Abstract
Morphogens are conserved, secreted signalling molecules that regulate the size, shape and patterning of animal tissues and organs. Recent experimental evidence has emphasized the fundamental role of tissue growth in expanding the expression domains of morphogens and their target genes, in generating morphogen gradients and in modulating the response of cells to morphogens. Moreover, the classic view of how morphogens, particularly through their concentration gradient, regulate tissue size during development has been revisited recently. In this review, we discuss how morphogens and tissue growth affect each other, and we attempt to integrate genetic and molecular evidence from vertebrate and invertebrate model systems to put forward the idea that the interaction between growth and morphogens is a general feature of highly proliferative tissues.
Keywords: morphogens, growth, limb development, Drosophila , vertebrate
See Glossary for abbreviations used in this article.
Glossary.
- Dpp
Decapentaplegic
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- Hh
Hedgehog
- Shh
Sonic hedgehog
- TGF-β
transforming growth factor-β
- Wg
Wingless
Introduction
Secreted signalling proteins of the Wnt, Hh, EGF, FGF and TGF-β families have been shown to act as morphogens to specify cell identities within tissues (reviewed in Benazet & Zeller, 2009; Gallet, 2011; Kutejova et al, 2009; Meinhardt, 2009; Wartlick et al, 2009). These morphogens are secreted into the extracellular medium, in which they bind to their receptors, triggering changes in gene expression. They are produced and released from a local source and spread to the rest of the tissue, forming a concentration gradient in which the highest morphogen levels are at the source. These gradients set the transcriptional state of target genes in discrete domains of gene expression as a function of their distance from the source according to morphogen-concentration thresholds. These domains are used to define cell identities and tissue pattern. The formation of the gradient and its interpretation by the receiving cells is precisely regulated to ensure proper patterning of the tissue. This regulation can occur at several levels, including the rates of synthesis, diffusion and degradation, as well as interaction with extracellular factors that can enhance or inhibit signal transduction in the receiving cells (reviewed in Kutejova et al, 2009). Thus, it is well accepted that morphogen gradients are precisely regulated in time and space. However, the way in which the expression and distribution of morphogens and the response of the signal-receiving cells are influenced by tissue growth is only starting to be understood. The impact of growth on the establishment and function of morphogen gradients adds a new layer of complexity in highly proliferative tissues. Studies of limb development, in both vertebrates and invertebrates, have been instrumental in our understanding of morphogen function, particularly of the way in which morphogens and tissue growth affect each other. Recent experimental evidence from Drosophila and vertebrate tissues has shed light on the contribution of tissue growth to the generation of morphogen gradients, the modulation of cellular responses to these gradients, and the expansion of the expression domains of morphogens and their target genes. In this review, we discuss recent findings that support the idea that the interplay between growth and morphogens is a general feature of highly proliferative and growing tissues.
Growth expands gene expression domains
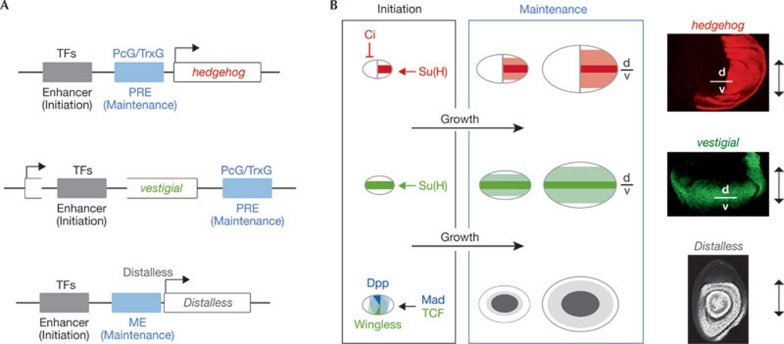
In mature vertebrate and invertebrate limb primordia, morphogens and their targets are expressed in large domains that consist of several thousand cells. It is pertinent to ask whether mechanisms exist that contribute to maintaining the expression domains of morphogens and their targets during the proliferative stages of limb primordia and, most importantly, whether proliferation per se contributes to the expansion of these expression domains. Three illustrative examples in Drosophila demonstrate that this is the case. Drosophila limb primordia are subdivided into compartments, adjacent cell populations that do not mix during the proliferative stages (García-Bellido et al, 1973). Stable subdivision into anterior and posterior compartments is a consequence of asymmetrical signalling by Hh from posterior to anterior cells (Dominguez et al, 1996; Tabata et al, 1995; Zecca et al, 1995). Only posterior cells express Hh and only anterior cells respond to it. Dissection of the cis-regulatory elements of the hh gene has revealed a new mechanism that contributes to the regulation of its expression in the developing wing (Fig 1; Pérez et al, 2011). This mechanism is based on the communication between an enhancer region and a Polycomb responsive element (PRE), both located upstream from hh. The enhancer region initiates gene expression in a posterior stripe that corresponds to the dorsal–ventral compartment boundary in the early wing primordium. The PRE functions as a memory module (Bejarano & Milan, 2009; Maurange & Paro, 2002) and contributes to maintaining and expanding the active transcriptional state of hh throughout the posterior compartment of the primordium. The identification of this mechanism was based on the observation that a large fraction of the cells that give rise to the mature wing are born at the dorsal–ventral boundary and displaced out of this domain by growth of the primordium. Once these cells leave the dorsal–ventral boundary they maintain hh expression, and this maintenance depends on the activity of Polycomb and Trithorax proteins (Pérez et al, 2011), which bind to the PREs and maintain the active or repressive transcriptional state of the adjacent gene. Thus, a ‘trigger-maintenance’ mechanism contributes to expansion of the expression domain of hh throughout the posterior compartment by tissue growth (Pérez et al, 2011).
Figure 1.
Growth contributes to expanding the expression domains of morphogens and their target genes. (A) Distinct molecular mechanisms contribute to the expansion and robust expression of morphogens and their target genes in highly proliferative Drosophila tissues. Transcription factors (TFs) that are active in restricted domains bind to cis-regulatory elements (enhancers) and activate or repress gene expression (note that the vestigial enhancer is located in an intron). The Polycomb responsive element (PRE) and maintenance element (ME) act as memory modules and contribute, together with tissue growth, to the expansion of the expression domain of these genes. (B) The ‘trigger-maintenance’ mechanism contributes to the expression of hedgehog (in red) and vestigial (in green) in the Drosophila wing, as well as Distalless (grey) in the Drosophila leg. hh and vestigial are initially expressed in a restricted pattern (dark red and dark green, respectively). As the tissue grows, new cells (light red and light green, respectively) inherit the expression of these genes by an epigenetic mechanism that depends on the activity of PcG and TrxG. In the case of Distalless, a leg trigger element integrates Wg and Dpp inputs (dark grey). As cells proliferate, Distalless binds to a maintenance element in an autoregulatory loop that sustains morphogen-independent expression in newly generated cells (light grey). Su(H), TCF and Mad are the nuclear effectors of the Notch, Wg and Dpp signalling pathways, respectively. d, dorsal; Dpp, Decapentaplegic; hh, hedgehog; PcG, Polycomb group proteins; Su(H), Suppressor of Hairless; TrxG, Trithorax group proteins; v, ventral; Wg, Wingless.
This trigger-maintenance mechanism contributes not only to morphogen expression, but also to the spread of morphogen-regulated gene expression. At the time the wing primordium consists of about 1,000 cells, Wg (the founding member of the Wnt family) is already expressed in a stripe corresponding to the dorsal–ventral compartment boundary. It spreads to form a gradient and sets the transcriptional state of target genes such as vestigial in graded domains (Fig 1; Neumann & Cohen, 1997; Zecca et al, 1996). Unexpectedly, once the expression of vestigial is initiated, its expression can be maintained even in the absence of Wg protein or in cells lacking the Wg receptor (Piddini & Vincent, 2009). These surprising results support the idea that vestigial expression is regulated not only by Wg, but also by other redundant mechanisms. Indeed, enhancer–PRE communication also seems to contribute to vestigial expression. The well-known Boundary-Enhancer of vestigial (Williams et al, 1993) initiates gene expression at the dorsal–ventral boundary, whereas a PRE located in the vestigial locus contributes to expansion—by means of tissue growth—of the expression domain of this gene at both sides of this boundary (Fig 1; Pérez et al, 2011). The first in vivo functional validation of a PRE was recently provided for a vertebrate gene (Sing et al, 2009). Thus, the role of enhancer–PRE communication in expanding the expression domains of morphogens and their target genes in the developing fly wing might open up new avenues for the identification of similar mechanisms in vertebrate tissues.
A similar trigger-maintenance mechanism has been shown to contribute to expansion of the expression domains of target genes in the fly leg primordium. Expression of Distalless in the leg primordium is based on separable cis-regulatory elements that initiate and maintain transcriptional activity (Fig 1; Estella et al, 2008). A leg-trigger element directly integrates Wg and Dpp inputs and is active only in cells receiving high levels of both signals, whereas a maintenance element sustains morphogen-independent expression. In the case of distalless, the maintenance element functions as an autoregulatory element by directly recruiting the Distalless protein itself. Thus, distinct molecular mechanisms contribute to the expansion and robust expression of morphogens and their target genes in highly proliferative Drosophila tissues. However, it is also worth noting that morphogen-expression domains do not always expand during tissue growth. For example, Dpp and Wg in Drosophila limbs and Shh in vertebrate limbs show an expression pattern that is restricted to certain domains, indicating the existence of negative-feedback loops (Chen & Struhl, 1996; Rulifson et al, 1996) or signalling events between adjacent cell populations (Niswander et al, 1994) that counteract the effects of tissue growth on gene expression.
Growth modulates the response to morphogens
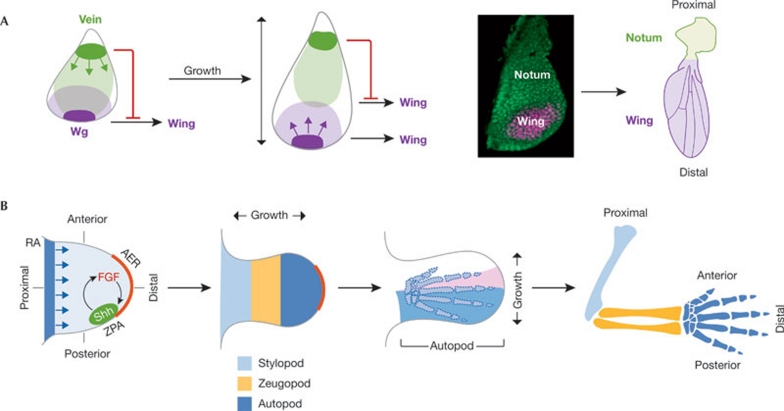
Morphogens are produced and secreted from localized sources within vertebrate and invertebrate limb primordia and they spread to the rest of the tissue, establishing a concentration gradient. As these primordia are highly proliferative, it is also relevant to assess whether the increase in distance from the source caused by tissue growth modulates the response to morphogens and, most importantly, whether this modulation has an important role in driving developmental decisions. Several examples in Drosophila and vertebrate limbs demonstrate that this is the case. The wing primordium of Drosophila contains the progenitors of the adult thoracic body wall (notum) and the adult wing (Bryant, 1975). The developmental decision between wing and body wall is made early in development, when the wing primordium consists of a few hundred cells. This decision is defined by the opposing activities of two secreted signalling molecules, Wg and the EGF-receptor ligand Vein—a homologue of vertebrate EGF—that are expressed at the most ventral and dorsal sides of the wing primordium, respectively (Fig 2A; Ng et al, 1996; Wang et al, 2000; Zecca & Struhl, 2002). The relative concentrations of Wg and Vein experienced by disc cells direct their fate to become part of the wing or body wall. Wg induces wing-fate specification and restricts the expression of Vein to the most dorsal side of the early wing primordium, whereas Vein antagonizes Wg activity. Notably, the expression of these two molecules is established long before the cells are competent to respond to them in the presumptive wing primordium (Wu & Cohen, 2002). This observation raises questions about the nature of the mechanism that underlies temporal regulation of the competency of the cells in the primordium, to decide between wing and notum fate specification. Studies in the Drosophila eye–head primordium have shed some light on this issue (Kenyon et al, 2003). In this developmental context, specification of eye and head cellular fates also depends on the opposing activities of two morphogens expressed in the opposite sides of the primordium. In this case, Dpp specifies the eye field and Wg specifies head structures. Tissue growth was proposed to pull the sources of these two counteracting morphogens apart, thus facilitating the response of the cells to the eye-inducing activity of Dpp. Experimental evidence from the wing primordium indicates that an analogous increase in the size of the tissue is also the mechanistic trigger in specification of the wing field (Rafel & Milan, 2008). In the early wing primordium, Vein reaches every cell in the primordium, blocks responsiveness to Wg and represses wing-fate specification (Fig 2A). An increase in the size of the tissue also increases the distance between the sources of Wg and Vein. Consequently, ventral cells do not receive sufficient levels of Vein to block Wg-mediated wing-fate specification. If tissue growth is experimentally inhibited, the ability of ventral cells to respond to Wg remains blocked by Vein and, as a result, the wing is not defined and a duplication of notum structures can be observed. Therefore, tissue growth controls eye- and wing-fate specification in an elegant manner by modulating the exposure of the cells to the activities of signalling molecules. Interestingly, loss and recovery of wings has occurred during the course of insect evolution (Whiting et al, 2003), suggesting that the developmental potential to generate a particular structure is maintained and that adaptive changes in animal size might drive some of these extraordinary, reversible transitions simply by modulating the cellular response to morphogens.
Figure 2.
Growth modulates the response to morphogens. (A) The developmental decision between wing and body wall is defined by the antagonistic activities of Wg and Vein, expressed at opposite sides of the wing primordium. Early in development, high levels of Vein block Wg-induced wing-fate specification. An increase in the size of the tissue pulls the sources of Wg and Vein apart, facilitating the response of the cell to the wing-inducing activity of Wg. Wg- and Vein-expressing cells are shown in dark purple or dark green, respectively, and the range of diffusion is depicted in light purple or light green. (B) Two signalling centres are found in the vertebrate limb primordium: FGFs are synthesized in the AER (in red), and Shh is produced in the ZPA. Through a positive feedback, Shh and FGFs maintain each others' expression, and the loop is crucial for producing a normal limb structure. Growth of the vertebrate limb-bud along the anterior–posterior axis and specification of the digits is regulated by Shh. Tissue growth helps to generate the Shh gradient and expands the Shh non-expressing domain. Cells far from the source, and thus receiving low levels of Shh, generate anterior digits. Cells exposed to higher levels of Shh and for a longer period generate posterior digits. Specification of cell-fate identities along the proximal–distal axis, in turn, is controlled by the antagonistic activities of retinoic acid and FGFs, which are expressed at opposite sides of the limb bud. Growth along the proximal–distal axis modulates the response of cells to FGFs and retinoic acid, and helps in generation of the proximal (stylopod), medial (zeugopod) and distal (autopod) segments of the adult limb. AER, apical ectodermal ridge; FGF, fibroblast growth factor; RA, retinoic acid; Shh, Sonic hedgehog; Wg, Wingless; ZPA, zone of polarizing activity.
Growth also modulates the response of the tissue to morphogens in vertebrate limbs. Two classic signalling centres are found in the vertebrate limb primordium (Fig 2B; reviewed in Zeller et al, 2009). The distal outgrowth of the primordium requires the activity of several FGFs that are synthesized in the apical ectodermal ridge (AER), and patterning and growth of the anterior–posterior axis of the limb relies on the activity of Shh, which is produced by a second signalling centre, the zone of polarizing activity (ZPA) in the posterior mesenchyme (reviewed in Towers & Tickle, 2009). Through a positive-feedback loop, Shh and FGFs maintain each others expression (Niswander et al, 1994), and this loop is crucial for producing a normal limb structure (Fig 2B). An increase in the distance between the AER and the ZPA cells breaks down the Shh–FGF loop so that its expression decreases and the limb stops growing (Scherz et al, 2004; Verheyden & Sun, 2008). Thus, growth promoted by the activity of Shh and FGF modulates the response of the tissue to these morphogens and contributes to the termination of limb-bud growth.
Growth of the vertebrate limb primordium along the anterior–posterior axis and specification of the digits is mediated by the activity of Shh diffusing from the ZPA (Fig 2B). Growth along the anterior–posterior axis is fundamental to specifying digits, as tissue-growth inhibition leads to the development of limbs with only posterior digits (Towers et al, 2008). This defect is attributed to the fact that every cell in the now reduced limb primordium is exposed to high levels of Shh for a longer period of time (Harfe et al, 2004; Towers et al, 2008). Growth promoted by Shh induces an expansion of the Shh non-expressing domain. Cells far from the source and receiving low levels of Shh generate anterior digits, whereas cells that are exposed to higher levels of Shh for a longer period of time generate posterior digits. In summary, tissue growth helps to generate the Shh gradient, which is fundamental to specifying digit identities along the anterior–posterior axis.
Although several models have been proposed to explain the proximal–distal patterning of vertebrate limbs, recent experimental evidence in the chicken limb primordium indicates that specification of proximal, medial and distal segments of adult limbs relies on the opposing activities of FGFs—expressed in the AER and acting as distalizers—and retinoic acid, produced in the body trunk and acting as a proximalizer (Fig 2B; Cooper et al, 2011; Rosello-Diez et al, 2011). In this context, growth of the limb primordium is proposed to modulate the response of cells to FGFs and retinoic acid and to help in generating the proximal, medial and distal segments of the adult limb. The initial limb primordium is maintained in a pluripotent state by the combination of distal—FGFs—and proximal—retinoic acid—signals that it simultaneously receives. As the primordium grows, the proximal limb is established as the region closer to the source of retinoic acid, and the developmental programmes determining the medial and distal segments are initiated in domains that grow beyond the influence of retinoic acid, but are still under the influence of FGFs.
Graded morphogen activity is not required for tissue growth
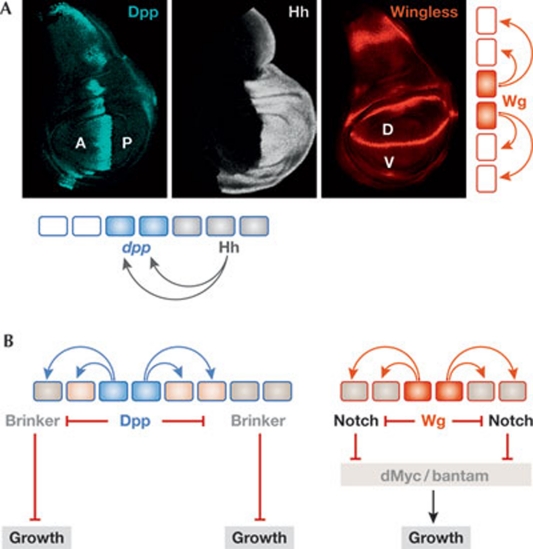
The role of morphogen gradients in specifying cell identities in a concentration-dependent manner is well established. By contrast, the contribution of morphogen gradients to the regulation of tissue growth remains the subject of dispute. In the fly wing primordium, Dpp and Wg morphogens are expressed in thin stripes corresponding to the compartment boundaries, from where they diffuse to the rest of the tissue to form a gradient (Fig 3A; Lecuit et al, 1996; Nellen et al, 1996; Neumann & Cohen, 1997; Zecca et al, 1996). Several models have been proposed to explain the role of these two morphogens as growth promoters, including the ‘steepness model’, by which the juxtaposition of cells sensing disparate levels of the morphogen promotes proliferative growth (Lawrence & Struhl, 1996; Rogulja & Irvine, 2005). However, recent experimental evidence indicates that the Dpp gradient scales with the size of the wing primordium and that the slope of the gradient does not change (Wartlick et al, 2011), thereby challenging the steepness model. Indeed, uniform expression of Dpp and Wg is sufficient to restore the tissue undergrowth caused by their depletion (Baena-Lopez et al, 2009; Schwank et al, 2008), further indicating that graded activity of Dpp and Wg is not required for tissue growth.
Figure 3.
Morphogens and tissue growth. (A) The Dpp (blue), Hh (grey) and Wg (red) morphogens are expressed in restricted domains. Dpp expression relies on the activity of Hh, which is expressed in posterior cells and acts as a short-range morphogen. Wg and Dpp act as long-range morphogens. (B) Dpp and Wg have a permissive role in promoting tissue growth in the Drosophila wing by inhibiting the expression or activity of proteins that repress growth. Dpp inhibits expression of the transcriptional repressor Brinker, which represses growth by an unknown mechanism, whereas Wg represses Notch activity, which is known to inhibit the expression of the proto-oncogene dMyc and the growth-promoting microRNA bantam. A, anterior; D, dorsal; Dpp, Decapentaplegic; P, posterior; V, ventral; Wg, Wingless.
If the graded activity of morphogens is not required for tissue growth, then how do these signalling molecules regulate this growth? Is their role in promoting growth instructive or permissive? In the Drosophila wing primordium, Dpp and Wg morphogens have been shown to have a permissive rather than an instructive role in promoting growth. The graded distribution of Dpp leads—through interaction with its receptor complex Punt–Thickvein—to graded activation of Mad/Medea, which in turn represses the transcription of brinker (reviewed in Affolter & Basler, 2007). This creates a gradient of Brinker expression that is reciprocal to the Dpp activity gradient (Fig 3B). Brinker is a transcriptional repressor that acts negatively to establish, in a dose-dependent manner, the precise expression domain of Dpp target genes such as spalt (Fig 4; Campbell & Tomlinson, 1999; Jazwinska et al, 1999; Minami et al, 1999). Therefore, Dpp regulates the expression of target genes by repressing brinker. Interestingly, the reduced size of the wing primordium observed in flies that are mutant for hypomorphic alleles of dpp is restored when combined with brinker mutants (Schwank et al, 2008). This evidence indicates that, as is the case for the expression of target genes involved in wing pattern formation, Dpp controls wing growth entirely through repression of brinker, although the target genes that are responsible remain unknown (Fig 3B).
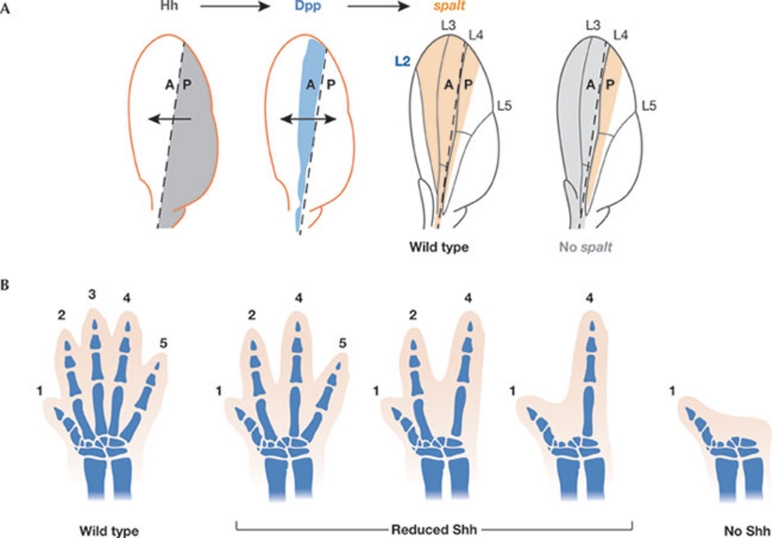
Figure 4.
Morphogen-induced subdomains contribute to regulation of final tissue size. (A) The subdivision of a growing tissue into secondary domains has a crucial role in tissue growth. Hh (grey) induces expression of Dpp (blue) in anterior cells. Spalt (orange) is a target of Dpp in the wing. The boundary between spalt-expressing and non-expressing cells defines the location of the longitudinal vein L2. Loss of spalt in the whole anterior compartment (grey) gives rise to smaller adult wings. Note that the anterior compartment consists of three intervein regions in wild-type wings, but only two in the absence of spalt activity. The boundary between the anterior (A) and posterior (P) compartments is shown by a dashed line. (B) Depletion of Shh activity at different times of limb primordium development reduces the size of the adult limb and causes loss of adult digits. Dpp, Decapentaplegic; Hh, Hedgehog; Shh, Sonic hedgehog.
Wg seems to control tissue growth in a similar manner to Dpp. Wg is expressed along the dorsal–ventral compartment boundary (Fig 3A) and spreads at both sides of this boundary to form a gradient (Neumann & Cohen, 1997; Zecca et al, 1996). The bantam microRNA and the dMyc proto-oncogene have a growth-promoting function in Drosophila tissues and have been shown to mediate the role of Wg in the growth of the wing primordium (Brennecke et al, 2003; Herranz et al, 2008). Wg controls the expression of these two genes through repression of the Notch signalling pathway (Fig 3B; Herranz et al, 2008). Whereas Notch acts as a repressor of bantam and dMyc, Wg has a permissive role in alleviating Notch-mediated repression of these two genes. Analogously to the relationship between Dpp and Brinker, reduced Notch activity completely rescues the strong reduction in bantam activity and dMyc mRNA levels caused by depletion of Wg activity (Herranz et al, 2008). Taken together, these results indicate that graded activity of Wg and Dpp is not required to induce tissue growth, and that these two morphogens have a permissive rather than an instructive role in this process.
In vertebrate limbs, the role of the Shh gradient in specifying digit identities and the contribution of gradients of retinoic acid and FGFs in specifying proximal, medial and distal segments is widely accepted. Shh induces the growth of the developing limb by regulating the expression of several growth and cell-cycle genes including N-Myc, cyclin D1 and cyclin D2 (Towers et al, 2008), and FGFs secreted from the AER exert a mitogenic effect on distal cells (Reiter & Solursh, 1982). However, the requirement for a morphogen gradient in this process has not been studied.
An instructive role for morphogens in anisotropic growth?
Growth of Drosophila and vertebrate limb primordia is anisotropic. Oriented cell divisions contribute to the generation of an elongated shape in the adult Drosophila wing (Baena-Lopez et al, 2005). Pointing in the same direction, recent computational and experimental quantifications performed in the vertebrate limb primordium suggest that distal elongation of this structure does not simply rely on the increased proliferation rates observed in regions close to the AER (Boehm et al, 2010). Actually, an important contribution comes from directional cell behaviours such as oriented cell divisions. As graded activity of morphogens is not required to induce tissue growth, at least in Drosophila, it would be interesting to address whether morphogen gradients have an instructive role in orienting cell divisions and driving anisotropic growth. Interestingly, ubiquitous expression of Dpp or Wg morphogens in the wing primordium gives rise to round adult wings (Garcia-Bellido, 2009).
Morphogen-induced subdomains regulate final tissue size
Graded activity of Shh morphogen specifies digit identities along the anterior–posterior axis of the vertebrate limb, and graded activity of Wg and Dpp morphogens induces the subdivision of the Drosophila leg and wing primordia into repetitive patterning structures such as the segments of the leg or intervein territories of the wing. These structures seem to be involved in regulating final tissue size. In Drosophila, clonal depletion of the Dpp target gene spalt compromises the specification of the longitudinal vein L2 and leads to smaller adult wings, mostly due to the absence of an intervein region (Fig 4B; de Celis et al, 1996). Fly legs lacking Dachshund or Distalless activity—transcription factors involved in the specification of certain leg segments, the expression of which is regulated by the combined activities of Wg and Dpp—are smaller than wild-type legs. This finding is mostly attributed to failure in the specification of certain leg segments (Cohen & Jürgens, 1989; Mardon et al, 1994). In both cases, synthesis at the source, diffusion and activity levels of the morphogens are not affected. Remarkably, there is no growth compensation from other territories. This observation supports the idea that wing interveins and leg segments behave as units of growth control.
In the 1950s, Bretscher & Tschumi reported a series of experiments demonstrating that localized treatment of amphibian limb primordia with mitotic inhibitors resulted in limbs with fewer digits (Bretscher & Tschumi, 1951). Later, in the 1980s, Alberch and colleagues reported similar observations and concluded that digit number is dependent on the size of the limb primordium (Alberch & Gale, 1983). More recently, Zhu and colleagues showed that digit identity is specified by Shh early in development. Shh acts during subsequent stages of limb development, mainly to ensure that a sufficient number of cells is generated to form a normal five-digit limb (Zhu et al, 2008). Removal of Shh during development gives rise to adult limbs with a reduced number of digits (Fig 4B). Similarly to Drosophila legs and wings, there is no growth compensation from other territories in experimentally treated amphibian and mouse limbs.
Taken together, these observations indicate that graded activity of morphogens in Drosophila limbs subdivides the tissue into several repetitive structures that behave as units of growth control. In vertebrate limbs, morphogen-promoted growth is required to generate a sufficient number of progenitor cells to produce the normal complement of digits. Failure in the specification of interveins, leg segments or digits, reduces the final size of the adult limb. Thus, the size of the adult limb seems to be regulated in a discrete rather than graded manner by the activity of morphogens. Consistent with this proposal, ectopic expression of Shh, Dpp or Wg induces a qualitative effect on tissue growth, which results in the appearance of ectopic digits in vertebrate limbs, ectopic veins in Drosophila wings and ectopic segments in Drosophila legs. Therefore, morphogen-mediated re-specification of tissue in new subdomains induces a concomitant discrete effect on final tissue size.
Conclusions
The function of morphogens remains a central topic in developmental biology. We have discussed the way in which morphogens and tissue growth affect each other. Tissue growth has a fundamental role in the function of morphogens, by generating their concentration gradients, modulating the cellular response to them and expanding their expression domains and those of their target genes. The contribution of morphogens to the regulation of tissue growth has recently been revisited. The graded activity of morphogens—a central part of early models of growth control by morphogens—is not an absolute requirement for tissue growth, and they seem to have a permissive function in growth by alleviating expression or activity of growth repressors. Remarkably, the combined loss of morphogens and morphogen-regulated growth repressors has minor consequences for tissue growth, suggesting that the tissue is able to grow even in the absence of morphogens. There is a vast amount of experimental evidence that demonstrates that morphogen gradients have a fundamental and direct role in subdividing vertebrate and invertebrate limbs into repetitive units—such as digits, interveins and leg segments—by specifying their differential identity in a concentration-dependent manner. Here, we propose that the role of morphogens as ‘growth-promoting’ molecules is probably dependent on their role in cell-fate specification, through the subdivision of the tissue into these repetitive units. Tissue growth within these units is regulated locally and finely controls the final size of the adult organ. That morphogens contribute to not only growth, but also organ shape by driving anisotropic growth, is still an attractive speculation (Sidebar A).
Sidebar A | In need of answers.
Does tissue growth contribute to the expansion of the expression domains of morphogens and their target genes?
Does tissue growth modulate the response to morphogens and, most importantly, does this modulation have an important role in driving developmental decisions?
Do morphogens and their gradients have an instructive role in driving tissue growth?
Do morphogens and their gradients have an instructive role in driving anisotropic growth?
Is the effect of morphogens on tissue growth quantitative or qualitative?

Andrés Dekanty

Marco Milán
Acknowledgments
We thank J. Casanova, J.J. Sanz-Ezquerro, J.P. Vincent and members of the lab for comments on the early drafts of this review and T. Yates for help in preparing the manuscript. A.D. is funded by a Juan de la Cierva postdoctoral contract, M.M. is an ICREA Research Professor and M.M.'s laboratory is funded by grants from the Dirección General de Investigación Científica y Técnica (BFU2010-21123/BMC), the Generalitat de Catalunya (2005 SGR 00118), the Consolider-Ingenio 2010 Program (CSD2007-00008) and the EMBO Young Investigator Programme.
Footnotes
The authors declare that they have no conflict of interest.
References
- Affolter M, Basler K (2007) The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat Rev Genet 8: 663–674 [DOI] [PubMed] [Google Scholar]
- Alberch P, Gale EA (1983) Size dependence during the development of the amphibian foot. Colchicine-induced digital loss and reduction. J Embryol Exp Morphol 76: 177–197 [PubMed] [Google Scholar]
- Baena-Lopez LA, Baonza A, Garcia-Bellido A (2005) The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol 15: 1640–1644 [DOI] [PubMed] [Google Scholar]
- Baena-Lopez LA, Franch-Marro X, Vincent JP (2009) Wingless promotes proliferative growth in a gradient-independent manner. Sci Signal 2: ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejarano F, Milán M (2009) Genetic and epigenetic mechanisms regulating hedgehog expression in the Drosophila wing. Dev Biol 327: 508–515 [DOI] [PubMed] [Google Scholar]
- Benazet JD, Zeller R (2009) Vertebrate limb development: moving from classical morphogen gradients to an integrated 4-dimensional patterning system. Cold Spring Harb Perspect Biol 1: a001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm B, Westerberg H, Lesnicar-Pucko G, Raja S, Rautschka M, Cotterell J, Swoger J, Sharpe J (2010) The role of spatially controlled cell proliferation in limb bud morphogenesis. PLoS Biol 8: e1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM (2003) bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113: 25–36 [DOI] [PubMed] [Google Scholar]
- Bretscher A, Tschumi P (1951) Gestufte Reduktion con chemisch behandelten Xenopus-Beinen. Revue Suisse Zoologie 58: 391–398 [Google Scholar]
- Bryant PJ (1975) Pattern formation in the imaginal wing disc of Drosophila melanogaster: fate map, regeneration and duplication. J Exp Zool 193: 49–77 [DOI] [PubMed] [Google Scholar]
- Campbell G, Tomlinson A (1999) Transducing the Dpp morphogen gradient in the wing of Drosophila: regulation of Dpp targets by brinker. Cell 96: 553–562 [DOI] [PubMed] [Google Scholar]
- Cohen SM, Jürgens G (1989) Proximal–distal pattern formation in Drosophila: cell autonomous requirement for Distal-less gene activity in limb development. EMBO J 8: 2045–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KL, Hu JK, ten Berge D, Fernandez-Teran M, Ros MA, Tabin CJ (2011) Initiation of proximal–distal patterning in the vertebrate limb by signals and growth. Science 332: 1083–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Struhl G (1996) Dual roles for Patched in sequestering and transducing Hedgehog. Cell 87: 553–563 [DOI] [PubMed] [Google Scholar]
- de Celis JF, Barrio R, Kafatos FC (1996) A gene complex acting downstream of dpp in Drosophila wing morphogenesis. Nature 381: 421–424 [DOI] [PubMed] [Google Scholar]
- Dominguez M, Brunner M, Hafen E, Basler K (1996) Sending and receiving the Hedgehog signal: control by the Drosophila Gli protein Cubitus interruptus. Science 272: 1621–1625 [DOI] [PubMed] [Google Scholar]
- Estella C, McKay DJ, Mann RS (2008) Molecular integration of wingless, decapentaplegic, and autoregulatory inputs into Distalless during Drosophila leg development. Dev Cell 14: 86–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet A (2011) Hedgehog morphogen: from secretion to reception. Trends Cell Biol 21: 238–246 [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A (2009) The cellular and genetic bases of organ size and shape in Drosophila. Int J Dev Biol 53: 1291–1303 [DOI] [PubMed] [Google Scholar]
- García-Bellido A, Ripoll P, Morata G (1973) Developmental compartmentalisation of the wing disk of Drosophila. Nat New Biol 245: 251–253 [DOI] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ (2004) Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118: 517–528 [DOI] [PubMed] [Google Scholar]
- Herranz H, Perez L, Martin FA, Milán M (2008) Wingless A, Notch double-repression mechanism regulates G1–S transition in the Drosophila wing. EMBO J 27: 1633–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinska A, Kirov N, Wieschaus E, Roth S, Rushlow C (1999) The Drosophila gene brinker reveals a novel mechanism of Dpp target gene regulation. Cell 96: 563–573 [DOI] [PubMed] [Google Scholar]
- Kenyon KL, Ranade SS, Curtiss J, Mlodzik M, Pignoni F (2003) Coordinating proliferation and tissue specification to promote regional identity in the Drosophila head. Dev Cell 5: 403–414 [DOI] [PubMed] [Google Scholar]
- Kutejova E, Briscoe J, Kicheva A (2009) Temporal dynamics of patterning by morphogen gradients. Curr Opin Genet Dev 19: 315–322 [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G (1996) Morphogens, compartments and pattern: lessons from Drosophila? Cell 85: 951–961 [DOI] [PubMed] [Google Scholar]
- Lecuit T, Brook WJ, Ng M, Calleja M, Sun H, Cohen SM (1996) Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature 381: 387–393 [DOI] [PubMed] [Google Scholar]
- Mardon G, Solomon NM, Rubin GM (1994) dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development 120: 3473–3486 [DOI] [PubMed] [Google Scholar]
- Maurange C, Paro R (2002) A cellular memory module conveys epigenetic inheritance of hedgehog expression during Drosophila wing imaginal disc development. Genes Dev 16: 2672–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt H (2009) Models for the generation and interpretation of gradients. Cold Spring Harb Perspect Biol 1: a001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M, Kinoshita N, Kamoshida Y, Tanimoto H, Tabata T (1999) brinker is a target of Dpp in Drosophila that negatively regulates Dpp-dependent genes. Nature 398: 242–246 [DOI] [PubMed] [Google Scholar]
- Nellen D, Burke R, Struhl G, Basler K (1996) Direct and long-range action of a DPP morphogen gradient. Cell 85: 357–368 [DOI] [PubMed] [Google Scholar]
- Neumann CJ, Cohen SM (1997) Long-range action of Wingless organizes the dorsal–ventral axis of the Drosophila wing. Development 124: 871–880 [DOI] [PubMed] [Google Scholar]
- Ng M, Diaz-Benjumea FJ, Vincent JP, Wu J, Cohen SM (1996) Specification of the wing by localized expression of wingless protein. Nature 381: 316–318 [DOI] [PubMed] [Google Scholar]
- Niswander L, Jeffrey S, Martin GR, Tickle C (1994) A positive feedback loop coordinates growth and patterning of the vertebrate limb. Nature 371: 609–612 [DOI] [PubMed] [Google Scholar]
- Pérez L, Barrio L, Cano D, Fiuza U, Muzzopappa M, Milán M (2011) Enhancer–PRE communication contributes to expanding the domains of gene expression in proliferating primordia. Development 138: 3135–3145 [DOI] [PubMed] [Google Scholar]
- Piddini E, Vincent JP (2009) Interpretation of the wingless gradient requires signaling-induced self-inhibition. Cell 136: 296–307 [DOI] [PubMed] [Google Scholar]
- Rafel N, Milán M (2008) Notch signalling coordinates tissue growth and wing fate specification in Drosophila. Development 135: 3995–4001 [DOI] [PubMed] [Google Scholar]
- Reiter RS, Solursh M (1982) Mitogenic property of the apical ectodermal ridge. Dev Biol 93: 28–35 [DOI] [PubMed] [Google Scholar]
- Rogulja D, Irvine KD (2005) Regulation of cell proliferation by a morphogen gradient. Cell 123: 449–461 [DOI] [PubMed] [Google Scholar]
- Rosello-Diez A, Ros MA, Torres M (2011) Diffusible signals, not autonomous mechanisms, determine the main proximodistal limb subdivision. Science 332: 1086–1088 [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Micchelli CA, Axelrod JD, Perrimon N, Blair SS (1996) wingless refines its own expression domain on the Drosophila wing margin. Nature 384: 72–74 [DOI] [PubMed] [Google Scholar]
- Scherz PJ, Harfe BD, McMahon AP, Tabin CJ (2004) The limb bud Shh–Fgf feedback loop is terminated by expansion of former ZPA cells. Science 305: 396–399 [DOI] [PubMed] [Google Scholar]
- Schwank G, Restrepo S, Basler K (2008) Growth regulation by Dpp: an essential role for Brinker and a non-essential role for graded signaling levels. Development 135: 4003–4013 [DOI] [PubMed] [Google Scholar]
- Sing A, Pannell D, Karaiskakis A, Sturgeon K, Djabali M, Ellis J, Lipshitz HD, Cordes SP (2009) A vertebrate Polycomb response element governs segmentation of the posterior hindbrain. Cell 138: 885–897 [DOI] [PubMed] [Google Scholar]
- Tabata T, Schwartz C, Gustavson E, Ali Z, Kornberg TB (1995) Creating a Drosophila wing de novo: the role of engrailed and the compartment border hypothesis. Development 121: 3359–3369 [DOI] [PubMed] [Google Scholar]
- Towers M, Tickle C (2009) Growing models of vertebrate limb development. Development 136: 179–190 [DOI] [PubMed] [Google Scholar]
- Towers M, Mahood R, Yin Y, Tickle C (2008) Integration of growth and specification in chick wing digit-patterning. Nature 452: 882–886 [DOI] [PubMed] [Google Scholar]
- Verheyden JM, Sun X (2008) An Fgf/Gremlin inhibitory feedback loop triggers termination of limb bud outgrowth. Nature 454: 638–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Simcox A, Campbell G (2000) Dual role for Drosophila epidermal growth factor receptor signaling in early wing disc development. Genes Dev 14: 2271–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartlick O, Kicheva A, Gonzalez-Gaitan M (2009) Morphogen gradient formation. Cold Spring Harb Perspect Biol 1: a001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartlick O, Mumcu P, Kicheva A, Bittig T, Seum C, Julicher F, Gonzalez-Gaitan M (2011) Dynamics of Dpp signaling and proliferation control. Science 331: 1154–1159 [DOI] [PubMed] [Google Scholar]
- Whiting MF, Bradler S, Maxwell T (2003) Loss and recovery of wings in stick insects. Nature 421: 264–267 [DOI] [PubMed] [Google Scholar]
- Williams JA, Paddock SW, Carroll SB (1993) Pattern formation in a secondary field: a hierarchy of regulatory genes subdivides the developing Drosophila wing disc into discrete subregions. Development 117: 571–584 [DOI] [PubMed] [Google Scholar]
- Wu J, Cohen SM (2002) Repression of Teashirt marks the initiation of wing development. Development 129: 2411–2418 [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G (1995) Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development 121: 2265–2278 [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G (1996) Direct and long-range action of a Wingless morphogen gradient. Cell 87: 833–844 [DOI] [PubMed] [Google Scholar]
- Zecca M, Struhl G (2002) Subdivision of the Drosophila wing imaginal disc by EGFR-mediated signaling. Development 129: 1357–1368 [DOI] [PubMed] [Google Scholar]
- Zeller R, Lopez-Rios J, Zuniga A (2009) Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat Rev Genet 10: 845–858 [DOI] [PubMed] [Google Scholar]
- Zhu J, Nakamura E, Nguyen MT, Bao X, Akiyama H, Mackem S (2008) Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev Cell 14: 624–632 [DOI] [PMC free article] [PubMed] [Google Scholar]