, indicating reduced white matter connectivity between these regions.58 A recent study by Gaffrey et al. focused on functional connectivity of the subgenual cingulate, but not the amygdala, using sub- sample of children with pre-school onset MDD from the same study and found reduced connectivity between the subgenual cingulate and prefrontal control regions.59 However, too date, no studies have examined amygdala resting state functional connectivity in children with early childhood onset MDD.
Amygdala Function in Children at Risk for MDD
Connections among regions involved in emotion regulation may also be altered in individuals at risk for developing MDD. Children with a parental history of MDD face a risk of developing MDD three times greater than same age peers with no parental history of MDD.60 Several behavioral and fMRI studies have been conducted with high-risk groups. These studies suggest that neural and behavioral differences related to emotion dysregulation (and similar to those found in adolescents and adults with MDD) are present in children who have never displayed MDD but are at high risk for developing the disease.61–63 However, to our knowledge, no studies have investigated differences in functional connectivity within a familial high-risk population. Studying resting state functional connectivity within emotion regulation networks in individuals at high-risk and low-risk for developing MDD, in addition to those with early-onset MDD, may inform our understanding of whether changes in the connectivity of these networks is a symptom of MDD or predates clinical onset of the disease.
Goals and Hypotheses of the Current Study
The present study used resting state functional connectivity to investigate amygdala connectivity in children with a past episode of C-MDD and those at risk for later MDD. We focused on amygdala connectivity because of its central role in affective processing, along with past work in adults with MDD suggesting disrupted amygdala connectivity.46, 51 Given the evidence for altered connectivity patterns among adults with MDD, we hypothesized that children who had an episode of MDD during early childhood and those at risk for MDD would also show reduced connectivity between the amygdala and prefrontal and cingulate regions thought to be important for the regulation of emotion. Our predictions were less clear in regards to positive connectivity between the amygdala and other limbic regions involved in the expression of emotional experience or the retrieval of emotional information, as the existing literature does not point to a clear hypothesis. As such, our investigation of differences in connectivity of the Positive Network between the three MDD groups was exploratory.
Method
Participants
Beginning in 2002 caregivers with children ages 3 to 6 years were recruited to participate in a longitiudinal study of preschool onset depression. Prospective data continues to be collected from the original sample at annual assessment waves conducted in the Early Emotional Development Program at Washington University School of Medicine in St. Louis, details of the recruitment process have been previously published.5 Participants (ages 7–11) in the current investigation included children with: 1) a history of MDD during early childhood (before age 8) (C-MDD; N=13); 2) a maternal history of affective disorders (M-MDD; N=11); 3) both maternal and C-MDD (CM-MDD; N=13); and 4) without either C-MDD or maternal history (CON; N=14). It is important to emphasize that placement in either C-MDD or CMMDD group was based on past MDD diagnosis and that although current depressive symptoms were included in subsequent analyses, diagnostic data from time of scan were not avaliable for these analyses. Additionally, children were placed in the CON group if they did not have a personal MDD history or maternal history of affective disorders, regardless of other diagnoses, although any comorbidies were included as covariates in the anlyses. The criteria for excluding participants based on movement and the number of participants excluded are discussed under fcMRI data processing. See Table 1 for relevant demographic and clinical characteristics. The C-MDD had significantly lower family income than all other groups (p<.05), but no other groups differed significantly. The C-MDD group had significantly lower maternal education (p<.05) than the M-MDD and CM-MDD groups, but no other groups differed significantly. Because education level and income are highly correlated (p<.0001), only maternal education level was used as a covariate in further analyses. The study was approved by the Washington University institutional review board. All parents/guardians gave written informed consent and all participants gave written or oral assent following a description of the risks and benefits of study participation.
Table 1.
Demographic and Clinical Characteristics of the Participant Groups
| Child Only MDD (C-MDD) | Maternal Only MDD (M-MDD) | Child and Maternal MDD (CM-MDD) | Neither Child/ Maternal MDD (CON) | |
|---|---|---|---|---|
| N=13 | N=11 | N=13 | N=14 | |
| Gender (% Male) | 38.5 | 45.5 | 61.5 | 42.9 |
| Age (in years)* | ||||
| Mean | 8.9 | 9.1 | 9.5 | 8.4 |
| SD | .62 | 1.0 | 1.1 | 0.9 |
| Range | 8–10 | 8–11 | 8–11 | 7–10 |
| Ethnicity (% white)* | 38.4 | 72.7 | 76.9 | 64.3 |
| Psychiatric Medication at Diagnostic Timepoint Prior to Scan (%)a | 30.8 | 18.2 | 23.1 | 14.3 |
| Family Income Level* b | 1.85 | 2.8 | 3.25 | 3.25 |
| Maternal Education Level* c | 4.79 | 5.73 | 6.69 | 5.93 |
| Co-Morbidities (%)** | ||||
| Mother Other Internalizing Diagnosis | 0 | 27.3 | 23.1 | 0 |
| Mother Externalizing Disorder Diagnosis | 23.1 | 18.2 | 0 | 7.1 |
| Child Other Internalizing Disorder Diagnosis | 53.8 | 9.1 | 69.2 | 7.1 |
| Child Externalizing Disorder Diagnoses | 61.5 | 36.4 | 76.9 | 14.3 |
| Child Depression Inventory (child form) total t-score (parent form) | 40.9 (2.558) | 44.8 (6.374) | 43.25 (6.369) | 40.9 (2.558) |
| Child Depression Inventory total t-score (parent form) | 44.2 (9.175) | 51.6 (7.351) | 50.17 (7.107) | 44.2 (9.175) |
| Timepoint One Depression Sum Score** | 2.50 (2.41) | 2.64 (2.618) | 6.31 (4.404) | 2.5 (2.410) |
| Avgerage Depression Sum Score** (timepoints 1,3,5) | 1.60 (1.68) | 2.67 (.9428) | 7.128 (2.577) | 1.595 (1.680) |
Note: MDD = major depressive disorder
The results were essentially identical if children on medications were excluded from the analyses, or if medication status was used as a covariate.
Family income per year was obtained at the first diagnostic wave using the following categories: 1 -($0-20k), 2-($20,001-40k), 3-($40,001-60K), and 4-($60,001+)
Maternal education level was obtained using the following categories: 1-some grade school, 2-completed grade school, 3-some high school, 4-completed high school, 5-some college or a 2-year degree, 6-completed a 4 year college degree, 7-some school beyond college, 8-completed a professional or graduate degree.
p<.05,
p<.01
Diagnosis and Individual Differences
Trained staff members conducted up to three annual in-person interviews with the participants and parents/guardians over the course of 4–6 years. These interviews included the Preschool-Age Psychiatric Assessment (PAPA), a semi-structured developmentally appropriate parent assessment of psychiatric symptoms with established reliability.64 PAPA interviews were audiotaped and reviewed for reliability as previously reported.5 The PAPA utilizes a computerized DSM-IV algorithm to derive MDD and other Axis-I diagnostic determinations. However the two-week duration of symptoms requirement for the diagnosis of MDD was omitted based on empirical data suggesting it may not be an appropriate duration threshold for younger children.4 Using information from the PAPA, children were classified as having a C-MDD history if the child met all symptom criteria for MDD or had anhedonia along with three other symptoms at one or more of the annual assessments. Maternal affective disorder history was determined using the FIGS, a widely used and reliable measure of family history of psychiatric disorder administered by trained research assistants.65 Participants were considered part of the maternal history group if their primary caregiver reported the child's biological mother as ever having MDD or Bipolar Disorder. Two mothers in the M-MDD group were diagnosed with bipolar disorder. In the control group, one reporter of maternal MDD/bipolar history was the child's biological father, one was an adoptive parent and in the C-MDD group one reporter was the child's guardian but not a biological or adoptive parent or a grandparent. All other reports were from the biological mother's about themselves. Participants were excluded from the following analyses if maternal affective disorder history was missing.
Along with the MDD module, all other relevant diagnostic modules of the PAPA were administered to determine whether the following disorders were present at any of the 3 study waves: Separation Anxiety Disorder, Post-Traumatic Stress Disorder, General Anxiety Disorder, Attention Deficit Hyperactivity Disorder, Conduct Disorder, Specific Phobia and Oppositional Defiant Disorder.The CON and M-MDD groups showed significantly fewer internalizing diagnoses than the C-MDD and CM-MDD groups. The CON group showed significantly fewer externalizing diagnoses than the PH-MDD and CM-MDD groups while the M-MDD group showed significantly fewer externalizing diagnoses than the CM-MDD group only. To control for possible influences of early childhood co-morbidities we included prior diagnosis of other internalizing and/or externalizing disorders as covariates in subsequent analyses.
At each annual diagnostic assessment, depression sum scores or depression severity scores (the total number of MDD symptoms endorsed in the PAPA) were calculated and used as a proxy for symptom intensity severity.66 To evaluate current depressive symptoms, the Child Depression Inventory (CDI) was administered on the same day as children's fMRI scanning. All children were older than age 6 at the time of scan, the lower age limit of the CDI.67 Children and caregivers completed the child and parent versions respectively. Scores from 7 children were not available (4 CON, 1 C-MDD, 1 M-MDD, 1 CM-MDD). Total CDI scores were computed for both informants and converted to T-scores. The Childhood Emotional Management Scale (CEMS) was used to measure children's ability to cope with, and regulate experiences of sadness.68 The CEMS was administered at the annual assessment prior to the scanning session. Diagnostic groups did not significantly differ in CEMS scores or in parent/child CDI scores (all p>.05). Children's sadness dysregulation and coping scores on the CEMS, MDD sum scores from T1, and the average of MDD sum scores across all three annual diagnostic waves (T1, T2, and T3) were used to investigate individual differences in connectivity associated with MDD severity and emotion regulation ability.
fMRI Scanning
Two resting state fMRI scans, each including 164 frames (each lasting ~6.8 minutes), were acquired using a 3T Tim TRIO Scanner at WUSM. Subjects were instructed to remain awake and rest with his/her eyes closed during the resting state scan. Data were acquired using an asymmetric spin-echo, echo-planar sequence, which was maximally sensitive to blood oxygenation level-dependent (BOLD) contrast (T2*) (repetition time [TR] = 2500 ms, echo time [TE] = 27 ms, field of view [FOV] = 256 mm, flip=90°, voxel size=4× 4× 4 mm, slices = 36).” Additionally a T1 structural image was acquired for alignment purposes using a sagittal MP-RAGE 3D sequence (TR=2400 ms, TE=3.16 ms, flip=8°; voxel size=1 × 1 × 1 mm). To facilitate registration of the T1 and functional scans, we also acquired a T2 image in the same space as the functional scans (TE=96ms, TR=5s, 189×256 acquisition matrix, 36 slices, 1.02×1×3mm voxels).
fcMRI Data Preprocessing
Prior to processing, the signal-to-noise values (mean across time points within a run, divided by the standard deviation across time points, computed for each slice) were examined for each resting state scan for each child. We considered a scan to be of usable quality if the mean signal-to-noise value across slices was 150 or greater, as our prior studies have suggested that this cut off predicts a number of other quality assurance indices. Using this criterion, both scans from 25 children were excluded (CON n=11; PH-MDD n=3; M-MDD n=3; CM-MDD n=8), one of the two scans from 11 children was excluded (CON n=3; PH-MDD n=2; M-MDD n=3; CM-MDD n=3), and both scans from all remaining children were used bringing our total number of subjects from 76 chidren scanned to 51 children for our analyses. We then compared signal-to-noise for the included children and scans across groups, and found no significant differences (F(3,50) = .504, p = .681). Data were then processed using the following steps prior to fcMRI analysis: 1) Compensation for slice-dependent time shifts; 2) Removal of first 5 images from each run during which fMRI signal was allowed to reach steady state; 3) Elimination of odd/even slice intensity differences due to interpolated acquisition; 4) Realignment of data acquired in each subject within and across runs to compensate for rigid body motion;69 5) Intensity normalization to a whole brain mode value of 1,000; 6) Registration of the 3D structural volume (T1) to the atlas representative template in the Talairach coordinate system70 using a 12-parameter affine transform and re-sampled to 1mm cubic representation;69, 71 and 7) Co-registration of the 3D fMRI volume to the T2, and the T2 to the participants structural image; and 8) transformation of the fMRI to atlas space using a single affine 12-parameter transform (derived from the combination of steps 6 and 7) that included a re-sampling to a 3 mm cubic representation. Finally, as described elsewhere,72, 73 to remove possible sources of spurious correlations, such as movement, scanner and physiological noise, a number of additional preprocessing steps were performed using in-house software written in Matlab (The Mathworks, Natick, MA). Briefly, the steps included: 1) spatial smoothing using a 6mm full-width half-maximum Gaussian filter; 2) high pass filtering at 0.009 Hz; 3) removal of several nuisance regressors including 6 rigid-body motion correction parameters, ventricle, whole-brain and deep white matter signal, as well as their first derivatives.72, 74, 75
Network Region Definition
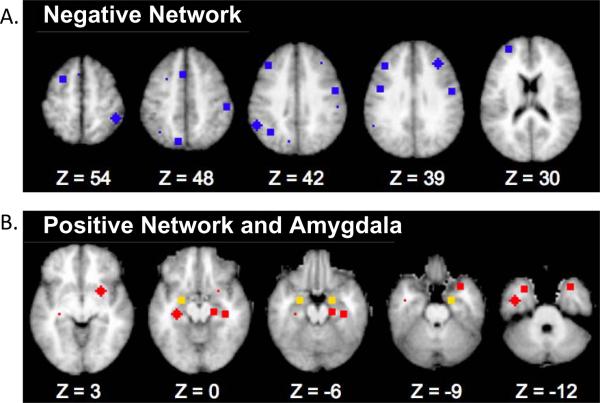
We were interested in examining the relationship between the amygdala and both cortical and limbic regions. To do this we identified networks of regions that showed both positive and negative functional correlations with both left and right amygdala using an independent data set of 24 healthy adults previously analyzed by our group.73 To do so, we first created seed maps of regions showing functional connections with the right and left amygdala respectively. Specifically, the time series for all voxels within either a left or right amygdala ROI (anatomically defined for each adult individual using FreeSurfer segmentation)76, 77 were extracted and correlated with all other voxels in the brain. We estimated group-level statistical significance by converting individual correlation maps to Fisher Z maps and computing a voxelwise one-sample t test (comparing the Fisher Z values against zero across the group). We thresholded these maps with a Z of 3 and 13 contiguous voxels to achieve a whole brain false positive rate of .05 for each of the two maps.78 We then identified all voxels that passed threshold in both maps, and created two networks of regions that included regions either positively or negatively correlated with the bilateral amygdala, respectively along with group-level amygdala regions of interest. These two networks are shown in Figure 1 and the coordinates for the centroid of each region are shown in Tables S1 and S2, available online. Overall, regions whose activity correlated positively with amygdala activity (“positive” network; see Table S1, available online) tended to be more inferior “limbic” regions, while regions whose activity was negatively correlated with amygdala activity (“negative” network; see Table S2, avaliable online) tended to be more dorsal frontal/parietal regions often associated with various forms of cognitive control.79–82 Of note, the maps for right and left amygdala seed correlations were highly similar, and the results would not have been substantively different if we had identified separate positive and Negative Networks for right and left amygdala and used those in subsequent analyses. A conjunction analysis was performed on two maps using either the left or right amygdala as seeds. This provided networks with the same regions for both right and left amygdala and allowed us to conduct symmetrical analyses with amygdala hemisphere as a factor and to increase the ease of presentation.
Figure 1.
Negative and Postive Network Regions of Interest (ROIs)
Note: Regions of interest comprising the A.) Negative Network (blue) and B.) Positive Network (red) along with the bilateral amygdala (yellow). See Tables S1 and S2, available online, for region coordinates and abbreviations.
ROI-ROI Correlation Computation
In our child sample we used the centroids of the regions, including the amygdala, listed in Tables S1 and S2, available online, to create spherical ROIS 12mm in diameter. These regions of interest were then masked by each child's individual FreeSurfer Segmentation so as to include only voxels falling within gray matter. We then extracted the timeseries from each of these ROIs and computed pairwise correlations between each ROI and the left and right amygdala separately.
Data Analysis
To compare connectivity between the groups, we conducted a repeated measures ANOVA for each network (positive and negative) with region (see Tables S1 and S2, available online) and hemisphere (right or left amygdala) as within-subject factors, and maternal affective disorder status and child MDD status as between-subject factors. To control for possible confounds, gender, age, ethnicity (Caucasian, African American or other), past diagnosis of any externalizing disorders (oppositional defiant disorder, conduct disorder, attention deficit hyperactivity disorder), diagnosis of any other internalizing disorders (generalized anxiety disorder, seasonal affective disorder, post-traumatic stress disorder), medication status at scan (whether child was taking psychotropic medications during the diagnostic time period prior to scan (see Table S3, avaliable online), and parent-reported CDI score were included as covariates. No significant differences in results from those presented with the full sample were found when children on medications were excluded in follow-up analyses. Because of our interest in changes in connectivity between children with personal and maternal affective disorders compared to control children, and in the interest of brevity we focused on main effects and interactions between these factors only.
Results
Within Network Connectivity
Negative Network
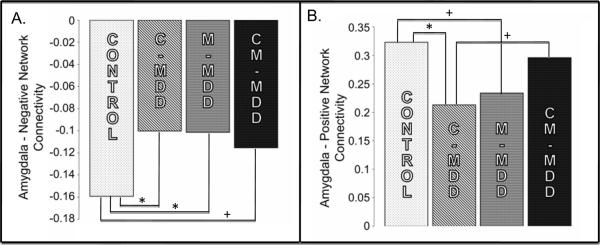
The ANOVA for the Negative Network did not reveal a significant main effect of either child MDD history (F(1,48)=1.809, p=.185) or maternal affective disorder history (F(1,48)=1.631, p=.208). However, there was a significant interaction between C-MDD history and maternal affective disorder history (F(1,48) = 4.805, p=0.033). This interaction remained significant after adding age, gender, ethnicity, maternal education level, past diagnoses of any internalizing or externalizing disorder, and parent CDI score as covariates (F(1,32) = 10.215, p=.003). Post hoc analyses performed to identify the source of this interaction revealed decreased negative connectivity in the groups with personal (C-MDD; p=0.012), maternal (M-MDD, p=0.02) and both histories (CM-MDD; trend level, p=0.064) when compared to controls (Figure 2a). In other words, all three groups with either or both a personal history of MDD or a maternal history of MDD showed reduced negative connectivity between the amygdala and regions such as dorsal prefrontal and parietal cortices compared to healthy children.
Figure 2.
Average Connectivity between the Amygdala and Negative and Positive Networks for all four groups
Note: Average functional connectivity between the amygdala and the A.) Positive Network and B.) Negative Network in each of the four groups. Control = Neither Maternal (M) nor Childhood Onset (C) major depressive disorder (MDD) History; CM-MDD = Both Maternal and Childhood Onset MDD History. * p<.05, + p<.10
Positive Network
The ANOVA for the Positive Network did not reveal a significant main effect of either C-MDD history, F(1,48)=.575, p=.452, or maternal affective disorder history, F(1,48)=.010, p=.922. However, there was a significant interaction between C-MDD history and maternal affective disorder history (F(1,48) = 7.349. p<0.01). This significant interaction remained after adding age, gender, ethnicity, maternal education level, past diagnoses of any internalizing or externalizing disorder, medication status and parent-reported CDI score as covariates (F(1,32) = 7.58 p=.01). Post hoc analyses performed to identify the source of this interaction revealed decreased positive connectivity within the C-MDD (p=0.014) group compared to controls, as well as a trend for decreased positive connectivity within the maternal MDD group compared to controls (p=0.058). There were,however, no significant (p=0.538) differences in positive connectivity between the control and CM-MDD groups. (Figure 2b)
Current Depression Severity
One important question is whether the relationship between either diagnosis of MDD in early childhood or risk for MDD was mediated through depressive symptomatology and impairment at the time of scan. To investigate this question, we examined the correlations between both parent and child CDI scores and the average connectivity for each network (positive or negative). None of these correlations were significant (all p>.09).
Relationship to MDD Severity
To determine whether differences in positive and negative amygdala connectivity were associated with individual differences in MDD severity, we computed correlations of the average positive and Negative Networks connectivity with MDD severity at the first assessment and average MDD severity across all assessments (see Figures S1 and S2, available online; Table 2). None of these correlations were significant in the sample as a whole, or when the sample was split by the presence or absence of child MDD. However, when the group was split based on the presence or absence of maternal affective disorder history, significant correlations were found within the no maternal MDD history group. As shown in Table 2, for children without maternal MDD history, the greater the MDD severity at both baseline and across diagnostic assessments, the lower the connectivity between bilateral amygdala and the regions with which the amygdala is normally positively correlated. A similar finding emerged from correlations with average negative connectivity, where significant correlations were found only in the no maternal history group. Average negative connectivity (see Table 2) was significantly positively correlated with MDD sum score at the first interview, and also showed a trend level correlation with average MDD sum score over all diagnostic time-points. Because the expected correlation for the Negative Network is negative in value, a positive correlation between MDD severity and negative connectivity means that greater MDD severity is associated with reduced negative connectivity between the amygdala and regions in the Negative Network.
Table 2.
Correlations Between Depression Severity and Positive (POS) and Negative (NEG) Amygdala Connectivity
| All Participants | Maternal History | No Maternal History | ||||
|---|---|---|---|---|---|---|
| POS | NEG | POS | NEG | POS | NEG | |
| T1 Depression Sum Score | −.22 | .22 | .10 | −.07 | −.52** | .47* |
| Average Depression Sum Score | −.13 | .20 | .20 | −.05 | −.39* | .32+ |
Note:
p<.05,
p<.01,
p<.10
Relationship to Emotion Regulation
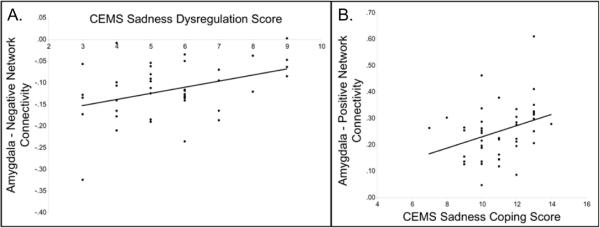
To evaluate the relationship between individual differences in connectivity and emotion regulation we correlated average Negative Network connectivity and CEMS sadness dysregulation scores as well as average Positive Network connectivity and CEMS sadness coping scores. There were no significant relationships between either network (positive or negative) and sadness inhibition scores. The average connectivity of the Negative Network with the amygdala was significantly positively correlated with sadness dysregulation (r=.391, p=.009) indicating that as a child's ability to regulate his/her sadness effectively decreases, so does Negative Network connectivity. The average connectivity of the Positive Network with the amygdala was significantly positively correlated with sadness coping (r=.33, p=.031) indicating that as a child's ability to cope with his/her sadness effectively increases so does Positive Network connectivity (see Figure S2, available online; Figure 3).
Figure 3.
Individuals Differences in Childhood Emotion Management Scale Scores and Negative/Positive Network Connectivity
Note: Scatterplot illustrating the relationship between individual differences in A.) the average Negative Network connectivity and Childhood Emotional Management Scale (CEMS) sadness dysregulation scores and B.) the average Positive Network connectivity and CEMS sadness coping scores
Discussion
Given the deficits in emotion regulation and identification displayed by children with and at increased risk for MDD and the role of the amygdala and associated regions in emotion regulation, the goal of this study was to investigate whether changes in resting state functional connectivity of regions associated with the amygdala are present in children with early childhood onset MDD (C-MDD, CM-MDD) and those at increased risk for developing the disease (M-MDD).61, 83 As hypothesized, when compared to controls, we found evidence of reduced connectivity between the bilateral amygdala and the associated Negative Network in children with C-MDD, children with a maternal history of MDD or bipolar disorder (MMDD) and children with both histories (CM-MDD). Children with either early onset MDD or maternal MDD history displayed reduced connectivity between the amygdala and the Positive Network compared to controls, whereas children with both histories did not significantly differ from controls. Reduced connectivity for both networks was associated with increased MDD symptom severity only for children without a maternal history of MDD or bipolar disorder. Additionally, increased connectivity between the amygdala and the negative and Positive Networks was associated with reports of increased ability to regulate and cope with sadness, respectively, across all groups.
Connectivity Between the Amygdala and Cognitive/Emotion Regulation Regions
As hypothesized, children with C-MDD, a maternal history of MDD/bipolar disorder or both histories displayed reduced connectivity between the amygdala and Negative Network. As reviewed above, the Negative Network consists of regions frequently associated with various aspects of cognitive control and emotion regulation.84 Additionally, stronger connectivity between dorsal frontal regions and the amygdala during emotion regulation is associated with more successful regulation of emotions.43 Previous work in adults with MDD has found reduced resting state functional connectivity between the amygdala and regions of the prefrontal and anterior cingulate corticies, interpreted as reflecting alterations in the neural systems normally supporting emotion regulation.51, 53 Also supporting this model, the reduced connectivity among adults with MDD was ameliorated following treatment with antidepressant medication.51, 53 Furthermore, the current findings are consistent with other studies investigating resting state functional connectivity in children with MDD. Gaffrey and colleagues found reduced connectivity between the subgenual cingulate and prefrontal control regions in children with preschool onset MDD in a different sub-set of the same study sample.59
Importantly, in our sample, the reduction in connectivity between the amygdala and cognitive control regions was found not only in children who experienced an episode of MDD during the early childhood period (prior to age 8), but also in those who have never been depressed but are at high risk based on a maternal history of depression. Furthermore, reduced connectivity was associated with reports of a decreased ability to regulate feelings of sadness on the CEMS across all groups. The relationship between Negative Network connectivity and emotion regulation is consistent with the interpretation that reduced connectivity between amygdala and control regions is associated with poor emotion regulation. Interestingly, for children without a maternal history of MDD or bipolar disorder, reduced connectivity was also associated with more severe depression. However, the finding that the relationship between MDD severity and Negative Network connectivity differed as a function of familial risk was unexpected. It is possible that maternal MDD impacts how the child's symptom severity is reported or expressed, and thus its relationship to functional connectivity. As such, further research is needed to confirm and understand this finding.
Taken together, these results suggest that reduction in resting state functional connectivity between the amygdala and the Negative Network may be a marker of increased risk for MDD and may also be associated with functional indicators of poor emotion regulation. In one sense, our results are somewhat surprising, as one might have expected to see main effects of either personal history of MDD or maternal history, rather than solely an interaction between personal and maternal history. However, the form of the observed interaction was that of an “odd man out”, such that children with maternal risk (whether or not they had a personal history) and children with a personal history (whether or not they have maternal risk) all showed similarly altered patterns of connectivity. These results suggest that altered patterns of connectivity in the networks we examined were more associated with risk for depression and did not discriminate between those children who actually developed depression and those who did not. If reduced connectivity of the Negative Network proves to be a risk factor for MDD, longitudinal studies investigating functional outcome both for children with MDD and those who have not developed the disorder are needed to determine if there is a direct relationship between resting state functional connectivity of these networks and future depressive episodes. Moreover, it would be beneficial to determine whether interventions specifically designed to improve emotion regulation skills have any effect on the resting state functional connectiivty of these networks, and whether they have any impact on preventing/ameliorating depressive symptoms particularly in children with reduced connectivity.
Connectivity Between the Amygdala and other Limbic Regions
Children with C-MDD and with maternal history of either MDD or bipolar disorder, but not those with both histories, displayed reduced connectivity between the amygdala and Positive Network. As compared to the research on connectivity between the amygdala and cognitive control regions, fewer studies have focused on the functional implications of the strength of connectivity between the amygdala and other limbic regions to which it is positively connected. Thus, there are less data upon which to develop hypotheses about alterations in connectivity between the amygdala and limbic system in C-MDD. increased connectivity between the amygdala and hippocampus has been implicated in increased recall of emotional stimuli over time.85 As such, the evidence for depressed negative memory bias in depressed individuals suggests that children with MDD could show increased connectivity between the amygdala and Positive Network.86 However, Chen and colleagues in 2008 found reduced positive connectivity between the amygdala and regions such as the hippocampus and parahippocampal gyrus in patients with MDD compared to controls.51 Our results were more in line with the findings of Chen and colleagues; in that we found reduced amygdala to limbic system connectivity in children with either a personal or maternal history of MDD. One possible hypothesis is that this reduction in connectivity could reflect either a failure to integrate emotional experience and memory, or possible inefficiency in utilizing recall of emotional memories as a tool to regulate or cope with emotion. This interpretation would be consistent with our finding that increased connectivity between the amygdala and Positive Network was associated with reports of better sadness coping. Unexpectedly, we did not find evidence for such reduced connectivity in the children with both a personal and a family history of MDD. This intriguing finding requires further work to clarify the interaction between C-MDD, risk for MDD, and connectivity between the amygdala and other limbic regions.
As noted above, we did not find any connectivity changes selectively associated with personal history of MDD. There are several potential explanations for this. First,it may be that connectivity changes are more associated with risk for depression, and that factors other than connectivity are associated with manifest illness (e.g., amplitude differences in functional responses to emotion processing or emotion regulation, other types of pathophysiological changes). Alternatively, it could be that connectivity changes in different networks that we did not examine (e.g., default mode network) do discriminate between children who develop depression and those at risk. Further work in larger samples examining a broader range of brain networks and both connectivity and functional brain reponses will be necessarily to distinguish between these possibilities.
The current study suggests that alterations in connectivity, evident in both high-risk groups and those with a very early episode of MDD during the early childhood period of development, may represent an early biological risk marker for MDD. This finding, combined with with other alterations in brain functional connectivity in children with MDD onset during the early childhood period, suggests that a neurobiological endophenotype may be evident prior to the onset of depressive symptoms and prior to the onset of childhood or adolescent onset MDD.59, 87 Future studies that investigate the longitudinal developmental course of these changes and their plasticity in response to early interventions may prove very fruitful for studies of the prevention and early intervention in MDD.
Interpretation of these results should be examined within the context of several limitations. First, while our study can address important questions regarding functional relationships between key regions involved in emotion processing and regulation in children with and at risk for MDD, our sample size was limited. Larger sample sizes will be needed to confirm and extend our results. Second, our groups were heterogenious in terms of medication status and ethnicity. A number of the children included in the analyses were medicated at the diagnostic timepoint prior to the time of the scan (see Table S3, avaliable online). However, we found reductions in connectivity between the amygdala and cognitive control regions both in children with C-MDD and those with only a maternal history of MDD, despite the fact that relatively few children in the maternal history only group were taking any medications. Further, all of the interactions between C-MDD and maternal history of MDD remained significant when controlling for medication status prior to scan, as well as when only examining children un-medicated at that diagnostic timepoint. Thus, we think it is unlikely that our results were substantially influenced by medication effects. Also, while ethnicity was included as a covariate in our analyses, our groups did differ in ethnic make-up such that the C-MDD group had fewer caucasians than other groups. However, this group does not display a different pattern of connectivity from either the M-MDD and CM-MDD groups, neither of which differs from each other or the control group in terms of ethnicity.
Third, our diagnostic information and group categorization was based on prior assessments and reports of maternal affective disorder history, and did not take into account paternal affective disorder history or current diagnostic status at time of scan. Although research suggests that maternal MDD history conveys stronger risk than paternal MDD history, future work investigating the influence of paternal MDD history on resting state functional connectivity of the amygdala would benefit from a more complete assessment of familial affective disorder history in both parents.88 In addition, our group of children with a personal history of MDD were heterogenous in terms of the number of prior MDD episodes and the types of comorbid disorders. Although the group differences in functional connectivity remained even when controlling for these individual differences, it will be important to examine their influence on functional connectivity in future work with larger samples. Finally, future work is needed to characaterize the developmental course of functional connectivity of the amygdala in healthy typically developing children. Our amygdala networks were defined using an independent dataset from adults rather than in the heatlhy control child sample so as not to bias us toward finding difference between the MDD and control children. However, it is possible that somewhat different networks of regions showing either positive or negative connectivity with the amygdala would be identified in a child sample, which should be explored in future studies.
In our sample of children 7–11 years old, reduced connectivity within networks both positively and negatively correlated with the amygdala was associated with both C-MDD and a maternal history of the disease. Moreover, reduced connectivity of the negative and Positive Networks was associated with reports of decreased capacity to regulate and cope with feelings of sadness, respectively. Future studies investigating the relationship between the resting state functional connectivity of these networks and clinical outcome are needed to determine whether connectivity of these networks has any predictive/diagnostic power, potentially indicating treatment responsivity in children with MDD and the development of future MDD in children at risk for this disorder.
Supplementary Material
Acknowledgments
Disclosure: Dr. Barch has received grants from the National Institute of Mental Health (NIMH), National Institute on Aging (NIA), the National Alliance for Research on Schizophrenia and Depression (NARSAD), Allon, Novartis, and the McDonnell Center for Systems Neuroscience. Dr. Repovs has served as a consultant on NIMH grants. Dr. Luby has received grants from the NIMH, Communities Healing Adolescent Depression and Suicide (CHADS), and NARSAD. Dr. Gaffrey has received funding from a Klingenstein Third Generation Foundation Postdoctoral Fellowship. Ms. Luking has received training support from an Integrative Graduate Education and Research Traineeship (IGERT) grant. Dr. Belden has received a grant from the NIMH. Dr. Botteron has received grants from the NIMH, the National Institutes of Health (NIH), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the National Institute of Child Health and Human Development (NICHD), CHADS, Simons Foundation and NARSAD.
The current study was funded by the National Institute of Mental Health, grants MH64769 (JL), MH090786 (JL, DB, KB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material cited in this article is available online.
References
- 1.Luby JL, Heffelfinger AK, Mrakotsky C, Hessler MJ, Brown KM, Hildebrand T. Preschool major depressive disorder: preliminary validation for developmentally modified DSM-IV criteria. J Am Acad Child Adolesc Psychiatry. 2002 Aug;41(8):928–937. doi: 10.1097/00004583-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Cole PM, Michel MK, Teti LO. The development of emotion regulation and dysregulation: a clinical perspective. Monogr Soc Res Child Dev. 1994;59(2–3):73–100. [PubMed] [Google Scholar]
- 3.Egger HL, Angold A. Common emotional and behavioral disorders in preschool children: presentation, nosology, and epidemiology. J Child Psychol Psychiatry. 2006 Mar–Apr;47(3–4):313–337. doi: 10.1111/j.1469-7610.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 4.Luby JL, Belden AC, Pautsch J, Si X, Spitznagel E. The clinical significance of preschool depression: impairment in functioning and clinical markers of the disorder. J Affect Disord. 2009 Jan;112(1–3):111–119. doi: 10.1016/j.jad.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luby JL, Si X, Belden AC, Tandon M, Spitznagel E. Preschool depression: homotypic continuity and course over 24 months. Arch Gen Psychiatry. 2009 Aug;66(8):897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joormann J, Gotlib IH. Emotion Regulation in Depression: Relation to Cognitive Inhibition. Cogn Emot. 2010 Feb 1;24(2):281–298. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson RA. Emotion regulation: a theme in search of definition. Monogr Soc Res Child Dev. 1994;59(2–3):25–52. [PubMed] [Google Scholar]
- 8.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005 May;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004 Oct;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008 Sep 25;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehring T, Tuschen-Caffier B, Schnulle J, Fischer S, Gross JJ. Emotion regulation and vulnerability to depression: spontaneous versus instructed use of emotion suppression and reappraisal. Emotion. 2010 Aug;10(4):563–572. doi: 10.1037/a0019010. [DOI] [PubMed] [Google Scholar]
- 12.Erk S, Mikschl A, Stier S, et al. Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci. 2010 Nov 24;30(47):15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eugene F, Joormann J, Cooney RE, Atlas LY, Gotlib IH. Neural correlates of inhibitory deficits in depression. Psychiatry Res. 2010 Jan 30;181(1):30–35. doi: 10.1016/j.pscychresns.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Ryan ND, Casey BJ. Altered emotional processing in pediatric anxiety, depression, and comorbid anxiety-depression. J Abnorm Child Psychol. 2005 Apr;33(2):165–177. doi: 10.1007/s10802-005-1825-z. [DOI] [PubMed] [Google Scholar]
- 15.Trosper SE, Buzzella BA, Bennett SM, Ehrenreich JT. Emotion regulation in youth with emotional disorders: implications for a unified treatment approach. Clin Child Fam Psychol Rev. 2009 Sep;12(3):234–254. doi: 10.1007/s10567-009-0043-6. [DOI] [PubMed] [Google Scholar]
- 16.Luby JL, Heffelfinger AK, Mrakotsky C, et al. The clinical picture of depression in preschool children. J Am Acad Child Adolesc Psychiatry. 2003 Mar;42(3):340–348. doi: 10.1097/00004583-200303000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Luby JL. Early childhood depression. Am J Psychiatry. 2009 Sep;166(9):974–979. doi: 10.1176/appi.ajp.2009.08111709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birmaher B, Arbelaez C, Brent D. Course and outcome of child and adolescent major depressive disorder. Child Adolesc Psychiatr Clin N Am. 2002 Jul;11(3):619–637. x. doi: 10.1016/s1056-4993(02)00011-1. [DOI] [PubMed] [Google Scholar]
- 19.Dunn V, Goodyer IM. Longitudinal investigation into childhood- and adolescence-onset depression: psychiatric outcome in early adulthood. Br J Psychiatry. 2006 Mar;188:216–222. doi: 10.1192/bjp.188.3.216. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs M, Obrosky DS, Gatsonis C, Richards C. First-episode major depressive and dysthymic disorder in childhood: clinical and sociodemographic factors in recovery. J Am Acad Child Adolesc Psychiatry. 1997 Jun;36(6):777–784. doi: 10.1097/00004583-199706000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Lewinsohn PM, Seeley JR, Buckley ME, Klein DN. Bipolar disorder in adolescence and young adulthood. Child Adolesc Psychiatr Clin N Am. 2002 Jul;11(3):461–475. vii. doi: 10.1016/s1056-4993(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 22.Fareri DS, Martin LN, Delgado MR. Reward-related processing in the human brain: developmental considerations. Dev Psychopathol. 2008 Fall;20(4):1191–1211. doi: 10.1017/S0954579408000576. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, LaBar KS, Smoski M, et al. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res. 2008 Jul 15;163(2):143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Wingen GA, van Eijndhoven P, Cremers HR, et al. Neural state and trait bases of mood-incongruent memory formation and retrieval in first-episode major depression. J Psychiatr Res. 2010 Jun;44(8):527–534. doi: 10.1016/j.jpsychires.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Suslow T, Konrad C, Kugel H, et al. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry. 2010 Jan 15;67(2):155–160. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Arch Gen Psychiatry. 2008 Nov;65(11):1275–1284. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007 Jan 15;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 28.Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009 Feb 10;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001 Nov 1;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 30.Lee BT, Seong Whi C, Hyung Soo K, et al. The neural substrates of affective processing toward positive and negative affective pictures in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007 Oct 1;31(7):1487–1492. doi: 10.1016/j.pnpbp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 31.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007 Aug 15;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gotlib IH, Sivers H, Gabrieli JD, et al. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005 Nov 7;16(16):1731–1734. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- 33.Fu CH, Williams SC, Cleare AJ, et al. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol Psychiatry. 2008 Sep 15;64(6):505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 34.Fu CH, Williams SC, Cleare AJ, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004 Sep;61(9):877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 35.Fales CL, Barch DM, Rundle MM, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008 Feb 15;63(4):377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dichter GS, Felder JN, Smoski MJ. Affective context interferes with cognitive control in unipolar depression: an fMRI investigation. J Affect Disord. 2009 Apr;114(1–3):131–142. doi: 10.1016/j.jad.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abler B, Erk S, Herwig U, Walter H. Anticipation of aversive stimuli activates extended amygdala in unipolar depression. J Psychiatr Res. 2007 Sep;41(6):511–522. doi: 10.1016/j.jpsychires.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 38.Thomas KM, Drevets WC, Dahl RE, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001 Nov;58(11):1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 39.Lau JY, Goldman D, Buzas B, et al. Amygdala function and 5-HTT gene variants in adolescent anxiety and major depressive disorder. Biol Psychiatry. 2009 Feb 15;65(4):349–355. doi: 10.1016/j.biopsych.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beesdo K, Lau JY, Guyer AE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009 Mar;66(3):275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halari R, Simic M, Pariante CM, et al. Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naive adolescents with depression compared to controls. J Child Psychol Psychiatry. 2009 Mar;50(3):307–316. doi: 10.1111/j.1469-7610.2008.01972.x. [DOI] [PubMed] [Google Scholar]
- 42.Monk CS, Telzer EH, Mogg K, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008 May;65(5):568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007 Dec;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995 Aug 25;269(5227):1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 45.LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. J Neurosci. 1995 Oct;15(10):6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 47.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002 Nov 15;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 48.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995 Oct;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 49.Stein JL, Wiedholz LM, Bassett DS, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007 Jul 1;36(3):736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 50.Roy AK, Shehzad Z, Margulies DS, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009 Apr 1;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen CH, Suckling J, Ooi C, et al. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology. 2008 Jul;33(8):1909–1918. doi: 10.1038/sj.npp.1301593. [DOI] [PubMed] [Google Scholar]
- 52.Anand A, Li Y, Wang Y, Gardner K, Lowe MJ. Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an FMRI study. J Neuropsychiatry Clin Neurosci. 2007 Summer;19(3):274–282. doi: 10.1176/appi.neuropsych.19.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anand A, Li Y, Wang Y, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005 May 15;57(10):1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 54.Anand A, Li Y, Wang Y, et al. Antidepressant effect on connectivity of the mood-regulating circuit: an FMRI study. Neuropsychopharmacology. 2005 Jul;30(7):1334–1344. doi: 10.1038/sj.npp.1300725. [DOI] [PubMed] [Google Scholar]
- 55.Dannlowski U, Ohrmann P, Konrad C, et al. Reduced amygdala-prefrontal coupling in major depression: association with MAOA genotype and illness severity. Int J Neuropsychopharmacol. 2009 Feb;12(1):11–22. doi: 10.1017/S1461145708008973. [DOI] [PubMed] [Google Scholar]
- 56.Matthews SC, Strigo IA, Simmons AN, Yang TT, Paulus MP. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J Affect Disord. 2008 Nov;111(1):13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 57.Lui S, Wu Q, Qiu L, et al. Resting-State Functional Connectivity in Treatment-Resistant Depression. Am J Psychiatry. 2011;168:642–648. doi: 10.1176/appi.ajp.2010.10101419. [DOI] [PubMed] [Google Scholar]
- 58.Cullen KR, Klimes-Dougan B, Muetzel R, et al. Altered white matter microstructure in adolescents with major depression: a preliminary study. J Am Acad Child Adolesc Psychiatry. 2010 Feb;49(2):173–183. e171. doi: 10.1097/00004583-201002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaffrey MS, Luby JL, Repovs G, et al. Subgenual cingulate connectivity in children with a history of preschool-depression. Neuroreport. 2010 Dec 29;21(18):1182–1188. doi: 10.1097/WNR.0b013e32834127eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weissman MM. Recent advances in depression across the generations. Epidemiol Psichiatr Soc. 2006 Jan–Mar;15(1):16–19. [PubMed] [Google Scholar]
- 61.Joormann J, Gilbert K, Gotlib IH. Emotion identification in girls at high risk for depression. J Child Psychol Psychiatry. 2010 May;51(5):575–582. doi: 10.1111/j.1469-7610.2009.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kujawa AJ, Torpey D, Kim J, et al. Attentional Biases for Emotional Faces in Young Children of Mothers with Chronic or Recurrent Depression. J Abnorm Child Psychol. 2011;1:125–135. doi: 10.1007/s10802-010-9438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monk CS, Klein RG, Telzer EH, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008 Jan;165(1):90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 64.Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A. Test-Retest Reliability of the Preschool Age Psychiatric Assessment (PAPA) J Am Acad Child Adolesc Psychiatry. 2006 May;45(5):538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- 65.Maxwell E. Health NIoM. Intramural Research Program, Clinical Neurogenetics Branch; Washington, DC: 1995. The Family Interview for Genetic Studies: Manual. [Google Scholar]
- 66.Luby JL, Mrakotsky C, Heffelfinger A, Brown K, Spitznagel E. Characteristics of depressed preschoolers with and without anhedonia: evidence for a melancholic depressive subtype in young children. Am J Psychiatry. 2004 Nov;161(11):1998–2004. doi: 10.1176/appi.ajp.161.11.1998. [DOI] [PubMed] [Google Scholar]
- 67.Kovacs M. The Children's Depression, Inventory (CDI) Psychopharmacol Bull. 1985;21(4):995–998. [PubMed] [Google Scholar]
- 68.Zeman J, Shipman K, Penza-Clyve S. Development and initial validation of the children's sadness management scale. Journal of Nonverbal Behavior. 2001 Fal;25(3):187–205. [Google Scholar]
- 69.Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997 Oct;6(3):156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- 70.Talairach J, Tournoux P, Musolino A. Anatomical Stereotaxic Studies of the Frontal-Lobe in the Management of the Epilepsies. Epilepsia. 1988 Mar–Apr;29(2):205–205. [Google Scholar]
- 71.Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004 Oct;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 72.Repovs G, Csernansky JG, Barch DM. Brain Network Connectivity in Individuals with Schizophrenia and Their Siblings. Biol Psychiatry. 2011 Dec 28;69(10):967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anticevic A, Repovs G, Barch DM. Resisting emotional interference: brain regions facilitating working memory performance during negative distraction. Cogn Affect Behav Neurosci. 2010 May;10(2):159–173. doi: 10.3758/CABN.10.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anticevic A, Repovs G, Barch DM. Emotional effects on attention, amygdala activity, and functional connectivity in Schizophrenia. Schizophrenia Bulletin; published online ahead of print March 17, 2011. doi: 10.1093/schbul/sbq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cole MW, Anticevic A, Repovs G, Barch D. Variable Global Dysconnectivity and Individual Differences in Schizophrenia. Biological Psychiatry. 2011;70(1):43–50. doi: 10.1016/j.biopsych.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 77.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002 Jan 31;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 78.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995 May;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 79.Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004 Nov;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 80.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004 Oct 15;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 81.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000 Oct;1(1):59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 82.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001 Jul;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 83.Luby JL, BA . Mood disorders and an Emotion Developmental Model of Depression. In: L JL, editor. Handbook of Preschool Mental Health: Development, Disorders and Treatment. Guilford Press; New York CIty: 2006. [Google Scholar]
- 84.Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M. How to Regulate Emotion? Neural Networks for Reappraisal and Distraction. Cereb Cortex. 2011;21(6):1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- 85.Ritchey M, Dolcos F, Cabeza R. Role of amygdala connectivity in the persistence of emotional memories over time: an event-related FMRI investigation. Cereb Cortex. 2008 Nov;18(11):2494–2504. doi: 10.1093/cercor/bhm262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leppanen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry. 2006 Jan;19(1):34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- 87.Gaffrey MS, Luby JL, Belden AC, Hirshberg JS, Volsch J, Barch DM. Association between depression severity and amygdala reactivity during sad face viewing in depressed preschoolers: An fMRI study. J Affect Disord. 2010 Sep 22;:364–370. doi: 10.1016/j.jad.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Currier D, Mann MJ, Oquendo MA, Galfalvy H, Mann JJ. Sex differences in the familial transmission of mood disorders. J Affect Disord. 2006 Oct;95(1–3):51–60. doi: 10.1016/j.jad.2006.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.