Abstract
One limitation for the study of chromosomal fragile sites is that they must be studied on metaphase spreads, after the breakage. We show here that bacterial lac operator (lacO) repeats are prone to spontaneous breakage, which when combined with a fluorescent lac repressor (lacR) has allowed us to track a fragile site through the cell cycle. By using this system, we show that Plk1-interacting checkpoint helicase (PICH) is already present at fragile sites during interphase, suggesting roles for this helicase beyond mitosis. In addition, we report that the oncogene Myc promotes the formation of anaphase bridges and micronuclei containing fragile-site sequences.
Keywords: anaphase bridge, PICH, common fragile site, lac operon, micronucleus
Introduction
Chromosomal fragile sites represent unstable regions of the genome, which are visualized in metaphase spreads as chromatid gaps, breaks or chromosomal rearrangements (Durkin & Glover, 2007). Although the existence of break-prone loci was observed previously, the term ‘fragile site’ was coined in 1970 with the analysis of a heritable fragile site on chromosome 16 that was present in several members of a large family (Magenis et al, 1970). On the basis of this work, most of the early efforts were oriented to the discovery of fragile sites that could be associated with familiar diseases, such as X-linked mental retardation (Harvey et al, 1977). In addition to its link to rare, heritable diseases, we now know that fragile-site breakage is also a characteristic of many cancer cells (Arlt et al, 2006). In summary, fragile-site breakage—also known as ‘expression’—has been associated with several human diseases.
One of the most important pieces of information about fragile sites is that their breakage can be stimulated in conditions of replicative stress (Sutherland, 1977, 1979; Glover, 1981). Besides its mechanistic relevance, this finding enabled easier testing of fragile-site expression in the laboratory, by growing cells in the presence of compounds that limit replication, such as low doses of the DNA polymerase inhibitor aphidicolin (APH). In addition to chemical agents, the presence of certain oncogenes can also be a source of replicative stress (Bartkova et al, 2005; Gorgoulis et al, 2005; Halazonetis et al, 2008). Moreover, oncogene-induced replicative stress has been found to occur preferentially at common fragile sites (CFSs; Tsantoulis et al, 2008). Therefore, the fact that oncogenes can generate replicative stress could explain the increased frequency of fragile site breakage detected in cancer (Dereli-Oz et al, 2011). Regardless of their association with disease, and although more than 100 fragile sites have been characterized in human cells, how, when and why fragile sites break is not completely understood.
The link between fragile sites and replicative stress indicates that conditions and sequences that perturb replication increase the fragility of a given locus. Accordingly, the chromatin at fragile sites has been shown to have features of compact chromatin (Jiang et al, 2009), and difficult-to-replicate repeats or AT-rich regions are frequently found at fragile sites (Durkin & Glover, 2007). Moreover, telomeres, which are naturally occurring repeats, have been recently identified as fragile sites (Martinez et al, 2009; Sfeir et al, 2009). On the basis of these properties, we explored whether the integration of tandem repeats of the lac operator (lacO) sequence from bacteria can generate a fragile site in mammalian cells, which when combined with a fluorescently tagged lac repressor (lacR), could be used for the visualization of fragile-site dynamics.
Results And Discussion
To analyse the fragility of a DNA sequence, we decided to focus on the lacO/lacR system from Escherichia coli. We used a previously characterized NIH-3T3 cell line that contains 256 repeats of the lacR-binding site stably integrated on chromosome 3 (3T3lacO; Soutoglou et al, 2007). When combined with a fluorescently labelled lacR, this system enables indirect visualization of the underlying lacO DNA sequence. Such a strategy has been used previously for the visualization of several nuclear activities at the lacO locus, such as RNA Pol II-mediated transcription (Darzacq et al, 2007) or DNA repair (Soutoglou et al, 2007). Given that the inserted locus is a large stretch of repeated small sequences and that the presence of lacR has been shown to promote replication blockade of lacO insertions (Payne et al, 2006), we explored whether this insertion could generate a fragile site in mammalian cells.
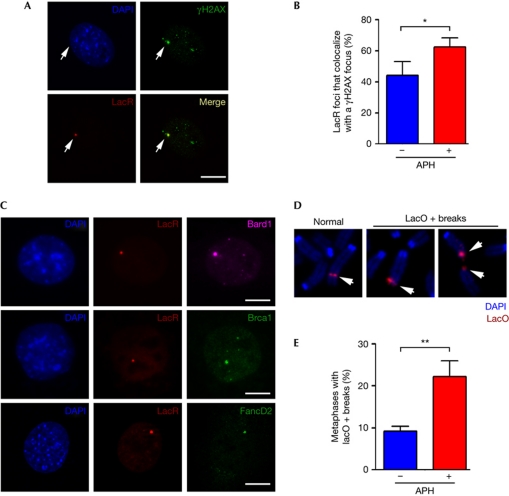
To determine whether evidence of DNA breakage could be found at the lacO locus, we first analysed the distribution of the phosphorylated form of histone H2AX (γH2AX)—a well-characterized marker of DNA double-strand breaks (Fernandez-Capetillo et al, 2004)—in 3T3lacO cells that had been transfected with a Cherry-tagged lacR. This experiment showed that 44% of the lacR spots that mark the lacO insertion colocalized with a well-defined γH2AX focus in the absence of exogenous DNA damage (Fig 1A,B). Moreover, the exposure of cells to conditions that promote fragile-site expression, such as low doses of APH, increased the percentage of lacR spots that colocalized with γH2AX (Fig 1B). Other markers of chromosomal breaks, such as Brca1 (28.2±7%) or Bard1 (24.7±4%), also colocalized with the focalized lacR signal in otherwise untreated cells (Fig 1C). In addition, a more-specific marker of fragile sites, such as FancD2 (Chan et al, 2009; Naim & Rosselli, 2009), also formed foci at the lacO locus (Fig 1C). It is noteworthy that the presence of FancD2 foci at the lacR spot was less abundant (6.3±1.2%) than the one observed for the previous proteins. Whereas these differences between the percentages could be due to several factors including antibody efficiencies, they could also reflect that FancD2 foci have been shown to mark fragile sites before the breakage (Chan et al, 2009; Naim & Rosselli, 2009). Altogether, these data suggest that the lacO locus is prone to spontaneous breakage in lacR-transfected 3T3lacO cells, and that this phenotype is exacerbated in conditions that promote fragile-site expression.
Figure 1.
Spontaneous DNA breakage at the lac operator locus. (A) Example of a lacR–Cherry-transfected 3T3lacO cell that was stained with a γH2AX-recognizing antibody. (B) Quantification of the percentage of lacR foci that colocalize with a well-defined γH2AX focus in 3T3lacO cells that had been grown in the absence or presence of aphidicolin (0.2 μM, 48 h). (C) Distribution of Brca1, Bard1 and FancD2 in lacR-transfected 3T3lacO cells. Scale bars, 2.5 μm. (D) FISH analysis of 3T3lacO metaphases with a DNA probe against the lacO insertion (red). Images show an intact chromosome 3 containing the insertion (left), and two examples of chromosomal breaks at the lacO locus. Both derivative fragments are present in the far right example. White arrowheads indicate the position of the lacO FISH signals. (E) Quantification of the percentage of metaphases that presented a chromosomal break at the lacO locus, as shown above. Numbers are shown for 3T3lacO cells that had been grown in the absence or presence of aphidicolin (0.2 μM, 48 h). Error bars indicate s.d. from three independent experiments. *P<0.05; **P<0.01; Student's t-test. APH, aphidicolin; DAPI, 4,6-diamidino-2-phenylindole; FISH, fluorescence in situ hybridization; lacO, lac operator; lacR, lac repressor.
To demonstrate breakage of the lacO insertion, we performed fluorescence in situ hybridization (FISH) analysis with a probe prepared from a plasmid containing the lacO repeats. FISH analyses from 3T3lacO cells revealed a significant number of metaphases showing spontaneous breaks involving the lacO insertion (Fig 1D,E). Chromosomal breaks in which the two resulting fragments harbouring the lacO sequence were still nearby were also detectable, suggesting that the break occurred within the insertion (Fig 1D). Importantly, all spontaneous breaks that could be observed on 3T3lacO cells involved a lacO-positive FISH signal at one end, which suggests that the lacO insertion was the most important source of increased spontaneous breakage in these cells. Finally, and similarly to our observations of foci in interphase cells, breaks containing lacO sequences were also induced by low doses of APH (Fig 1E). Altogether, these results indicate that the lacO repeats are prone to spontaneous breakage in mammalian cells in a manner that resembles the behaviour of CFS.
An important paradox about fragile sites is that they are conserved throughout evolution, which is counterintuitive given that, in principle, they pose a potential threat to genomic integrity. Data derived from two recent works indicate that their conservation might be due to the fact that their presence might be necessary for proper chromosome cohesion (Chan et al, 2009; Naim & Rosselli, 2009). As DNA sequences at fragile sites are difficult to replicate, cells could frequently go into mitosis with unreplicated DNA or catenated sister chromatids at these loci. Such structures would provide an extra layer of inter-sister chromatid cohesion, which would then be normally dissolved in mitosis by a network that involves topoisomerases and the Bloom (BLM) helicase. In agreement with this view, culture conditions that promote fragile-site expression derive into frequent anaphase bridges that are enriched in BLM (Chan et al, 2007). We next evaluated whether the frequent breakage observed at the lacO locus could fit with the recently proposed model for endogenous fragile sites. We reasoned that if the lacO insertion behaves as a bona fide fragile site, it should be frequently (1) improperly segregated into daughter cells and (2) observed at anaphase bridges. In addition, both phenotypes should be aggravated in conditions that promote fragile-site expression.
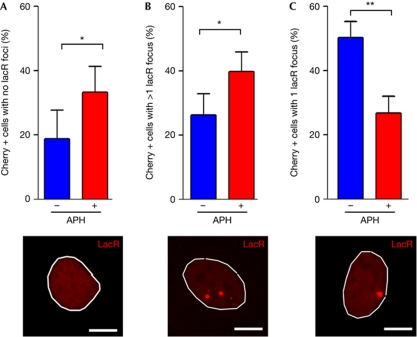
To analyse the segregation of the lacO locus, 3T3lacO cells were transfected with lacR, and the number of lacR spots present in each cell was scored 48 h after transfection. This analysis showed that 18% of the cells that were positive for Cherry lacked a detectable lacRC spot (Fig 2A). Whereas the absence of a lacR spot could not only be explained by an imbalanced segregation of the lacO locus, a similar percentage of Cherry-positive cells presented more than one lacR focus (Fig 2B). Moreover, and consistent with these phenotypes being associated with fragile-site dynamics, they were all aggravated in the presence of APH. The percentage of 3T3lacO cells that had one lacR focus decreased in the presence of the drug (Fig 2C). At the same time, the percentage of Cherry-positive cells that had either no detectable lacR focus or several lacR foci increased when cells were cultured in the presence of APH (Fig 2A–C). In summary, these data show that the lacO insertion is prone to improper segregation, a phenotype that is enhanced in conditions that promote fragile-site expression.
Figure 2.
Deficient segregation of the lac operator insertion. Percentages of 3T3lacO cells that had been transfected with lacR for 48 h, were positive for Cherry and had (A) zero, (B) more than one or (C) one lacR focus. Cells were grown in the absence or presence of aphidicolin (0.2 μM, 48 h). Representative examples of each case are shown below the graphs. Scale bars, 2.5 μm. Error bars indicate s.d. from three independent experiments. *P<0.05; **P<0.01; Student's t-test. APH, aphidicolin; lacO, lac operator; lacR, lac repressor.
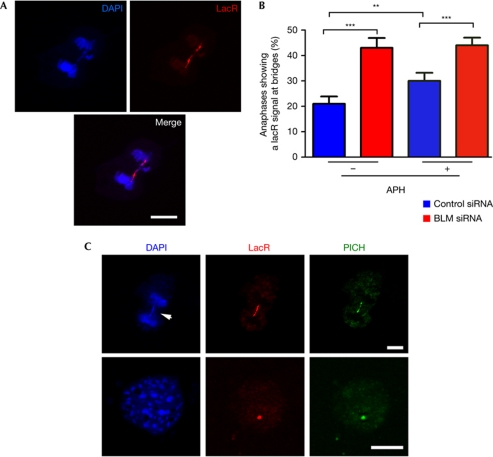
As mentioned above, a recent study revealed that CFSs are frequently found in anaphase bridges (Chan et al, 2009), indicating that fragile-site breakage could be the consequence of the resolution of inter-sister chromatid linkages during mitosis. In agreement with the lacO insertion behaving as a fragile site, the analysis of mitotic figures in 3T3lacO cells showed that the lacR signal was observed at an anaphase bridge in around one-fifth of all anaphases (Fig 3A,B). The percentage of lacR-positive anaphase bridges was enhanced in the presence of low doses of APH, reinforcing its link with the ontogeny of fragile sites (Fig 3B). Co-transfection of lacR with a green fluorescent protein-tagged H2B allowed us to track this phenomenon in living cells (supplementary Movie 1), which showed that the lacO sequence underwent significant stretching and contraction at anaphase bridges. We believe that this example illustrates the mechanical forces that might affect stability of fragile sites in mitosis.
Figure 3.
PICH coats anaphase bridges at the lac operator locus. (A) Example of an anaphase from 3T3lacO cells that had been transfected with lacR for 48 h, presenting an anaphase bridge that is positive for lacR (red). Scale bars, 2.5 μm. (B) Quantification of the percentage of anaphases showing a lacR signal at an anaphase bridge. Cells were transfected with lacR for 48 h, and then grown in the presence or absence of aphidicolin (0.2 μM, 48 h), or subsequently transfected with BLM-targeting siRNAs for an additional 48 h. Error bars indicate s.d. from three independent experiments. **P<0.01; ***P<0.001; Student's t-test. (C) Representative examples of the distribution of PICH (green) and lacR (red) in an anaphase bridge (top) and an interphase cell (bottom) obtained from 3T3lacO cells that had been transfected with lacR for 48 h. Scale bars, 2.5 μm. The white arrowhead indicates the presence of DAPI at the bridge. APH, aphidicolin; BLM, Bloom syndrome protein; DAPI, 4,6-diamidino-2-phenylindole; lacO, lac operator; lacR, lac repressor; PICH, Plk1-interacting checkpoint helicase; siRNA, small-interfering RNA.
Previous studies have shown that the BLM helicase localizes to and suppresses anaphase bridges (Chan et al, 2007). Further analyses showed that BLM was particularly relevant for the suppression of anaphase bridges involving chromosomal fragile sites (Chan et al, 2009; Naim & Rosselli, 2009). In agreement with this model, RNA interference-mediated knockdown of BLM increased the percentage of anaphases that presented a lacR-positive bridge signal on 3T3lacO cells (Fig 3B). It is noteworthy that the anaphase bridges containing lacO repeats presented DNA at the bridge that was detectable by 4,6-diamidino-2-phenylindole (DAPI) staining. However, a recent study reported a distinct class of anaphase bridges that showed no obvious signal for DAPI, but which were detectable with an antibody that recognizes the Plk1-interacting checkpoint helicase (PICH; Chan et al, 2007). These structures are known as ultrafine bridges. Nevertheless, to what extent ultrafine bridges are distinct from other anaphase bridges remains to be clarified. For instance, BLM was shown to suppress both DAPI-positive and DAPI-negative anaphase bridges (Chan et al, 2007). In agreement with this idea, 67% of the anaphase bridges involving lacO repeats were also recognized by the PICH antibody, regardless of the presence of DAPI (Fig 3C). Interestingly, a significant fraction (27±2%) of lacR foci colocalized with a distinct PICH spot on interphase cells (Fig 3C), suggesting that PICH might recognize fragile sites at other stages besides mitosis.
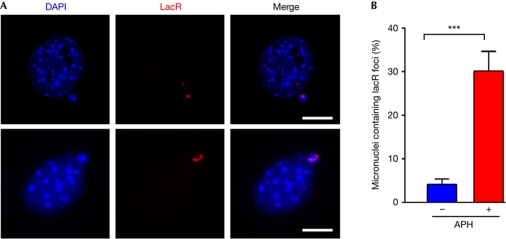
One limitation in the study of anaphase bridges is the short duration of mitosis. However, anaphase bridges have been shown to frequently derive into micronuclei on daughter cells (Hoffelder et al, 2004), so that the presence of micronuclei could be used as a surrogate marker of past anaphase bridges. In agreement with anaphase bridge data, 3T3lacO cells had an increased number of micronuclei containing lacR signals, even more so when cells were cultured in the presence of APH (Fig 4A,B). It is noteworthy that some of these micronuclei presented several lacR foci (Fig 4A), reinforcing the link between a deficient segregation as the origin of micronuclei. Together, these data indicate that the mechanism of breakage of the lacO insertion is similar to that reported for CFS, and suggest that the role of PICH in dealing with fragile sites might extend from that observed at mitosis.
Figure 4.
Aphidicolin induces micronuclei containing lac operator repeats. (A) Examples of 3T3lacO cells that had been transfected with lacR for 48 h, presenting micronuclei that are positive for lacR (red). The bottom panels show an example containing several lacR signals on the same micronucleus. Scale bars, 2.5 μm. (B) Quantification of the percentage of micronuclei containing lacR signals. Cells were transfected with lacR for 48 h, and then grown in the presence or absence of aphidicolin (0.2 μM, 48 h). Error bars indicate s.d. from three independent experiments. ***P<0.001; Student's t-test. APH, aphidicolin; DAPI, 4,6-diamidino-2-phenylindole; lacO, lac operator; lacR, lac repressor.
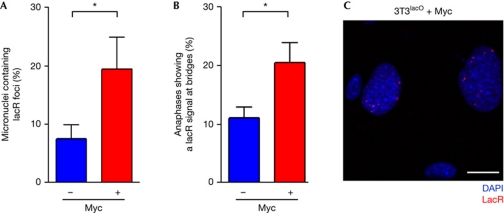
As mentioned, several oncogenes have been shown to be a source of replicative stress, which would limit the expansion of early malignant lesions through the activation of the DNA damage response (DDR; Halazonetis et al, 2008). In particular, the Myc oncogene has been shown to generate significant amounts of replicative stress (Herold et al, 2009). In the context of this model, we reasoned that the replicative stress induced by the oncogenes might not be intrinsically distinct from that induced by APH and could therefore also promote the formation of anaphase bridges or micronuclei containing lacO sequences. In agreement with this, transduction of 3T3lacO cells with a Myc-expressing retrovirus led to an significant increase in the number of micronuclei and anaphase bridges that presented lacR signals (Fig 5A,B). In addition, the presence of Myc led to the appearance of cells (12±2.5%) containing large numbers of lacR foci, which were not found on control cells (Fig 5C). Altogether, these data indicate that the stability of the lacO integration site is perturbed by the presence of the Myc oncogene.
Figure 5.
Myc increases the instability of lac operator repeats. (A) Quantification of the percentage of micronuclei containing lacR signals in 3T3lacO cells that had been infected with Myc overexpressing retroviruses for 72 h. (B) Quantification of the percentage of anaphases showing a lacR signal at an anaphase bridge in 3T3lacO cells that had been infected with Myc overexpressing retroviruses for 72 h. Control cells were infected with retroviruses derived from the empty vector. (C) Example from Myc-infected 3T3lacO cells that illustrates the presence of multiple lacR signals in these cells. LacR was transfected 24 h before the analysis. Error bars indicate s.d. from three independent experiments. *P<0.05; Student's t-test. DAPI, 4,6-diamidino-2-phenylindole; lacO, lac operator; lacR, lac repressor.
We present data here to illustrate that the introduction of several tandem repeats of bacterial lacO provides a traceable fragile site in mammalian cells. As mentioned before, the lacR has been shown to promote a replication block at lacO sequences (Payne et al, 2006), so it is possible that the repressor could not only mark, but also facilitate the expression of this fragile site. In fact, while we were resubmitting this work, an independent study in yeast has shown that the introduction of lacO sequences also generates a fragile site in Schizosaccharomyces pombe, which was stimulated by the lacR (Sofueva et al, 2011). Our study extends these findings and shows the breakage of the fragile site in metaphase chromosomes in mammalian cells. Moreover, our work shows that the PICH helicase might have a more active role on fragile sites beyond mitosis, and for the first time reveals that oncogenes can stimulate the generation of anaphase bridges and micronuclei containing fragile-site sequences. Altogether, we believe that the system provides a powerful platform for investigating the biology of fragile sites. The system could, for example, be used to obtain an in cellulo view of new proteins and chromatin modifications that mark fragile sites in interphase cells or anaphase bridges. A more-comprehensive characterization of the mechanisms responsible for resolving anaphase bridges remains a key question for future studies of the origin of fragile-site breakage.
Finally, we note that many mammalian studies are exploiting the power of this lacO/lacR system to bring factors to a known chromatin location. For instance, 3T3lacO cells were previously used to claim that a DNA break was not necessary to activate the DDR (Soutoglou & Misteli, 2008). The strategy used was to bring DDR factors to the lacO sequence by tethering them to the lacR, which showed an activation of the DDR by the presence of lacR foci that colocalized with some of the factors analysed here, such as γH2AX. However, although it is possible that bringing DDR factors to chromatin might activate a DDR, the data presented here should be taken into account when interpretting these observations. Given the wide use of this strategy among cell biologists, we believe that the inherent fragility of the lacO insertion should be considered in determining the suitability of such an approach.
Methods
Cells and plasmids. Cells (3T3lacO) and the Cherry-conjugated lacR expression plasmid have been described before and were kindly provided by T. Misteli (Soutoglou et al, 2007). The plasmid was transfected using Lipofectamine 2000 (Invitrogen), following the manufacturer's instructions. BLM-targeting validated small-interfering RNA sequences were obtained from Invitrogen, transfected at 20 nM with Lipofectamine 2000, and knockdown was verified by quantitative reverse transcription polymerase chain reaction 48 h after transfection.
Immunofluorescence. The primary antibodies used in this work were γH2AX (Upstate Biotechnology), FANCD2 (Novus Biologicals), Brca1 (gift from A. Nussenzweig, National Cancer Institute, USA), PICH (gift from I. Hickson, University of Copenhagen, Denmark) and Bard1 (gift from J. Chen, MD Anderson, USA). For immunofluorescence, secondary antibodies conjugated with Alexa 647 or Alexa 488 (Molecular Probes) were used at 1:250. The lacR signal coming from the lacR–Cherry construct was imaged without extra antibodies. Image acquisition was done by using a Zeiss Axioimager Z1 microscope equipped with an Apotome system of structured illumination.
Live-cell imaging. For live-cell imaging, 3T3lacO cells that had been previously transfected with a human H2B–enhanced green fluorescent protein expression plasmid (Addgene) were cultured in DMEM (Lonza) supplemented with 10% FCS and antibiotics. Cells were grown on glass-bottomed P96-Well plates (Greiner Bio-One 96-Well Microplates) and maintained in a 37°C environmental chamber with CO2. All live microscopy was carried out on a Leica Workstation AF6000, with a × 63 objective. The acquisition was done every 30 s in 10 different Z-axis plan. The images from the same acquisition time were merged and cut using the Leica Las AF software (v.1.3).
lacO FISH. FISH of the lacO sequences was performed as described previously (Soutoglou et al, 2007). Briefly, 3T3lacO cells were incubated in colcemid (Invitrogen) for 3 h, swollen in pre-warmed 50 mM KCl, fixed in methanol/acetic acid (3:1), and air-dried on slides overnight. FISH was performed with a probe labelled with biotin-dUTP generated from a plasmid containing the lacO insertion DNA (gift from T. Misteli). The FISH probe was precipitated in 50% formamide, 20% dextran sulphate and 2 SSC, and hybridized to slides containing metaphase spreads for 48 h at 37°C. Slides were then washed three times in 50% formamide in 2 × SSC at 45°C, three times in 0.1 × SSC at 60°C and incubated for 45 min at 37°C with avidin–fluorescein isothiocyanate (1:200) diluted in 4 × SSC, 0.1% Tween20. The slides were finally washed three times in 4 SSC, 0.1% Tween20 at 45°C and preserved in DAPI-containing mounting media. The imaging was done as described above.
Myc overexpression. pBabe-puro-MycER (Addgene) was retrovirally transduced into 3T3lacO cells following standard procedures. Infected cells were selected with 1.5 μg/ml puromycin for 2–3 days and subsequently grown in the presence of 4-hydroxytamoxifene for 48 h for Myc activation. The empty pBabe-puro vector (Addgene) was used as a control.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank T. Misteli and I. Hickson for providing reagents. Work in O.F.'s laboratory is supported by grants from the Spanish Ministry of Science (CSD2007-00017 and SAF2008-01596), EMBO Young Investigator Programme and the European Research Council (ERC-210520).
Author Contributions: A.J. conducted all of the experiments. O.F.-C. supervised the research and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Arlt MF, Durkin SG, Ragland RL, Glover TW (2006) Common fragile sites as targets for chromosome rearrangements. DNA Repair (Amst) 5: 1126–1135 [DOI] [PubMed] [Google Scholar]
- Bartkova J et al. (2005) DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434: 864–870 [DOI] [PubMed] [Google Scholar]
- Chan KL, North PS, Hickson ID (2007) BLM is required for faithful chromosome segregation and its localization defines a class of ultrafine anaphase bridges. EMBO J 26: 3397–3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KL, Palmai-Pallag T, Ying S, Hickson ID (2009) Replication stress induces sister-chromatid bridging at fragile site loci in mitosis. Nat Cell Biol 11: 753–760 [DOI] [PubMed] [Google Scholar]
- Darzacq X, Shav-Tal Y, de Turris V, Brody Y, Shenoy SM, Phair RD, Singer RH (2007) In vivo dynamics of RNA polymerase II transcription. Nat Struct Mol Biol 14: 796–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereli-Oz A, Versini G, Halazonetis TD (2011) Studies of genomic copy number changes in human cancers reveal signatures of DNA replication stress. Mol Oncol [Epub 20 May] doi:10.1016/j.molonc.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin SG, Glover TW (2007) Chromosome fragile sites. Annu Rev Genet 41: 169–192 [DOI] [PubMed] [Google Scholar]
- Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A (2004) H2AX: the histone guardian of the genome. DNA Repair (Amst) 3: 959–967 [DOI] [PubMed] [Google Scholar]
- Glover TW (1981) FUdR induction of the X chromosome fragile site: evidence for the mechanism of folic acid and thymidine inhibition. Am J Hum Genet 33: 234–242 [PMC free article] [PubMed] [Google Scholar]
- Gorgoulis VG et al. (2005) Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434: 907–913 [DOI] [PubMed] [Google Scholar]
- Halazonetis TD, Gorgoulis VG, Bartek J (2008) An oncogene-induced DNA damage model for cancer development. Science 319: 1352–1355 [DOI] [PubMed] [Google Scholar]
- Harvey J, Judge C, Wiener S (1977) Familial X-linked mental retardation with an X chromosome abnormality. J Med Genet 14: 46–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold S, Herkert B, Eilers M (2009) Facilitating replication under stress: an oncogenic function of MYC? Nat Rev Cancer 9: 441–444 [DOI] [PubMed] [Google Scholar]
- Hoffelder DR, Luo L, Burke NA, Watkins SC, Gollin SM, Saunders WS (2004) Resolution of anaphase bridges in cancer cells. Chromosoma 112: 389–397 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lucas I, Young DJ, Davis EM, Karrison T, Rest JS, Le Beau MM (2009) Common fragile sites are characterized by histone hypoacetylation. Hum Mol Genet 18: 4501–4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenis RE, Hecht F, Lovrien EW (1970) Heritable fragile site on chromosome 16: probable localization of haptoglobin locus in man. Science 170: 85–87 [DOI] [PubMed] [Google Scholar]
- Martinez P, Thanasoula M, Munoz P, Liao C, Tejera A, McNees C, Flores JM, Fernandez-Capetillo O, Tarsounas M, Blasco MA (2009) Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev 23: 2060–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim V, Rosselli F (2009) The FANC pathway and BLM collaborate during mitosis to prevent micro-nucleation and chromosome abnormalities. Nat Cell Biol 11: 761–768 [DOI] [PubMed] [Google Scholar]
- Payne BT, van Knippenberg IC, Bell H, Filipe SR, Sherratt DJ, McGlynn P (2006) Replication fork blockage by transcription factor-DNA complexes in Escherichia coli. Nucleic Acids Res 34: 5194–5202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL, de Lange T (2009) Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 138: 90–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofueva S, Osman F, Lorenz A, Steinacher R, Castagnetti S, Ledesma J, Whitby MC (2011) Ultrafine anaphase bridges, broken DNA and illegitimate recombination induced by a replication fork barrier. Nucleic Acids Res [Epub 16 May] doi:10.1093/nar/gkr340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutoglou E, Dorn JF, Sengupta K, Jasin M, Nussenzweig A, Ried T, Danuser G, Misteli T (2007) Positional stability of single double-strand breaks in mammalian cells. Nat Cell Biol 9: 675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutoglou E, Misteli T (2008) Activation of the cellular DNA damage response in the absence of DNA lesions. Science 320: 1507–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland GR (1977) Fragile sites on human chromosomes: demonstration of their dependence on the type of tissue culture medium. Science 197: 265–266 [DOI] [PubMed] [Google Scholar]
- Sutherland GR (1979) Heritable fragile sites on human chromosomes I. Factors affecting expression in lymphocyte culture. Am J Hum Genet 31: 125–135 [PMC free article] [PubMed] [Google Scholar]
- Tsantoulis PK et al. (2008) Oncogene-induced replication stress preferentially targets common fragile sites in preneoplastic lesions. A genome-wide study. Oncogene 27: 3256–3264 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.