Abstract
The present study demonstrates that serotonin (5-hydroxytryptamine, 5-HT)-containing axons project to two sets of neurons in the dorsolateral pons that have been implicated in salt appetite regulation. These two neuronal groups are the pre-locus coeruleus (pre-LC) and a region in the parabrachial nucleus termed the external lateral-inner subdivision (PBel-inner). Neurons in both regions constitutively express the transcription factor Forkhead protein2 (FoxP2), and become c-Fos activated after prolonged sodium depletion. They send extensive projections to the midbrain and forebrain, including a strong projection to the ventral tegmental area (VTA) – a reward processing site. The retrograde neuronal tracer cholera toxin β-subunit (CTb) was injected into the VTA region; this was done to label the cell bodies of the pre-LC and PBel-inner neurons. After one week, the rats were killed and their brainstems processed by a triple-color immunofluorescence procedure. The purpose was to determine whether the CTb-labeled pre-LC and PBel-inner neurons, which also had FoxP2 immunoreactive nuclei, received close contacts from 5-HT axons. Neurons with these properties were found in both sites. Since the origin of this 5-HT input was unknown, a second set of experiments was carried out in which CTb was injected into the pre-LC or lateral PB. One week later, the rats were perfused and the brainstems from these animals were analyzed for the presence of neurons that co-contained CTb and tryptophan hydroxylase (synthetic enzyme for 5-HT) immunoreactivity. Co-labeled neurons were found mainly in the area postrema and to a lesser degree, in the dorsal raphe nucleus. We propose that the 5-HT inputs to the pre-LC and PBel-inner may modulate the salt appetite-related functions that influence the reward system.
Keywords: area postrema, dorsal raphe nucleus, parabrachial nucleus, pre-locus coeruleus, salt appetite, ventral tegmental area
INTRODUCTION
Serotonin (5-hydroxytryptamine, 5-HT)-containing central neurons have been implicated in a wide variety of neurological functions including the regulation of sodium appetite (Reis, 2007). Drug-induced depletion of central serotonin stores induces an increase in sodium appetite (Lima et al., 2004). In addition, third ventricle injections of 5-HT pharmacological agents that activate 5-HT2B/2C and 5-HT3 receptors cause a decrease in sodium appetite in sodium-depleted rats, but this drug treatment does not affect salt intake in normal animals (Castro et al., 2003). These global experiments raise the possibility that 5-HT acts centrally to modulate sodium intake, but the site of action remains unknown.
The parabrachial nucleus (PB), a brainstem region implicated in a range of visceral functions, is one site where focal injections of 5-HT drugs affect salt appetite. For example, when the nonselective 5-HT1/2 antagonist methysergide is injected bilaterally into the lateral PB, rats increase their sodium intake (Menani and Johnson, 1995, Colombari et al., 1996, De Gobbi et al., 2000, Menani et al., 2000, Andrade-Franze et al., 2010, Davern and McKinley, 2010). Bilateral activation of 5-HT1A receptors in the PB increases sodium appetite (De Gobbi et al., 2005), yet a similar treatment with pharmacological agents that act on 5-HT2A and 5-HT2c receptors inhibits sodium ingestion (De Gobbi et al., 2007). Finally, Tanaka and co-workers (2004) measured the levels of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) in the PB following sodium intake and depletion. 5-HT and 5-HIAA levels increased following the saline intake, and both decreased after sodium depletion (Tanaka et al., 2004). Collectively, when one considers all of these data, a strong case can be developed to suggest that 5-HT has a modulatory function in the PB that affects salt appetite. These 5-HT agents also modulate other visceral functions including those that affect the cardiovascular system (Menani and Johnson, 1995).
The exact site(s) in the PB complex where these pharmacological agents act is unknown. There are two sites in the dorsolateral pons that become c-Fos activated after prolonged sodium depletion (Geerling and Loewy, 2007, Geerling et al., 2011). One is a subregion within the PB, which is termed the PB external lateral –inner division subdivision (PBel-inner) and the other is the nearby pre-locus coeruleus (pre-LC). Only about 55–65% of the neurons in these two respective sites become Fos-activated eight days after sodium deprivation (Geerling et al., 2011). The reason(s) for this remains unclear, but several possibilities may explain these findings. One of these is that the sodium depletion was incomplete, and perhaps it would require longer periods of sodium deprivation than one week to get maximal Fos labeling in these two cell groups. Another possibility is that other functional types of neurons exist within these two regions, besides those that become Fos-activated after sodium depletion. While we have assumed in this and previous studies (Geerling et al., 2011, Shin et al., 2011) that the majority of neurons in these two sites contribute to an ascending projection system that regulates salt appetite, some of these neurons may not be activated by sodium depletion but respond to other types of visceral afferent information. For example, Figure 3 in a paper published by Rocha and Herbert (1996) shows that c-Fos activated neurons are present in areas in the dorsolateral pons that appear to correspond to pre-LC and PBel-inner; these cells were activated after 60 min of hypotension (Rocha and Herbert, 1996), and thus, raise the possibility that these or a separate set of neurons respond to cardiovascular information.
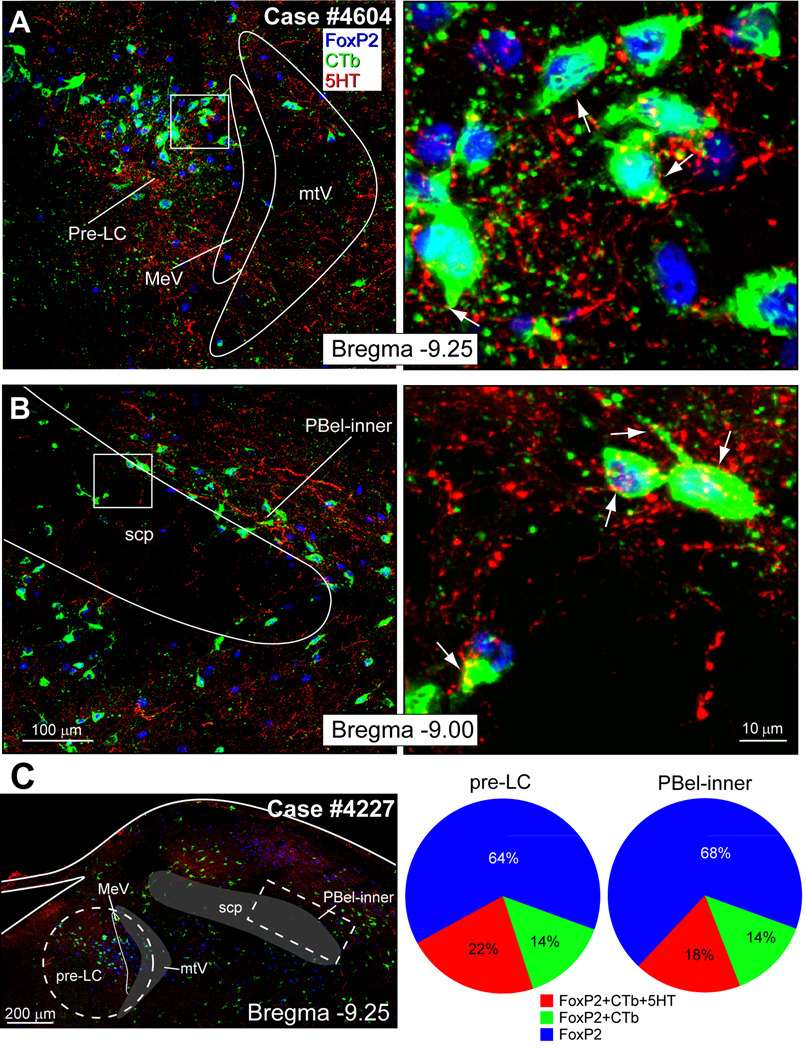
Fig. 3.
A. Confocal digital images of the pre-locus coeruleus showing that CTb-retrogradely labeled neurons (green), with FoxP2+ nuclei (blue), receive 5-HT (red or yellow) inputs. B. Confocal images of the parabrachial external lateral-inner division showing CTb-labeled neurons (green) near or embedded in the superior cerebellar peduncle (scp) that project to the VTA, and receive 5-HT close contacts (red or yellow). C. White dashed lines outline the areas in the pre-LC region (circle) and PBel-inner (rectangle) selected used for cell counts. The pie charts show that approximately 50% of the FoxP2+ neurons that project to the ventral tegmental area receive 5-HT close contacts.
Both the pre-LC and PBel-inner contribute to an afferent pathway that projects to numerous midbrain and forebrain sites (Shin et al., 2011). Neurons in both of these regions constitutively express the transcription factor Forkhead protein2 (FoxP2) (Geerling et al., 2011), and this property provides an unique cellular marker for the immunohistochemical localization of these two groups (Geerling et al., 2011). Even with the limitations described above, the analysis of the inputs and outputs of the FoxP2+ neurons in both of these cell groups represents a new approach for deciphering the connections of the PB region.
Few reports have been published describing the source(s) of the 5-HT input to the PB region, but one of them found that the 5-HT neurons of the area postrema project to the lateral PB (Lanca and van der Kooy, 1985). Surprisingly, little is known about the distribution or origin of the 5-HT inputs to the PB or the nearby pre-LC beyond the report by Steinbusch who analyzed the distribution of 5-HT fibers in the rat brain (Steinbusch, 1981). This issue is now important in light of the pharmacological data discussed above.
Because the 5-HT system has been implicated as a neurotransmitter in reward processing (Kranz et al., 2010), the present experiments were designed to selectively analyze two subsets of FoxP2+ neurons localized in the pre-LC and PBel-inner that project to the ventral tegmental area (VTA) – an area implicated as part of the reward system. Here we show that 5-HT axons make ‘close contacts’ with the pre-LC and PBel-inner neurons that project to the VTA. Then, we found two brainstem sites which contain 5-HT neurons that project to these dorsolateral pontine areas; one originates from the area postrema (AP) and the other arises from the dorsal raphe nucleus (DR).
EXPERIMENTAL PROCEDURES
All animal procedures were approved by the Washington University School of Medicine Animal Care Committee, conformed to NIH guidelines, and were performed on Sprague-Dawley rats (male and female; 250–350g; Charles River Labs, Wilmington, MA) under sodium pentobarbital (50 mg/kg, intraperitoneal injections) anesthesia. At the termination of each experiment, anesthetized rats were killed by transcardiac perfusion with 200 ml of saline, followed by 500 ml of 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH = 7.4). Brains were removed and stored in this fixative for 1–2 weeks.
Double-color immunofluorescence histochemistry: Distribution of 5-HT axons in relationship to the FoxP2 neurons in the pre-LC and PB
Brainstems from three normal female rats (250–300g) were cut in the transverse plane at 50 µm on a freezing microtome and the sections were stored as a 1-in-5 series in 0.1 M sodium phosphate buffer (pH = 7.4) containing 0.1% sodium azide. Sections through the PB region were processed by a double-color immunofluorescence method for 5-HT and FoxP2. Sections were incubated overnight in a solution containing rabbit anti-5-HT (1:1,000; S-5545; Sigma, St. Louis, MO) and sheep anti-FoxP2 (1:5,000; AF5647; R&D Systems, Minneapolis, MN). The antibodies were added to a solution containing in 0.1 M sodium phosphate buffer (pH = 7.4), 5% donkey serum, and 0.3% Triton X-100 (Sigma). This solution was used in all the subsequent primary and secondary antibody solutions described in this report. The sections were washed in potassium phosphate buffered saline (KPBS; 0.01 M; pH = 7.4) and transferred a solution of secondary antibodies Cy3-donkey anti-rabbit (1:500; Jackson ImmunoResearch, West Grove, PA) and Cy2-donkey anti-sheep (1:200; Jackson), washed in KPBS (2×), mounted on glass slides, and coverslipped using a fade-retardant glycerol mount solution containing sodium azide and n-propyl gallate.
The polyclonal 5-HT antibody used here was raised in rabbit against 5-HT creatinine sulfate conjugated to bovine serum albumin, and was found to be optimal for immunostaing of 5-HT axons. The polyclonal sheep antibody to FoxP2 has been used in previous publications from our laboratory (Stein and Loewy, 2010, Geerling et al., 2011).
CTb injections in the ventral tegmental area
Anesthetized rats (n=43) were placed in a stereotaxic apparatus, their skull was leveled, and then, a dorsal craniotomy was performed. Using an operating microscope, glass micropipettes (tip ≈ 25 µm) were backfilled under direct visualization with a 0.1% CTb solution made in distilled water (Product # 104; List Biological, Campbell, CA). The pipettes were mounted on a micromanipulator and advanced into the VTA using stereotaxic coordinates taken from a rat brain atlas (Paxinos and Watson, 2005). The coordinates were: bregma = −4.1 mm, lateral = 1.1 mm and deep = 7.2 mm.
CTb was iontophoresed for 30 minutes using 7 µA on/off positive pulses delivered from a Midguard precision current source (Stoelting, Wood Dale, IL). The pipette was left in place for 5 minutes and then removed. The wound was closed in layers. The rats were allowed to survive for one week and were re-anesthetized, killed by vascular perfusion, and their brains were removed and stored in fixative for one week.
Transverse frozen sections (50 µm) were cut through the region of the CTb injection site. A 1-in-5 series midbrain sections were incubated for 16 h in a goat polyclonal antiserum directed against CTb (1:25,000; Product #703; List Biologicals, Campbell, CA), washed in KPBS, and colorized by the ABC-DAB method (Vectastain kit PK-4000, Vector Labs, Burlingame, CA), mounted on glass slides, air-dried, counter-stained with 0.1% thionin, and coverslipped.
CTb + FoxP2 double-immunofluorescence method for the analysis of the injection sites
Once the CTb injection sites had been prepared by the DAB and examined, the key cases in which the injection had been confined to either the pre-LC or PBel-inner were immunostained by a method allowing for the visualization of CTb and FoxP2+.
Another group of sections from the 1-in-5 series through the injection site was incubated in a solution containing rabbit anti-FoxP2 (1:10K, Abcam) and goat anti-CTb (1:25K; List) overnight, then washed in KPBS, and transferred to a solution of Cy2-donkey anti-rabbit (1:500; Jackson) and Cy3-donkey anti-goat (1:500; Jackson) for 2 hours, washed, mounted on glass slides, air-dried, and coverslipped. Separate images for the CTb injection site (green) and the FoxP2+ neurons (red) were taken with a Nikon fluorescence microscope. The images were superimposed in Abode Photoshop and these images were redrawn and converted into line drawings using Abode Illustrator in order to establish whether CTb had spread into the region containing the FoxP2+ neurons.
Triple-color immunofluorescence histochemistry: immunostaining of 5-HT axons in relationship to the CTb-labeled FoxP2 neurons
In those cases that had a CTb injection in the VTA, an additional series of 1-in-5 sections from the pontomedullary level of the brainstem were processed by triple-color immunohistochemical procedure that was designed to show CTb, FoxP2, and 5-HT (Krout et al., 2003). This triple-color method was originally developed by Ferri and co-workers (Ferri et al., 1999).
Free-floating sections were incubated for 16h at room temperature in a mixture of two antibodies: mouse monoclonal anti-5-HT (1:4,000; PH8 monoclonal antibody; MAB #5278; Chemicon/Millipore, Temecula, CA) and sheep anti-FoxP2 (1:5000; AF5647; R&D Systems, Minneapolis, MN). (Note: We refer to the 5-HT cell body staining obtained with this monoclonal antibody as “5-HT” immunostaining, but this antibody is directed against tryptophan hydroxylase, the enzyme which synthesizes 5-HT. None of the catecholamine-containing brainstem neurons were immunostained with this antibody.) The sections were washed in KPBS, followed by a free avidin solution (5mg/50ml KPBS; A-9275; Sigma) for 40 min; KPBS rinse, 10% formaldehyde in KPBS for 40 min, KPBS rinse, put in a free biotin solution (1 mg/50ml KPBS; B-4501, Sigma), and then into biotinylated donkey anti-rabbit (1:250; Jackson) for 3 hours, KPBS, followed by ABC complex for 1 hour. After this, the sections were briefly washed in KPBS, placed in a Cy3-strepavidin solution (1:250, Jackson) for 2 hours, KPBS, and then, transferred to Cy5-donkey anti-sheep (1:500, Jackson) for 2 hours. Next, they were washed and subsequently transferred to a solution containing chicken anti-CTb (1:2000; 16 h; Abcam, Cambridge, MA), and then, we treated the sections with the sequence of avidin-biotin blocking steps described above, and finally, the sections were moved into a biotinylated donkey anti-chicken solution (1:250, Jackson), followed by 1 hour in ABC, washed, and then Alexa 488-strepavidin (1:250, Invitrogen, Carlsbad, CA) for 3 hours, washed, mounted, dried, and coverslipped with a non-fade glycerol mountant.
Quantitative analysis of 5-HT fibers contacting the FoxP2+ pre-LC and PBel-inner projection neurons
A quantitative analysis of the pre-LC and PB regions was made (Fig. 3E). Three types of samples were made: the number of neurons that were FoxP2+ only, double-labeled (viz., FoxP2+ and CTb+ only), or triple-labeled (FoxP2+ and CTb+ with 5-HT close contacts). Cell counts were made on two out of the three consecutive sections from the 1-in-5 series through the optimal parts of the pre-LC and PBel-inner. For PBel-inner cell counts, the first two rostral sections were used; the two caudal sections were used for the cell counts of the pre-LC. Thus, the middle section was counted for both samples.
The two sections containing the pre-LC or PBel-inner were imaged using an Olympus Fluoview FV500b laser-scanning confocal microscope. The sections were spaced 200 µm apart, and z-stack images acquired. These were montaged and the regions of interest were analyzed using MetaMorph software (Molecular Devices, Sunnyvale, CA). As shown in figure 3E, a region of interest was created that encompassed either the pre-LC or PBel-inner. In the case of the pre-LC, a circle with a diameter of 500 µm was positioned over the montage that included lateral part of the periventricular gray matter with its outermost edge going through the mesencephalic tract of the trigeminal nerve. FoxP2+ neurons are concentrated mainly in the pre-LC proper, but some extended laterally into the mesencephalic nucleus of the trigeminal nerve and its tract. For the analysis of the PBel-inner, a rectangle measuring 450 µm × 200 µm was overlaid on the montage, and it was aligned parallel to the ventrolateral border of the superior cerebellar peduncle. Separate counts for the three types of cells were made; these included single labeled FoxP2+ neurons, co-labeled (FoxP2+ and CTb+) neurons, and triple-labeled (FoxP2+, CTb+, and 5HT axons) neurons using the ‘Manually Count Objects’ tool in the MetaMorph program. Systematic counts for each section through the complete z-stack were made, and each type of neuron was identified and then, recorded in a spreadsheet. The mean and standard error of the mean were calculated.
Origin of the 5HT neurons that project to the pre-LC and PBel-inner regions
In order to determine the origin of the 5-HT inputs to pre-LC and PBel-inner, a series of CTb injections were made as described above, except the injections were made over a 15 min period. For the pre-LC experiments (n=75), the following coordinates were used: interaural = −0.23 mm; lateral = 1.00 mm; and deep = 5.50 mm. For the PBel-inner experiments (n=30), the following coordinates were used: bregma = −9.2 mm; lateral = 2.2 mm; deep = 5.50 mm. The rats were allowed to survive 5–7 days, anesthetized, perfused, and their brains stored in fixative for one week before histochemical processing. Sections through the injection sites were immunostained as described above. Then, in the successful cases (pre-LC cases = 4; PBel cases = 3), a complete 1-in-5 series section through the brainstem was prepared for a double-color immunohistochemical method for visualization of 5-HT and CTb.
Transverse sections of the brainstem were cut on a freezing microtome at a thickness of 50 µm and stored in buffer as a 1-in-5 series. At a later date, one series of sections was incubated overnight at room temperature in a mixture of two antibodies: mouse monoclonal anti-tryptophan hydroxylase (1:4000, Chemicon/Millipore) and rabbit anti-CTb (1:25K; B65927R; Biodesign, Saco, Maine). The sections were washed in KPBS, and transferred to biotinylated donkey anti-mouse (1:500: Jackson) for 3 hours, washed in KPBS, reacted with Cy3-streptavidin (1:500, Jackson) for 3 hours, washed again, and placed in Cy2 donkey anti-rabbit (1:250, Jackson) for 3 hours, washed, mounted glass slides, air-dried, and coverslipped with non-fade glycerol mountant.
Digital Images
Brightfield images were taken on a Nikon microscope using CCD camera and Nikon ACT-1 software (v2.62). Image cropping, resizing and adjustments in brightness, contrast, sharpness, and color balance were performed using Adobe Photoshop CS3.
Confocal imaging was performed with an Olympus Fluoview FV500b laser-scanning microscope. Images were acquired as multiple individual stacks using either 20× (NA 1.17) or 40× (NA 1.35) oil objective lens. For the 20× and 40× oil lenses, images were taken in steps of 0.621 or 0.311 µm, respectively, through the full thickness of the 50 µm transverse tissue section. All images were taken at resolution of 1024 × 1024 pixels or 2048 × 2048 pixels; one pixel in the X–Y plane was the minimum unit of resolution which covered an area roughly 0.6 × 0.6 µm. The z-frames for the images were collapsed into a two-dimensional, maximum-projection image to produce individual tiles. Finally, individual two-dimensional projection images were aligned and photomontages were constructed using Adobe Photoshop, with adjustments in brightness and contrast as necessary.
All manipulations of confocal stacks, z-frame projections, and pseudocoloration were performed using MetaMorph software (Molecular Devices, Sunnyvale, CA, USA). Uniform brightness and contrast adjustments were performed in Photoshop. Line drawings of the cytoarchitectonic boundaries were added to all the photomicrographs in Adobe Illustrator.
Terminology
The term ‘close contact’ has been used in this paper to describe 5-HT labeled boutons (~1–3 µm in diameter) abutting on cell bodies or dendrites of CTb retrogradely labeled neurons. These regions were visualized in one or more z-planes and no unlabeled pixels were found between them. Nevertheless, it is important to note that a given contact seen with the confocal microscope is not necessarily a synapse, and documentation that a particular ‘close contact’ is a synapse requires electron microscopic evidence. Thus, the findings presented in this report need to be confirmed in the future at the ultrastructural level.
RESULTS
Distribution of 5-HT axons in relationship to the FoxP2 neurons in the pre-LC and PB
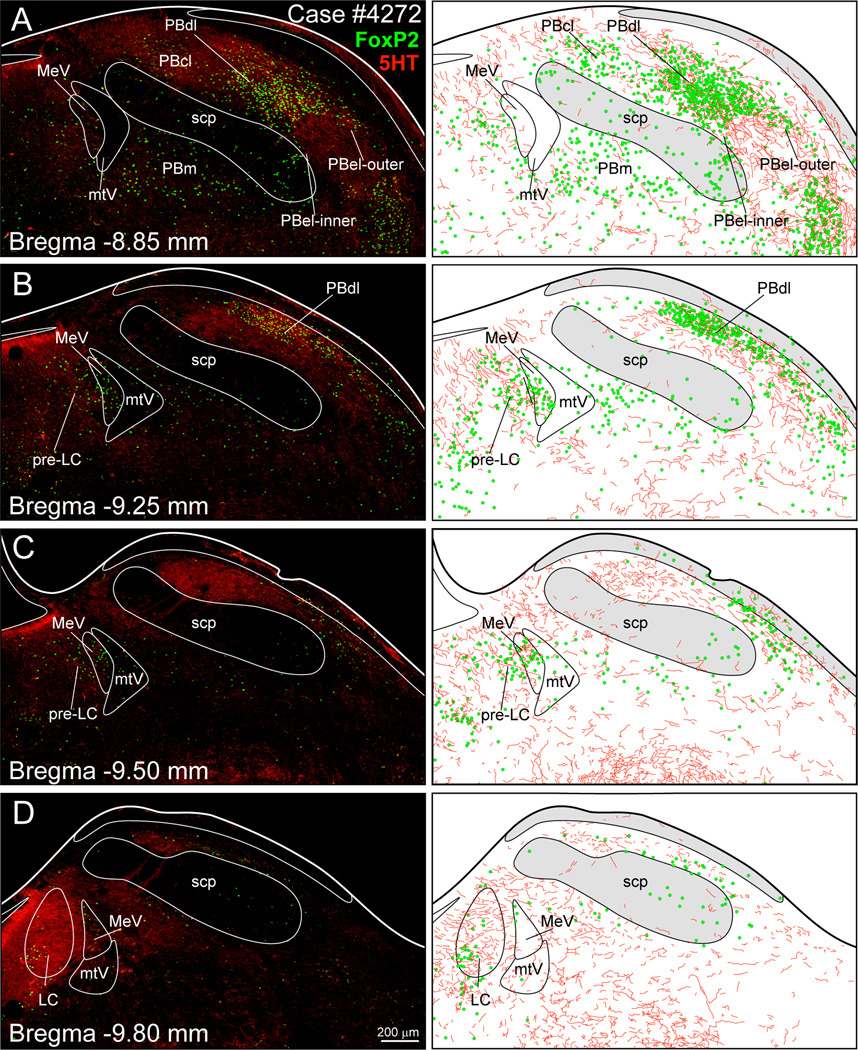
Figure 1 presents a series of digital images and the accompanying line drawings to show the distribution of 5-HT axons in the PB and pre-LC in relationship to the FoxP2+ neurons. The axons were widely distributed throughout rostral-caudal levels of the PB. The density of the 5-HT fibers was the greatest in the locus coeruleus (Fig. 1D), with the lateral PB containing a substantial concentration of 5-HT axons, especially in the PB dorsal lateral and PB external lateral- outer subnuclei (Figs. 1A–D). The pre-LC region contained a moderate number of 5-HT axons that were distributed within the area containing the FoxP2+ nuclei (Figs. 1B and C).
Fig. 1.
Distribution of 5-HT axons (red) in relationship to FoxP2+ neurons (green) in the dorsolateral pons. A dense concentration of 5-HT+ fibers was found in the lateral parabrachial area and the locus coeruleus (LC), with lesser amounts in the pre-locus coeruleus (pre-LC). See list for abbreviations.
5-HT axons make close contacts with CTb-labeled FoxP2 neurons
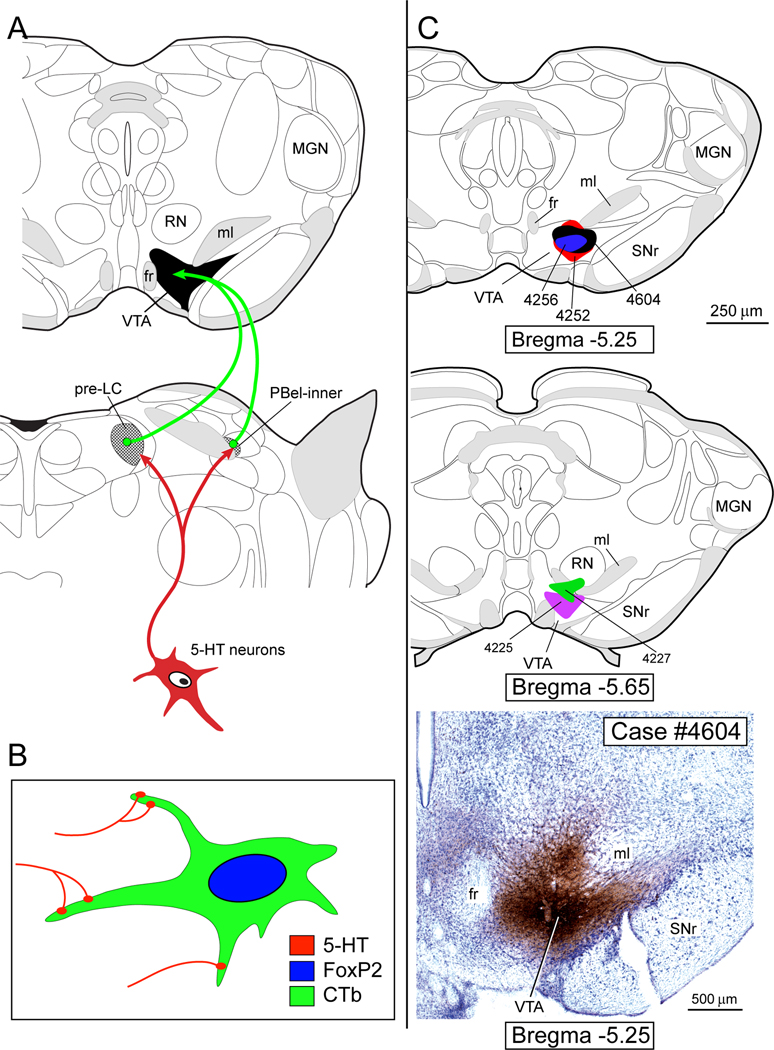
Figure 2A provides a schematic drawing of the neural pathway that has been analyzed in the present study. Figure 2B illustrates the hypothetical neurons we sought to identify in the pre-LC and PBel-inner. The upper two drawings in Figure 2C presents two different levels of the midbrain to illustrate the CTb injection sites, and the bottom panel of Figure 2C shows a photomicrograph of the injection site from Case #4604.
Fig. 2.
A. Proposed 5-HT neural pathway that makes close contacts with the parabrachial and pre-LC neurons that project to the ventral tegmental area (VTA). Brain drawings modified from a rat brain atlas (Paxinos and Watson, 2005). B. Hypothesized 5-HT inputs onto the FoxP2+ neurons that project to the VTA. C. Upper two panels illustrate 5 cases in which CTb was injected in the VTA. Lower panel shows photomicrograph of VTA injection site. Drawing modified from (Swanson, 1998). See list for abbreviations.
Digital photoimages of the pre-LC and PBel-inner regions from Case #4604 are shown in Figures 3A and 3B, respectively. Figure 3A (right panel) presents several examples of the pre-LC neurons with 5-HT close contacts. Figure 3B (right panel) contains three neurons in the PBel-inner with 5-HT-containing close contacts. Figure 3C (left panel) shows two areas outlined by white dashed lines; these are the areas in the pre-LC and PBel-inner, respectively that were selected for cell counts. Cell counts were collected for three types of neurons: FoxP2, FoxP2 & CTb, and FoxP2 & CTb with 5-HT close contact fibers. The pie charts in Figure 3C summarize these findings. The raw data for 5 different cases are presented in Table 1. In the pre-LC, 36% of the neurons that express FoxP2 also were co-labeled with CTb. In the PBel-inner, 32% of the neurons were co-labeled (FoxP2+ & CTb). Over 50% of these co-labeled neurons in both regions also received 5-HT close contacts.
Table 1.
5-HT inputs to the FoxP2 neurons of the pre-locus coeruleus and parabrachial exterior lateral-inner subdivision that project to the VTA. CTb was injected into the VTA in order to retrogradely label subsets of neurons in the pre-LC and PBel-inner. Some of the CTb labeled neurons were also FoxP2+.
| Pre-LC | PBel-inner | |||||
|---|---|---|---|---|---|---|
| Rat # | FoxP2(+), CTb(−), 5HT(−) |
FoxP2(+), CTb(+), 5HT(−) |
FoxP2(+), CTb(+), 5HT(+) |
FoxP2(+), CTb(−), 5HT(−) |
FoxP2(+), CTb(+), 5HT(−) |
FoxP2(+), CTb(+), 5HT(+) |
| 4225 | 103 | 45 | 45 | 62 | 20 | 24 |
| 4227 | 135 | 26 | 24 | 122 | 19 | 26 |
| 4252 | 118 | 18 | 36 | 81 | 9 | 12 |
| 4256 | 91 | 27 | 52 | 69 | 11 | 15 |
| 4604 | 126 | 14 | 42 | 45 | 16 | 22 |
| Mean | 112 | 29 | 39 | 84 | 15 | 19 |
| SEM | 10 | 6 | 6 | 13 | 3 | 3 |
Origin of the 5-HT inputs to the pre-LC and lateral PB
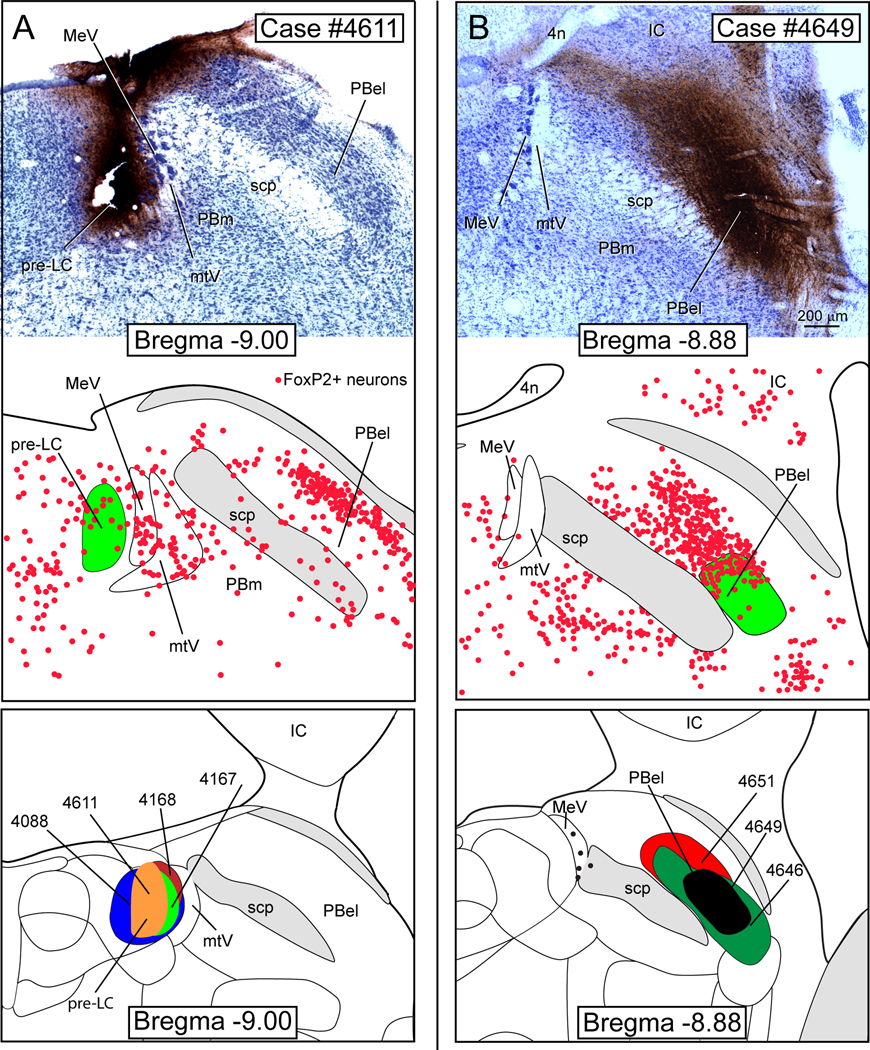
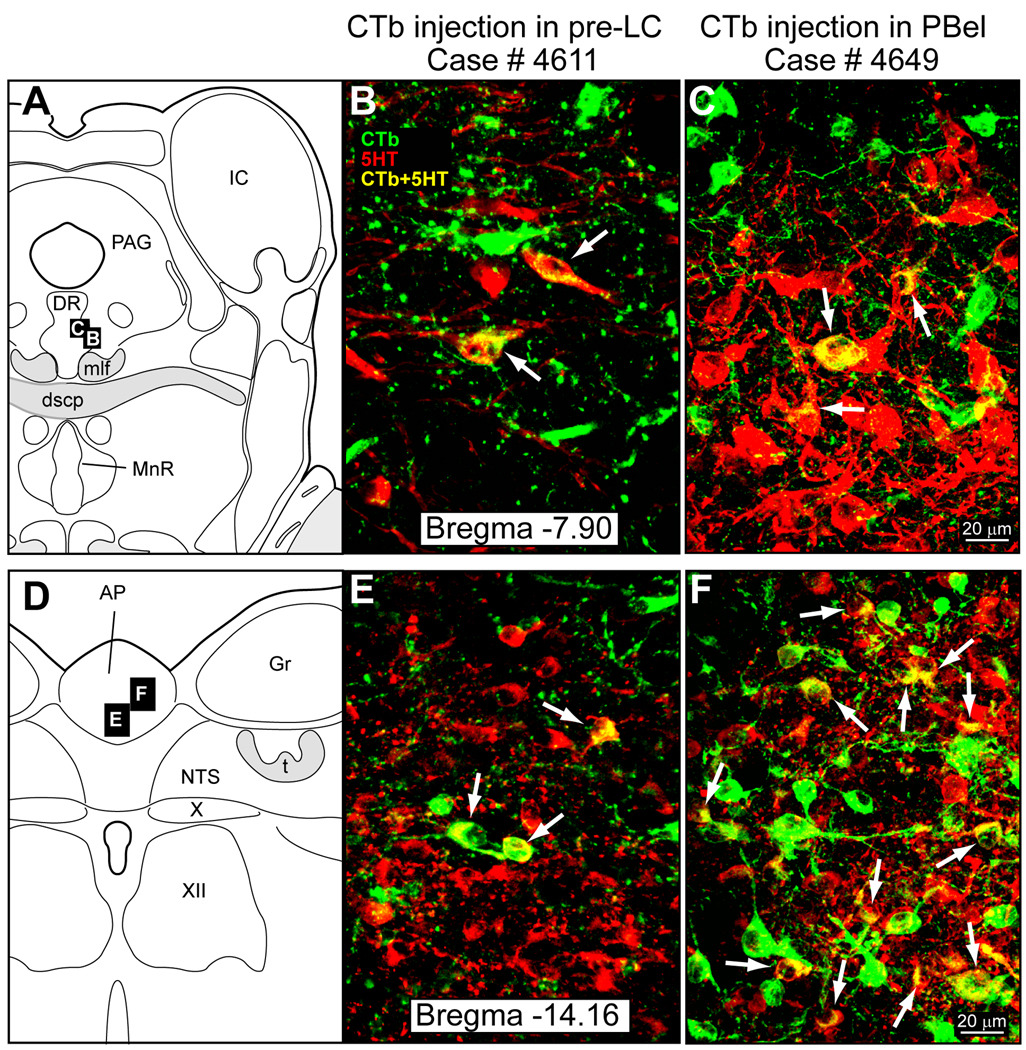
Figure 4 illustrates two separate retrograde tracing experiments. Figure 4A presents a photomicrograph from a case in which CTb was injected in the pre-LC and Figure 4B shows another case in which CTb was injected in the lateral PB. The brainstem from both groups of rats was analyzed for the presence of co-labeled 5-HT and CTb neurons. These were found almost exclusively in the AP and DR (Fig. 5). Because it was of critical importance for the present study to demonstrate that the CTb injections overlapped with the FoxP2+ neurons in the pre-LC or PBel-inner, the injection sites were immunostained by a two-color immunostaining method that permitted us to view both with separate filters and then, construct diagrams and images in Abode Illustrator showing the localization of the injection site in relationship to the FoxP2+ neurons. The middle panels in Figures 4A and 4B show the respective CTb injections sites (green) and the FoxP2+ neurons in the pre-LC and PB. The pre-LC injection site involved the core of the FoxP2+ neurons, but was highly restricted and did not encroach on the FoxP2+ neurons in the mesencephalic trigeminal nucleus or its tract (Fig. 4A middle panel). The lateral PB injection site was centered within a part of a dense cluster of FoxP2+ neurons localized in the PBel-inner. The lower panels of Figure 4A and 4B illustrate the cases used to determine the 5-HT input to these two areas.
Fig. 4.
A. CTb injection in the pre-LC region for case #4611. The middle panel shows the injection site in relationship to the local FoxP2+ (red dots) neurons. The lower panel illustrates the four cases which had CTb injections in the pre-LC. B. CTb injection in lateral PB for case #4649. The middle panel shows the CTb injection site in relationship to the FoxP2+ neurons. Lower panel shows the three cases which had CTb injections in the lateral PB. Drawings modified from Swanson (1998).
Fig. 5.
A) Serotonergic neurons in the dorsal raphe nucleus project to B) the pre-LC and C) the PBel. D) 5-HT neurons in the area postrema project to E) the pre-LC and F) the PBel. Drawings modified from Swanson (1998),
Slight variations in the rostral-caudal position of the CTb injection sites, especially for the PBel experiments, caused significant differences in the overall number of the CTb labeled cell bodies in the AP. CTb injections in the PBel that was co-extensive with the caudal part of the inferior colliculus (bregma = −8.88 to −9.00 mm) resulted in considerably more CTb-labeled neurons in the AP than injections placed 250–500 µm caudal to this level. Differences in the DR labeling pattern were not detected. Because the longitudinal length of the pre-LC is only about 300–400 µm, we did not have a sufficient sample size to determine whether regional variations in the placement of the CTb injections would produced different retrograde labeling patterns in the AP or DR.
Although we did not perform systematic cell counts, our overall impression was the number of CTb-labeled DR neurons was less than found in the AP. Perhaps, AP neurons send efferent projections to a few select PB subnuclei, whereas the DR innervates a diverse group of PB neurons. We did not have sufficient data to compare the AP and DR projections to the pre-LC.
Figures 5 presents examples of photomicrographs of tryptophan hydroxylase-immunoreactive (=5-HT) neurons co-labeled with CTb. The upper panel shown in Figure 5A shows co-labeled 5-HT & CTb neurons from a case in which CTb was injected into the pre-LC (Fig. 5B) and PBel (Fig. 5C). Co-labeled AP neurons are shown from experiments in which CTb was injected into the pre-LC (Fig. 5E) or PBel (Fig. 5F).
The 5-HT projections to the pre-LC and PBel originate mainly from the DR and AP. In one pre-LC case (case#4088), however, a few co-labeled cells were found in the median raphe nucleus. Also, some co-labeled neurons were localized in the medial longitudinal fascicle for both sets of experiments (data not shown), but the number was quite small (2 cells per case). Non-5HT neurons originating from the AP and DR also project to the pre-LC and PBel.
CTb injections in both the pre-LC and PBel also resulted in retrograde labeling in other brain regions besides the AP and DR. Since the goal of this study was to study the 5-HT inputs into the pre-LC/PBel-inner, we did not completely map the brains from these animals. However, we did examine the forebrain circumventricular organs (CVO). CTb-labeled neurons were localized in the organ vasculosum of the lamina terminalis (OVLT) in three of the pre-LC cases and three of the PBel cases. The subfornical organ (SFO) was not labeled in any of the cases. Finally, the median preoptic nucleus (MnPO), which receives inputs from the OVLT and SFO, also contained CTb-labeled neurons in the two of the pre-LC cases and three of the PBel cases.
DISCUSSION
This study shows that FoxP2+ neurons in the pre-LC and PBel-inner, which project to the VTA, receive inputs from the 5-HT neurons that reside in the AP and DR. We will first review previous anatomical studies, and then, briefly consider the pharmacological studies that have suggested that the 5-HT inputs to the PB may inhibit sodium appetite.
Previous anatomical studies
Earlier anatomical tracing studies reported that the AP and DR project to the dorsolateral pons, but did not precisely define these connections. For example, anterograde axonal tracing studies demonstrated that the DR projects to the locus coeruleus, the area immediately rostral to the locus coeruleus (presumably the pre-LC), and the lateral PB (Vertes and Kocsis, 1994). An earlier retrograde cell body tracing study reported that the 5-HT neurons in the AP project to the PB, without defining which PB subnuclei receive this input (Lanca and van der Kooy, 1985). Other retrograde tracing studies have described the inputs to the locus coeruleus, and one important contribution studied an area which may have been the pre-LC; this study referred to this region as the peri-mesencephalic trigeminal nucleus (Luppi et al., 1995). These investigators found that the DR projected to the pre-LC area, but did not find that the AP provided an input as well (Luppi et al., 1995).
Using an anterograde tracing method, we demonstrated that the AP provides a weak projection to the FoxP2+ neurons lying in the region of the pre-LC, PBel-inner, and PB dorsal lateral subnucleus (Stein and Loewy, 2010). It is important to note that we were extremely cautious in this study and did not use cases in which the anterograde tracer (PHAL) had spread into the ventral part of the AP. This was done to prevent labeling of the underlying neurons of the nucleus of solitary tract because they also project to pre-LC and PBel. Thus, the data presented by Stein and Loewy (2010) underestimates the density of innervation in the pre-LC, PBel-inner, and PBdl that arises from the AP.
Functional studies implicating the 5-HT system in sodium appetite regulation
The first experiments indicating that specific groups of 5-HT brainstem neurons were involved in sodium appetite were published by Franchini and co-workers (Franchini et al., 2002). First, they examined whether 5-HT neurons of the raphe system and AP became activated after 24 hours of sodium depletion and reported that Fos-expression in these sites had decreased. In their second experiment, Fos-expression in the 5-HT neurons of the DR, median raphe, raphe pallidus, and AP were analyzed following sodium repletion in salt-deprived rats. Unlike sodium depletion experiments, sodium repletion caused Fos-expression of the 5-HT of the raphe system and AP, suggesting that these neurons were activated during the process of sodium intake to set limits on salt intake, and avoid excess sodium consumption. Thus, the 5-HT neurons may function like a brake to slow down ongoing sodium intake.
Rats with lesions in the AP (Contreras and Kosten, 1981, Hyde and Miselis, 1984, Edwards et al., 1993, Curtis et al., 1999) or DR (Olivares et al., 2003, Cavalcante-Lima et al., 2005) consume more NaCl than normal animals. No experiments, to our knowledge, have been published that selectively lesion the 5-HT neurons in these sites and then, examine the effect on sodium ingestion.
Recent pharmacological studies reported that when the selective 5-HT1A receptor agonist 8-hydroxy-2(n-dipropylamino) tetralin-HBr (8-OH-DPAT) was injected into the DR of the rats, it caused an increase sodium consumption (Fonseca et al., 2009). Presumably, this response was due to disinhibition. Although the mechanism is unknown, these data suggest that the 8-OH-DPAT inhibited the 5-HT DR projection system that targets the pre-LC and PB lateral.
The 5-HT input to the pre-LC and PBel-inner is weak and considerably less than what has been found in many forebrain regions innervated by the DR (Vertes, 1991). This does not mean that a weak 5-HT projection to particular area is not important. 5-HT, like other monoamines, may exert its effect by volume transmission (also referred to as paracrine secretion) on local neurons. This type of neural communication is dependent on the diffusion and flow of neurotransmitters or neuromodulators in the extracellular space (Fuxe et al., 2007, Agnati et al., 2010). 5-HT can diffuse distances of 20 µm and still be highly effective as a transmitter (Bunin and Wightman, 1998). This means that the relatively weak innervation is less of an issue than knowing the affinity values of the various 5-HT receptors that are present in a particular brain region (Daws, 2009). Clearly, an important line of future research regarding the role of the PB complex (including the pre-LC) in salt appetite will depend on determining the presence and localization of specific classes of 5-HT receptors on the FoxP2+ neurons. In addition, it will be important to explore whether other types of transporters, such as organic cation transporters and plasma membrane monoamine transporters which clear 5-HT from the extracellular space, exist in or near these FoxP2+ cell groups because they could also affect the actions of 5-HT at these sites as well (Daws, 2009).
Finally, the 5-HT system may interact with other types of transmitters in the PB complex, and one of them may be GABA. Activation of GABAB receptors in the PB with baclofen, a GABAB receptor agonist, causes an increase in sodium ingestion (De Oliveira et al., 2011). Further, bilateral PB microinjections with the GABAA drug muscimol produces an increase in NaCl intake, which can be blocked by bicuculline. Whether the 5-HT afferent system described in this report contribute to this system, or function independently remains unknown.
CONCLUSION
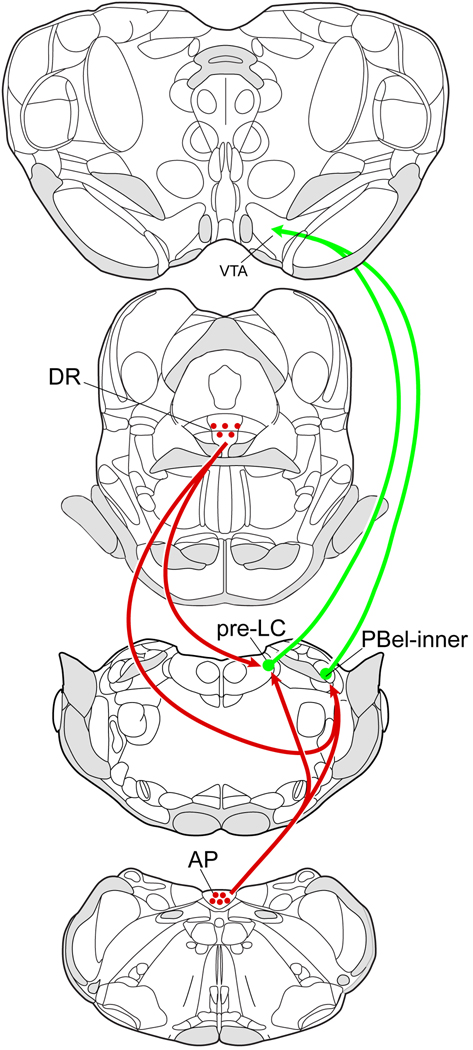
In summary, we demonstrate that a subset of FoxP2+ neurons in the pre-LC and PBel-inner that project to the VTA receive close contacts from 5-HT axons (Fig. 6). The origin of this input comes from the 5-HT neurons found in the AP and DR. These 5-HT inputs may provide inhibitory regulation of the FoxP2+ dorsolateral pontine neurons that have been implicated in sodium appetite regulation, and possibly other systems including those modulating cardiovascular and neuroendocrine functions.
Fig. 6.
Proposed 5-HT pathways projecting to the dorsolateral pontine FoxP2+ neurons implicated in sodium appetite regulation. Drawings modified from (Paxinos and Watson, 2005).
Highlights.
Serotonin-containing axons are distributed in the parabrachial nucleus and pre-locus coeruleus
5-HT axons project to FoxP2 neurons of the parabrachial nucleus and pre-locus coeruleus
Some of the FoxP2 neurons project to the ventral tegmental area which may be linked to the reward system
5-HT inputs to FoxP2 dorsolateral pontine neurons may modulate salt appetite-related functions
ACKNOWLEDGMENTS
Grant sponsor:
National Heart, Lung, and Blood Institute of the National Institutes of Health – Grant number: HL025449, Bakewell Imaging Center Fund, and National Institutes of Health – Grant number: NS057105 Neuroscience Blueprint Core Grant.
We thank Xay van Nguygen for excellent technical assistance, Dennis Oakley for help with the confocal microscopy, and Marcy Hartstein for constructing the computer graphic drawings.
ABBREVIATIONS
- 4n
trochlear nerve
- 5-HT
5-hydroxytryptamine
- X
dorsal vagal nucleus
- XII
hypoglossal nucleus
- AP
area postrema
- CTb
cholera toxin – β subunit
- DR
dorsal raphe nucleus
- dscp
decussation of the superior cerebellar peduncle
- fx
fornix
- Gr
gracile nucleus
- IC
inferior colliculus
- LC
locus coeruleus
- ml
medial lemniscus
- MeV
mesencephalic trigeminal nucleus
- MGN
medial geniculate nucleus
- mlf
medial longitudinal fasciculus
- MnR
median raphe nucleus
- mtV
tract of the mesencephalic trigeminal nucleus
- NTS
nucleus of the solitary tract
- OVLT
organum vasculosum of the lamina terminalis
- PAG
periaqueductal gray matter
- PBcl
parabrachial nucleus, central lateral subnucleus
- PBdl
parabrachial nucleus, dorsal lateral subnucleus
- PBel
parabrachial nucleus, external lateral subnucleus
- PBel-inner
parabrachial nucleus, external lateral subnucleus – inner portion
- PBel-outer
parabrachial nucleus, external lateral subnucleus – outer portion
- PBl
parabrachial nucleus, lateral region
- PBm
parabrachial nucleus, medial subnucleus
- PBvl
parabrachial nucleus, ventral lateral subnucleus
- pre-LC
pre-locus coeruleus
- RN
red nucleus
- SC
superior colliculus
- scp
superior cerebellar peduncle
- SFO
subfornical organ
- SNr
substantia nigra, pars reticulata
- t
solitary tract
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
Literature Cited
- Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. Understanding wiring and volume transmission. Brain Res Rev. 2010;64:137–159. doi: 10.1016/j.brainresrev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Andrade-Franze GM, Andrade CA, De Luca LA, Jr, De Paula PM, Menani JV. Lateral parabrachial nucleus and central amygdala in the control of sodium intake. Neuroscience. 2010;165:633–641. doi: 10.1016/j.neuroscience.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Bunin MA, Wightman RM. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. J Neurosci. 1998;18:4854–4860. doi: 10.1523/JNEUROSCI.18-13-04854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro L, Athanazio R, Barbetta M, Ramos AC, Angelo AL, Campos I, Varjao B, Ferreira H, Fregoneze J, de Castro e, Silva E. Central 5-HT2B/2C and 5-HT3 receptor stimulation decreases salt intake in sodium-depleted rats. Brain Res. 2003;981:151–159. doi: 10.1016/s0006-8993(03)03015-4. [DOI] [PubMed] [Google Scholar]
- Cavalcante-Lima HR, Badaue-Passos D, Jr, de-Lucca W, Jr, Lima HR, Costa-e-Sousa RH, Olivares EL, Cedraz-Mercez PL, Reis RO, Medeiros MA, Cortes WS, Reis LC. Chronic excitotoxic lesion of the dorsal raphe nucleus induces sodium appetite. Braz J Med Biol Res. 2005;38:1669–1675. doi: 10.1590/s0100-879x2005001100015. [DOI] [PubMed] [Google Scholar]
- Colombari DS, Menani JV, Johnson AK. Forebrain angiotensin type 1 receptors and parabrachial serotonin in the control of NaCl and water intake. Am J Physiol. 1996;271:R1470–R1476. doi: 10.1152/ajpregu.1996.271.6.R1470. [DOI] [PubMed] [Google Scholar]
- Contreras RJ, Kosten T. Changes in salt intake after abdominal vagotomy: evidence for hepatic sodium receptors. Physiol Behav. 1981;26:575–582. doi: 10.1016/0031-9384(81)90127-x. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Huang W, Sved AF, Verbalis JG, Stricker EM. Impaired osmoregulatory responses in rats with area postrema lesions. Am J Physiol. 1999;277:R209–R219. doi: 10.1152/ajpregu.1999.277.1.R209. [DOI] [PubMed] [Google Scholar]
- Davern PJ, McKinley MJ. Forebrain regions affected by lateral parabrachial nucleus serotonergic mechanisms that influence sodium appetite. Brain Res. 2010;1339:41–48. doi: 10.1016/j.brainres.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Daws LC. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther. 2009;121:89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gobbi JI, Barbosa SP, De Luca LA, Jr, Thunhorst RL, Johnson AK, Menani JV. Activation of serotonergic 5-HT(1A) receptors in the lateral parabrachial nucleus increases NaCl intake. Brain Res. 2005;1066:1–9. doi: 10.1016/j.brainres.2005.04.055. [DOI] [PubMed] [Google Scholar]
- De Gobbi JI, Martinez G, Barbosa SP, Beltz TG, De Luca LA, Jr, Thunhorst RL, Johnson AK, Vanderlei Menani J. 5-HT2 and 5-HT3 receptors in the lateral parabrachial nucleus mediate opposite effects on sodium intake. Neuroscience. 2007;146:1453–1461. doi: 10.1016/j.neuroscience.2007.03.004. [DOI] [PubMed] [Google Scholar]
- De Gobbi JIF, De Luca LA, Menani JV. Serotonergic mechanisms of the lateral parabrachial nucleus on DOCA-induced sodium intake. Brain Research. 2000;880:131–138. doi: 10.1016/s0006-8993(00)02784-0. [DOI] [PubMed] [Google Scholar]
- De Oliveira LB, Kimura EH, Callera JC, De Luca LA, Jr, Colombari DS, Menani JV. Baclofen into the lateral parabrachial nucleus induces hypertonic sodium chloride and sucrose intake in rats. Neuroscience. 2011;183:160–170. doi: 10.1016/j.neuroscience.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Edwards GL, Beltz TG, Power JD, Johnson AK. Rapid-onset "need-free" sodium appetite after lesions of the dorsomedial medulla. Am J Physiol. 1993;264:R1242–R1247. doi: 10.1152/ajpregu.1993.264.6.R1242. [DOI] [PubMed] [Google Scholar]
- Ferri GL, Ferri GL, Gaudio RM, Castello FI, Tirolo C, Chiolerio FM. Multiple biotin-aavidin amplification for multiple immunostaining. Appl Immunohistochem Mol Morphol. 1999;7:73–80. [Google Scholar]
- Fonseca FV, Mecawi AS, Araujo IG, Almeida-Pereira G, Magalhaes-Nunes AP, Badaue-Passos D, Jr, Reis LC. Role of the 5-HT(1A) somatodendritic autoreceptor in the dorsal raphe nucleus on salt satiety signaling in rats. Exp Neurol. 2009;217:353–360. doi: 10.1016/j.expneurol.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Franchini LF, Johnson AK, de Olmos J, Vivas L. Sodium appetite and Fos activation in serotonergic neurons. Am J Physiol Regul Integr Comp Physiol. 2002;282:R235–R243. doi: 10.1152/ajpregu.00766.2000. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Dahlstrom A, Hoistad M, Marcellino D, Jansson A, Rivera A, Diaz-Cabiale Z, Jacobsen K, Tinner-Staines B, Hagman B, Leo G, Staines W, Guidolin D, Kehr J, Genedani S, Belluardo N, Agnati LF. From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Res Rev. 2007;55:17–54. doi: 10.1016/j.brainresrev.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Sodium deprivation and salt intake activate separate neuronal subpopulations in the nucleus of the solitary tract and the parabrachial complex. J Comp Neurol. 2007;504:379–403. doi: 10.1002/cne.21452. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Stein MK, Miller RL, Shin JW, Gray PA, Loewy AD. FoxP2 expression defines dorsolateral pontine neurons activated by sodium deprivation. Brain Res. 2011;1375:19–27. doi: 10.1016/j.brainres.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde TM, Miselis RR. Area postrema and adjacent nucleus of the solitary tract in water and sodium balance. Am J Physiol. 1984;247:R173–R182. doi: 10.1152/ajpregu.1984.247.1.R173. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Kasper S, Lanzenberger R. Reward and the serotonergic system. Neuroscience. 2010;166:1023–1035. doi: 10.1016/j.neuroscience.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Krout KE, Mettenleiter TC, Loewy AD. Single CNS neurons link both central motor and cardiosympathetic systems: a double-virus tracing study. Neuroscience. 2003;118:853–866. doi: 10.1016/s0306-4522(02)00997-1. [DOI] [PubMed] [Google Scholar]
- Lanca AJ, van der Kooy D. A serotonin-containing pathway from the area postrema to the parabrachial nucleus in the rat. Neuroscience. 1985;14:1117–1126. doi: 10.1016/0306-4522(85)90281-7. [DOI] [PubMed] [Google Scholar]
- Lima HR, Cavalcante-Lima HR, Cedraz-Mercez PL, Costa ESRH, Olivares EL, Badaue-Passos D, Jr, Medeiros MA, Cortes WS, Reis LC. Brain serotonin depletion enhances the sodium appetite induced by sodium depletion or beta-adrenergic stimulation. An Acad Bras Cienc. 2004;76:85–92. doi: 10.1590/s0001-37652004000100008. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience. 1995;65:119–160. doi: 10.1016/0306-4522(94)00481-j. [DOI] [PubMed] [Google Scholar]
- Menani JV, De Luca LA, Jr, Thunhorst RL, Johnson AK. Hindbrain serotonin and the rapid induction of sodium appetite. Am J Physiol Regul Integr Comp Physiol. 2000;279:R126–R131. doi: 10.1152/ajpregu.2000.279.1.R126. [DOI] [PubMed] [Google Scholar]
- Menani JV, Johnson AK. Lateral parabrachial serotonergic mechanisms: angiotensin-induced pressor and drinking responses. Am J Physiol. 1995;269:R1044–R1049. doi: 10.1152/ajpregu.1995.269.5.R1044. [DOI] [PubMed] [Google Scholar]
- Olivares EL, Costa ESRH, Cavalcante-Lima HR, Lima HR, Cedraz-Mercez PL, Reis LC. Effect of electrolytic lesion of the dorsal raphe nucleus on water intake and sodium appetite. Braz J Med Biol Res. 2003;36:1709–1716. doi: 10.1590/s0100-879x2003001200013. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Burlington, MA: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- Reis LC. Role of the serotoninergic system in the sodium appetite control. An Acad Bras Cienc. 2007;79:261–283. doi: 10.1590/s0001-37652007000200009. [DOI] [PubMed] [Google Scholar]
- Rocha MJ, Herbert H. c-fos expression in the parabrachial nucleus following cardiovascular and blood volume changes. J Hirnforsch. 1996;37:389–397. [PubMed] [Google Scholar]
- Shin JW, Geerling JC, Stein MK, Miller RL, Loewy AD. FoxP2 brainstem neurons project to sodium appetite regulatory sites. J Chem Neuroanat. 2011 doi: 10.1016/j.jchemneu.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MK, Loewy AD. Area postrema projects to FoxP2 neurons of the pre-locus coeruleus and parabrachial nuclei: brainstem sites implicated in sodium appetite regulation. Brain Res. 2010;1359:116–127. doi: 10.1016/j.brainres.2010.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Amsterdam: Elsevier; 1998. [Google Scholar]
- Tanaka J, Hayashi Y, Yamato K, Miyakubo H, Nomura M. Involvement of serotonergic systems in the lateral parabrachial nucleus in sodium and water intake: a microdialysis study in the rat. Neurosci Lett. 2004;357:41–44. doi: 10.1016/j.neulet.2003.12.040. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Projections of the dorsal raphe nucleus to the brainstem: PHA-L analysis in the rat. J Comp Neurol. 1994;340:11–26. doi: 10.1002/cne.903400103. [DOI] [PubMed] [Google Scholar]