Abstract
The thiol-ene reaction serves as a more oxygen tolerant alternative to traditional (meth)acrylate chemistry for forming photopolymerized networks with numerous desirable attributes including energy absorption, optical clarity, and reduced shrinkage stress. However, when utilizing commercially available monomers, many thiol-ene networks also exhibit decreases in properties such as glass transition temperature (Tg) and crosslink density. In this study, hybrid organic/inorganic thiol-ene resins incorporating silsesquioxane (SSQ) species into the photopolymerized networks were investigated as a route to improve these properties. Thiol- and ene-functionalized SSQs (SH-SSQ and allyl-SSQ, respectively) were synthesized via alkoxysilane hydrolysis/condensation chemistry, using a photopolymerizable monomer [either pentaerythriol tetrakis(3-mercaptopropionate) (PETMP) or 1,3,5-triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione (TATATO)] as the reaction solvent. The resulting SSQ-containing solutions (SSQ-PETMP and SSQ-TATATO) were characterized, and their incorporation into photopolymerized networks was evaluated.
Keywords: organic-inorganic hybrid, thiol-ene, silsesquioxane, methacrylate, photopolymerization
Introduction
Photopolymerization is employed industrially in applications ranging from dental restorative composites to polymer coatings, as a means to rapidly and efficiently convert liquid, multifunctional monomer resins to crosslinked polymer networks.1–3 Depending on the final application requirements, the ultimate properties of the resulting networks (e.g., degree of crosslinking, glass transition temperature, mechanical properties) vary significantly based on parameters such as the monomer type (including repeat unit structure and degree of functionality) and formulation as well as the incorporation of additives, including the commonly added inorganic fillers.
Multifunctional acrylate and methacrylate [i.e., (meth)acrylate] monomers have been the foundation for free-radically cured polymer resins used extensively over the last 30+ years.4 These classical (meth)acrylate photopolymerizations proceed rapidly by means of a chain-growth mechanism to form densely crosslinked networks.1, 4, 5 The use of light to induce this transformation affords several advantages, including spatial and temporal control of the polymerization process, as well as enabling the use of resin compositions that are solvent free. Despite their utility, however, these monomer systems still have significant limitations, such as oxygen inhibition when cured under ambient conditions1, 6 and challenges resulting from polymerization-induced volumetric shrinkage and stress.7, 8
An alternative approach for photopolymerization chemistry utilizes multifunctional thiol and ene monomers that form polymer networks through the step-growth, addition/chain transfer, radical-mediated “thiol-ene” click reaction.9 Also a light-mediated reaction, these thiol-ene radical polymerizations are characterized by several advantageous properties relative to traditional (meth)acrylate systems, including reduced oxygen inhibition, delayed gelation, reduced polymerization shrinkage stress and the formation of more homogeneous polymer network structures.10 Consequently, these thiol-ene materials provide an attractive alternative to (meth)acrylate resin systems and have been investigated in such areas as the production of tack-free polymer coatings and improved dental restoratives.10, 11 However, the polymerization of most commercially available thiol and ene monomer pairs yields networks with reduced mechanical properties (such as glass transition temperature (Tg) and crosslink density) as compared to the more common (meth)acrylate networks.
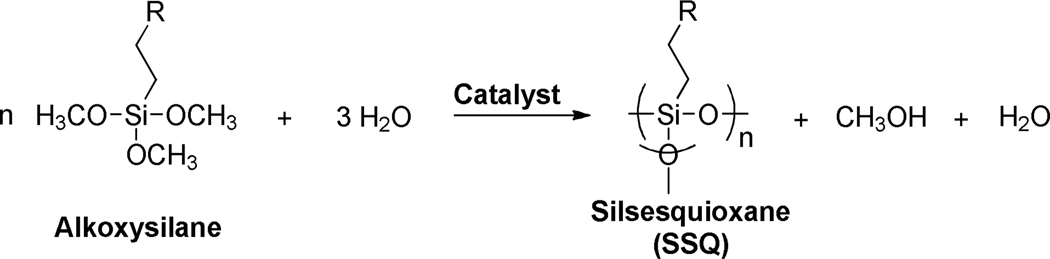
In this study, an approach to address thiol-ene material property reductions, through the preparation of silsesquioxane (SSQ)-containing hybrid organic/inorganic thiol-ene resins, was investigated. SSQs are silicon-based species composed of an RSiO1.5 repeat unit structure that is typically derived from either trichloro- or trialkoxysilane monomers (Scheme 1).12–15 These monomers contain a single organic substituent ‘R’ covalently attached to the silicon center, as well as the three chloro- or alkoxy- groups that are subsequently able to react, forming Si-O-Si backbones. Depending upon the synthetic conditions employed, condensed SSQ species with varying molecular weights (from small cyclic and oligomeric species to polymeric) can be prepared.16 In this manner, employing the condensation route with either a thiol or ene-functionalized trimethoxysilane as the starting monomer provides a facile route by which to obtain multifunctional thiol or ene species capable of subsequent incorporation into photopolymerized/crosslinked thiol-ene-based networks.
Scheme 1.
Hydrolysis/condensation “sol-gel” reaction of alkoxysilanes to form silsesquioxanes (SSQs).
Research in the field of “hybrid” materials (broadly defined) is expansive, with numerous studies investigating methods to synergistically combine the attributes of organic and inorganic materials to yield improvements in properties such as refractive index, thermal stability, or abrasion resistance.13 Particularly relevant as background for this work is previous research combining thiol-ene chemistry and silicon-containing materials. This includes reports in the mid-1990s by Rose17 and Moszner et al.18 detailing the use of the photoinitiated thiol-ene reaction as an alternative chemistry for curing neat silicone (polysiloxane) materials. More recently, work from Hoyle and colleagues19 investigated the incorporation of functionalized POSS (polyoligomeric silsesquioxanes) cages into thiol-ene networks. For thiol-ene resins containing up to 5 mol% vinyl-functionalized POSS, rapid photopolymerization kinetics were maintained and the resulting networks possessed the same Tg, improved scratch resistance, and decreased flame spread compared to a neat thiol-ene control.
Additional relevant work has been described by Sangermano and coworkers, who have published their investigations of hybrid thiol-ene-based systems. First, in an effort to increase mechanical and thermal network properties, Sangermano et al. incorporated from 5–20 wt% of thiol-functionalized zirconium oxoclusters into UV-cured trimethylolpropane-tris-(3-mercaptopropanoate):allyl pentaerythritol thiol:ene resins.20 The oxocluster-containing resins achieved rapid curing and high functional group conversions, with the resulting networks exhibiting up to a 10 °C increase in Tg (from −5 °C for the neat resin, up to 5 °C for the highest loading of clusters). In addition, a slight increase in crosslink density was obtained, along with enhancements in thermal stability and pencil hardness.20 Subsequently, Sangermano reported additional hybrid materials composed of trimethylolpropane-tris-(3-mercaptopropanoate): trimethylolpropane triallyl ether base resin, but this time incorporating a tetraethoxyorthosilicate (TEOS) sol-gel precursor21 and 3-mercaptopropyltrimethoxysilane coupling agent.22 Using a two-step cure procedure, the compositions were first photopolymerized to form the thiol-ene network and then placed in a humid, acidic, heated environment to enable the sol-gel condensation. The final dual-cured films were transparent, and for those containing up to 50% original TEOS, Tg’s were increased as much as 12 °C (from −42°C to −30°C). In addition, surface hardness values of the films also improved with increasing inorganic content.22
For the work investigated in this study, we focused particularly on the incorporation of thiol or ene-functionalized SSQ species as a means to form glassy (i.e., Tg’s above room temperature) organic/inorganic thiol-ene networks with significantly enhanced crosslink densities. In addition, to limit post-gelation volumetric changes in the network, which are known to be problematic for bulk light-cured materials, the hybrid SSQ species were pre-condensed (and volatile by-products removed) prior to incorporation into the thiol-ene resins. Specifically, the SSQ species were prepared by the hydrolysis/condensation reactions of either thiol- or ene-functionalized alkoxysilanes. To obtain the SSQs in a single synthetic step as well-dispersed species, their syntheses were carried out utilizing a compatible (i.e., miscible, with matched chemical functionality) photopolymerizable monomer [either pentaerythriol tetrakis(3-mercaptopropionate) (PETMP) or 1,3,5-triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione (TATATO)] as the reaction solvent. Employing alkoxysilane condensation chemistry provides a flexible synthetic avenue through which to prepare SSQs with an array of functionalities, sizes, and morphologies. For example, SSQ species ranging from branched, fractal polymeric morphologies to highly condensed particulate structures can be obtained from acid or base-catalyzed reactions, respectively.16 Additionally, numerous organo-functionalized alkoxysilanes are commercially available, which, by virtue of their substituents, can form condensed SSQ species with the organo-functionality present throughout. For instance, hydrolysis/condensation of 3-mercaptopropyltrimethoxysilane yields SSQs possessing the thiol functionality, whereas, utilizing an ene-functionalized alkoxysilane reactant yields the analogously-functionalized ene species. The presence of these reactive organo-functional groups throughout the SSQ species provides numerous reactive handles with which to covalently incorporate the SSQ components, thus forming a well-integrated, crosslinked network.
In this work, thiol- and ene-functionalized SSQs (i.e., SH-SSQ and allyl-SSQ) were first synthesized via alkoxysilane hydrolysis/condensation chemistry, with the appropriate photopolymerizable monomer (PETMP or TATATO, respectively) used as the reaction solvent. The resulting SSQ-containing solutions (SSQ-PETMP or SSQ-TATATO, respectively) were characterized by NMR spectroscopy and size exclusion chromatography. Finally, the effect of SSQ incorporation into light-cured thiol-ene-based resin systems, therein forming hybrid thiol-ene-based networks, was evaluated.
Experimental
Materials
Unless otherwise specified, all materials were used as received. Trimethoxysilanes, 3-mercaptopropyltrimethoxysilane (SHTMS) and allyl trimethoxysilane (allyl-TMS), were purchased from Gelest, Inc. Pentaerythriol tetrakis(3-mercaptopropionate) (PETMP) and 1,3,5-triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione (TATATO) were obtained from Evans Chemetics LP and Sartomer Company Inc., respectively. Irgacure 819 (phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide) was from Ciba Specialty Chemicals (Tarrytown, NY) and Q1301, an N-nitrosophenylhydroxyamine aluminum salt, was from Wako Pure Chemicals (Osaka, Japan). α,α-Dimethoxy-α-phenylacetophenone, Irgacure 651 initiator (I651), was obtained from BASF. Bisphenol A ethoxylate dimethacrylate (BisEMA) and triethylene glycol dimethacrylate (TEGDMA) were generously donated by Esstech Inc. (Essington, PA).
Instrumentation
1H and 29Si-gHMBC NMR spectra were obtained on either a Varian 500 MHz or Bruker 300 MHz spectrometer, and referenced using the solvent residual peak. A Nicolet Magna-IR infrared spectrometer, equipped with a custom-built horizontal sample chamber, was utilized for photopolymerization conversion measurements. A TA Instruments Q800 dynamic mechanical analyzer was used to determine elastic modulus and tan δ values. An EXFO Acticure lamp equipped with either 365 nm or 400–500 nm filter (as specified subsequently) and connected to an optical light guide, was used to deliver the curing light to the resin compositions. For the curing/preparation of all mechanical testing specimens (e.g. DMA, flexural properties) a collimating lens was placed at the end of the light guide to promote uniform irradiation across the sample surface. The curing light irradiation intensity was measured at the sample surface using an International Light IL1400A radiometer.
Synthesis of SSQ-PETMP (72:28 wt% SH-SSQ:PETMP composition)
PETMP (2.00 g, 4.10 × 10−3 mol) was added to a glass jar equipped with a magnetic stir bar. 3-mercaptopropyltrimethoxysilane (4.08 × 10−2 mol, 8.00 g) was then added and the resulting mixture was stirred until homogeneous. A stoichiometric amount of water (equal to the moles of methoxy groups from the SHTMS) as a 0.2 M dilute hydrochloric acid solution (2.20 g of solution; 1.22 × 10−1 mol of H2O) was added, resulting in a small exotherm. The reaction was left to stir at ambient temperature, with the mixture becoming increasingly cloudy as the reaction progressed. After stirring for 24 hours, volatile reaction by-products were removed by rigorously vacuum drying the mixture to a constant weight. This yielded the product, a thiol-functional SSQ/photopolymerizable monomer mixture (SH-SSQ:PETMP), as a clear, colorless viscous liquid (6.98 g, 97% yield neglecting endgroups).
Synthesis of SSQ-TATATO (70:30 wt% allyl-SSQ:TATATO composition)
The synthetic approach utilized to prepare SSQ-PETMP was also used for the synthesis of ene-functionalized SSQ species, except the starting materials and quantities were as follows: TATATO (2.00 g, 8.02 × 10−3 mol) and allyltrimethoxysilane (8.00 g, 4.93 × 10−2 mol) were stirred until homogeneously mixed, and then 0.2 M HCl solution (2.68 g) was subsequently added. Following reaction and drying, the colorless allyl-SSQ:TATATO liquid was obtained (6.34 g, 96% yield neglecting endgroups).
GPC Analysis of SH-SSQ:PETMP solutions
A Viscotek triple detector gel permeation chromatography (GPC) system, equipped with Viscotek VE 3580 refractive index detector and Viscotek 270 dual detector (containing 90° and 7° light scattering plus capillary bridge viscometer detectors) was used for characterization of the SH-SSQ:PETMP solutions. The mobile phase was HPLC grade tetrahydrofuran at a flow rate of 1 mL/min through three sequentially connected columns (Viscotek Viscogel GMHR-L, M, and N). Samples were prepared in HPLC grade THF to produce a ~5 g/L solution, where 10 µL of sample was injected for each analysis.
Resin formulation and photopolymerization
For the binary thiol-ene resin study, SSQ-PETMP solutions, containing up to 100 wt% SH-SSQ, were mixed with TATATO to form resins with a 1:1 thiol:ene functional group stoichiometry. These mixtures were compared to a neat PETMP:TATATO control, also with 1:1 thiol:ene stoichiometry. All resins contained 0.1 wt% α,α-dimethoxy-α-phenylacetophenone [Irgacure 651 (I651) initiator] and were cured with 1 mW/cm2 of 365 nm light. All compositions for the ternary resin study contained 0.3 wt% loading of Irgacure 819 (I819) initiator and 0.035 wt% of the aluminum tris-nitroso inhibitor, Q1301. The SSQ-containing ternary resins each consisted of 60:40 wt% BisEMA:thiol/ene content with a 2:1 molar ratio of thiol:ene functional groups. These were compared to a 60:40 wt% BisEMA:PETMP/TATATO ternary control and a 70:30 wt% BisEMA:TEGDMA dimethacrylate control. Polymerization conversion was monitored by Fourier-transform infrared spectroscopy (FTIR) in the Near-IR region. Specifically, the decrease in the peak area of the characteristic methacrylate and ene C-H stretches was followed as the polymerization progressed. Since the BisEMA/TEGDMA (peak max = 6167 cm−1) and TATATO (6133 cm−1) peaks were partially overlapped in the FTIR spectra of the ternary resin systems, they were deconvoluted by fitting to Gaussian curves using a Matlab-based procedure, enabling the area reduction of each resonance to be independently determined.
Dynamic mechanical analysis
Elastic modulus and tan δ measurements were made on rectangular samples prepared by injecting each resin composition into a 0.8 mm thick silicone rubber mold sandwiched between two glass slides. Binary thiol-ene resins were UV-cured (1 mW/cm2, 365 nm, 15 min), while resins for the ternary methacrylate:thiol-ene study were irradiated with visible light (100 mW/cm2, 400–500 nm, 600 sec.). The cured specimens were finished with 400 grit sandpaper, then placed between tension clamps in the DMA. Binary thiol-ene and ternary methacrylate:thiol-ene samples were subjected to a temperature ramp heating/cooling protocol (1 °C per minute) under an oscillatory strain of 1 Hz. Reported values were from the third and second heating scans for the binary thiol-ene and ternary methacrylate:thiol:ene samples, respectively.
Shrinkage stress measurements
Evaluation of polymerization-induced shrinkage stress was performed with a cantilever beam-based tensometer23 (American Dental Association Foundation) equipped with an aluminum bar. Cylindrical glass rods (6 mm diameter) were polished on both ends with a 180 grit sandpaper wheel, then one end of each bar (designated the resin contact end) was immersed in freshly prepared acidic Piranha solution. After rinsing with de-ionized water and wiping dry, each resin contact end was brushed with Fusion dental bonding solution to promote surface adhesion. Glass rods were then secured with collets to the tensometer base and aluminum bar, respectively, with treated rod ends facing each other, and a 1 mm gap in between. For each run, resin was injected into the gap between rods, and then cured with ca. 100 mW/cm2 of 400–500 nm filtered light from an EXFO Acticure lamp connected by fiber optic light guide to the opposite end of the bottom glass rod. As the polymerization proceeded, deflection of the aluminum beam was measured by a linear variable differential transformer (LVDT) and converted to a shrinkage stress value based on a prepared calibration curve. The resulting shrinkage stress values were monitored for 600 sec. of light exposure.
Results and Discussion
Synthesis and characterization of SSQ solutions
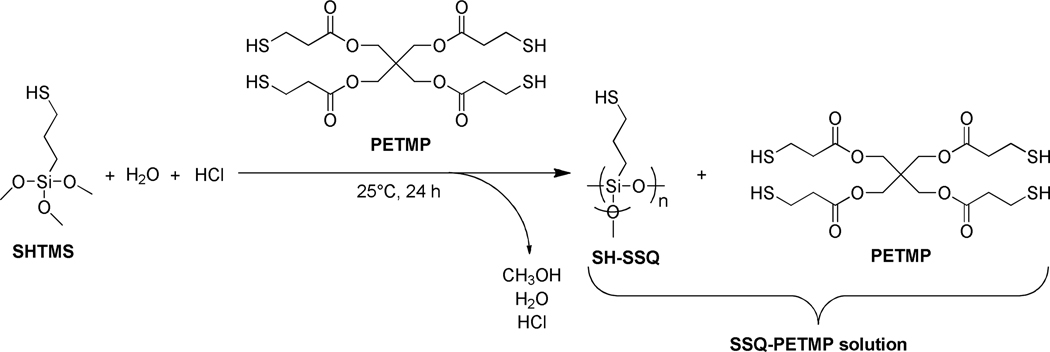
The SSQ species were prepared through the hydrolysis/condensation of thiol- or ene-functionalized trimethoxysilanes. The syntheses were carried out using a compatible photopolymerizable monomer (e.g., PETMP or TATATO) as the reaction solvent (Scheme 2). Performing the synthesis in this manner yielded, in one step and in a single reaction vessel, the SSQ species with controlled concentration as a solution in the corresponding monomer. This approach was envisioned as a means to obtain the SSQs as well-dispersed species, thereby minimizing any aggregation. Since the synthetic procedures and subsequent characterization were analogous for both thiol (SH-SSQ) and ene (allyl-SSQ) species, information only for the thiol-functionalized system is discussed in further detail.
Scheme 2.
Synthesis scheme for SSQ-PETMP (SH-SSQ in PETMP).
Specifically, 3-mercaptopropyltrimethoxysilane (SHTMS) was first mixed with PETMP, forming a miscible, colorless liquid. After a catalytic amount of aqueous hydrochloric acid was added to initiate the SHTMS reaction, the mixture became cloudy and white as the reaction proceeded. Following vacuum drying to remove the volatile byproducts, the final SSQ-PETMP solution (i.e., SH-SSQ + PETMP) was obtained as a clear, colorless, viscous liquid. Since no capping agents were added to any residual, uncondensed silanols in the synthesized SSQs, the potential for further condensation with time was possible. This reaction was evidenced by an increase in the SSQ solution viscosity with storage time. However, with rigorous vacuum drying post-synthesis, the SSQ solutions could be stored and utilized for several weeks without any significant viscosity increase.
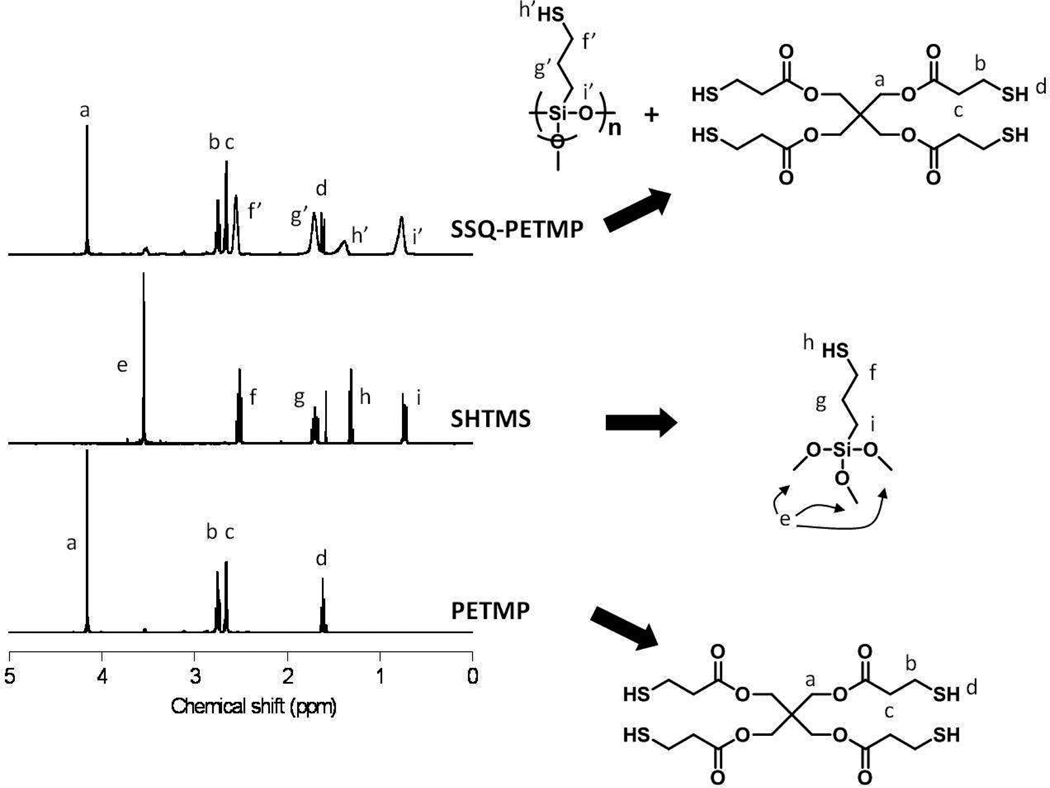
The 1H NMR spectrum of the SSQ-PETMP solution (Figure 1) showed that the PETMP resonances were unchanged during the synthesis, thus demonstrating the monomer’s stability to the alkoxysilane hydrolysis/condensation conditions. Furthermore, the remaining peaks in the product spectrum were indicative of successful SSQ formation. In particular, the decrease of the large CH3O- resonance at 3.6 ppm, which signified a high degree of SHTMS methoxy group hydrolysis, was confirmed. Additionally, the other peaks from SHTMS were still present, although broadened and less distinct, consistent with the formation of oligomeric SH-SSQ species through condensation reactions.
Figure 1.
1H NMR spectra of SSQ-PETMP with starting materials SHTMS and PETMP. The significant decrease of the 3.6 ppm resonance demonstrates the high degree of SHTMS hydrolysis, while broadening of the remaining resonances is consistent with SSQ formation.
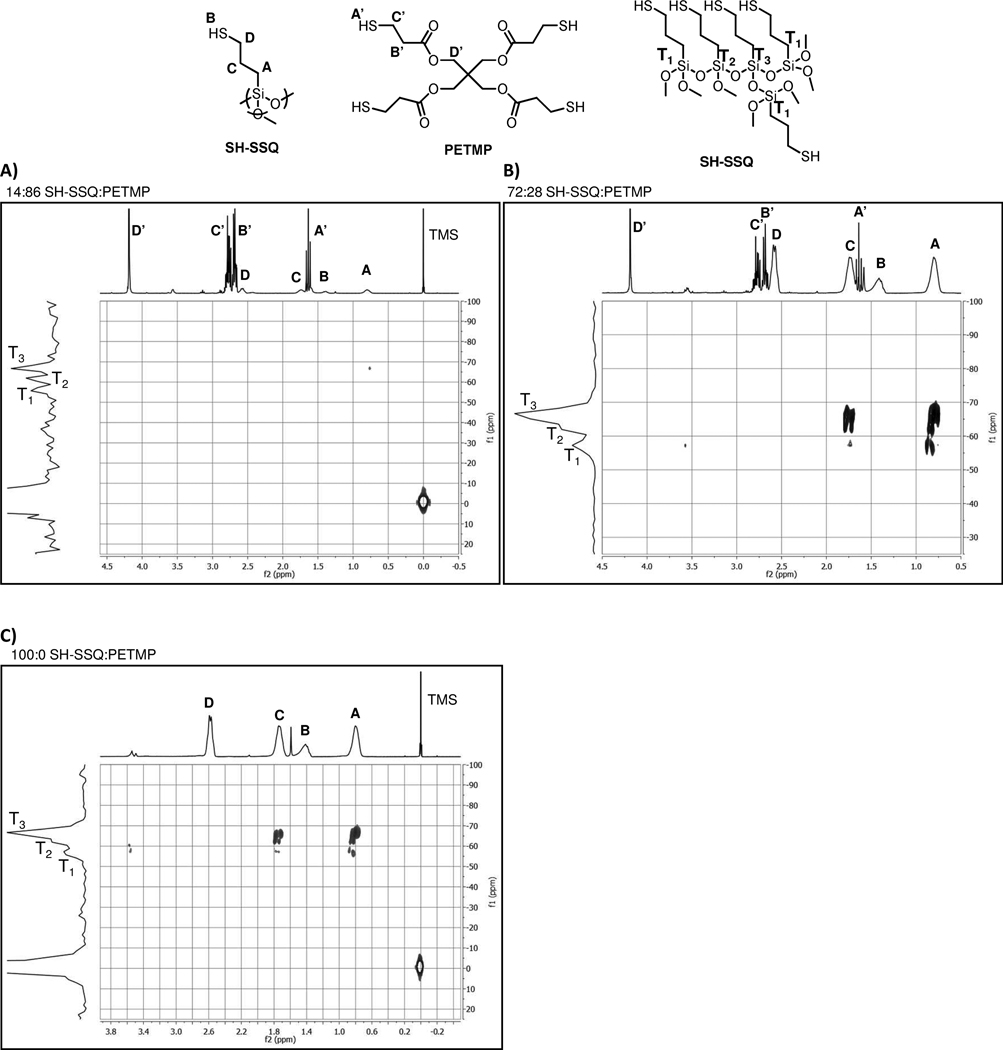
29Si-gHMBC NMR spectroscopy was also employed to characterize further the silicon-containing species in the SSQ-PETMP solutions. In particular, this technique provides details regarding substitution around the silicon centers (i.e., number of Si-O-Si bonds formed), as determined by indirect detection through hydrogens attached to adjacent carbons. As shown in Figure 2, the information for the low ratio SH-SSQ:PETMP solution is limited by the low intensity of the silicon signals. However, as the amount of SH-SSQ in the solutions increases, relative amounts of T1 (singly Si-O-Si reacted Si centers), T2 (doubly Si-O-Si reacted Si centers), and T3 (triply Si-O-Si reacted Si centers) are apparent in the 29Si-gHMBC spectra. In particular, at higher SH-SSQ loadings, the majority of silicons appear as condensed T2 or T3 centers.
Figure 2.
29Si-gHMBC spectra for SSQ-PETMP solutions: A) 14:86 wt% SH-SSQ:PETMP. B) 72:28 wt% SH-SSQ:PETMP. C) 100:0 wt% SH-SSQ:PETMP.
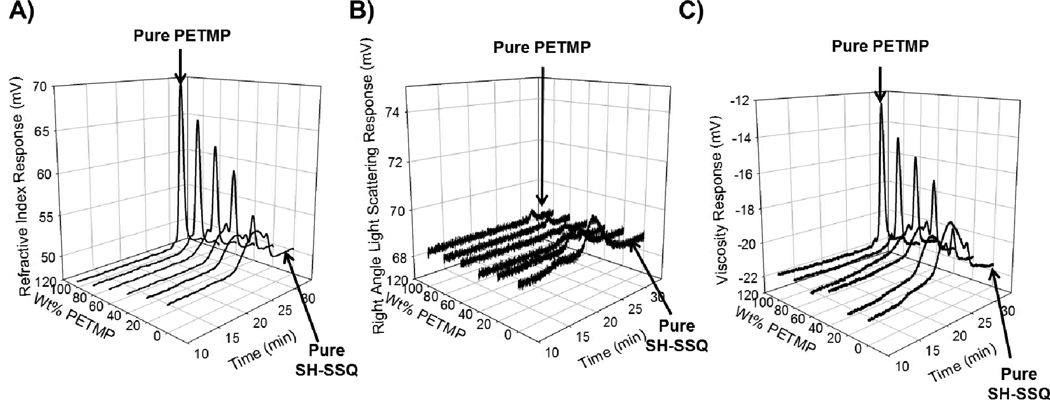
The six different solutions containing varying ratios of SH-SSQ:PETMP were also examined by GPC. Chromatograms of the different solutions were plotted using the three different detectors (Figure 3), and PETMP and SSQ were resolved. The retention time of PETMP was approximately 29 minutes shown by the peak appearing in the chromatograms of the sample containing 0:100 wt% SH-SSQ:PETMP, while the peak is not present in the chromatograms of the sample with 100:0 wt% SH-SSQ:PETMP. As shown from the chromatograms, a broad peak at higher molecular weight (lower elution volume) relative to PETMP is clearly evident for the sample containing SH-SSQ species, suggesting a polydisperse population; however, due to the limited resolution of the column separation, quantitative data for size and structure could not be obtained.
Figure 3.
GPC chromatograms of different ratios of SH-SSQ:PETMP solutions (0:100, 14:86, 30:70, 49:51, 72:28, 100:0): using: A) refractive index, B) 2-angle light scattering, and C) intrinsic viscosity detectors.
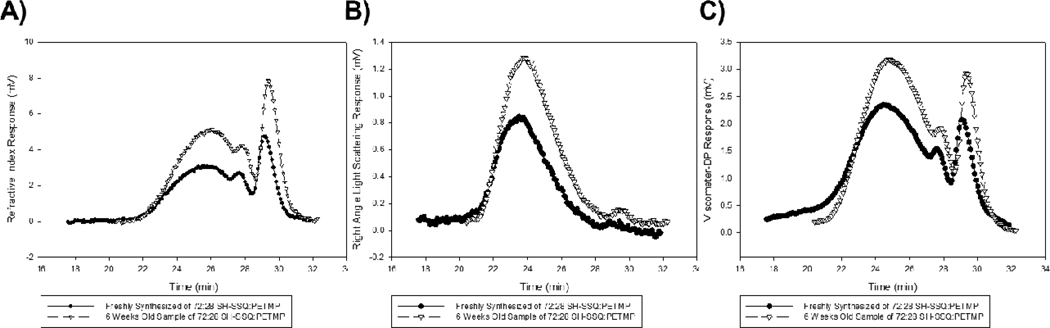
Since residual silanol (Si-OH) groups on the SSQ species were not capped post-synthesis, the potential existed for further condensation (resulting in increased molecular weight and viscosity) upon storage. However, with vigorous vacuum drying of the as-synthesized solutions to remove residual HCl and reaction byproducts, the resulting SSQ solutions exhibited stability for several weeks. GPC analysis of a freshly prepared sample of 72:28 wt% SH-SSQ:PETMP solution exhibited an identical chromatogram to a sample subsequently re-analyzed 6 weeks later. Thus, all SSQ solutions were used for formulation and experiments within that timeframe to ensure consistency.
Photocuring and property evaluation of SSQ-containing networks
SSQ-containing binary thiol-ene networks
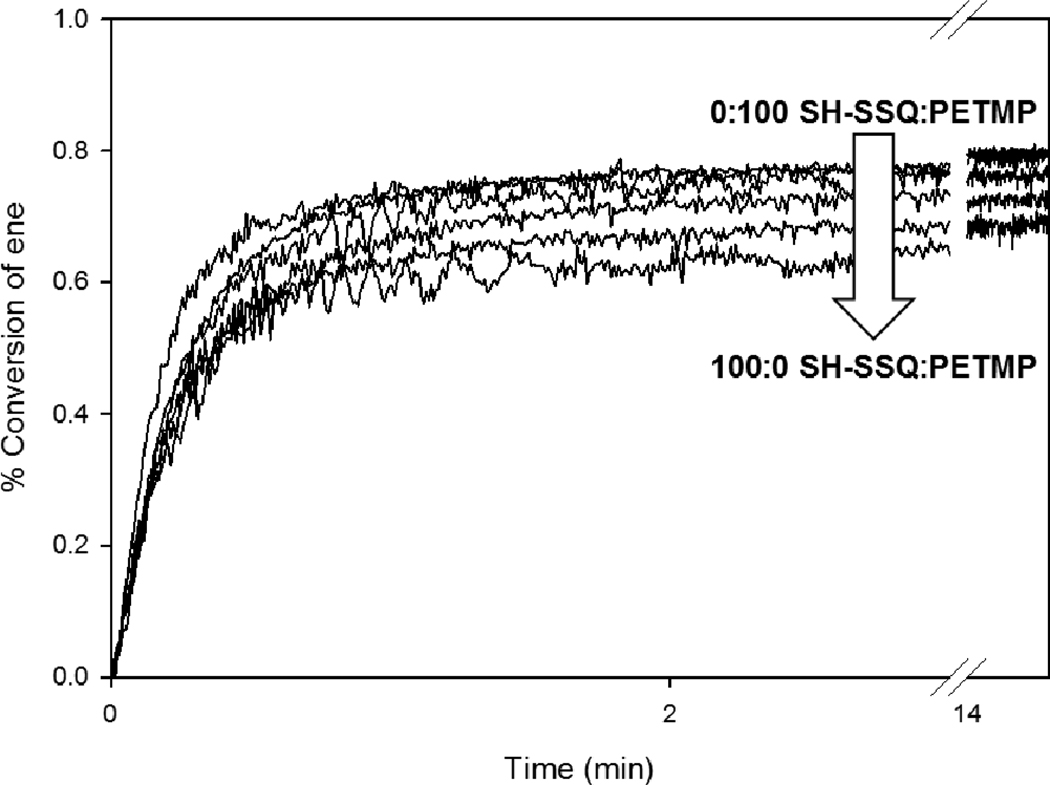
To examine the effect of incorporating SSQ species into binary thiol-ene photopolymerized networks, the previously discussed series of SSQ-PETMP solutions (containing 0–100 wt% SH-SSQ and prepared by varying the initial weight ratio of SHTMS:PETMP utilized in the synthesis) was formulated into polymerizable thiol-ene resins. To each SSQ-PETMP solution, a requisite amount of TATATO was added to obtain a 1:1 stoichiometric ratio of thiol:ene functional groups. Additionally, 0.1 wt% Irgacure 651 (I651) was added to each resin as a photoinitiator. This approach resulted in a series of formulated thiol-ene resins with SH-SSQ loadings varying from 0 wt% (i.e., the PETMP:TATATO control resin) to 61 wt% (in which case, 100% of thiol functionalities present were derived from SH-SSQ species). The resins were subsequently cured with 1 mW/cm2 of 365 nm light. As shown in Figure 5, the polymerization rates remained rapid for all compositions, even as the loading of SH-SSQ was increased. However, the ultimate final conversions of the resins decreased with increasing SH-SSQ content. For example, for the neat PETMP:TATATO control (0 wt% SH-SSQ), the final ene conversion was ca. 78%, whereas vitrification further limited the conversion of the 61 wt% SH-SSQ resin to ca. 67%.
Figure 5.
Photopolymerization kinetics, obtained from FT-IR spectroscopy, for hybrid thiol-ene networks. Solutions of SH-SSQ:PETMP (0:100, 14:86, 30:70, 49:51, 72:28, 100:0) were mixed with TATATO to produce a 1:1 molar ratio of thiol:ene, and 0.1 wt% Irgacure 651 was added. Samples were cured with an EXFO Acticure with 365 nm filter at 1 mW/cm2 intensity for 15 min.
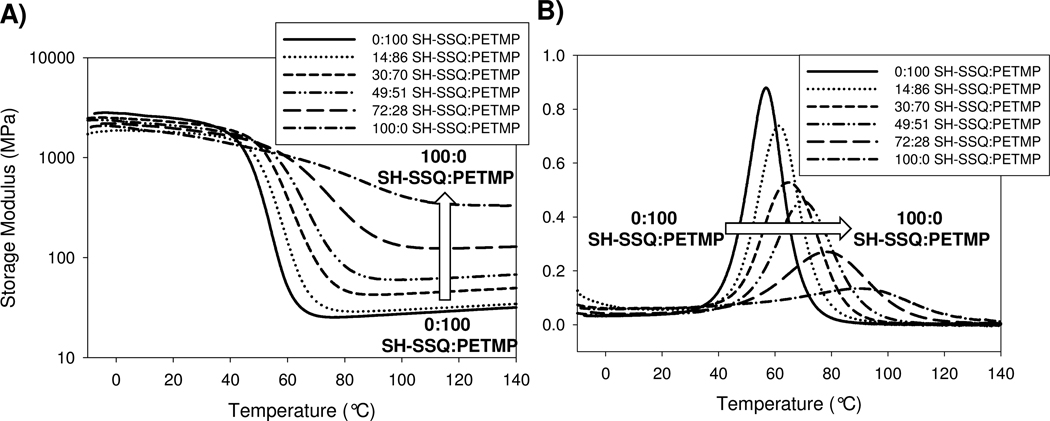
The network properties of the cured hybrid samples were also investigated by dynamic mechanical analysis (DMA), and results are shown in Figure 6. DMA testing demonstrated that significant increases in rubbery storage modulus (which correlates to crosslink density) were realized as the SH-SSQ loading increased (Figure 6A). Additionally, glass transition temperatures rose by as much as 30°C with high SH-SSQ loadings (Figure 6B). These results indicate that the SH-SSQ species function as multifunctional thiol reactants with an average thiol functionality greater than that of the tetrathiol, PETMP. This approach enables the preparation of networks with higher crosslink densities, which correspondingly restricts segmental mobility in the network, thus raising the Tg’s and improving the strength and modulus of the material. These results are supported by previously reported studies of epoxy-polyhedral oligomeric silsesquioxane nanocomposites, where higher loadings of amine increased the crosslink density observed by DMA.24
Figure 6.
Dynamic mechanical analysis data for hybrid binary thiol-ene networks: A) storage modulus and B) tan δ.
SSQ-containing ternary methacrylate:thiol-ene networks
Methacrylate:thiol-ene “ternary” resins have also been investigated as a route to combine the favorable mechanical properties of methacrylate-based systems with the reduced oxygen inhibition and improved shrinkage characteristics of thiol-ene polymerizations.25 Recent studies of the flexural and shrinkage stress properties of visible light-cured ethoxylated Bisphenol A dimethacrylate (BisEMA):PETMP/TATATO ternary mixtures revealed that the flexural properties were significantly reduced for resins with greater than 30 wt% thiol-ene content.26, 27 Consequently, the addition of SSQs to these ternary systems was investigated as a strategy to improve the network characteristics and mechanical properties when a large relative amount of thiol-ene is incorporated.
Specifically, 60:40 wt% BisEMA:PETMP/TATATO resins were modified by substituting either SSQ-PETMP for the PETMP component or SSQ-TATATO for the TATATO component. The SSQ-PETMP and SSQ-TATATO solutions utilized in the study were prepared with 80 wt% functionalized alkoxysilane (SHTMS or allylTMS) and 20 wt% monomer solvent (PETMP or TATATO) in the initial synthesis reaction. After alkoxysilane reaction and vacuum drying, final compositions of the SSQ solutions (assuming 100% condensation and neglecting endgroups) were 72:28 wt% SH-SSQ:PETMP (for SSQ-PETMP) and 70:30 wt% allyl-SSQ:TATATO (for SSQ-TATATO), owing to methoxy group mass loss from condensation. These SSQ solutions were subsequently used in the preparation of 60:40 wt% BisEMA:SSQ-PETMP/TATATO and 60:40 wt% BisEMA:PETMP/SSQ-TATATO resins. 70:30 wt% BisEMA:TEGDMA and neat 60:40 wt% BisEMA:PETMP/TATATO mixtures were also prepared as control systems. Complete resin compositions are shown in Table 1.
Table 1.
Resin compositions and flexural sample conversions for SSQ-containing methacrylate:thiol/ene resins study
| Composition (w/w)* | Conversion (%) Methacrylate† | Conversion (%) TATATO (SSQ-TATATO)† |
|---|---|---|
| 70:30 BisEMA:TEGDMA | 72 ± 3 | -- |
| 60:40 BisEMA:PETMP/TATATO‡ | 98 ± 1 | 75 ± 2 |
| 60:40 BisEMA:SSQ-PETMP/TATATO‡ | 95 ± 1 | 61 ± 2 |
| 60:40 BisEMA:PETMP/SSQ-TATATO ‡ | 99 ± 1 | (75 ± 2) |
with 0.3 wt% I819, 0.035 wt% Q1301
2:1 mol ratio of thiol:ene functional groups
For 2 × 2 × 25 mm flexural property samples: EXFO Acticure, 400–500 nm filter, collimating lens, 100 mW/cm2, 600 sec.
All resins, which contained 0.3 wt% Irgacure 819 (I819) initiator and 0.035 wt% Q1301 inhibitor, were cured under analogous conditions with visible light (100 mW/cm2, 400–500 nm, collimating lens, 600 sec.). The polymerization of DMA and flexural property specimens (2 × 2 × 25 mm samples) was monitored in the near-IR region during curing to determine the methacrylate and ene conversions. The BisEMA (peak max = 6167 cm−1) and TATATO (6133 cm−1) peaks, although partially overlapped, could be analyzed by fitting the convoluted peak to two Gaussian curves, and monitoring the decrease in their respective areas with time. For the BisEMA:PETMP/SSQ-TATATO mixture, although three different unsaturated functional groups (i.e., methacrylate of BisEMA, allyl of TATATO, and allyl of SSQ) were present, the signals for the TATATO and allyl-SSQ double bonds were coincident (at ~6125 cm−1), thus, only an overall SSQ-TATATO conversion could be obtained.
The final C=C conversion values are shown in Table 1. All three 60:40 wt% methacrylate:thiol/ene resins (both the neat control and the two SSQ-containing) showed high methacrylate conversions (>95%), which were significantly increased over that of 70:30 wt% BisEMA:TEGDMA (72 ± 3%). Among the ternary resins, the neat mixture and that containing the ene-functionalized SSQs achieved higher TATATO (or SSQ-TATATO) conversions (75 ± 2%) than the resin with thiol-functional SSQ (61 ± 2%).
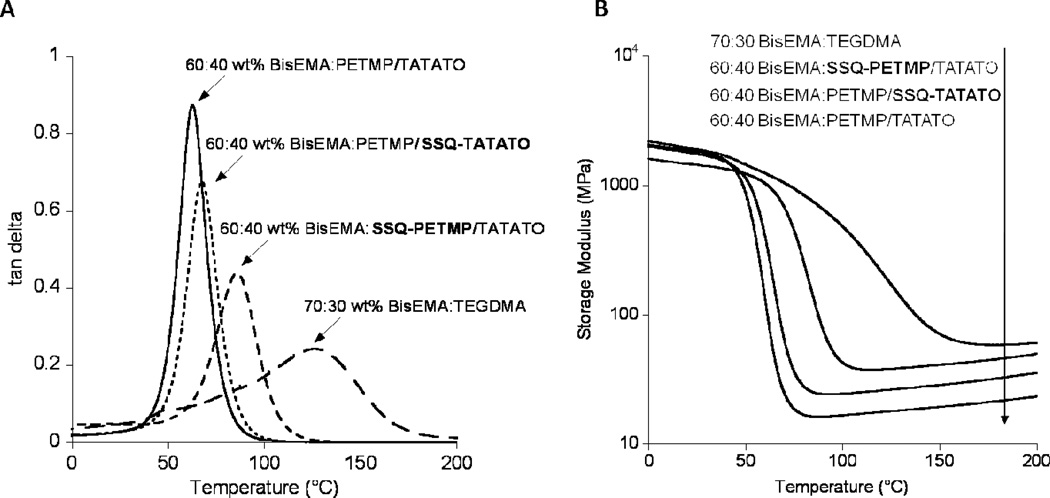
Using tan δ maximum values obtained from DMA temperature ramps (1 °C/min, second heating scan), glass transition temperatures (Tg’s) were determined for the cured resins. Consistent with the observations in the binary resin study, for both the SSQ-containing ternary resins, Tg’s were enhanced over that of the unmodified ternary BisEMA:PETMP/TATATO resin (69°C for BisEMA:SSQ-PETMP/TATATO, 56°C for BisEMA:PETMP/SSQ-TATATO, and 54°C for BisEMA:PETMP/TATATO (Figure 7)). The SSQ-PETMP resin, which contained a higher percentage of SSQ due to the 2:1 thiol:ene ratios used, demonstrated a larger relative Tg increase compared to the SSQ-TATATO resin. Although Tg values for the ternary resins were not as high as the BisEMA:TEGDMA resin (Tg = 120°C), the tan δ peak widths (FWHH) were much narrower, indicating the formation of significantly more homogeneous networks compared to the pure methacrylate resin.
Figure 7.
Dynamic mechanical analysis results illustrating increases in A) Tg (maximum in tan δ) and B) rubbery storage modulus (crosslink density) for photopolymerized SSQ-containing methacrylate-thiol-ene networks compared to neat methacrylate and methacrylate-thiol-ene networks.
By employing the theory of rubbery elasticity, the relative crosslink densities (XLDs) of the network compositions could also be determined from DMA measurements, by comparing their rubbery plateau storage modulus values.28 The BisEMA:PETMP/SSQ-TATATO composition possessed a significantly enhanced crosslinking density relative to BisEMA:PETMP/TATATO (Figure 7). Even higher still was the crosslink density of the BisEMA:SSQ-PETMP/TATATO composition, which approaches the high crosslink density value of the pure dimethacrylate formulation, i.e., 70:30 wt% BisEMA:TEGDMA.
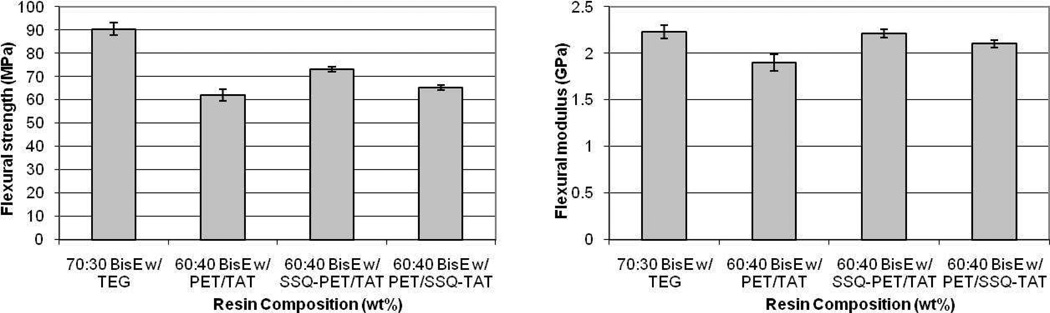
Subsequent flexural property testing of the cured resins revealed that SSQ-containing ternary resins exhibited modest, but statistically significant increases in flexural strength relative to the control ternary system. Although still possessing diminished flexural strength compared to the 70:30 wt% BisEMA:TEGDMA dimethacrylate control (Figure 8, at left), the 60:40 wt% BisEMA:SSQ-PETMP/TATATO resin exhibited a ca. 10 MPa flexural strength increase relative to neat 60:40 wt% BisEMA:PETMP/TATATO. Also improved over that of neat 60:40 wt% BisEMA:PETMP/TATATO were the flexural moduli of the ternary resins containing SSQ-TATATO and SSQ-PETMP. In fact, the 60:40 wt% BisEMA:SSQ-PETMP/TATATO resin possessed a flexural modulus equivalent to 70:30 wt% BisEMA:TEGDMA (Figure 8, at right).
Figure 8.
Flexural strength (at left) and modulus (at right) properties for 70:30 wt% BisEMA:TEGDMA and 60:40 wt% methacrylate:thiol-ene resins.
Shrinkage stress measurements were also carried out using a cantilever beam-based tensometer technique coupled with FTIR spectroscopic monitoring in the Near-IR region, to simultaneously capture both stress and reactive functional group conversion data for the curing resins.23 Shown in Table 2 are the average final shrinkage stresses and conversions obtained for triplicate runs of each composition (except 70:30 BisEMA:TEGDMA, for which only two runs were recorded). The 70:30 BisEMA:TEGDMA resin displayed the highest shrinkage stress (0.93 MPa), despite having the lowest final methacrylate conversion (68%). By comparison, shrinkage stresses for the ternary resins were 40–50% lower, with methacrylate conversions of 90% or higher and TATATO conversions ranging from 57–73%. Among the ternary resins, BisEMA:SSQ-PETMP/TATATO shrinkage stress (0.57 ± 0.02 MPa) was slightly higher than the other two ternary resins, which were 0.44 ± 0.02 MPa and 0.47 ± 0.01 MPa, respectively, for 60:40 BisEMA:PETMP/TATATO and 60:40 BisEMA:PETMP/SSQ-TATATO. Pairing the storage modulus results (Figure 7B) with the tensometer measurements (Table 2) reveals that the incorporation of SSQ species into ternary methacrylate:thiol-ene networks is an effective way to increase the ultimate crosslink densities while retaining favorable, decreased shrinkage stresses relative to pure methacrylate networks.
Table 2.
Shrinkage stress and C=C conversion values for tensometer experiments
| Resin Compositions (w/w)† | Shrinkage Stress (MPa)^ | Final Conversion (%) Methacrylate |
Final Conversion (%) TATATO (SSQ-TATATO) |
|---|---|---|---|
| 70:30 BisEMA:TEGDMA* | 0.93 (± 0.03*) | 68 ± 2 | -- |
| 60:40 BisEMA:PETMP/TATATO‡ | 0.44 ± 0.02 | 94 ± 1 | 71 ± 2 |
| 60:40 BisEMA:PETMP/SSQ-TATATO‡ | 0.47 ± 0.01 | 96 ± 1 | (73 ± 2) |
| 60:40 BisEMA:SSQ-PETMP/TATATO‡ | 0.57 ± 0.02 | 90 ± 1 | 57 ± 1 |
With 0.3 wt% Irgacure 819 initiator and 0.035 wt% Q1301 inhibitor
2:1 mol ratio of thiol:allyl functional groups
Curing conditions: EXFO Acticure, 400–500 nm filter, 33–35 mW/cm2, 600 sec.
Only 2 runs performed for this composition
Conclusions
The preparation of hybrid organic/inorganic networks, obtained by the incorporation of SSQ species into photopolymerizable thiol-ene based resins, was investigated. First, a straightforward synthetic scheme was developed to prepare both thiol- and ene-functionalized SSQ species from the hydrolysis/condensation of organo-functional trimethoxysilanes in the presence of photopolymerizable monomers. The resulting SSQ “solutions” were subsequently incorporated into both binary (thiol-ene) and ternary (methacrylate:thiol-ene) photopolymerizable resin compositions. Investigations of the cured network properties indicated that SSQ species function as highly multifunctional reactants, which increase the crosslink densities (and by extension, glass transition temperatures and moduli) of the final materials. Studies of the binary hybrid networks demonstrated as much as 30°C enhancements in glass transition temperature, accompanied by increases in rubbery storage modulus (i.e., the crosslink density) of up to nearly 10 times. Even in ternary methacrylate:thiol-ene resins, where SSQ loadings were below that of the binary resins, increases in Tg and XLD were still obtained. The effect was more pronounced for SSQ-PETMP-containing networks, likely because the species were both incorporated in higher loadings than the SSQ-TATATO solutions, as well as indicated to be more highly condensed (i.e., higher molecular weight, therefore a higher average number of functional groups per molecule) by size exclusion chromatography. Furthermore, these network property enhancements were obtained while retaining polymerization shrinkage stresses well below those of traditional methacrylate-based resins. Therefore, this strategy provides a route to improve the network characteristics of thiol-ene based photopolymers, without significantly compromising their favorable shrinkage stress characteristics.
Supplementary Material
Figure 4.
Comparison of chromatograms of 72:28 wt% SH-SSQ:PETMP solutions: freshly synthesized (—●—) and sample after 6 weeks (—▽—) as analyzed by A) refractive index, B) 2-angle light scattering, and C) intrinsic viscosity detectors. Signal baselines are normalized by baseline subtraction.
Acknowledgements
The authors thank Dr. Rich Shoemaker (UCB NMR lab), Dr. Christopher Kloxin, and Dr. Tai Yeon Lee for helpful insight and discussions. This work was partially funded by the I/UCRC Photopolymerization Center. Additional support was provided by NIH/NIDCR awards DE018233-0142 and F32DE019072. This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Institute of Dental & Craniofacial Research.
Footnotes
Supporting Information Available:
Comparison of 1H NMR spectra of samples prior to filtration and after filtration for GPC analysis. This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kloosterboer JG. Adv. Polym. Sci. 1988;84:1–61. [Google Scholar]
- 2.Decker C. Polymer International. 2002;51(11):1141–1150. [Google Scholar]
- 3.Decker C. Prog. Polym. Sci. 1996;21(4):593–650. [Google Scholar]
- 4.Decker C. Polymer International. 1998;45(2):133–141. [Google Scholar]
- 5.Cramer NB, Stansbury JW, Bowman CN. Journal of Dental Research. 2011;90(4):402–416. doi: 10.1177/0022034510381263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Brien AK, Bowman CN. Macromolecules. 2006;39(7):2501–2506. [Google Scholar]
- 7.Francis LF, McCormick AV, Vaessen DM, Payne JA. Journal of Materials Science. 2002;37(22):4717–4731. [Google Scholar]
- 8.Lu H, Stansbury JW, Bowman CN. Dent. Mater. 2004;20(10):979–986. doi: 10.1016/j.dental.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Hoyle CE, Bowman CN. Angewandte Chemie, International Edition. 2010;49(9):1540–1573. doi: 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- 10.Hoyle CE, Lee TY, Roper T. J. Polym. Sci., Part A: Polym. Chem. 2004;42(21):5301–5338. [Google Scholar]
- 11.Lu H, Carioscia JA, Stansbury JW, Bowman CN. Dent. Mater. 2005;21(12):1129–1136. doi: 10.1016/j.dental.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Baney RH, Itoh M, Sakakibara A, Suzuki T. Chem. Rev. 1995;95(5):1409–1430. [Google Scholar]
- 13.Kickelbick G. Hybrid Materials: Synthesis, Characterization, and Applications. Weinheim: Wiley-VCH; 2007. p. 498. [Google Scholar]
- 14.Cordes DB, Lickiss PD, Rataboul F. Chemical Reviews. 2010;110(4):2081–2173. doi: 10.1021/cr900201r. [DOI] [PubMed] [Google Scholar]
- 15.Kawakami Y, Kakihana Y, Miyazato A, Tateyama S, Hoque MA. Adv. Polym. Sci. 2010;235:185–228. (Silicon Polymers) [Google Scholar]
- 16.Brinker CJ, Scherer GW. Sol-gel science: The Physics and Chemistry of Sol-Gel Processing. San Diego: Academic Press, Inc.; 1990. p. 908. [Google Scholar]
- 17.Rose K. Photo-crosslinked polysiloxanes - properties and applications. In: Auner N, Weis J, editors. Organosilicon Chemistry II: From Molecules to Materials. Weinheim: Wiley-VCH; 1996. pp. 649–653. [Google Scholar]
- 18.Moszner N, Schoeb W, Rheinberger V. Polymer Bulletin (Berlin) 1996;37(3):289–295. [Google Scholar]
- 19.Clark TS, Hoyle CE, Nazarenko S. J. Coat. Technol. Res. 2008;5(3):345–351. [Google Scholar]
- 20.Sangermano M, Gross S, Priola A, Rizza G, Sada C. Macromol. Chem. Phys. 2007;208(23):2560–2568. [Google Scholar]
- 21.Iler RK. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry. New York: Wiley; 1979. p. 892. [Google Scholar]
- 22.Sangermano M, Colucci G, Fragale M, Rizza G. React. Funct. Polym. 2009;69(9):719–723. [Google Scholar]
- 23.Lu H, Stansbury JW, Dickens SH, Eichmiller FC, Bowman CN. Journal of Biomedical Materials Research, Part B: Applied Biomaterials. 2004;71B(1):206–213. doi: 10.1002/jbm.b.30088. [DOI] [PubMed] [Google Scholar]
- 24.Choi J, Kim SG, Laine RM. Macromolecules. 2004;37(1):99–109. [Google Scholar]
- 25.Lee TY, Carioscia J, Smith Z, Bowman CN. Macromolecules. 2007;40(5):1473–1479. [Google Scholar]
- 26.Cramer NB, Couch CL, Schreck KM, Boulden JE, Wydra R, Stansbury JW, Bowman CN. Dent. Mater. 2010;26(8):799–806. doi: 10.1016/j.dental.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cramer NB, Couch CL, Schreck KM, Carioscia JA, Boulden JE, Stansbury JW, Bowman CN. Dent. Mater. 2010;26(1):21–28. doi: 10.1016/j.dental.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairbanks BD, Scott TF, Kloxin CJ, Anseth KS, Bowman CN. Macromolecules. 2009;42(1):211–217. doi: 10.1021/ma801903w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.