Abstract
A patient with benign prostatic hyperplasia presented with chronic lower urinary tract symptoms despite prior surgery and continued medical therapy. Using a MR-guided transperineal approach, two cryoprobes were placed into the transition zone of the prostate gland, and two cryoablation freeze-thaw cycles were performed. Ten weeks after treatment, the frequency of nocturia had decreased from once every 1.5 hours to once per night, urinary peak flow rates had increased from 5.1 mL/s to 10.3 mL/s, and post-void residual urinary bladder volume had decreased from 187 mL to 58 mL. Improved flow rates and symptoms remained stable 16 weeks after treatment.
Introduction
Benign prostatic hyperplasia (BPH) is a common age-related medical condition, present histologically in the transition zone of the prostate gland in 50% of men by age 50 (1, 2). In half of men greater than 60 years of age, BPH results in lower urinary tract symptoms, ranging from voiding difficulty and urine storage problems to renal insufficiency, recurrent urinary tract infections and acute urinary retention. Depending on the severity of symptoms, initial treatment options typically include active surveillance and medical management with alpha-adrenergic blockade and 5-alpha reductase inhibitors (1). Surgical or minimally invasive therapies are appropriate for patients for whom medical management fails, or in patients who do not want or cannot tolerate medical therapy. While transurethral resection of the prostate (TURP) remains the gold standard for surgical treatment, alternative minimally invasive treatment methods, including endoscopic laser prostatectomy, transurethral microwave therapy (TUMT) and transurethral needle ablation (TUNA) generally have fewer co-morbidities (2–4).
Percutaneous transrectal ultrasound-guided cryosurgery of the prostate gland has been used to treat prostate cancer; recently, focal prostate cryosurgery has been developed as a minimally invasive technique to treat localized prostate cancer (5, 6). To date, however, percutaneous cryoablation for BPH has only been reported in one small case series, with sub-optimal results (7). Recently, MRI guidance has been investigated as a way to improve focal cryosurgery (8, 9). We hypothesize that coupling freezing with MRI guidance and monitoring enables BPH ablation that is tailored to the size and extent of hyperplasia causing the symptoms. We present a case in which lower urinary tract symptoms secondary to BPH were refractory to medical and surgical management, but were improved by MR guided transperineal prostate cryoablation.
Case Report
The study was approved by the Institutional Review Board. All risks, benefits and alternatives to therapy were discussed with the patient, and informed consent was obtained prior to inclusion in the study. The patient was a 61-year-old man with chronic lower urinary tract symptoms due to BPH. Despite prior transurethral incision of the prostate and continued medical therapy with a combination of an alpha adrenergic blocker and 5-alpha reductase inhibitor, the patient had severe lower urinary tract symptoms. His complaints included urinary frequency and a poor urine stream. He had a high American Urological Association (AUA) symptom score with poor flow rates, a high bother score of 4 (“mostly dissatisfied”), and a modest post void residual volume of urine (Table 1) (10). Transrectal ultrasound (TRUS) imaging of his prostate confirmed prostatic hypertrophy, with a total prostate volume of 67 mL, and a transition zone volume of 46 mL. His serum Prostate Specific Antigen was 2.6 ng/mL, and sextant transrectal needle biopsy had demonstrated benign prostatic glands and stroma with no evidence of carcinoma.
Table 1. Quantitative change in symptoms after BPH cryoablation.
The American Urological Association (AUA) has developed the International Prostate Symptom Score (IPSS) based on patient responses to questions about incomplete bladder emptying, frequent voiding, hesitancy, incontinence, weak stream, straining, nocturia, and impact on quality of life to grade the severity of patient symptoms. The AUA IPSS categories are grouped as mild (0–7), moderate (8–19) and severe (20–35). The bother score, discussed in the case report, is part of the IPSS, and asks “If you were to spend the rest of your life with your prostate symptoms just as they are now, how would you feel about that?” and scores responses from 0, “delighted”, to 6, “terrible” (10).
| AUA Symptom Score | Peak Flow (mL/s) | |
|---|---|---|
| Before MRI cryoablation | 23 (severe) | 5.1 |
| 10 weeks after cryoablation | 13 (moderate) | 10.3 |
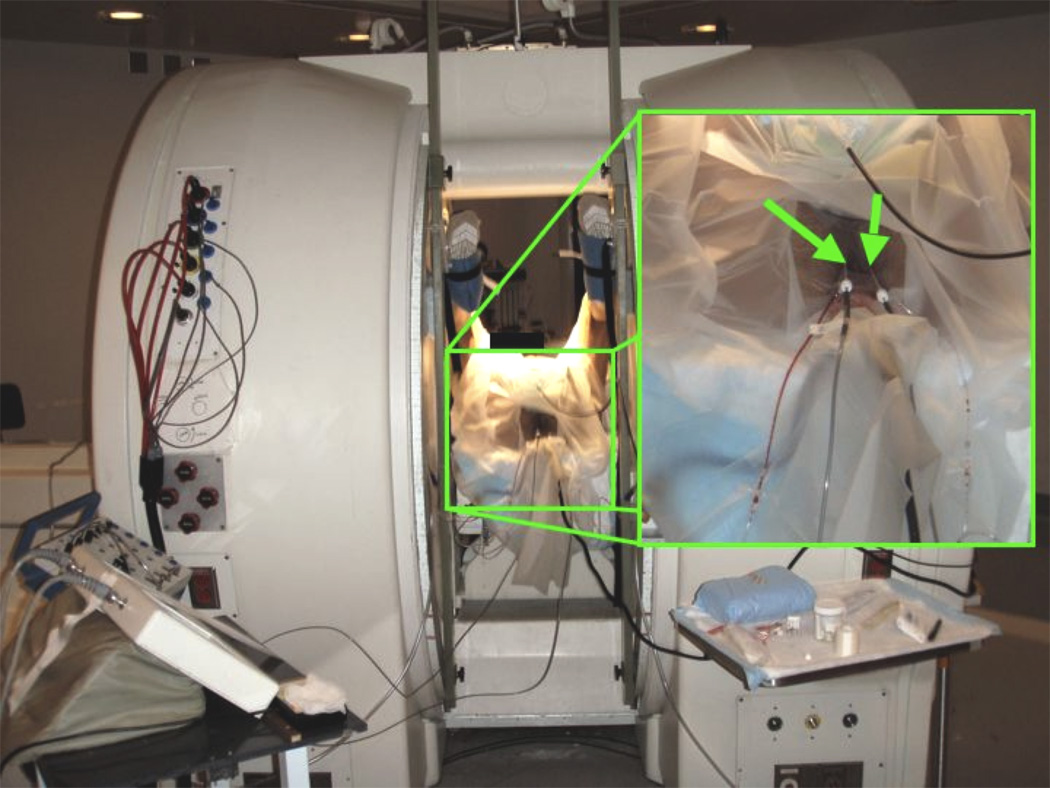
MRI-guided focal cryoablation was performed as an outpatient procedure. The patient was positioned in the dorsal lithotomy position within a vertically open 0.5T MR scanner (Signa-SP, GE Healthcare, Waukesha, WI) (Figure 1). A custom rigid endorectal coil and a custom 16 cm square surface coil placed anterior to the pubis were used for imaging. A single entry site on each side of the perineum was chosen using T2 fast spin echo (FSE) imaging guidance (parameters were: coronal/axial: 2/2.9 s repetition time (TR), 81/86 ms echo time (TE), 20 cm field of view (FOV), 122 kHz bandwidth, 256 × 128 matrix, 4 mm slice thickness (ST), 5 mm slice spacing, echo train length (ETL) of 20, 29 s scan duration). Local anesthesia was administered before and during probe placement. A 17 gauge disposable MRI-compatible Argon-powered cryoprobe with a sharp pencil tip (IceSeed, Galil Medical, Arden Hills, MN), within a 5 Fr introducer sheath (11 cm Pinnacle, Terumo Interventional Systems, Somerset, NJ) connected to vacuum suction, was inserted through the perineum. Using a free-hand technique, the cryoprobe and vascular sheath were advanced into the hyperplastic transition zone of the right side of the prostate gland, lateral to the urethra, pausing intermittently to confirm needle position using axial T2 FSE MRI. Another probe was similarly inserted into the transition zone to the left of the urethra. Separate probes were used on each side of the prostate in order to enable simultaneous bilateral ablations to minimize treatment time. Final T2 FSE MR confirmed that the probes were located in the enlarged transition zone, at least 1 cm from the urethra, rectum, urinary bladder, and periprostatic neurovascular bundles.
Figure 1. Patient in lithotomy position in vertically open MR scanner during treatment.
Green arrows indicate sheathed cryoprobes.
Prior to cryoablation, the target transition zone was biopsied using a MRI compatible biopsy needle (18 gauge 15 cm SABD, US Biopsy, Franklin, IN) inserted coaxially through each sheath. Histopathology later revealed benign prostatic tissue and stroma with no malignancy.
For cryoablation, each vascular sheath was retracted to expose 2 cm of the cryoprobe tip, while still enclosing the shaft of the probe. Two cycles of freezing and thawing were done with MR monitoring. Moderate conscious sedation was administered when the patient reported discomfort during the first thaw cycle.
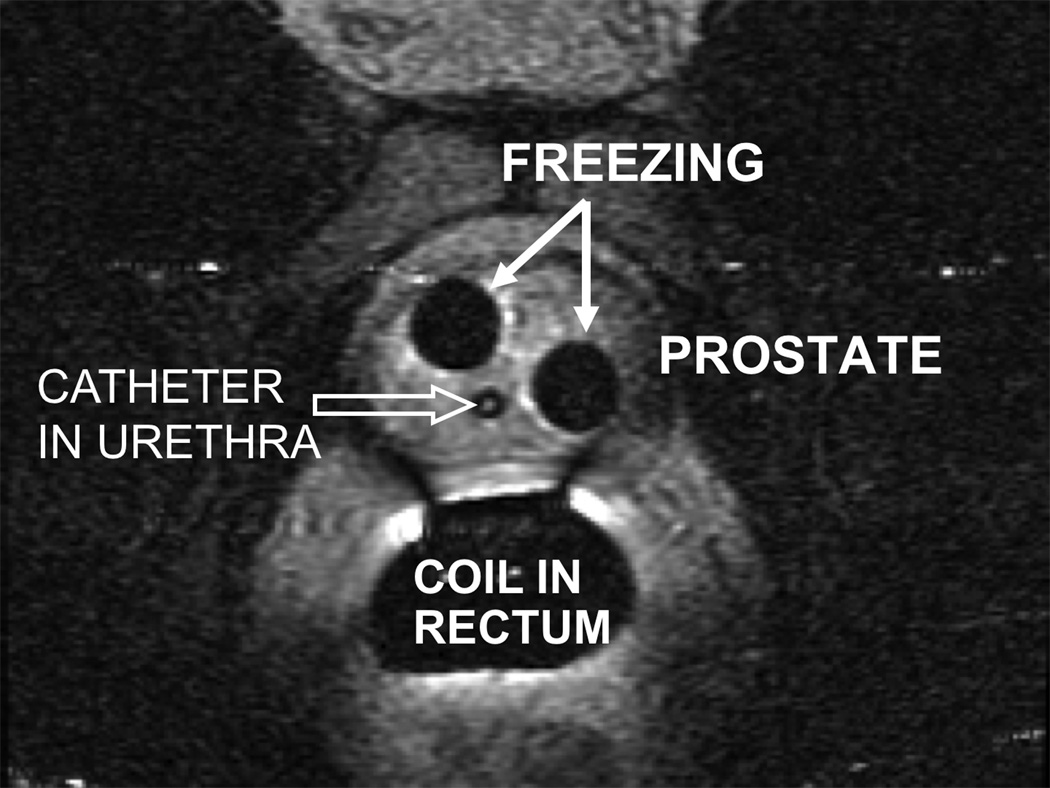
The first freeze cycle lasted for 10 minutes, and was followed by an active 5 minute thaw using high pressure helium. The second freeze cycle lasted for 5 minutes, followed by an active 3 minute thaw and removal of the cryoprobes. No urethral warming device was utilized, as axial T2 FSE MRI was continuously repeated every 2–3 seconds to monitor the extent of the iceball (Figure 2), with the goal to approach but not freeze the immediate periurethral region, and to avoid injury to other vital structures including the rectum, neurovascular bundles and bladder base.
Figure 2. MRI during freezing cycle.
Axial T2 weighted image of the prostate obtained during the freezing cycle. Two signal voids (white arrows) correspond to the iceballs formed around the probes and reveal the maximum extent of freezing. A Foley catheter is visible in the urethra (open arrow)
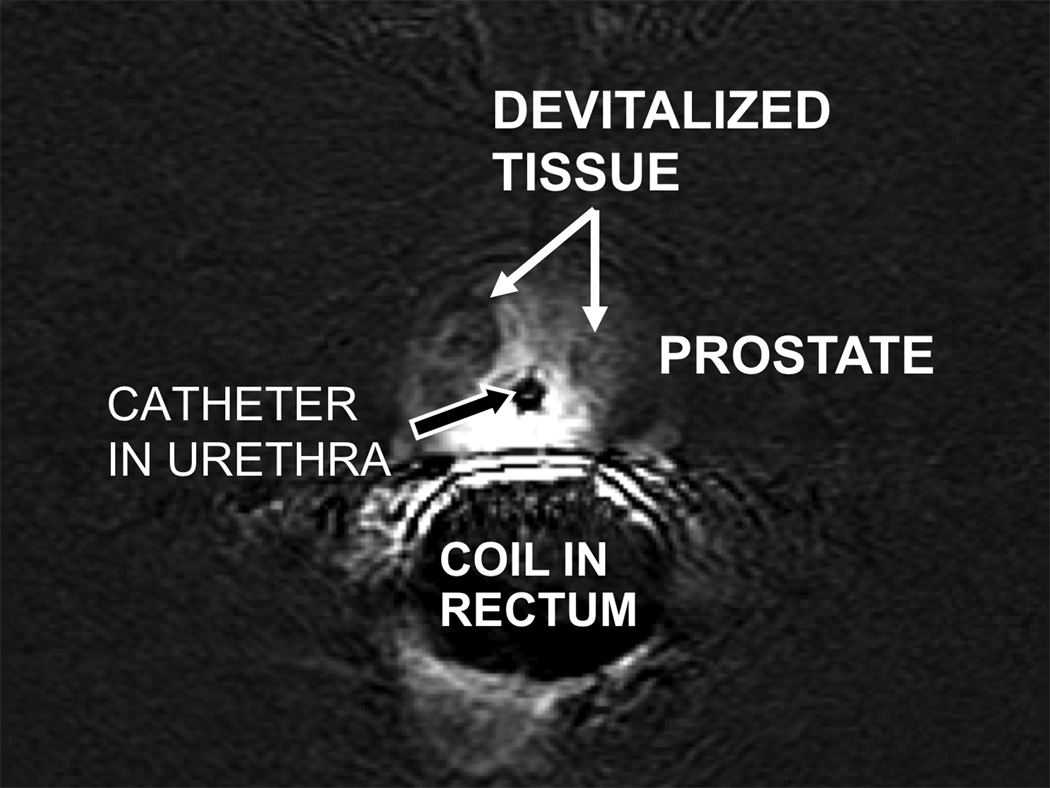
Immediate pre- and post-contrast 3D RF-spoiled T1 weighted gradient recalled echo imaging (33 ms TR, 3 ms TE, 25° flip angle, 20 cm field of view, 122 kHz bandwidth, 256 × 160 matrix, 2.6 mm slice thickness, 2.6 mm slice spacing, 196 s scan duration) was performed to image the extent of devitalized tissue (Figure 3) (7). Although the overall enhancement of the prostate was heterogeneous, subtracted contrast-enhanced images demonstrate ovoid, hypoenhancing areas on either side of the urethra that appear to correspond to the treated areas.
Figure 3. Post treatment subtracted contrast enhanced images.
Axial 3D T1 weighted image obtained before and 5 minutes after intravenous injection of 15 mL of Magnevist. Pre- and post-contrast images were subtracted using a GE Advantage workstation. There is reduced contrast enhancement in the areas that were ablated (white arrows).
The entire procedure took less than 3.5 hours, from the patient’s entry into the MRI suite to his transfer to the recovery area. Placement of the two probes required approximately 1.5 hours, while patient positioning, the freeze-thaw cycles, and post-treatment imaging each required approximately 30 minutes. The patient’s Foley catheter was removed and he successfully voided prior to discharge home. Several hours after discharge, the patient returned to the hospital with acute urinary retention requiring placement of a Foley catheter; this was maintained for one week, and then discontinued without residual urinary retention.
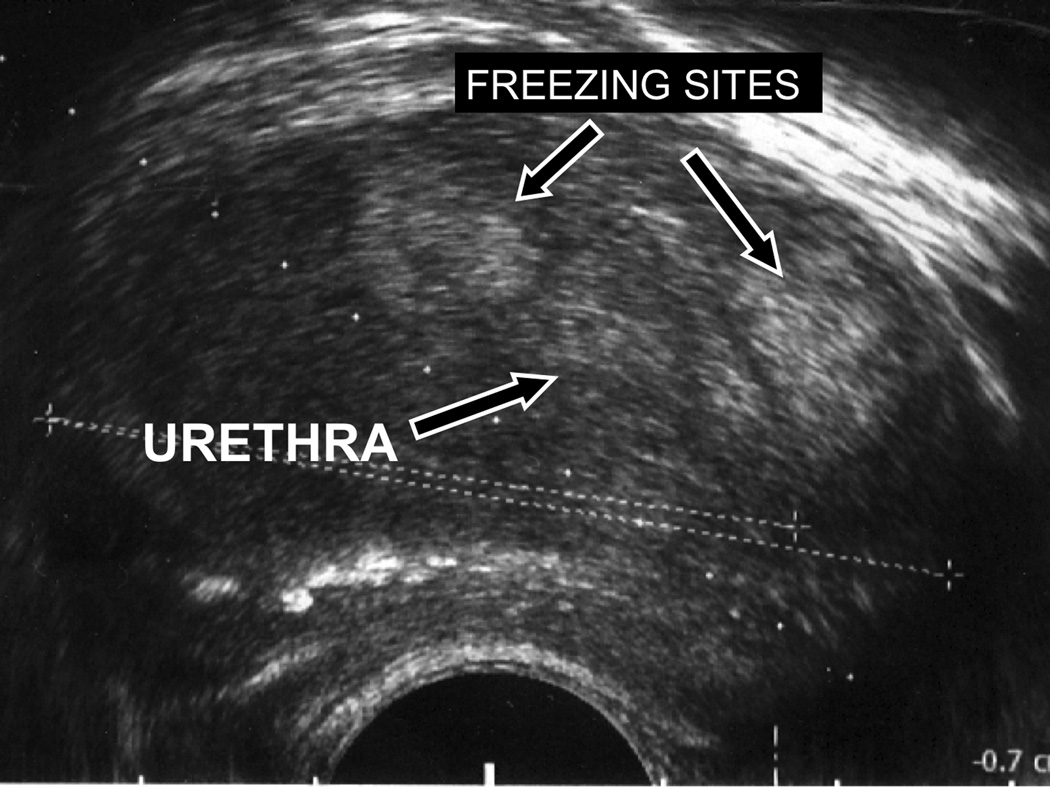
The patient was instructed to stop his alpha adrenergic blocker and 5-alpha reductase inhibitor post treatment. Ten weeks after treatment, his daytime urinary frequency had decreased from every 1.5 hours to every 2–4 hours, and frequency of nocturia had decreased from every 1.5 hours to once per night. His symptom score decreased by 43% and now indicated moderate symptoms, and his bother score had decreased from 4, “mostly dissatisfied”, to 1, “pleased”. Although the force of his urine stream had not subjectively improved, urinary urgency had decreased and his peak flow rate had increased by 102% (Table 1). He reported being able to empty his bladder adequately, and subjective voided urinary volumes had increased. His postvoid residual volume was 31% of the pretreatment value, and his voided urine volume had increased by 30%. The patient noticed improvement in these symptoms beginning approximately 4 weeks after treatment. TRUS revealed that his prostate volume was 67 mL, with a transition zone volume of 49 mL. TRUS of the prostate revealed two echogenic, circumscribed parenchymal foci, which appear similar in configuration to the sites of cryoablation (Figure 4). The patient’s symptomatic improvement persisted at a 16 week follow-up visit, when his peak flow rate was 9.9 mL/s, increased by 94% from the pre-treatment value, and his postvoid residual volume was 84 mL, decreased to 45% of pretreatment value.
Figure 4. Post treatment TRUS.
Axial transrectal ultrasound of the prostate gland using a 7.5 MHz biplanar endorectal ultrasound probe. Two echogenic foci are present in the gland antero-lateral to the urethra, and appear similar in configuration to the sites of cryoablation seen on the MR images during freezing, as well as the areas of devitilization seen on the subtracted post-contrast images.
Discussion
Focal prostate cryoablation has been used in preliminary studies to treat localized prostate cancer (5, 6). The complications of generalized prostate cryoablation, including erectile dysfunction, rectourethral fistula, incontinence, urinary retention, and pain are less frequent with focal cryoablation. However, focal prostate cryoablation for prostate cancer is limited by the ability of imaging and biopsy to accurately determine the extent of disease within the gland. The potential application of this method for treatment of localized prostate cancer awaits investigation through prospective clinical trials.
Transperineal transrectal ultrasound-guided cryosurgery for BPH has been previously reported in the Chinese-language literature in a series of 21 patients (7). Response was favorable with average urinary flow rates increasing from 3.8 ± 2.1 mL/s to 17.0 ± 5.8 ml/s. No rectal fistula occurred. However, urethral injury remained prevalent, with 15 of 20 patients reporting up to 1 week of hematuria, and 4 patients developing mild incontinence. Thus even though the probes avoided the urethra, and 40°C saline was irrigated through the bladder and urethra during the procedure, some urethral freezing likely occurred. Urethral injury in this study underscores the limitations of TRUS guidance of cryosurgery. Once the iceball begins to form, deeper visualization is obscured by sonographic shadowing from the edge of the iceball closest to the transducer (11). While TRUS guidance is sufficient to visualize the rectal wall and avoid rectal complications, it does not visualize the entire urethra sufficiently to protect it during BPH ablation (8).
Focal MR-guided cryoablation of the prostate transition zone was used to treat a patient with lower urinary tract symptoms refractory to surgical and medical therapy. MR guidance and treatment monitoring has several advantages. MR monitoring of cryoablation shows the full extent of freezing in 3 dimensions without shadowing artifacts (8, 9, 11). MRI is particularly attractive for monitoring cryosurgery because there is no radiation and the prostate zonal anatomy is clearly seen on T2-weighted images (8). Intraprocedural MR monitoring allows visualization and control of the extent of freezing near critical structures. Furthermore, MRI methods, including T2- and diffusion-weighted imaging, and contrast-enhanced imaging can be used to monitor the effects of cryoablation on the target tissue immediately after ablation (8).
Notably, TRUS at 10 weeks revealed no change in the patient’s total prostate or transition zone volume. The relation of the size of the prostate and patients’ symptoms remains controversial (12). Initially, cryotherapy may improve symptoms by destroying the alpha receptors in the prostate and bladder neck (13). Ultimately, cryotherapy also results in decreasing the size of the prostate after complete involution of the treated area, although it may take 4 to 6 months for complete involution of the treated areas (14).
Several limitations are present in this study. The treatment utilized a vertically open MR scanner that is not generally available. While providing for easier access to the patient, cryoprobe insertion time was in part prolonged by the slow imaging speed of the scanner; the scanner is also limited by relatively low resolution imaging. However, this case does demonstrate the potential of MR guided cryoablation for treatment of BPH and issues of patient and cryoprobe positioning for this procedure in more conventional wide-bore scanners can be overcome by adapting computer-aided and robotic remote probe positioning and manipulation strategies (9, 15).
In summary, our result shows that MR guided prostate cryoablation targeting the transition zone of the prostate gland may relieve lower urinary tract symptoms resulting from BPH. Like TUMT or TUNA, this approach is minimally invasive, but it spares the urethra entirely, theoretically minimizing complications such as urethral injury or bladder neck contractures. Like TUNA and TURP, it is an ablative modality with the potential for a durable effect (2–4). Although only a minority of patients with symptomatic BPH are refractory to medical and/or surgical management, the large number of men with BPH implies that a still sizable population of patients might benefit from an alternative approach. Studies with more patients and longer clinical follow-up are needed to determine the efficacy and safety of this treatment approach.
Acknowledgments
Supported by NIH R01 CA092061.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest and financial disclosures: None
Contributor Information
Pejman Ghanouni, Department of Radiology, Stanford University Hospital and Clinics, 300 Pasteur Dr, Rm H1307, MC 5621, Stanford, CA 94305
Harcharan Gill, Department of Urology, Stanford University Hospital and Clinics, 875 Blake Wilbur Dr, MC 5118, Stanford, CA 94305
Elena Kaye, Department of Electrical Engineering, Stanford University, Stanford, CA 94305
Kim Butts Pauly, Departments of Radiology and Bioengineering, Stanford University, Stanford, CA 94305
Bruce Daniel, Department of Radiology, Stanford University Hospital and Clinics, 300 Pasteur Dr, Rm H1307, MC 5621, Stanford, CA 94305
References
- 1.Auffenberg GB, Helfand BT, McVary KT. Established medical therapy for benign prostatic hyperplasia. Urol Clin North Am. 2009;36:443–459. doi: 10.1016/j.ucl.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Turini GA, Pareek G. Update on minimally invasive therapies for benign prostatic hyperplasia. Med Health R I. 2009;92:336–338. [PubMed] [Google Scholar]
- 3.Burke N, Whelan JP, Goeree L, et al. Systematic review and meta-analysis of transurethral resection of the prostate versus minimally invasive procedures for the treatment of benign prostatic obstruction. Urology. 2010;75:1015–1022. doi: 10.1016/j.urology.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 4.d'Ancona FC. Nonablative minimally invasive thermal therapies in the treatment of symptomatic benign prostatic hyperplasia. Curr Opin Urol. 2008;18:21–27. doi: 10.1097/MOU.0b013e3282f20157. [DOI] [PubMed] [Google Scholar]
- 5.Finley DS, Pouliot F, Miller DC, Belldegrun AS. Primary and salvage cryotherapy for prostate cancer. The Urologic clinics of North America. 2010;37:67–82. doi: 10.1016/j.ucl.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen CT, Jones JS. Focal therapy in the management of localized prostate cancer. BJU Int. 2011;107:1362–1368. doi: 10.1111/j.1464-410X.2010.09975.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang AX, Wang D, Tong W. Cryosurgical ablation for aged patients with severe benign prostate hyperplasia: a report of 21 cases. Zhonghua Nan Ke Xue. 2007;13:421–423. [PubMed] [Google Scholar]
- 8.van den Bosch MA, Josan S, Bouley DM, et al. MR imaging-guided percutaneous cryoablation of the prostate in an animal model: in vivo imaging of cryoablation-induced tissue necrosis with immediate histopathologic correlation. J Vasc Interv Radiol. 2009;20:252–258. doi: 10.1016/j.jvir.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdelaziz S, Esteveny L, Renaud P, et al. Design considerations for a novel MRI compatible manipulator for prostate cryoablation. Int J Comput Assist Radiol Surg. 2011 doi: 10.1007/s11548-011-0558-4. Electronic publication ahead of printing. [DOI] [PubMed] [Google Scholar]
- 10.AUA guideline on management of benign prostatic hyperplasia. Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170:530–547. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- 11.Tatli S, Acar M, Tuncali K, Morrison PR, Silverman S. Percutaneous cryoablation techniques and clinical applications. Diagn Interv Radiol. 2010;16:90–95. doi: 10.4261/1305-3825.DIR.1922-08.0. [DOI] [PubMed] [Google Scholar]
- 12.Steele GS, Sullivan MP, Sleep DJ, Yalla SV. Combination of symptom score, flow rate and prostate volume for predicting bladder outflow obstruction in men with lower urinary tract symptoms. The Journal of urology. 2000;164:344–348. [PubMed] [Google Scholar]
- 13.Perachino M, Bozzo W, Puppo P, Vitali A, Ardoino S, Ferro MA. Does transurethral thermotherapy induce a long-term alpha blockade? An immunohistochemical study. Eur Urol. 1993;23:299–301. doi: 10.1159/000474616. [DOI] [PubMed] [Google Scholar]
- 14.Leibovici D, Zisman A, Lindner A, Stav K, Siegel YI. PSA elevation during prostate cryosurgery and subsequent decline. Urol Oncol. 2005;23:8–11. doi: 10.1016/j.urolonc.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Rossi MR, Tanaka D, Shimada K, Rabin Y. Computerized planning of prostate cryosurgery using variable cryoprobe insertion depth. Cryobiology. 2010;60:71–79. doi: 10.1016/j.cryobiol.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]