Abstract
Covalent attachment of small ubiquitin-like modifier (SUMO) to proteins regulates many processes in the eukaryotic cell. This reaction is similar to ubiquitination and usually requires an E3 ligase for substrate modification. However, only a few SUMO ligases have been described so far, which frequently facilitate sumoylation by bringing together the SUMO-conjugating enzyme Ubc9 and the target protein. Ubc9 is an interaction partner of the transcription factor Krox20, a key regulator of hindbrain development. Here, we show that Krox20 functions as a SUMO ligase for its coregulators—the Nab proteins—and that Nab sumoylation negatively modulates Krox20 transcriptional activity in vivo.
Keywords: Krox20, Nab, SUMO ligase, Ubc9
Introduction
Sumoylation is the post-translational modification of proteins by the small ubiquitin-like modifier (SUMO), and regulates many processes in the eukaryotic cell (Gareau & Lima, 2010). SUMO is a 100-amino-acid polypeptide that is covalently attached to the ε-amino group of a lysine residue of target proteins, commonly included in the consensus ΨKxE/D (Ψ: large hydrophobic residue). The reaction, similarly to ubiquitination, involves transfer from the conjugating enzyme Ubc9 to the target protein and frequently requires the concourse of a SUMO ligase. These ligases often facilitate transfer by simultaneously binding to Ubc9 and to the target protein. In comparison with ubiquitination, few SUMO ligases have been described so far (Gareau & Lima, 2010). Indeed, identification of new SUMO ligases remains a challenge, and will shed light on the mechanisms and regulation of the sumoylation process.
From a previous two-hybrid screening, we identified the SUMO-conjugating enzyme Ubc9 as a partner of the transcription factor Krox20 (Garcia-Dominguez et al, 2006), a key regulator of hindbrain development (Schneider-Maunoury et al, 1993), where it participates in the control of the expression of various genes, including its own and genes encoding its coregulators Nab and the tyrosine kinase receptor EphA4 (Desmazieres et al, 2009). Development of the vertebrate hindbrain involves a transient segmentation process that generates 7–8 segments or rhombomeres (r) along the anterior–posterior axis (Lumsden & Krumlauf, 1996). Krox20 is expressed in r3 and r5 and is required for the formation and maintenance of these segments (Schneider-Maunoury et al, 1993; Voiculescu et al, 2001). Besides three zinc fingers for DNA binding, Krox20 contains the R1 motif, the surface for interaction with Nab1 and Nab2, initially identified as corepressors (Russo et al, 1995; Svaren et al, 1996). It has been reported that the repressive activity of Nab2 is partly due to interaction with CHD4 (Srinivasan et al, 2006).
In this report, we show that Krox20 functions as a ligase for the sumoylation of its coregulators, the Nab proteins. SUMO modification of Nab2 negatively modulates Krox20 transcriptional activity. Thus, sumoylation adds to the list of mechanisms involved in Krox20 autoregulation.
Results
Krox20 interacts with Ubc9
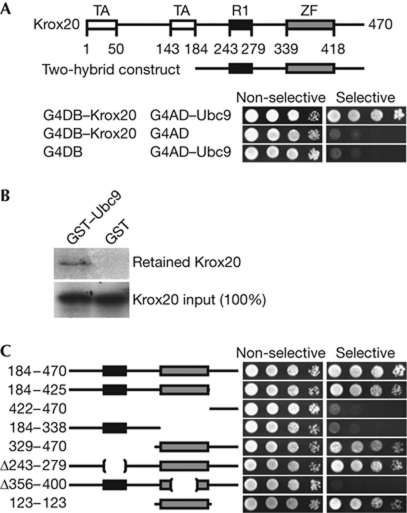
From a previous two-hybrid screening based on Krox20 (Garcia-Dominguez et al, 2006), we isolated seven clones corresponding to the SUMO-conjugating enzyme Ubc9 (Fig 1A). Pull-down experiments with purified glutathione S-transferase (GST) or a GST–Ubc9 fusion, and in vitro translated Krox20, demonstrated a direct and specific interaction between Krox20 and Ubc9 (Fig 1B). We mapped the interaction surface in Krox20 by using the yeast two-hybrid assay. Analysis indicated that the zinc-finger domain was necessary and sufficient for Ubc9 binding (Fig 1C).
Figure 1.
The zinc-finger domain of Krox20 mediates interaction with Ubc9. (A) Growth of yeast transformed with the indicated constructs was tested on non-selective and selective media. Bait and prey constructs were based on Gal4 DNA-binding domain (G4DB) and Gal4 activation domain (G4AD) vectors, respectively. (B) Pull-down experiments were carried out with immobilized purified glutathione S-transferase (GST) or a GST–Ubc9 fusion and in vitro translated, radioactively labelled Krox20. (C) Deletion constructs of Krox20 were tested for interaction with Ubc9 by yeast two-hybrid screening as indicated in A. TA, transactivation domain; ZF, zinc finger.
Krox20 functions as a SUMO ligase
Protein interaction with Ubc9 often leads to sumoylation of the interacting protein. However, we have previously reported that Krox20 is not sumoylated (Garcia-Dominguez et al, 2006). Ubc9 also interacts with SUMO ligases to facilitate sumoylation of target proteins. We therefore speculated that Krox20 might recruit Ubc9 to function as a ligase in the sumoylation of other proteins, and the Krox20 coregulators Nab1 and Nab2 (Russo et al, 1995; Svaren et al, 1996) represent good candidates. Indeed, amino-acid sequence analysis revealed two conserved sumoylation consensus sites in each protein. A two-hybrid assay did not reveal direct interaction between Nab2 and Ubc9 (not shown).
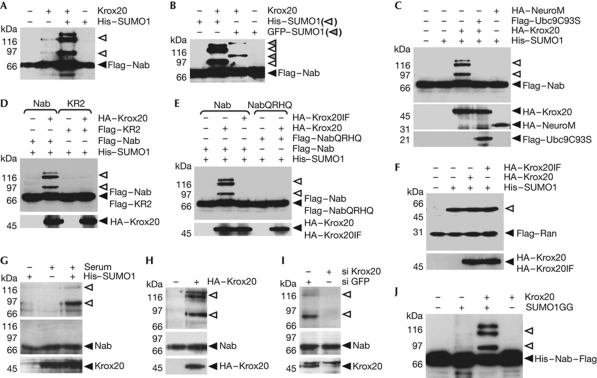
To test sumoylation of Nab proteins, we performed sumoylation assays in 293T cells transfected with Flag-tagged Nab expression constructs and analysed the cell extracts by western blot analysis. Transfection of Nab2 resulted in detection of a single band. However, when Nab2 and Krox20 expression vectors were cotransfected, up to three additional bands were observed, consistent with the presence of two sumoylation consensus sites (Fig 2A). Nab1 was also sumoylated in the presence of Krox20 (supplementary Fig S1A online). As Nab1 and Nab2 are highly homologous and have been shown to display similar functions (Svaren et al, 1996), we restricted the following experiments to Nab2, referred to as Nab. In the cell, SUMO1 is mostly bound to proteins, resulting in a reduced free SUMO1 pool (Gareau & Lima, 2010). The addition of low amounts of a histidine-tagged SUMO1 (His–SUMO1) expression vector in cotransfections resulted in increased modification of Nab (Fig 2A). Overexpression of the protein inhibitor of activated STAT (PIAS) proteins did not significantly modify sumoylation (data not shown). Green fluorescent protein–SUMO1 was also efficiently conjugated to Nab (Fig 2B), but not His–SUMO2 (supplementary Fig S1B online). In addition, Nab sumoylation was prevented by a dominant-negative version of Ubc9 (C93S; Fig 2C). Nab was specifically modified in the presence of Krox20 as a non-related transcription factor (NeuroM) had no effect (Fig 2C). As expected, a double mutant of the putative target lysines (K379RK517R (KR2)) was not SUMO-modified by Krox20 (Fig 2D). The mutation did not affect Nab nuclear localization nor its interaction with Krox20 (supplementary Fig S2 online).
Figure 2.
Krox20 mediates Nab sumoylation. (A–F) 293T cells were transfected with Flag–Nab or Flag–RanGAP1-C-ter (Flag–Ran) expression vectors and the constructs indicated at the top of each panel. Flag-tagged proteins were detected by western blot. Black arrowheads indicate non-modified proteins, and other arrowheads indicate modified proteins. Bottom panels correspond to inputs of the indicated proteins. (A,B) Nab is sumoylated in the presence of Krox20, and sumoylation is enhanced if low amounts of histidine-tagged small ubiquitin-like modifier 1 (His–SUMO1) or green fluorescent protein (GFP)–SUMO1 are transfected. (C) Nab is specifically sumoylated by Krox20, as the addition of NeuroM does not affect Nab sumoylation state; however, dominant-negative Ubc9 (C93S) interferes with Nab sumoylation. (D) The Nab K379RK517R (KR2) protein is not sumoylated. (E) Krox20 I268F and Nab Q64RH95Q mutants, affected in their ability to interact with each other, are ineffective as ligase and target in the sumoylation reaction, respectively. (F) Sumoylation of RanGAP1-C-ter is not altered by the presence of Krox20 or the I268F mutant. (G–I) Sumoylation of endogenous Nab was analysed in P19 cells in pull-down experiments with anti-Nab antibodies. (G) Endogenous Nab is sumoylated following His–SUMO1 transfection and serum stimulation, which results in the expression of Krox20. (H) Sumoylation of endogenous Nab was also observed after transfection of a Krox20 expression construct under normal growth conditions. (I) Nab sumoylation was prevented by transfection of Krox20 short interfering RNA (siRNA; si), but not by a control GFP siRNA, under serum stimulation conditions. Upper panels in G–I show pulled His–SUMO1–Nab, revealed with Nab antibodies, whereas lower panels show 1.5% input of the indicated proteins. Note that Krox20 antibodies also reveal a nonspecific upper band. (J) In vitro sumoylation assays with 300 ng of purified Flag-tagged Nab were carried out in the presence or the absence of mature SUMO (SUMO1GG) and 15 ng of Krox20 as indicated. Sumoylated products corresponded to 52.96%±2.74 (mean±s.d.) of loaded protein, as determined by chemiluminescence measurement from three independent western blots. HA, haemagglutinin epitope tag.
We next investigated the involvement of Krox20–Nab interaction in Nab sumoylation. The single mutation I268F in Krox20 and the double mutation Q64RH95Q in Nab have been shown to abrogate Krox20–Nab interaction (Svaren et al, 1998). We found that both mutations prevented sumoylation (Fig 2E). By contrast, neither Krox20 nor the I268F mutant showed any effect in general sumoylation as monitored by modification of the carboxy-terminal part of RanGAP1 (RanGAP1-C-ter), as a control (Fig 2F).
To investigate sumoylation of endogenous Nab we used P19 cells, as they express Krox20 and Nab. In this line, constitutive levels of Nab protein were detected under normal growth conditions, whereas Krox20 expression required serum stimulation (Fig 2G; supplementary Fig S3 online). We were not able to observe sumoylation of endogenous Nab with endogenous SUMO1. Thus, we decided to transfect P19 cells with low amounts of the His–SUMO1 expression vector to analyse Nab sumoylation after pull-down of the His-tagged products. As shown in Fig 2G, although Nab-sumoylated forms could not be detected in the input (1.5% of the total), they were observed in the precipitates. Moreover, the presence of these bands was strongly reinforced after serum stimulation; that is, upon induction of Krox20 (Fig 2G). Sumoylation of endogenous Nab was also observed after transfection of a Krox20 expression vector without serum stimulation (Fig 2H). Finally, knockdown of induced Krox20 by a combination of two short interfering RNA (siRNA) molecules also prevented sumoylation of endogenous Nab (Fig 2I).
To definitively demonstrate that Krox20 functions as a SUMO ligase for Nab, we performed in vitro sumoylation assays with purified proteins produced in bacteria. As illustrated in Fig 2J, sumoylation of Nab occurred in vitro and was indeed dependent on the presence of Krox20. Furthermore, comparison of the number of molecules between input Krox20 (0.3 × 10−12 mol) and sumoylated Nab (2.78 × 10−12 mol, as estimated by measure of chemiluminescence from western blots and comparing with the amount of loaded protein, Fig 2J) indicated a much larger number of sumoylated Nab molecules, suggesting that Krox20 was functioning in a catalytic manner.
Nab mediates SUMO recruitment to the chromatin
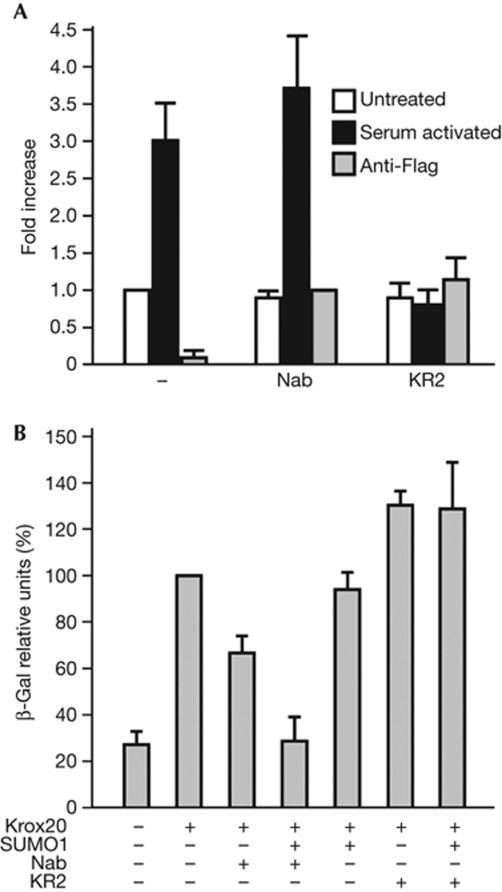
We next investigated whether SUMO can be recruited to a Nab-regulated sequence in the context of chromatin. We chose to examine the Id4 promoter because it has been previously shown to be regulated by the Krox20–Nab complex (Mager et al, 2008). In P19 cells, serum addition led to a threefold increase in SUMO levels associated to the Id4 promoter (Fig 3A). To investigate whether Nab sumoylation was involved, cells were transfected with wild-type Nab or the KR2 mutant immediately after serum deprivation. Whereas in the presence of wild-type Nab the increase of SUMO levels were similar to those observed in the absence of transfection, with the KR2 mutant serum-mediated accumulation of SUMO on the Id4 promoter was impaired, presumably due to competition with the endogenous Nab (Fig 3A).
Figure 3.
Sumoylation of Nab contributes to SUMO recruitment to chromatin and modulates Krox20 transcriptional activity. (A) Levels of small ubiquitin-like modifier 1 (SUMO1) associated with the Id4 promoter were determined by chromatin immunoprecipitation experiments in P19 cells transfected with Flag-tagged wild-type Nab or the KR2 mutant or not transfected (−). Levels of SUMO were determined on cells growing normally (white bars) or subjected to serum stimulation (black bars). Fold increase in SUMO levels were normalized to the value determined in non-transfected untreated cells. Flag levels were also determined as a control (grey bars) and fold increase was normalized to the value of Nab-transfected cells. (B) A lacZ reporter construct responsive to Krox20 was tested in P19 cells cotransfected with the indicated expression constructs. Relative units of β-galactosidase (β-gal) were normalized to the value of cells cotransfected with Krox20 alone (100%). Values are means of three independent experiments±s.d.
Nab sumoylation modulates Krox20 activity in vivo
To evaluate whether Nab sumoylation has an impact on Krox20 transcriptional control, we took advantage of a lacZ-based reporter of Krox20 activity (Garcia-Dominguez et al, 2006). This construct was transfected in P19 cells together with Krox20, SUMO1 and Nab expression constructs. The analysis demonstrated that Nab efficiently repressed Krox20 transcriptional activity in the presence of SUMO, whereas the KR2 mutant resulted in increased reporter activity, suggesting a dominant-negative effect (Fig 3B).
To investigate the possible modulation of Krox20 activity by Nab sumoylation in vivo, we turned to the hindbrain, in which Krox20 regulates various genes, including itself and Nab genes, in r3 and r5 (Mechta-Grigoriou et al, 2000; Giudicelli et al, 2001; Chomette et al, 2006; Desmazieres et al, 2009). Furthermore, in gain-of-function experiments, Nab was shown to repress Krox20 activity, suggesting the existence of a negative-feedback regulatory loop (Mechta-Grigoriou et al, 2000).
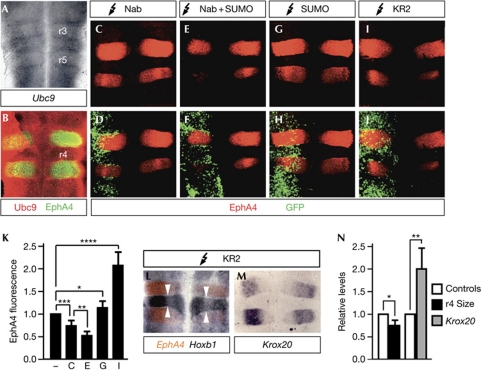
Electroporation of chick embryos allows gain-of-function analysis in the hindbrain. We first confirmed the expression of Ubc9 in this structure by in situ hybridization and immunofluorescence analyses (Fig 4A,B). We analysed the consequences of Nab and SUMO1 misexpression on the expression of the r3/r5 marker EphA4, which is under direct control of Krox20 (Theil et al, 1998). As electroporation only affects one side, the other constitutes a control. Limited repression of EphA4 after electroporation of Nab expression vectors has been previously reported (Desmazieres et al, 2009) and was confirmed in our experiments (Fig 4C,D,K). We observed that coelectroporation with Nab and SUMO expression vectors led to more severe repression (Fig 4C–H,K). By contrast, electroporation of the KR2 mutant led to upregulation of EphA4 (Fig 4I–K). Analysis of the expression of the r4 marker Hoxb1, together with EphA4, by double in situ hybridization showed that electroporation of Nab KR2 also resulted in reduced r4 size (Fig 4L,N), possibly associated with r3 and/or r5 expansion. Finally, expression of Krox20 itself appeared upregulated after KR2 electroporation, similarly to EphA4 (Fig 4M,N). Together, these data support an involvement of Nab sumoylation in the repression of Krox20 activity in the hindbrain.
Figure 4.
Nab sumoylation regulates Krox20 target genes in vivo. Flat-mounted chick hindbrains were analysed by (A) in situ hybridization using a Ubc9 antisense RNA probe or by (B) immunofluorescence using Ubc9 and EphA4 antibodies. (C–J) The neural tube of chick embryos was electroporated with constructs expressing the proteins indicated at the top of each panel. Green fluorescent protein (GFP) was used to monitor electroporation. Electroporated hindbrains were processed for (C–J) immunofluorescence using EphA4 antibodies, or (L,M) in situ hybridization using EphA4, Hoxb1 and Krox20 RNA probes. Arrowheads in L delimit rhombomere (r) 4. Electroporations were performed on the left side of embryos. (K) Fluorescence signals of EphA4 hybridization in C, E, G and I were measured using the MetaMorph software. For that, regions of the same area encompassing r3–r5 were defined on both electroporated and control sides. (N) Size of r4 was determined by measuring the Hoxb1-positive area using the ImageJ application, and intensity of the Krox20 hybridization signal was measured with the MetaMorph software on inverted grey-scale-converted images. Relative levels were normalized with respect to those on the control side (−). Values correspond to the mean±s.d. of 5–8 samples from three independent experiments. Statistical significance was analysed using Student's t-test: (K) *P=0.13, **P<0.025, ***P<0.005 and ****P<0.001; (N) *P<0.025 and **P<0.005. SUMO, small ubiquitin-like modifier.
Discussion
In this work, we report that Krox20 functions as a ligase in the sumoylation reaction of its coregulators, the Nab proteins. Ligase activity of Krox20 is based on the following observations: (i) overexpression of Krox20 under limiting SUMO availability leads to sumoylation of transfected Nab; (ii) Krox20 is able to recruit Ubc9 and Nab through different domains; (iii) physical interaction between the ligase and the target is critical for modification; (iv) sumoylation of endogenous Nab can also be observed in cultured cells and is dependent on Krox20 expression; and (v) Krox20 promotes Nab sumoylation in vitro and functions in a catalytic manner. To our knowledge, this constitutes the first example of a transcription factor functioning as a ligase for the sumoylation of its own coregulators. Our results support a role of Krox20 in locally recruiting Ubc9 for the sumoylation of other components in its transcriptional complex, namely the Nab proteins, contributing to our understanding of how the specificity of target sumoylation might be achieved. This raises the possibility that other factors might be sumoylated using Krox20 as a ligase, an exciting hypothesis considering the role of Krox20 in various developmental systems (Schneider-Maunoury et al, 1993; Topilko et al, 1994). Our findings also represent a stimulus for the identification of new ligases. Different types of ligase do not share significant homology. Similarly, no significant homology was observed between Krox20 and previously described ligases. These have in common the ability to interact with Ubc9, in many cases through apparently unrelated domains. The zinc-finger domain of Krox20 binds to Ubc9. Intriguingly, different zinc-based structures have been reported to be involved in Ubc9 binding and ligase function (Garcia-Dominguez et al, 2008).
Several pieces of evidence are consistent with a role of Nab sumoylation in vivo: (i) Nab sumoylation modulates Krox20 transcriptional activity in a reporter assay in cultured cells; (ii) SUMO recruitment to the Id4 promoter is dependent on Nab sumoylation sites; and (iii) altered Nab sumoylation affects the expression of Krox20 target genes in the hindbrain. Despite the numerous roles attributed to SUMO in eukaryotic cells, a function in transcriptional repression stands out (Garcia-Dominguez & Reyes, 2009). Accordingly, our results support a role of Nab sumoylation in transcriptional repression. Srinivasan et al (2006) have reported that CHD4 participates in, but does not account for, full repression activity associated with Nab2, supporting the involvement of additional mechanisms, for example sumoylation. Indeed, the fact that both wild-type Nab and the KR2 mutant interact equally with CHD4 (supplementary Fig S1C online) suggests that sumoylation is not involved in CHD4 recruitment.
It has been proposed that Krox20 mediates expansion of r3 and r5 territories by recruiting cells from adjacent even-numbered territories (Giudicelli et al, 2001). Subsequently, other mechanisms should limit Krox20 activity to restrict expansion of r3 and r5. Induction by Krox20 of its own corepressors, the Nab proteins, has been proposed as one of these mechanisms (Mechta-Grigoriou et al, 2000). In agreement with this hypothesis, interference with the interaction between Krox20 and Nab proteins leads to delayed downregulation of Krox20 target genes (Desmazieres et al, 2008, 2009). In this report, we show that interfering with Nab sumoylation also leads to altered expression of Krox20 target genes and to modifications in the size of rhombomeres. Together, our data are consistent with Nab sumoylation limiting Krox20 activity and the extension of Krox20-positive territories, in agreement with the proposed role of Nab proteins. However, experiments conducted in the mouse have shown that double Nab knockout or knockin of the I268F mutation in Krox20 do not lead to major defects in hindbrain patterning (Le et al, 2005; Desmazieres et al, 2008). This suggests the existence of redundant mechanisms for the limitation of the expansion of Krox20-positive territories.
In conclusion, we have revealed an intriguing novel activity of the Krox20 transcription factor, as a SUMO ligase for its coregulators Nab. Nab sumoylation affects Krox20 transcriptional activity, establishing an additional loop in the complex control of its own activity and expression by Krox20.
Methods
Plasmid constructs, protein production and purification, yeast two-hybrid and pull-down assays. Details for plasmid constructs and protein production and purification are provided in supplementary information online. The yeast two-hybrid assay was described previously (Garcia-Dominguez et al, 2006). Pull-down experiments were carried out with GST or GST–Ubc9 proteins loaded on Glutathione Sepharose 4B beads (GE Healthcare) and in vitro translated Krox20, as previously described (Garcia-Dominguez et al, 2006), or from 2 × 107 His–SUMO1-transfected cells under denaturating conditions (6 M urea) using His-Select Nickel Affinity Gel (Sigma) as indicated by the manufacturer.
Cell culture, transfection, reporter and sumoylation assays and western blot. 293T and P19 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 7% fetal bovine serum and α-modified Eagle's medium supplemented with 7.5% calf and 2.5% fetal bovine sera (PAA), respectively. For serum stimulation, P19 cells were deprived of serum for 48 h and harvested 2 h after serum re-addition. Transient transfections of plasmids or siRNA molecules were performed with Lipofectamine 2000 or Oligofectamine (Invitrogen), 36 or 48 h before harvesting the cells, respectively. The sequences of the siRNAs are provided in supplementary Table S1 online. For sumoylation assays in cells, we used 0.5 μg of RSV–Flag–Nab2, 0.15 μg of RSV–His–SUMO1/2 and 1 μg of other constructs. β-galactosidase activity of reporter construction was determined using a chemiluminescent assay (Roche). The cytomegalovirus promoter-driven luciferase expression vector pGL4.51 (Promega) was used for normalization. For western blot, cell extracts were prepared in 8 M urea, 10 mM Tris–HCl, pH8.0, and analysed using the ECL procedure (GE Healthcare). A ChemiDocXRS apparatus (Bio-Rad) was used for chemiluminescence measurement. Antibodies and in vitro sumoylation assay are detailed in supplementary information online.
Chromatin immunoprecipitation and quantitative PCR. Chromatin immunoprecipitation experiments were conducted on P19 cells. A total of 107 cells fixed in 1% formaldehyde for 10 min at 37°C were used in each experiment. The D-11 SUMO1 antibody (sc-5308, Santa Cruz Biotechnology) was used for chromatin precipitation. Quantitative PCR was used for analysis of the Id4 promoter and determination of gene expression levels, as detailed in supplementary information online. Sequence of primers is provided in supplementary Table S1 online.
In ovo electroporation, immunofluorescence and in situ hybridization. Electroporation, preparation of embryos for immunofluorescence and in situ hybridization were conducted as described previously (Giudicelli et al, 2001). Eggs were incubated at 38°C for 30 h (Hamburger & Hamilton stage 8–9) for electroporation and embryos were recovered after 24 h. For electroporation monitoring, the green fluorescent protein expression vector pEGFP-N1 (Clontech) was used at a concentration of 0.3 μg/μl. Other constructs were electroporated at a concentration of 1 μg/μl. Protocols for immunofluorescence and in situ hybridization have been described previously (Garcia-Dominguez et al, 2006). Probes and antibodies are detailed in supplementary information online. Fluorescent images were acquired on a Leica confocal microscope.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank A. Rojas and P. Gilardi-Hebenstreit for critical reading of the manuscript. We also thank personnel in the microscopy facility at CABIMER for assistance. P.G.-G. and F.J.-V. were recipients of JAE predoc (CSIC) fellowships. F.G.-C. was recipient of a JAE intro (CSIC) fellowship. This work was supported by BFU2009-10986/BMC (MICINN) and Intramural 200920I085 (CSIC) grants.
Footnotes
The authors declare that they have no conflict of interest.
References
- Chomette D, Frain M, Cereghini S, Charnay P, Ghislain J (2006) Krox20 hindbrain cis-regulatory landscape: interplay between multiple long-range initiation and autoregulatory elements. Development 133: 1253–1262 [DOI] [PubMed] [Google Scholar]
- Desmazieres A, Decker L, Vallat JM, Charnay P, Gilardi-Hebenstreit P (2008) Disruption of Krox20–Nab interaction in the mouse leads to peripheral neuropathy with biphasic evolution. J Neurosci 28: 5891–5900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmazieres A, Charnay P, Gilardi-Hebenstreit P (2009) Krox20 controls the transcription of its various targets in the developing hindbrain according to multiple modes. J Biol Chem 284: 10831–10840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Dominguez M, Reyes JC (2009) SUMO association with repressor complexes, emerging routes for transcriptional control. Biochim Biophys Acta 1789: 451–459 [DOI] [PubMed] [Google Scholar]
- Garcia-Dominguez M, Gilardi-Hebenstreit P, Charnay P (2006) PIASxβ acts as an activator of Hoxb1 and is antagonized by Krox20 during hindbrain segmentation. EMBO J 25: 2432–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Dominguez M, March-Diaz R, Reyes JC (2008) The PHD domain of plant PIAS proteins mediates sumoylation of bromodomain GTE proteins. J Biol Chem 283: 21469–21477 [DOI] [PubMed] [Google Scholar]
- Gareau JR, Lima CD (2010) The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat Rev Mol Cell Biol 11: 861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudicelli F, Taillebourg E, Charnay P, Gilardi-Hebenstreit P (2001) Krox-20 patterns the hindbrain through both cell-autonomous and non cell-autonomous mechanisms. Genes Dev 15: 567–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le N, Nagarajan R, Wang JY, Svaren J, LaPash C, Araki T, Schmidt RE, Milbrandt J (2005) Nab proteins are essential for peripheral nervous system myelination. Nat Neurosci 8: 932–940 [DOI] [PubMed] [Google Scholar]
- Lumsden A, Krumlauf R (1996) Patterning the vertebrate neuraxis. Science 274: 1109–1115 [DOI] [PubMed] [Google Scholar]
- Mager GM, Ward RM, Srinivasan R, Jang SW, Wrabetz L, Svaren J (2008) Active gene repression by the Egr2.NAB complex during peripheral nerve myelination. J Biol Chem 283: 18187–18197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechta-Grigoriou F, Garel S, Charnay P (2000) Nab proteins mediate a negative feedback loop controlling Krox-20 activity in the developing hindbrain. Development 127: 119–128 [DOI] [PubMed] [Google Scholar]
- Russo MW, Sevetson BR, Milbrandt J (1995) Identification of NAB1, a repressor of NGFI-A- and Krox20-mediated transcription. Proc Natl Acad Sci USA 92: 6873–6877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Maunoury S, Topilko P, Seitandou T, Levi G, Cohen-Tannoudji M, Pournin S, Babinet C, Charnay P (1993) Disruption of Krox-20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain. Cell 75: 1199–1214 [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Mager GM, Ward RM, Mayer J, Svaren J (2006) NAB2 represses transcription by interacting with the CHD4 subunit of the nucleosome remodeling and deacetylase (NuRD) complex. J Biol Chem 281: 15129–15137 [DOI] [PubMed] [Google Scholar]
- Svaren J, Sevetson BR, Apel ED, Zimonjic DB, Popescu NC, Milbrandt J (1996) NAB2, a corepressor of NGFI-A (Egr-1) and Krox20, is induced by proliferative and differentiative stimuli. Mol Cell Biol 16: 3545–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaren J, Sevetson BR, Golda T, Stanton JJ, Swirnoff AH, Milbrandt J (1998) Novel mutants of NAB corepressors enhance activation by Egr transactivators. EMBO J 17: 6010–6019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil T, Frain M, Gilardi-Hebenstreit P, Flenniken A, Charnay P, Wilkinson DG (1998) Segmental expression of the EphA4 (Sek-1) receptor tyrosine kinase in the hindbrain is under direct transcriptional control of Krox-20. Development 125: 443–452 [DOI] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P (1994) Krox-20 controls myelination in the peripheral nervous system. Nature 371: 796–799 [DOI] [PubMed] [Google Scholar]
- Voiculescu O, Taillebourg E, Pujades C, Kress C, Buart S, Charnay P, Schneider-Maunoury S (2001) Hindbrain patterning: Krox20 couples segmentation and specification of regional identity. Development 128: 4967–4978 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.