Abstract
The current study prospectively examined trajectories of change in symptoms of irritability, hyperactivity, and social withdrawal, as well as predictors of such behaviors from age 9 to 18 for youths with autism spectrum disorder (ASD) and a comparison group with nonspectrum developmental delays. Children with more severe core features of autism had consistently higher irritability and hyperactivity scores over time than those with broader ASD and nonspectrum delays. Across all diagnoses, behaviors related to hyperactivity showed the greatest improvement. Social withdrawal worsened with age for a substantial proportion of youths with ASD but not for the nonspectrum comparison group. Compared with nonspectrum youths, children with ASD showed greater heterogeneity in trajectories for maladaptive behaviors.
In addition to the core features of autism spectrum disorder (ASD), children and adolescents with ASD are at risk for developing various maladaptive behaviors and difficulties beyond those defining the disorder. Comorbid or co-occurring (used interchangeably) behaviors commonly include symptoms of hyperactivity, irritability, aggression, oppositional conduct, self-injury, depression, anxiety, and other socially unacceptable behaviors (e.g., Howlin, 2007; Lecavalier, 2006; Shattuck et al., 2007; Simonoff et al., 2008). Maladaptive behaviors have received less attention in the scientific literature than core features of ASD. However, they are important to study for a number of reasons.
First, most research has found comorbid maladaptive behaviors to be more prevalent among children with ASD than comparison groups, including those with Down Syndrome, Fragile X, Williams Syndrome, cerebral palsy, intellectual disability, and language impairment, as well as typically developing children (e.g., Blacher & McIntyre, 2006; Capone, Grados, Kaufmann, Bernad-Ripoli, & Jewell, 2005; Gadow, Devincent, Pomeroy, & Azizian, 2005; Gillot, Furniss, & Walter, 2001; Tonge & Einfield, 2003). In a population-derived sample of 112 children with ASD age 10 to 14, 70% had at least one comorbid disorder, most notably, social anxiety (29%), attention deficit hyperactivity disorder (28%), or oppositional defiant disorder (28%), among others (Simonoff et al., 2008).
Second, if untreated, the negative effects of maladaptive behaviors can be pervasive and long-lasting. The long-term negative impact of both externalizing and internalizing problems on the well-being of typically-developing populations in terms of academic achievement, social competence, and adult psychiatric outcome has been well-documented (e.g., Ansary, 2009; Galera, Melchior, Chastang, Bouvard, & Fombonne, 2009; Reef, Diamantopoulou, vanNeurs, Verhulst, & Van der Ende, 2010). Research on children with intellectual disabilities and ASD has reported similar findings (e.g., Chadwick, Piroth, Walker, Bernard, & Taylor, 2000; de Bildt, Sytema, Kraijer, Sparrow, & Minderaa, 2005; Kim, Szartmari, Bryson, Streiner, & Wilson, 2000). Likewise, maladaptive behaviors such as social withdrawal, hyperactivity, and irritability are associated with lower quality of life for adults with ASD and intellectual disability with respect to social integration, leisure activities, health and security, and self-determination (Gerber, Baud, Giroud, & Caminati, 2008).
Third, such behaviors may not necessarily follow the same trajectory as ASD core features over the life course. Comorbid behaviors such as irritability, hyperactivity, and social withdrawal show low to moderate correlations with core symptoms of ASD (Lecavalier, 2006). While for many people with ASD there is a general tendency for core symptoms of ASD to abate somewhat in adolescence and adulthood (see Seltzer, Shattuck, Abbeduto, & Greenberg, 2004, review), some maladaptive behaviors such as depression and anxiety appear to peak in adolescence, at least in verbally able children and adolescents with ASD (Ghazuiddin, Ghaziuddin, & Greden, 2002; Howlin, 2007). Consequently, interventions for young people with ASD may therefore be less effective if secondary maladaptive behaviors are not taken into account and treatment strategies refined accordingly.
Despite their prevalence, co-occurring maladaptive behaviors present with a high degree of variability within ASD. A small body of research has begun to examine predictors of such behaviors in school-aged children and young adults. Although studies are too few in number to reach firm conclusions, the prevalence of maladaptive behaviors in ASD appears to be greater among children who are younger, more cognitively impaired, and taking psychotropic medications (Estes, Dawson, Sterling, & Munson, 2007; Lecavalier, 2006; Lounds-Taylor & Seltzer, 2010; Shattuck et al., 2007). Research on children with intellectual and learning disabilities without ASD supports these findings (e.g., Brown, Aman, & Havercamp, 2002; Chadwick, Kusel, & Cuddy, 2008; Ono, 1997).
Nevertheless, some internalizing behaviors, particularly those related to depression and anxiety, may be more common in older children and adolescents (Lecavalier, 2006; Tonge & Einfield, 2003) who are more able in terms of IQ (Estes et al., 2007; Tonge & Einfield, 2003) and/or have fewer core symptoms of ASD (Gadow et al., 2005; Kanai et al., 2004). It is possible that higher rates of internalizing difficulties may be related to increasing awareness of one’s disability and higher social expectations from adults and peers as these children enter adolescence and young adulthood compared to those who are younger or less cognitively able. These social pressures could also interact with the physiological changes associated with puberty.
Few studies on ASD have specifically examined the impact of pubertal development on maladaptive behaviors in adolescence. Based on parental retrospective report (Kobayashi, Murata, & Yoshinaga, 1992) and clinical observation (Gillberg & Steffenberg, 1987), several research teams estimated that as many as one-third of children with ASD experience a deterioration in functioning for several years or more with the onset of puberty, marked by an increase in aggression, obsessions, destructiveness, and repetitive behaviors. Decreases in cognitive and language skills were also noted. Moreover, the increase in maladaptive behaviors around the time of puberty appeared to be related to more severe intellectual disability and epilepsy. Girls had a tendency to be affected by deterioration over a longer period of time (Gillberg & Steffenberg, 1987). Because a majority of children with ASD in these studies did not experience such a decline, one possibility is that there was an interaction between pubertal onset and other vulnerability factors.
In sum, adolescence has been proposed by some researchers and clinicians as an especially difficult time for a substantial proportion of young people with ASD. The cross-sectional nature of many studies makes it difficult to determine the extent to which findings may primarily reflect differences in age cohorts and recruitment (e.g., children with ASD and behavior problems have more contact with professionals than those with ASD who are doing well). Because some internalizing disorders such as depression and anxiety tend to overlap with those of core social deficits in ASD, at least when described in questionnaires, the longitudinal examination of patterns of change in such behaviors over time offers important information about secondary maladaptive behaviors in ASD.
Unfortunately, prospective longitudinal studies employing standardized measures at more than two points in time are rare (but see Lounds-Taylor & Seltzer, 2010; Tonge & Einfield, 2003). In addition, existing longitudinal studies of individuals with ASD have frequently targeted either those in the upper (e.g., Kanai et al., 2004) or lower ranges of IQ (Tonge & Einfield, 2003), making comparisons across studies and subgroups within ASD difficult. Longitudinal studies with more diverse samples may provide further clarification on the presentation and patterns of change in various maladaptive behaviors for young people with a broad range of cognitive abilities and ASD symptoms.
The aim of the current study was to begin bridging the gaps in the scientific literature on maladaptive behaviors in ASD, with a focus on the transitional period spanning mid-childhood through late adolescence. Trajectories of change in symptoms of irritability, hyperactivity, and social withdrawal were prospectively examined as well as some key predictors of such behaviors for youths with ASD and a Nonspectrum comparison group with developmental disabilities.
Method
Participants
Eligible participants were consecutive referrals younger than 37 months of age from agencies across North Carolina and metropolitan Chicago serving very young children with delays. Of the 221 families who were originally referred, 214 were seen. Participants consisted of 192 children (162 males, 30 females) referred for evaluation for possible autism and 22 developmentally-delayed children without ASD (DD: 10 males, 12 females) recruited from sources that referred to autism clinics. The autism referral group was comprised of children from four North Carolina state-funded autism centers (n=111) and a Chicago autism clinic within a private university hospital (n=81). Exclusion criteria included moderate to severe sensory impairments, cerebral palsy, or poorly-controlled seizures. Seventy-five percent of the 214 participants received diagnoses at age 2 which placed them on the autism spectrum, while the remainder had other developmental delays. The Nonspectrum group consisted of the 22 DD children (above) as well as children from the autism referral group who did not receive an ASD diagnosis. These children presented with some degree of intellectual disability or language delay (91%) or other disabilities such as severe ADHD. There was a mix of children from rural and urban areas. Ethnic minorities, the majority of whom were African American, accounted for a sizeable proportion of the sample (33%).
Of the original 214 participants, five were lost to follow-up after the initial assessment and the remainder were lost due to geographical relocation, unreachable status, or refusal to participate. Although African American families with less education were lost to the study at a higher rate than Caucasians and families with more education, attrition was not related to diagnosis, gender, or IQ at the initial assessment. By age 18, 142 youths from the original sample and their families continued to participate at least in part. The current study includes the 116 youths for whom we have complete data on maladaptive behaviors for a minimum of two time points (Mean number of assessments=14; S.D.=4.56) between age 9 and 18.
Measures/Procedures
A battery of diagnostic and psychometric instruments was administered in person when the children were ages 2, 5 (for North Carolina participants only), and 9 free of charge. In addition, a log of early childhood educational and intervention treatments as well as a history of seizures was obtained through more frequent interviews with and diaries completed by caregivers. Verbal feedback and a written report were provided to families after each assessment. Caregivers were asked to provide information on maladaptive behaviors, pubertal development, medication usage, and seizure activity through self-administered instruments when the youths were 9 and then every four months through mailed questionnaires when their children were between 13 and 18 years-old. The measures used in the present study are described below.
Diagnostic instruments
The Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994) is a comprehensive, standardized parent interview designed to distinguish children with ASD from non-ASD and developmentally-delayed populations. The Autism Diagnostic Observation Schedule (ADOS; Lord, Risi, Lambrecht et al., 2000) acquires diagnostic information through direct observation of the child by a trained clinician. An algorithm calculates summary scores for each instrument. Prior to administration of the instruments, all members of the research clinical team established inter-rater reliability exceeding 90% exact agreement (kappa > 0.70) for all items on the ADI-R and 80% exact agreement (kappa > 0.60) on codes for the ADOS for three consecutive administrations before the study began. Reliability was maintained through consensus coding approximately every sixth administration with a second rater who was blind to referral status. Clinicians administered the test batteries without knowledge of previous assessments whenever possible.
At each assessment, an overall best-estimate diagnosis of Autism, Broader Autism Spectrum Disorder (BASD), or other Nonspectrum disability was based on all available information. Immediately after conducting the assessment, and prior to calculating the diagnostic algorithms, the primary clinician dictated a two-page summary, along with ratings for overall “probability of an Autism diagnosis” (ranging from 1 = lowest to 15 = highest). The summary information, in addition to videotapes, was then used in deciding a consensus diagnosis. Children with Autism had the highest calibrated severity scores on the ADOS (Autism M=8.14, S.D.=1.42; BASD M=5.89, S.D.=2.46; Nonspectrum M=3.29; S.D.=2.31) and the highest probability ratings (Autism M=14.6, S.D.=0.73; BASD M=9.4, S.D.=1.55; Nonspectrum M=2.6, S.D.=1.68).
Previous research with this sample showed that clinicians’ ratings of diagnosis at age 9, using all available information, provided a useful categorical measure that surpassed, as well as summarized, the standardized diagnostic instruments in predicting later outcome (e.g., Lord et al., 2006). There are a number of ways to break down what are likely several continuous dimensions that contribute to diagnostic severity. Our purpose was to avoid reifying distinctions between different DSM IV-based autism spectrum disorders and to identify relatively easily interpretable ways of representing diversity between and within potential subsets, building on previous research with this sample (Anderson, Oti, Lord, & Welch, 2009; Anderson et al, 2007) and other studies (e.g., Szatmari et al., 2009). The current study used the most recent best-estimate diagnostic classification taken from the age 9 assessment. For three participants not seen at age 9, the age 5 diagnosis was used.
Psychometric instruments
For purposes of the current study, the most recent verbal IQ (VIQ) and nonverbal IQ (NVIQ) were taken from the age 9 assessment (or age 5 in three cases). Cognitive test selection followed a standard hierarchy designed for use when the children could not achieve a basal score or achieved ceiling scores: Wechsler Intelligence Scale for Children Third Edition (WISC-III; Wechsler, 1991; n=27); Differential Abilities Scale (DAS; Elliott, 1990 ; n=51) ; Mullen Scales of Early Learning (Mullen, 1995; n=36); and other (n=2). Ratio IQs were calculated when raw scores fell outside the ranges for deviation scores.
Maladaptive Behavior Measure
The Aberrant Behavior Checklist (ABC: Aman, 1994) is a 58-item checklist designed for use with children and adults with developmental delays. Caregivers were asked to rate their children’s maladaptive behaviors in the past four weeks on a four-point scale ranging from: (0) “not at all a problem” to (3) “the problem is severe in degree.” The three subscales of interest have been well-established in factor analytic studies (e.g., Aman, Singh, Stewart, & Field, 1985; Marshburn & Aman, 1992; Rojahn & Helsel, 1991): Lethargy/Social Withdrawal (16 items), Hyperactivity (16 items), and Irritability (15 items). We used the unweighted totals when data were present for all 16 items. For the Irritability subscale and for cases missing up to two items on any given subscale, individual items were weighted accordingly to allow for comparability in scores across person and subscale (i.e., 16 divided by the number of non-missing items). The Speech (4 items) and Stereotypies (7 items) subscales were not analyzed in the current study due to the small number of items on each. In addition to the standard ABC items, a question about current medications was also administered.
Pubertal Onset Measure
The Pubertal Development Scale (PDS; Petersen, Crockett, Richards, & Boxer, 1988) served as the measure of pubertal onset in the current study. Every four months parents were asked to rate various characteristics on a four-point scale from “no development” to “development complete.” For girls, pubertal onset was defined as one or more of the following characteristics: breast development, growth of body hair, or the start of menarche. Boys were considered pubescent if parents reported some growth in body hair, facial hair, or voice changes.
Seizure Measures
Information on the history of nonfebrile seizures from age 2 to 18 was obtained through parental report using a variety of methods, including in-person interviews as part of a larger assessment battery, a seizure interview administered over the phone (Spence et al., 2005), medical records, and a seizure log (Kuhn, Allen, & Shriver, 1995) that was part of the mailed packets sent to parents every four months.
Treatment Measure
Two raters established reliability and coded the treatment data. For the purpose of this paper, individual speech therapy was defined in terms of the total number of therapy hours received through age 5. Due to the relatively small number of families participating in mentored, parent-implemented structured teaching (MPST) (a home teaching program primarily modeled after the TEACCH extended diagnostic services; see Mesibov, Shea, & Schopler, 2005) and Applied Behavior Analysis (ABA) (n=17 and n=22 respectively), the number of hours in out-of-school treatment was collapsed into categories of “none” vs. “some.”
Other Measures
Several instruments were administered when the youths were approximately age 18, including parent report measures of internalizing and externalizing behaviors for youths with VIQs above 50 (Child Behavior Checklist: CBCL; Achenbach & Rescorla, 2000; or Adult Behavior Checklist: ABCL; Tenneij & Koot, 2007) or below 50 (Developmental Behavior Checklist: DBCL; Enfield & Tonge, 2002). A youth self-report measure of loneliness for the more able participants (Asher Loneliness Scale; Asher, Hymel, & Renshaw, 1984) was also administered.
Analyses
Growth curve analysis with SAS Proc Mixed was used to examine changes in comorbid behaviors from age 9 to 18. A separate intercept and slope were calculated for each child as a control for the high correlations among repeated measures on the same individuals over time. The Autism, BASD, and Nonspectrum diagnostic groups were compared with respect to: 1) relative starting points at 9 years of age (intercept); and 2) rate and pattern of change over time (linear and quadratic slopes). Other covariates added as fixed effects included: age at testing, gender, ethnicity, mother’s education, hours of individual treatment through age 5, most recent verbal or nonverbal IQ, pubertal onset, psychotropic medication usage (ever), and seizure onset. Onset of puberty and onset of seizures were allowed to vary over time (i.e., before puberty/before first seizure=0; after onset=1). All covariates were initially considered as predicting both the intercept and slope(s). The main effects and slopes of covariates which did not reach significance with or without the inclusion of other predictors, and did not affect the covariates that were retained, were dropped from analysis in order to reduce the number of parameters.
The estimates for both the covariance and beta parameters were obtained by restricted maximum likelihood methods so that results would be less biased (Verbeke & Molenberghs, 2000). To test for group differences in slopes and intercepts, we used t-statistics for each parameter, calculated as the ratio of the parameter estimate divided by the standard error. To examine whether rate of change in maladaptive behaviors over time differed significantly from “0,” we used t-tests for linear combinations of variables representing the slopes.
Because there are no existing clinical guidelines for interpreting problem behaviors as measured by the Aberrant Behavior Checklist, the effect size for changes in mean scores over time was calculated using the standardized mean difference (SMD) method: SMD=(Time 1 behavior score – Time 2 behavior score)/pooled standard deviation (see Cohen, 1988). We used the widely accepted guidelines of Cohen (1988) for interpreting the effect size where 0.2 is small, 0.5 is medium, and 0.8 is large. For the current study, medium to large effect sizes were considered clinically relevant.
A mixture modeling procedure called TRAJ (Jones, Nagin, & Roeder, 2001) was used to assess variability in outcomes within diagnoses. Intended to complement growth curve analysis, PROC TRAJ is an exploratory procedure written for use in SAS that identifies linear and nonlinear patterns in longitudinal data and then classifies the sample into groups. We ran the procedure using an uncensored, normal distribution for maladaptive behavior scores from age 9 to 18 to see what subgroupings would emerge within the sample as a whole. We first compared the absolute value of the Bayesian Information Criterion (BIC) between respective models (smaller indicates a better fit) to determine the optimal number of groups (Jones et al., 2001).
Results
Preliminary findings
Predictor variables
Table 1 includes the sample characteristics expected to affect the presentation of comorbid behaviors for those with diagnoses of Autism, BASD, and Nonspectrum DDs. Chi square analyses were conducted to test for differences in group proportions, while analysis of variance was used to test for group differences in means. The diagnostic groups did not differ by age, race, age of pubertal onset, or lifetime prevalence of seizures. The Autism and BASD subsamples included a higher percentage of male youths and parents with college educations compared to the Nonspectrum DD group. Children with Autism had significantly lower IQs than those with BASD and Nonspectrum DDs. However, despite the lower IQs for the Autism group as a whole, individual NVIQ and VIQ scores were quite variable, ranging from 2 to 126 and 3 to 115 respectively. Nearly a quarter of the sample reported a lifetime history of seizures. Of those ever reporting seizures, onset occurred after age 9 for 33% (not shown on table). This represents a smaller proportion of the total sample than expected, including 9% of children with Autism (n=6), 4% of youths with BASD (n=1), and 4% of those with Nonspectrum DDs (n=2). In addition, youths with Autism received more hours of early childhood intervention treatment than other groups, and a greater proportion were treated with psychotropic medications.
Table 1.
| Sample Characteristics | Most Recent Diagnosis | Test Statistic | |||
|---|---|---|---|---|---|
| Autism N=65 |
BASD1 N=27 |
Nonspectrum2 N=24 |
Chi Square |
F | |
| Age of Behavioral Assessment | |||||
| first | 9-9 (2.22) | 9-7 (2.12) | 9-9 (1.63) | 0.31 | |
| last | 18-1 (1.86) | 18-0 (2.37) | 18-9 (2.38) | 0.01 | |
| % Ethnic Minority | 20 | 22 | 21 | 0.06 | |
| % Male Participants | 90a | 82a | 54b | 14.90 | |
| Mother’s Education | |||||
| % high school or less | 14 | 18 | 25 | 1.57 | |
| % some college | 20a | 15a | 42b | 6.02 | |
| % college degree | 66a | 67a | 33b | 8.59 | |
| Nonverbal IQ | 53 (36.43)a | 85 (31.12)b | 74 (32.44)b | 11.56 | |
| Verbal IQ | 36 (26.72)a | 84 (34.84)b | 72 (32.05)b | 28.68 | |
| % Nonfebrile Seizures (ever) | 22 | 25 | 25 | 0.26 | |
| % Puberty Onset < Age 10 | 32 | 24 | 41 | 1.69 | |
| % Psychotropic Medication (ever) | 79a | 56b | 63ab | 5.52 | |
| Treatment Hrs. thru Age 5 | 741 (942.34)a | 475 (680.79)ab | 168 (149.00)b | 4.88 | |
The Broader Autism Spectrum Disorder (BASD) group includes those who did not meet strict criteria for an autism diagnosis.
The Nonspectrum group includes all of the DD children as well as children referred for possible autism who did not receive an ASD diagnosis.
Letter superscripts denote significant group differences (i.e., no shared letters across two groups indicates groups differences significant at p < .05).
Outcome variables
Table 2 highlights selected items from the Lethargy/Social Withdrawal, Hyperactivity, and Irritability subscales of the Aberrant Behavior Checklist. The two most frequently endorsed items at age 9 for each subscale by diagnosis provide descriptive information on the contents of the subscales as well as illustrate the types of items endorsed across diagnosis. Of particular note, the most frequently endorsed items on the Lethargy/Social Withdrawal subscale, regardless of diagnosis, were those capturing social withdrawal behaviors such as “prefers solitary activities,” “difficult to reach,” and “seeks isolation from others.” Items emphasizing a more physical dimension (e.g., listless, sluggish, inactive) were consistently among the least frequently endorsed and are not shown on Table 2.
Table 2.
Percentages of Most Frequently Endorsed ABC Items at Age 9 by Subscale and Diagnosis
| Subscale |
Autism (n=65) % |
BASD (n=27) % |
Nonspectrum (n=24) % |
|---|---|---|---|
| Lethargy/Social Withdrawal | |||
| Seeks isolation from others | 65a | 44ab | 371,2,3b |
| Prefers solitary activities | 742 a | 482 ab | 33b |
| Difficult to reach | 751 a | 37ab | 371,2,3b |
| Preoccupied, stares into space | 60a | 521 a | 371,2,3 a |
| Hyperactivity | |||
| Does not pay attention to instructions | 851 a | 56b | 622 b |
| Impulsive | 762 a | 771,2 a | 54b |
| Easily distractible | 74a | 771,2 a | 671 a |
| Irritability | |||
| Demands must be met now | 72a | 481 b | 50b |
| Temper tantrums | 771 a | 41b | 42b |
| Outbursts when not get own way | 752 a | 33ab | 581,2 a |
| Irritable and whiny | 61a | 442 a | 581,2 a |
Numeric superscripts indicate ranking of individual items by diagnosis. N=116
Letter superscripts denote significant group differences in proportions (i.e., no shared letters across two groups indicates chi square is significant at p < .05).
Finally, although there was a general trend for the Autism group to have more maladaptive behaviors on the selected items at age 9 compared with the other groups, there was substantial overlap among the diagnostic groups in the most frequently endorsed items. Subsequent analyses sought to uncover patterns of change over time for each subscale, including the role of various predictive factors.
Trajectory Analysis by Diagnosis
Differences by diagnosis
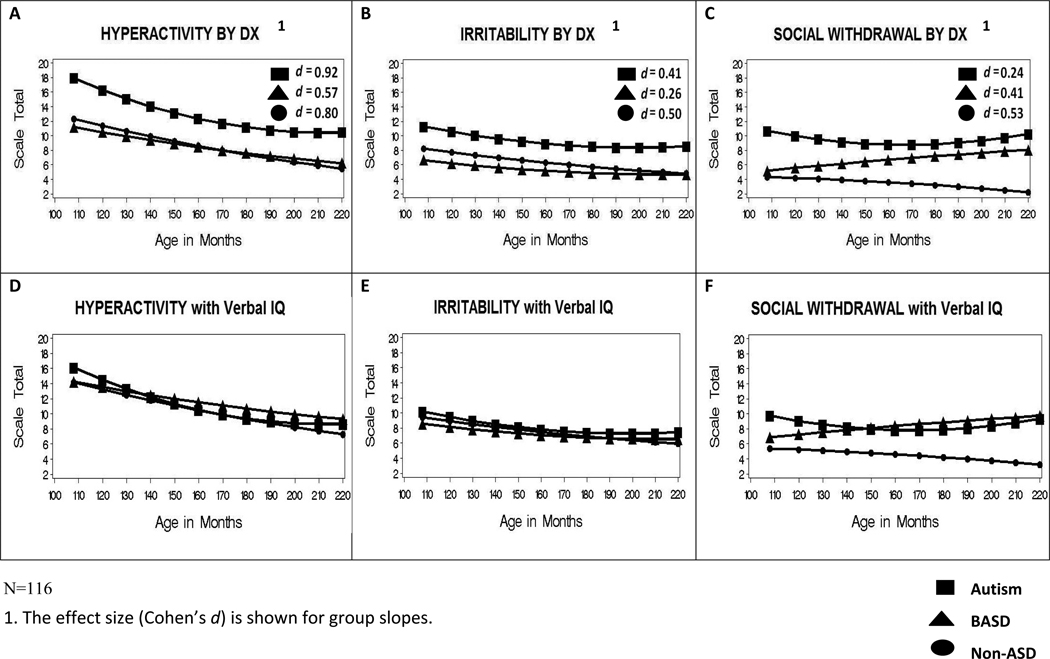
We examined growth trajectories of maladaptive behaviors over multiple time points between ages 9 and 18. Figures 1a through 1c graphically depict patterns of change across the three subscales by most recent diagnosis prior to adding any other predictors of outcome. Overall, the Hyperactivity subscale (Figure 1a) showed the greatest improvement over time; scores decreased for all three diagnostic groups (βAUT_quad_slope=.07, p < .001; βBASD_slope=−.04, p < .01; βNONASD_slope=−.06, p < .001). However, youths with Autism improved at a faster rate than the other two groups (i.e., the quadratic slope was significant) until approximately age 15, when scores began to level off. As expected, children with Autism had significantly higher Hyperactivity scores both at age 9 and 18 (β age 9=18.14; β age 18=10.69) than children with BASD (βage 9=11.17, p < .01; βage 18=6.24, p < .05) and Nonspectrum DDs (βage 9=12.31, p < .05; βage 18=5.48, p < .01). The 8-point drop in Hyperactivity scores from 9 to 18 for youths with Autism represents change with a large effect size. Medium and large effect sizes were observed for the slopes representing change over time in the BASD and Nonspectrum groups respectively.
Figure 1.
Patterns of Change in Maladaptive Behaviors (by Diagnostic Group)
Significant decreases in Irritability (Figure 1b) over time were also present for the Autism (βquad_slope=.04, p < .01) and Nonspectrum (βlinear_slope=−.03, p < .05) groups though changes were less dramatic than for Hyperactivity. Youths in the BASD group, whose scores were low at the outset, showed no significant change over time. The BASD and Nonspectrum groups did not differ from each other in their Hyperactivity and Irritability scores at various time points, nor in the rate or pattern of change.
Results for Social Withdrawal (Figure 1c) differed from the other two subscales. Perhaps most noteworthy, is the finding that difficulties with Social Withdrawal for those with BASD not only became increasingly elevated in comparison with their Nonspectrum counterparts but also with respect to those with Autism. Although there were no differences between the BASD and Nonspectrum groups at age 9, Social Withdrawal for the youths with BASD increased at a rate that was significantly different from “0” as they moved into adolescence (βslope=.02, p < .05). At the same time, the trajectory of this group differed significantly from the pattern of improvement over time for the Nonspectrum youths (βslope_difference=−.76, p < .05). Moreover, at age 9, BASD youths had lower scores on this subscale relative to the Autism group (βBASD=5.20, βAUT=10.85, p < .001) but, by age 18, scores for the two groups no longer differed significantly. As with the other two subscales, the quadratic effect for the Autism group was again significant (βquad_slope=.06, p < .001), showing an initial decrease in scores, with a trend for increasing Social Withdrawal after age 13. While the increase in Withdrawal during the teen years had a relatively small effect size overall for those with an Autism diagnosis, scores continued to worsen through age 18. Given the substantial differences between diagnostic groups in VIQ, the results displayed in Figures 1a–1c left unanswered the question of whether and to what extent similar patterns of change remain after taking verbal intellectual ability into consideration.
Accounting for IQ differences
Figures 1d–1f graphically depict patterns of change across the three subscales by most recent diagnosis after controlling for VIQ. In contrast to Figures 1a–1c, when the lower VIQs of the Autism group were taken into account, all group differences in Hyperactivity (Figure 1d) and Irritability (Figure 1e) at age 9 and 18 diminished to statistical nonsignificance. Otherwise, the rate and pattern of change (i.e., the linear and quadratic slopes) remained very similar for these two subscales. The quadratic effect for the Autism group remained, indicating a somewhat steeper decline in Irritability and Hyperactivity symptoms over time compared to the other two diagnostic groups.
In Figure 1f, differences in Social Withdrawal between those with Autism and BASD were further minimized when VIQ was controlled. Specifically, once the advantage of a higher group IQ was statistically removed for youths with BASD, scores for both groups converged at both age 9 and 18. At the same time, Social Withdrawal for the Autism (after age 13) and BASD groups continued to increase at a higher rate through the teenage years compared to youths with Nonspectrum DDs regardless of IQ (p < .01). Again, verbal IQ did not affect the rate or pattern of change.
Accounting for other covariates
Finally, Table 3 displays the final models including the effects of the various predictors for each subscale. An Autism diagnosis and lower VIQ consistently predicted more maladaptive behaviors of all types (as noted above). Likewise, youths with lower NVIQs generally had more maladaptive behaviors; however, this covariate was not included in the full models due to high colinearity with VIQ. Participants taking psychotropic prescription medications had higher levels of Irritability and Hyperactivity at age 9. Puberty onset was associated with higher levels of Irritability and with greater increases in Social Withdrawal/Lethargy at older ages. The effect of seizures on outcome was unique to the Social Withdrawal subscale. While seizure onset was associated with higher Social Withdrawal scores, the negative impact of seizures diminished with age.
Table 3.
Growth Models for Changes in Problem Behaviors from Age 9 to 18
| Predictors | Social Withdrawal | Irritability | Hyperactivity |
|---|---|---|---|
| Coefficient (S.E.) | Coefficient (S.E.) | Coefficient (S.E.) | |
| Fixed Effects | |||
| Intercept | 9.84 (.85) *** | 8.02 (1.33) *** | 13.51 (1.66) *** |
| Age of Behavioral Assessment | −0.10 (0.02) *** | −0.09 (0.02) *** | −0.15 (0.02) *** |
| Dx: | |||
| Autistic1 | ------ | ------ | ------ |
| BASD | −4.48 (1.65) ** | −2.37 (1.93) | −1.66 (2.47) |
| Nonspectrum | −4.85 (1.65) ** | −0.89 (1.95) | −1.88 (2.49) |
| Verbal IQ | −1.89 (0.58) ** | −2.13 (.66) ** | −3.39 (0.80) *** |
| Seizure Onset (Y/N)2 | 4.34 (1.07) *** | ------ | ------ |
| Pubertal Onset (Y/N)2 | −0.76 (0.81) | 1.44 (0.55) ** | ------ |
| Psychotropic Medication (Ever)2 | ------ | 2.82 (1.22) * | 3.79 (1.48) * |
| Linear Slopes: | |||
| Age*Autism1 | ------ | ------ | ------ |
| Age*BASD | 0.13 (0.03) *** | 0.05 (0.03) | 0.09 (0.03) ** |
| Age*Nonspectrum | 0.06 (0.03) | 0.03 (0.04) | 0.07 (0.04) |
| Age*seizure onset | −0.05 (0.01) *** | ------ | ------ |
| Age*pubertal onset | 0.04 (0.02) * | ------ | ------ |
| Quadratic Slopes:3 | |||
| Age2 | 0.06 (0.01) *** | 0.05 (0.01) *** | 0.07 (0.01) *** |
| Age2*Aut1 | ------ | ------ | ------ |
| Age2*BASD | −0.08 (0.01) *** | −0.03 (0.02) | −0.06 (0.02) * |
| Age2*Non | −0.07 (0.02) ** | −0.03 (0.02) | −0.06 (0.03) * |
| Variance | Variance | Variance | |
| Random Effects | |||
| Intercept | 24.73*** | 35.41*** | 71.16*** |
| Slope3 | 0.26*** | 0.26*** | 0.30*** |
N=116
p<.001,
p<.01,
p<.05
Dotted line indicates reference group.
Dotted line indicates parameter was omitted from the final model.
Slope parameters are multiplied by 100.
Gender, race, mother’s education, and hours of individual treatment were consistently non-significant predictors of outcome. No three-way interactions reached significance. Results were unaltered when extreme observations with studentized residuals larger than 3 were removed from the models. The analyses that follow examined variability and patterns of change when the data were not grouped a priori by diagnosis.
Post Hoc Trajectory Analysis
Patterns of change across subscales
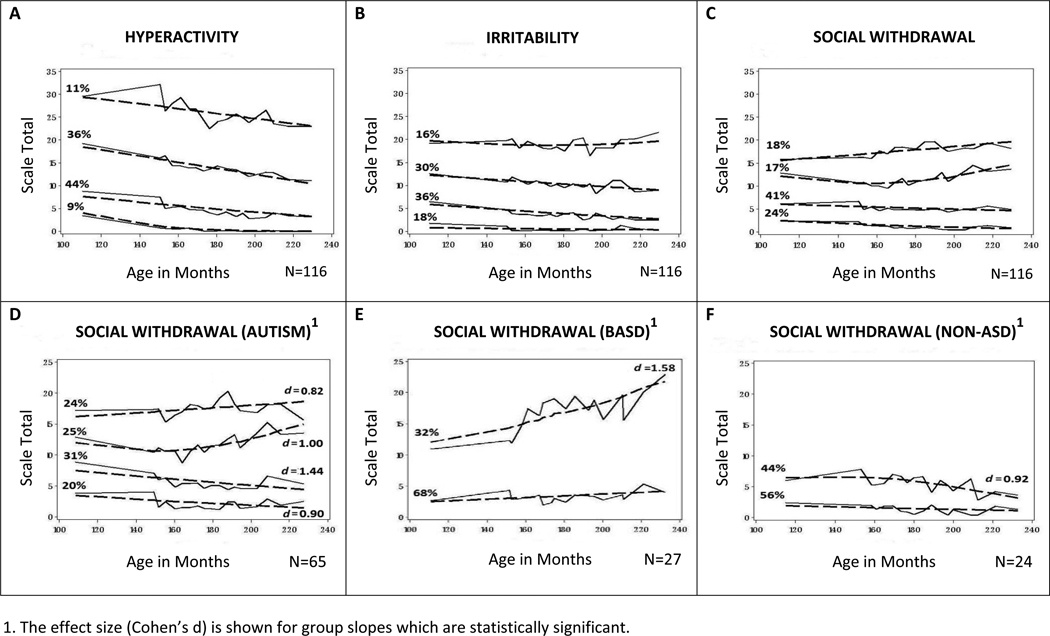
Growth trajectories in comorbid behaviors were further examined with mixture modeling using the PROC TRAJ procedure to discern subgroupings and patterns of change emerging naturally from the data over the nine year period. Visual support for the goodness of model fit is shown in Figures 2a–2c in which discrepancies between observed (solid) and expected (dotted) lines are small. The mean probability of an individual’s placement into assigned groups was high, ranging from .93 to .99 across subscales.
Figure 2.
Patterns of Change in Maladaptive Behaviors (by Trajectory Group)
Similar patterns of change occurred across time and subscale in the post hoc analyses as compared with the growth trajectories by diagnosis. Also apparent in all three subscales are the nonintersecting lines between higher and lower scoring groups. On average, children with the most severe maladaptive behaviors at 9 continued to have the highest scores at age 18. While this pattern was present when the sample was grouped by diagnosis in Figures 1a–1c, it was more pronounced in Figures 2a–2c when participants were grouped purely by trajectory. It underscores the importance of behavior in mid-childhood in predicting behavior in later teen years.
Relationship between post hoc groupings and diagnosis
Post hoc trajectory analyses provided additional information that is otherwise masked in the a priori groupings by diagnosis. (To avoid redundancy, an analysis of covariates is not reported here). While youths with Autism made up a disproportionate number of those assigned to one or both of the two highest scoring trajectory groups on each of the three subscales (p < .05 or less), Figures 2a–2c reveal considerable diversity within diagnostic groups. Depending on the subscale, more than one-third to approximately one-half of the Autism subsample was assigned to one of the two lower trajectory groups in Figures 2a–2c. For these youths, maladaptive behavior scores either decreased with time or remained consistently low over the nine-year period.
To better highlight diverse patterns of change in Social Withdrawal behaviors, Figures 2d–2f visually display the range of outcomes separately for each of the three diagnostic categories. For the Nonspectrum group, scores of the high and low trajectory groups began to converge with time (Figure 2f). Social Withdrawal either decreased (for those with initially higher scores) or remained consistently very low. In contrast, a clear bimodal distribution, fanning out over time, emerged for the Autism and BASD groups (Figures 2d and 2e). Those who were placed into the subgroups with increases in Social Withdrawal behaviors from age 9 to 18, constitute about one-third and one-half of the BASD and Autism groups respectively, while the remaining subgroups showed significant decreases (i.e., the bottom two Autism groups in Figure 2d) or maintained consistently low levels of comorbid behaviors over time (i.e., the lower trajectory group within BASD in Figure 2e). Notably, all significant changes in the slopes had a large effect size within each of the diagnostic groups.
Consistency of scores across subscales
Finally, there was a moderate to high association between individual scores across the three subscales. Correlations between scores across pairs of the three subscales for the entire sample ranged from .64 to .69 at age 9 (p < .001) and .43 to .73 at age 18 (p < .001). In Figures 2a–2c, almost a third of the Autism subsample (32%) was placed in one of the two trajectory groups with the most maladaptive behaviors across all three subscales, compared with 11% and 4% of the BASD and Nonspectrum groups respectively. More than half of the BASD and Nonspectrum groups (59% and 58% respectively) fell in either of the two trajectory groups with the fewest maladaptive behaviors across the three subscales, compared with 22% of those with Autism.
Post Hoc Follow Up on Social Withdrawal in ASD
The relationship between the ABC Social Withdrawal subscale and other conceptually-related measures administered at age 18 was examined primarily to provide more insight into the nature of social withdrawal in the current sample but also to confirm the validity of the construct as measured by the ABC. (A few individual items on the subscale, although not frequently observed in the current sample, relate more specifically to physical lethargy and inactivity).
Correlations between the ABC Social Withdrawal subscale and other measures administered at roughly the same time period were consistently moderate to high. We did not control for age or VIQ because these child characteristics were used in determining which measure to administer. A higher ABC Lethargy/Social Withdrawal score was related to greater social withdrawal (CBCL/ABCL Social Withdrawal subscale, r=.66, p < .001), general social problems (DBCL Social Relations subscale, r=.47, p < .01; CBCL/ABCL Friends subscale, r=−.61, p < .001), other internalizing behaviors, including depression (CBCL Internalizing, r=.49, p < .001; DBCL Depression subscale, r=.48, p < .01), and self-reports of greater loneliness by more able youths (Asher Loneliness Scale, r=.55, p < .01).
Discussion
In the current study, analyses of patterns of change by diagnosis extend our understanding of maladaptive behaviors in ASD and other neurodevelopmental delays, with a number of key findings. As expected, children with Autism consistently had more externalizing problem behaviors related to irritability and hyperactivity, as well as more social withdrawal at age 9, than those with BASD and Nonspectrum DDs. While the calls for special attention to the needs of individuals with ASD are sometimes questioned (Parsons, Lewis, & Ellins, 2009), these results support greater severity of maladaptive behaviors and, therefore, more need for treatment and support for youths with Autism. It is important to note, however, that, except for social withdrawal, the high rates of behavioral difficulty in Autism were primarily accounted for by severity of intellectual disabilities rather than by core ASD features. This is consistent with findings from other studies (Gillberg &Steffenberg, 1987).
In keeping with expectations for a general pattern of improvement in ASD over time in core symptoms (e.g., Seltzer et al., 2004) and general social and cognitive skills (Anderson et al, 2007; Anderson et al., 2009), the overall trend was one of decreasing irritability and hyperactivity problems with age, particularly for youths with an Autism diagnosis. At age 9, over one-third (36%) of our ASD sample would have met the lowest threshold on the Irritability subscale required for inclusion into various clinical trial studies targeting children with externalizing problem behaviors (minimum cutoff criteria ranged from 12 to 18; e.g., Aman et al., 2010; Arnold et al., 2006; Rezaei et al., 2010; Woodard et al., 2007). By age 18, approximately one-quarter (26%) would have met the same criteria.
Changes in social withdrawal over time by diagnosis followed a different pattern over time that was not related to IQ. Specifically, withdrawal actually increased with age for a substantial minority in both autism spectrum groups, while this trend was not observed for the Nonspectrum group. Moreover, the increase was greater for youths with broader ASD, despite a less severe presentation of core social symptoms, than for those with Autism. Although the pattern of increasing withdrawal in the present study may appear contrary to Lound-Taylor & Seltzer’s (2010) finding that internalizing symptoms decreased over time for high school-aged youths with ASD, results from the two studies are not directly comparable because the latter used a more general measure encompassing social withdrawal, repetitive behaviors, self-injury, and inattention.
In the present study, the 10-point increase in social withdrawal for one-third of the children with BASD was substantial enough to be of clinical concern. The increase over time was also substantial for one-half of the Autism group. Clinical trial studies of children with ASD have reported significant changes in Social Withdrawal by as little as 2.2 points (relative to controls; Arnold et al., 2006) and 4.4 points (change > 0; Posey et al., 2006). Problems with social withdrawal were predicted to be greater for youths with ASD compared to those with Nonspectrum DDs because these symptoms overlap with diagnostic criteria. However, the pattern of increasing withdrawal over time suggests the presence of other factors during adolescence which may exacerbate social difficulties associated with ASD.
Findings from various studies suggest that a number of vulnerability factors in adolescence may contribute to subsequent increases in withdrawal for children with ASD. In the present study, seizure onset predicted higher levels of social withdrawal, although the effects of seizures diminished with age. Onset of puberty was associated with greater increases in social withdrawal, especially at older ages, as well as more problems related to irritability regardless of diagnosis. For young people with ASD, physiological changes associated with puberty may be particularly detrimental when combined with increased social expectations in adolescence from adults and peers, and the greater awareness of one’s disability that often comes with age and/or higher cognitive abilities. Some research has shown that young people diagnosed with Aspergers who perceived themselves as more dissimilar to peers reported higher levels of depression (Butzer & Konstantareas, 2003; Hedley & Young, 2006). Other studies have documented greater loneliness in more able children with Autism compared to typically developing peers, despite a relatively high rate of social initiations (Bauminger, Shulman, & Agam, 2003).
Likewise, in the present sample, social withdrawal at age 18 for youths was associated with mood and social relationship problems such as greater self-reported feelings of loneliness in more cognitively able youths, more depressive symptoms in adolescents with lower IQs, and greater difficulties with friendships. The social demands of adolescence may be especially challenging for more able youths with ASD who may be more likely to find themselves among typically developing peers in and outside of the classroom setting. While increases in general social skills with age are observed for many youths with ASD, gains do not keep pace with those of typically developing children in most cases (Anderson et al., 2009; Klin et al., 2007). The increasing gap in the level of social skills attainment puts them at a further disadvantage as they approach puberty and later adolescence.
Despite worsening problems with social withdrawal for a substantial proportion of children with Autism and BASD in the current study, it is important to emphasize the diversity of outcome within our ASD sample. Compared with the Nonspectrum youths, children with ASD showed greater heterogeneity and range of trajectories for the various maladaptive behaviors. While an increase in social withdrawal over time was clear for a substantial proportion of children with ASD, in contrast, approximately one-half of the Autism and two-thirds of the BASD groups showed significant decreases or maintained consistently low levels of problems related to the same behaviors. Improvement and low scores over time for irritability and hyperactivity reached similar or higher proportions (respectively). A wide diversity of cognitive and adaptive behavior outcomes within ASD has been documented in previous analyses with the same sample (Anderson et al., 2007; Anderson et al., 2009).
There are a number of limitations and caveats to this study. Because all children in our study were referred for diagnosis under age 3, our findings may not be representative of children first diagnosed with ASD at later ages. Moreover, children in our sample, diagnosed 16 to 18 years ago, in all likelihood have more severe problems than children diagnosed with ASD today in part due to greater awareness and broadening of diagnostic criteria to include less severe presentation of symptoms in recent years (Fombonne, 2007). In addition, because attrition was greater in more socially disadvantaged families, the effects of demographic variables may have been underestimated.
Finally, our outcome measure was based solely on parental report. However, our confidence in the validity of our results was increased by the finding that parent reports of maladaptive behaviors related to other similar and conceptually relevant measures, including a youth self-report measure. In subsequent analyses with this sample, we will examine the extent to which parent reports of comorbid behaviors corroborate with those of teachers.
In summary, results are both encouraging in the extent of improvements and a reminder of the need for a continued focus on interventions aimed at facilitating social skills in ASD across development. Our findings support the need for attention to maladaptive behaviors starting in early childhood, given that those with the highest scores in mid-childhood, regardless of diagnosis, continued to have more maladaptive behaviors related to hyperactivity, irritability, and social withdrawal relative to their peers many years later.
The current study also highlights the need for services and interventions aimed at enhancing social functioning for adolescents and young adults with ASD who are reaching critical developmental milestones at the same time they remain socially disadvantaged relative to peers. While there are now many models of social skills specifically geared toward adolescents with ASD, outcome measures have not addressed issues of social withdrawal and other maladaptive behaviors, with a few notable exceptions (Horner et al., 2002; Koegel et al., 1992). Two recent papers (Kanne et al., in press; Klin et al., 2007) have highlighted that social deficits, as measured on diagnostic instruments such as the ADI-R and ADOS, and social adaptation in ASD are not the same. Social withdrawal, and, perhaps, loneliness, may be a third aspect of social functioning (Lord, 1993). Judging from the increase in social withdrawal from mid-childhood to young adulthood for a substantial proportion of participants in the present study, the need for continued services and support seems clear. Other research has noted unmet needs for young adults with ASD, particularly in social areas (Eaves & Ho, 2008). We hope to shed more light on this issue as our study participants age into their adult years.
Acknowledgments
We thank Kathy Welch for statistical consultation and Shanping Qiu for technical assistance with the data. Also, thanks to the research assistants Jessie Liang, Lindsay Harvey, and Jennifer Mason, who were available to help in many ways. Thank you, Mary Yonkovit, for your expert help with spelling checking and editing. Finally, we thank all of the study participants who made this research possible.
This work was supported by grants from the National Institutes of Mental Health (MH081873), the National Institute of Child Health and Human Development (U 19 HD 035482), and Autism Speaks (dated January 11th, 2008) to Dr. Lord.
| Full Grant Number Source of Funds |
Title of Project | Total Project Period |
Principal Investigator |
|---|---|---|---|
| NICHD U19 HD35482 | The Neurobiology and Genetics of Autism | 06/01/97–5/31/07 | Catherine Lord |
| Autism Speaks | Longitudinal Bridge Grant | 01/11/08–02/11/2008 | Catherine Lord |
| NIMH R01 MH081873-01A1 | Longitudinal Studies of Autism Spectrum Disorders: 2 to 23 | 09/01/08–05/31/13 | Catherine Lord |
Footnotes
Previous Presentations: Parts of this work were presented at the International Meeting for Autism Research (IMFAR) in Philadelphia, Pennsylvania in May, 2010.
Conflict of Interest: Although Dr. Lord receives royalties from the ADI-R and ADOS, no royalties were received for use of these instruments in this study.
References
- Achenbach T, Rescorla L. Manual for the Aseba forms and profiles. Burlington VT: University of Vermont Center for Children, Youth and Families; 2000. [Google Scholar]
- Aman M. Instruments for assessing treatment effects in developmentally disabled populations. Assessment in Rehabilitation and Exceptionality. 1994;1:1–20. [Google Scholar]
- Aman MG, Kasper W, Manos G, Mathew S, Marcus R, Owen R, Mankoski R. Line-item analysis of the Aberrant Behavior Checklist: Results from two studies of aripiprazole in the treatment of irritability associated with autistic disorder. Journal of Child and Adolescent Psychopharmacology. 2010;20(5):415–422. doi: 10.1089/cap.2009.0120. [DOI] [PubMed] [Google Scholar]
- Aman MG, Kristen S, Lam L, Collier-Crispin A. Prevalence and patterns of use of psychoactive medicines among individuals with autism in the Autism Society of Ohio. Journal of Autism and Developmental Disorders. 2003;33(5):527–534. doi: 10.1023/a:1025883612879. [DOI] [PubMed] [Google Scholar]
- Aman MG, Singh NN, Stewart AW, Field CJ. Psychometric characteristics of the Aberrant Behavior Checklist. American Journal of Mental Deficiency. 1985;89:492–502. [PubMed] [Google Scholar]
- Anderson DK, Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Patterns of growth in verbal abilities among children with autism spectrum disorder. Journal of Consulting and Clinical Psychology. 2007;75(4):594–604. doi: 10.1037/0022-006X.75.4.594. [DOI] [PubMed] [Google Scholar]
- Anderson DK, Oti RS, Lord C, Welch K. Patterns of growth in adaptive social abilities among children with autism spectrum disorders. Journal of Abnormal Child Psychology. 2009;37:1019–1034. doi: 10.1007/s10802-009-9326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansary NS. Distress and academic achievement among adolescents of affluence: A study of externalizing and internalizing problem behaviors and school performance. Development and Psychopathology. 2009;21(1):319–341. doi: 10.1017/S0954579409000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold LE, Aman MG, Cook AM, Witwer AN, Hall KL, Thompson S, Ramadan Y. Atomoxetine for hyperactivity in autism spectrum disorders: Placebo-controlled crossover pilot trial. American Adademy of Child and Adolescent Psychiatry. 2006;45(10):1196–1205. doi: 10.1097/01.chi.0000231976.28719.2a. [DOI] [PubMed] [Google Scholar]
- Asher SR, Hymel S, Renshaw PD. Loneliness in children. Child Development. 1984;55(4):1456–1464. [Google Scholar]
- Bauminger N, Shulman C, Agam G. The link between perceptions of self and of social relationships in high-functioning children with autism. Journal of Developmental and Physical Disabilities. 2004;16(2):193–214. [Google Scholar]
- Blacher J, McIntyre LL. Syndrome specificity and behavioural disorders in young adults with intellectual disability: Cultural differences in family impact. Journal of Intellectual Disability Research. 2006;50(3):184–198. doi: 10.1111/j.1365-2788.2005.00768.x. [DOI] [PubMed] [Google Scholar]
- Brown EC, Aman MG, Havercamp SM. Factor analysis and norms for parent ratings on the Aberrant Behavior Checklist-Community for young people in special education. Research in Developmental Disabilities. 2002;23(1):45–60. doi: 10.1016/s0891-4222(01)00091-9. [DOI] [PubMed] [Google Scholar]
- Butzer B, Konstantareas MM. Depression, temperament and their relationship to other characteristics in children with Asperger’s disorder. Journal on Developmental Disabilities. 2003;101:67–72. [Google Scholar]
- Capone GT, Grados MA, Kaufmann WE, Bernad-Ripoli S, Jewell A. Down syndrome and comorbid autism-spectrum disorder: Characterization using the Aberrant Behavior Checklist. American Journal of Medical Genetics. 2005;134(A):373–380. doi: 10.1002/ajmg.a.30622. [DOI] [PubMed] [Google Scholar]
- Chadwick O, Kusel Y, Cuddy M. Factors associated with the risk of behaviour problems in adolescents with severe intellectual disabilities. Journal of Intellectual Disability Research. 2008;52(10):864–876. doi: 10.1111/j.1365-2788.2008.01102.x. [DOI] [PubMed] [Google Scholar]
- Chadwick O, Piroth N, Walker J, Bernard S, Taylor E. Factors affecting the risk of behaviour problems in children with severe intellectual disability. Journal of Intellectual Disability Research. 2000;44(2):108–123. doi: 10.1046/j.1365-2788.2000.00255.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences, second edition. Hillsdale NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- deBildt A, Sytema S, Kraijer D, Sparrow S, Minderaa R. Adaptive functioning and behaviour problems in relation to level of education in children and adolescents with intellectual disability. Journal of Intellectual Disability Research. 2005;49(9):672–681. doi: 10.1111/j.1365-2788.2005.00711.x. [DOI] [PubMed] [Google Scholar]
- Eaves L, Ho H. Young adult outcome of autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38:739–747. doi: 10.1007/s10803-007-0441-x. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential Abilities Scale (DAS) San Antonio, TX: Psychological Corporation; 1990. [Google Scholar]
- Enfield SL, Tonge BJ. Manual for the Developmental Behaviour Checklist (DBC) 2nd ed. Clayton, Victoria: Centre for Developmental Psychiatry, Monish University; 2002. [Google Scholar]
- Estes AM, Dawson G, Sterling L, Munson J. Level of intellectual functioning predicts patterns of associated symptoms in school-age children with autism spectrum disorder. American Journal on Mental Retardation. 2007;112(6):439–449. doi: 10.1352/0895-8017(2007)112[439:LOIFPP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiological surveys of pervasive developmental disorders. In: Volkmar F, editor. Autism and Pervasive Developmental Disorders. New York: Cambridge University Press; 2007. pp. 33–68. [Google Scholar]
- Gadow KD, Devincent CJ, Pomeroy J, Azizian A. Comparison of DSM-IV symptoms in elementary school-age children with PDD versus clinical and community samples. Autism. 2005;9(4):392–415. doi: 10.1177/1362361305056079. [DOI] [PubMed] [Google Scholar]
- Galera C, Melchior M, Chastang JF, Bouvard MP, Fombonne E. Childhood and adolescent hyperactivity-inattention symptoms and academic achievement 8 years later: the GAZEL Youth study. Psychological Medicine. 2009;39:1895–1906. doi: 10.1017/S0033291709005510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber F, Baud M, Giroud G, Caminati G. Quality of life of adults with pervasive developmental disorders and intellectual disabilities. Journal of Autism and Developmental Disorders. 2008;38:1654–1665. doi: 10.1007/s10803-008-0547-9. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin M, Ghaziuddin N, Greden J. Depression in persons with autism: Implications for research and clinical care. Journal of Autism and Developmental Disorders. 2002;32(4):299–306. doi: 10.1023/a:1016330802348. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Steffenburg S. Outcome and prognostic factors in infantile autism and similar conditions: A population-based study of 46 cases followed through puberty. Journal of Autism and Developmental Disorders. 1987;17(2):273–287. doi: 10.1007/BF01495061. [DOI] [PubMed] [Google Scholar]
- Gillott A, Furniss F, Walter A. Anxiety in high-functioning children with autism. Autism. 2001;5:277–286. doi: 10.1177/1362361301005003005. [DOI] [PubMed] [Google Scholar]
- Hedley D, Young R. Social comparison processes and depressive symptoms in children and adolescents with Aspergers syndrome. Autism. 2006;10:139–153. doi: 10.1177/1362361306062020. [DOI] [PubMed] [Google Scholar]
- Horner R, Carr E, Strain P, Todd A, Reed H. Problem behavior interventions for young children with autism: A research synthesis. Journal of Autism and Developmental Disorders. 2002;32(5):423–446. doi: 10.1023/a:1020593922901. [DOI] [PubMed] [Google Scholar]
- Howlin P. The outcome in adult life for people with ASD. In: Volkmar F, editor. Autism and pervasive developmental disorders. Cambridge; NY: Cambridge University Press; 2007. pp. 269–306. [Google Scholar]
- Jones B, Nagin D, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29:374–393. [Google Scholar]
- Kanai C, Koyama T, Kato S, Miyamoto Y, Hirokazu O, Kurita H. Comparison of high-functioning atypical autism and childhood autism by Childhood Autism Rating Scale - Tokyo Version. Psychiatry and Clinical Neurosciences. 2004;58:217–221. doi: 10.1111/j.1440-1819.2003.01220.x. [DOI] [PubMed] [Google Scholar]
- Kanne S, Gerber A, Quirmbach L, Sparrow S, Cicchetti D, Saulnier C. The role of adaptive behavior in autism spectrum disorders: Implications for functional outcome. Journal of Autism and Developmental Disorders. doi: 10.1007/s10803-010-1126-4. (In press) [DOI] [PubMed] [Google Scholar]
- Kim JA, Szatmari P, Bryson SE, Streiner DL, Wilson FJ. The prevalence of anxiety and mood problems among children with autism and Asperger syndrome. Autism. 2000;42:117–132. [Google Scholar]
- Klin A, Saulnier C, Sparrow S, Cicchetti D, Volkmar F, Lord C. Social and communication abilities and disabilities in higher functioning individuals with autism spectrum disorders: The Vineland and ADOS. Journal of Autism and Developmental Disorders. 2007;37(4):748–759. doi: 10.1007/s10803-006-0229-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi R, Murata T, Yoshinaga K. A Follow-Up Study of 201 Children with Autism in Kyushu and Yamaguchi Areas, Japan. Journal of Autism and Developmental Disorders. 1992;22(3):395–411. doi: 10.1007/BF01048242. [DOI] [PubMed] [Google Scholar]
- Koegel L, Koegel R, Hurley C, Frea W. Improving social skills and disruptive behavior in children with autism through self-management. Journal of Applied Behavior Analysis. 1992;25(2):341–353. doi: 10.1901/jaba.1992.25-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn BR, Allen KD, Shriver MD. Behavioral management of children's seizure activity. Intervention guidelines for primary-care providers. Clinical Pediatrics. 1995;34(11):570–575. doi: 10.1177/000992289503401101. [DOI] [PubMed] [Google Scholar]
- Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: Relative prevalence, effects of subject characteristics, and empirical classification. Journal of Autism and Developmental Disorder. 2006;36:1101–1114. doi: 10.1007/s10803-006-0147-5. [DOI] [PubMed] [Google Scholar]
- Lord C. The complexity of social behavior in autism. In: Baron-Cohen S, Tager-Flusberg H, Cohen D, editors. Understanding other minds: Perspectives from autism. Oxford, U.K.: Oxford University Press; 1993. pp. 292–316. [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Archives of General Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook E, Leventhal B, DiLavore P, Rutter M. The ADOS-G (Autism Diagnostic Observation Schedule-Generic): A standard measure of social and communication deficits associated with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, LeCouteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lounds-Taylor J, Seltzer M. Changes in the autism behavioral phenotypeduring the transition to adulthood. Journal of Autism and Developmental Disorders. 2010;40:1431–1446. doi: 10.1007/s10803-010-1005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshburn EC, Aman MG. Factor validity and norms for the Aberrant Behavior checklist in a community sample of children with mental retardation. Journal of Autism and Developmental Disorders. 1992;22(3):357–373. doi: 10.1007/BF01048240. [DOI] [PubMed] [Google Scholar]
- Mesibov G, Shea V, Schopler E. The TEACCH approach to autism spectrum disorders. New York, NY: Spring-Science-Business Media; 2004. [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service Inc.; 1995. [Google Scholar]
- Ono Y. Assessment of behavior disorder for persons with mental retardation in residential facilities by Japanese version of the Aberrant Behavior Checklist. Japanese Journal on Developmental Disabilities. 1997;19:168–177. [Google Scholar]
- Parsons S, Lewis A, Ellins J. The views and experiences of parents and children with autistic spectrum disorder about educational provision: Comparisons of parents with children with other disabilities from an online survey. European Journal of Special Needs Education. 2009;24(1):37–58. [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Posey DJ, Wiegand RE, Wilkerson J, Maynard M, Stigler KA, McDougle CJ. Open-label atomoxetine for attention-deficit/hyperactivity disorder symptoms associated with high-functioning pervasive developmental disorders. Journal of Child and Adolescent Psychopharmacology. 2006;16(5):599–610. doi: 10.1089/cap.2006.16.599. [DOI] [PubMed] [Google Scholar]
- Reef J, Diamantopoulou S, van Neurs I, Verhulst F, Van der Ende J. Predicting adult emotional and behavioral problems from externalizing problem trajectories in a 24-year longitudinal study. European Child & Adolescent Psychiatry. 2010;19:577–585. doi: 10.1007/s00787-010-0088-6. [DOI] [PubMed] [Google Scholar]
- Rezaei V, Mohammadi MR, Ghanizadeh A, Sahraian A, Tabrizi M, Rezazadeh SA, Akhondzadeh A. Double-blind, placebo-controlled trial of risperidone plus topiramate in children with autistic disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:1269–1272. doi: 10.1016/j.pnpbp.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Rojahn J, Helsel WJ. The Aberrant Behavior Checklist with children and adolescents with dual diagnosis. Journal of Autism and Developmental Disorders. 1991;21:17–28. doi: 10.1007/BF02206994. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism, Research Reviews. Mental Retardation and Developmental Disabilities. 2004;10:234–247. doi: 10.1002/mrdd.20038. [DOI] [PubMed] [Google Scholar]
- Shattuck P, Seltzer MM, Greenberg JS, Ormond GI, Bolt D, Kring S, Lounds J, Lord C. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. Journal of Autism and Developmental Disorders. 2007;37:1735–1747. doi: 10.1007/s10803-006-0307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Spence SJ, Caplan R, Sullivan A, Qiu S, Gotham K, Lord C. The relationship of epilepsy and IQ in autism spectrum disorders and no spectrum developmental delay. 2005 Unpublished manuscript. [Google Scholar]
- Szatmari P, Bryson S, Duku E, Vaccarella L, Zwaigenbaum L, Bennett T, Boyle M. Similar developmental trajectories in autism and Asperger syndrome: From early childhood to adolescence. Journal of Child Psychology and Psychiatry. 2009;50(12):1459–1467. doi: 10.1111/j.1469-7610.2009.02123.x. [DOI] [PubMed] [Google Scholar]
- Tenneij NH, Koot HM. A preliminary investigation into the utility of the Adult Behavior Checklist in the assessment of psychopathology in people with low IQ. Journal of Applied Research in Intellectual Disabilities. 2007;20(5):391–400. [Google Scholar]
- Tonge BJ, Einfeld SL. Psychopathology and intellectual disability: The Australian child to adult longitudinal study. International Review of Research in Mental Retardation. 2003;26:61–91. [Google Scholar]
- Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children-3rd Edition. San Antonio, TX: Psychological Corp.; 1991. [Google Scholar]
- Woodard C, Groden J, Goodwin M, Bodfish J. A placebo double-blind pilot study of dextromethorphan for problematic behaviors in children with autism. Autism. 2007;11(1):29–41. doi: 10.1177/1362361307070989. [DOI] [PubMed] [Google Scholar]