Abstract
R-spondins are secreted Wnt signalling agonists, which regulate embryonic patterning and stem cell proliferation, but whose mechanism of action is poorly understood. Here we show that R-spondins bind to the orphan G-protein-coupled receptors LGR4 and LGR5 by their Furin domains. Gain- and loss-of-function experiments in mammalian cells and Xenopus embryos indicate that LGR4 and LGR5 promote R-spondin-mediated Wnt/β-catenin and Wnt/PCP signalling. R-spondin-triggered β-catenin signalling requires Clathrin, while Wnt3a-mediated β-catenin signalling requires Caveolin-mediated endocytosis, suggesting that internalization has a mechanistic role in R-spondin signalling.
Keywords: endocytosis, LGR4, LGR5, R-spondin, Wnt
Introduction
Wnts have a critical role in development and disease, and understanding their complex signalling mechanisms and biological roles is of wide interest (Nusse, 2005; Grigoryan et al, 2008). Besides Wnts, secreted R-spondins (Rspo1–4; roof plate-specific spondin) potently enhance β-catenin signalling (Kazanskaya et al, 2004; Kim et al, 2005). They are involved in embryonic patterning and differentiation in frogs and mice (Kazanskaya et al, 2004, 2008; Aoki et al, 2006; Blaydon et al, 2006; Parma et al, 2006). R-spondins are also implicated in human disease and hold therapeutic promise as potent stem cell growth factors (Kim et al, 2005; Blaydon et al, 2006; Parma et al, 2006; Zhao et al, 2009). R-spondins synergize with Wnts and Frizzled (Fz), and require the presence of Wnts to activate β-catenin signalling (Kazanskaya et al, 2004; Nam et al, 2006; Kim et al, 2008).
The mechanism of R-spondin signalling is poorly understood and notably the identity of their receptor is controversial. Frizzled, LRP6 and Kremen have been variably proposed and refuted (Wei et al, 2007, see also Fig 1A; Nam et al, 2006; Binnerts et al, 2007). We have recently shown that Rspo3 binds to syndecans, probably as co-receptors, to transduce Wnt/PCP signalling (Ohkawara et al, 2011).
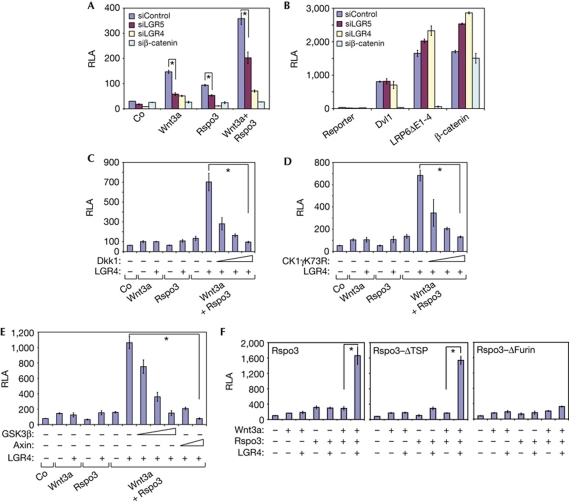
Figure 1.
LGR4 and LGR5 are new regulators of Wnt and R-spondin signalling. (A–F) Wnt luciferase reporter assays in HEK293T cells stimulated with the indicated constructs or Wnt3a and/or Rspo3-ΔC, or Rspo3-ΔC+ΔTSP, or Rspo3-ΔC+ΔFurin-conditioned medium, in the presence of the indicated small interfering RNAs. Co, control medium. RLA, relative luciferase activity. TSP, thrombospondin. Error bars indicate s.d. values, n=3; * indicates P<0.001 by Student's t-test.
In a genome-wide small interfering RNA (siRNA) screen for R-spondin receptors, we have identified leucine-rich repeat containing G-protein-coupled receptor 5 (LGR5). Here we show that LGR4 and LGR5 function as R-spondin receptors in Wnt/β-catenin and Wnt/PCP signalling. Furthermore, we provide evidence for an important role of endocytosis, where R-spondin-triggered β-catenin signalling requires Clathrin, while Wnt3a-mediated β-catenin signalling requires Caveolin-mediated internalization.
Results And Discussion
LGR4 and LGR5 promote R-spondin signalling
In a search for an R-spondin receptor we carried out a genome-wide siRNA screen (Cruciat et al, 2010). In brief, human embryonic kidney (HEK293T) cells were transfected with siRNA pools targeting about 18,500 human genes and analysed for transcription of a Wnt-responsive luciferase reporter. Cells were stimulated with six different Wnt activators, two of which were recombinant Rspo3, and transfected Wnt1+Rspo3. When the hits were sorted for genes with high score in both R-spondin regimes, and low in the other four Wnt stimulations, the orphan leucine-rich repeat containing G-protein-coupled receptor 5 was the number one hit (supplementary Table S1 online). LGR4 and LGR5 are related orphan receptors characterized by the presence of seven transmembrane domains and an extracellular domain containing leucine-rich repeat motifs, implicated in stem cell biology (Barker & Clevers, 2010; Mustata et al, 2011). Knock down of LGR4 and LGR5 by siRNA inhibited Wnt/β-catenin signalling in TOPFLASH reporter assays when stimulated by Wnt3a, Rspo3 or their combination (Fig 1A). In contrast, siLGR4/5 did not inhibit TOPFLASH reporter stimulated intracellularly by constitutively active LRP6 (LRP6ΔE1-4), Dvl1 or β-catenin (Fig 1B). The siLGR4 and -5 effects were rescued by co-transfection with LGR4 cDNA, attesting specificity (supplementary Fig S1A,B online). Limiting doses of Wnt3a and Rspo1 strongly synergized in Wnt signalling when co-transfected with LGR4 or LGR5 (supplementary Fig S2A,B online). R-spondin–LGR4 signalling was inhibited by the LRP6 antagonist Dkk1, dominant-negative Casein kinase 1γ, GSK3β and Axin (Fig 1C–E). This indicates that the R-spondin–LGR4 complex functions upstream or at the level of LRP6 signalling. Consistent with this, R-spondin–LGR4/5 signalling was inhibited by siLRP6 (supplementary Fig S2C,D online).
R-spondins contain two Furin type domains, which are essential for Wnt/β-catenin signalling (Kazanskaya et al, 2004), and a thrombospondin type 1 domain (TSP1), which in the case of Rspo3 mediates PCP signalling and binds to syndecans (Ohkawara et al, 2011). Rspo3 deletion mutants lacking the TSP1 domain signalled with LGR4, while ΔFurin mutants failed to activate the reporter (Fig 1F). We conclude that Rspo3 signalling by the β-catenin pathway requires LGR4 and LGR5, and is mediated by the Furin domains.
LGR4 and LGR5 bind to R-spondins
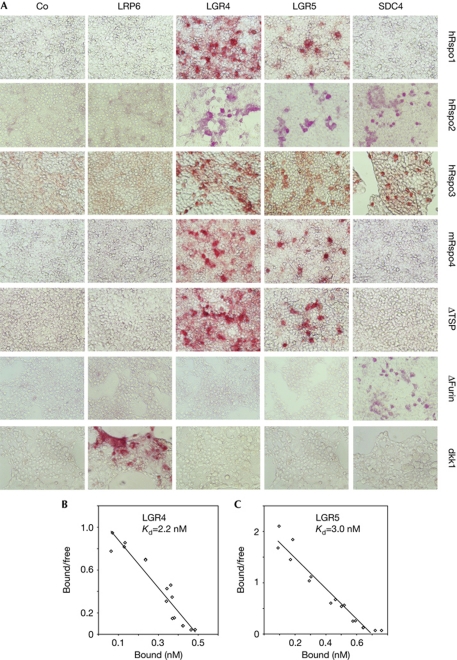
As LGR4 and LGR5 are transmembrane proteins and are required for Rspo3 signalling epistatically at the Wnt receptor level, we tested for direct interaction. In cell surface binding assays, alkaline-phosphatase (AP) fusion proteins of Rspo1,2,3,4 bound to cells transfected with LGR4 and -5 (Fig 2A). Weaker binding was found with LGR5-transfected cells, but this might reflect its low expression (not shown) rather than a major difference in affinity (see below). Rspo2 and Rspo3 bound to syndecan 4 (SDC4), while no R-spondin binding was detected with LRP6-transfected cells, which however bound recombinant Dkk1. Rspo3 deletion mutants lacking the TSP1 domain also bound to LGR4/5-transfected cells, while ΔFurin mutants failed to bind, consistent with the requirement of the Furin domains in Wnt reporter assays. The reverse was true for binding of Rspo3 deletion mutants to SDC4-transfected cells. Using immunopurified LGR4 and -5 and purified recombinant hRspo3–AP, we determined their apparent Kd as 2.2 and 3.0 nM, respectively (Fig 2B,C). Taken together with the functional data, these results indicate that LGR4 and LGR5 function as R-spondin receptors.
Figure 2.
R-spondins bind to LGR4 and LGR5. (A) Cell surface binding assay. Cells were transfected with the indicated plasmids and subjected to binding assays with conditioned medium containing alkaline-phosphatase (AP) fusion proteins of R-spondins-ΔC, Dkk1 or hRspo3-ΔC deletion constructs (ΔTSP and ΔFurin). (B,C) Scatchard plot analysis of in vitro binding assay using LGR4, LGR5 and hRspo3-ΔC–AP. Co, control medium; SDC4, syndecan 4; TSP, thrombospondin.
Clathrin endocytosis is required for Rspo3 signalling
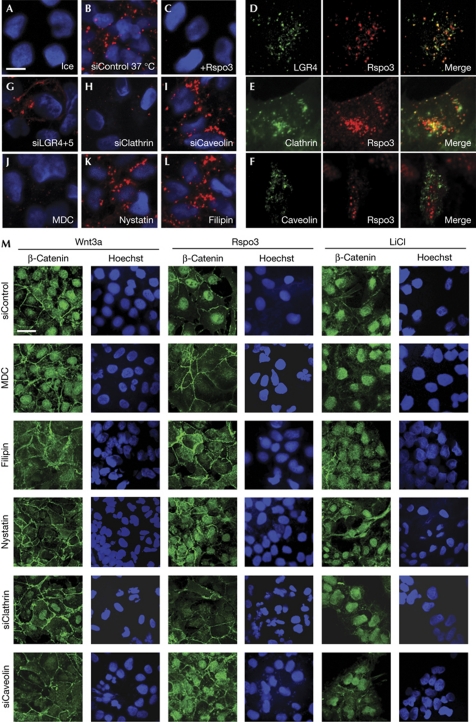
There is a good evidence that Wnt signalling proceeds by an endocytic compartment and that Wnt–receptor complex internalization is an essential step in both canonical and non-canonical Wnt signalling (reviewed in Kikuchi & Yamamoto (2007)). Furthermore, Rspo3 PCP signalling requires Clathrin-mediated endocytosis (Ohkawara et al, 2011). We therefore asked whether Rspo3 and LGR4 are co-internalized and whether endocytosis is also essential for R-spondin signalling by the β-catenin pathway. Internalization assays with recombinant Rspo3–horseradish peroxidase (HRP) fusion protein showed that the protein is endocytosed within 1 h of application (Fig 3A,B) and this was competed by unlabelled Rspo3 (Fig 3C). Rspo3 colocalized in vesicular structures with LGR4–green fluorescent protein (GFP, Fig 3D). Internalized Rspo3 colocalized with Clathrin, but not with Caveolin endocytic vesicles (Fig 3E,F). Furthermore, Rspo3 endocytic vesicles were positive for the early endosomal markers EEA1, but not the late endosomal marker LAMP (supplementary Fig S3A,B online). Rspo3 endocytosis was partially blocked by siLGR4+5 (Fig 3G). Consistent with colocalization, siClathrin and the Clathrin inhibitor monodansylcadaverine (MDC) blocked Rspo3 internalization, unlike inhibitors of Caveolin-mediated endocytosis including filipin, nystatin and siCaveolin (Fig 3H–L).
Figure 3.
Clathrin endocytosis is required for Rspo3 signalling. (A–L) Clathrin is colocalized with Rspo3 and is required for Rspo3 internalization. Confocal microscopy of HepG2 cells treated for 1 h with horseradish peroxidase (HRP)-tagged Rspo3-ΔC on ice (A), or at 37°C (B,D–L), or in the presence of Flag-tagged Rspo3-ΔC (C). HRP-tagged Rspo3-ΔC was visualized by tyramide signal amplification without or with coimmunofluorescence of anti-green fluorescent protein (GFP) antibody against overexpressed xtLGR4-EYFP (D), Clathrin–GFP (E) and Caveolin–GFP (F). Where indicated, cells were pretreated with small interfering RNAs (siRNAs) for 4 days (B,G–I) or with endocytic inhibitors for 1 h (J–L). (M) Clathrin is required for Rspo3-induced nuclear β-catenin accumulation. Confocal microscopy of NTERA2 cells incubated for 1 h with endocytic inhibitors or pretreated with the indicated siRNAs for 3 days. Cells were then treated for 4 h with Wnt3a, or Wnt3a together with Rspo3, or 50 mM LiCl. Scale bars indicate 10 μm. MDC, monodansylcadaverine.
We tested whether Rspo3 internalization was required for Wnt signalling. As a short-term signalling read-out we monitored β-catenin nuclear accumulation. Cells were stimulated either with Wnt3a or Rspo3 in the presence of a tonic Wnt3a dose, which by itself did not induce nuclear β-catenin (not shown). Nuclear β-catenin accumulation following Wnt3a stimulation was inhibited by the Caveolin endocytic inhibitors filipin and nystatin, as well as by siCaveolin treatment (Fig 3M; supplementary Fig S4A,B online). Conversely, Rspo3 signalling was inhibited by MDC and siClathrin. Nuclear β-catenin accumulation following LiCl stimulation, which blocks GSK3 intracellularly, was unaffected by any treatment. We confirmed these findings by monitoring soluble β-catenin levels (supplementary Fig S4C online).
Taken together, these results indicate (i) that Rspo3 and LGR4 are co-internalized, (ii) that internalization occurs by Clathrin-mediated endocytosis, which (iii) is an obligatory step in Rspo3 β-catenin signalling. In contrast, Wnt3 signalling requires Caveolin-mediated internalization.
LGR4 and LGR5 mediate PCP signalling in Xenopus
We recently showed that Rspo3 amplifies not only β-catenin, but also Wnt/PCP signalling, namely in Xenopus embryos (Ohkawara et al, 2011). To test whether LGR4 and LGR5 are required for R-spondin PCP signalling we analysed Xenopus tropicalis embryos. LGR4 and -5 are maternally expressed and their zygotic transcripts increased after gastrula stage (supplementary Fig S5A online). LGR4 was expressed in all three germ layers, while LGR5 was expressed predominantly in endoderm (supplementary Fig S5B online).
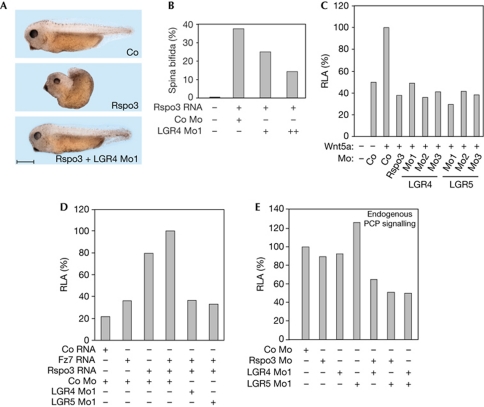
A hallmark of Rspo3 signalling in early Xenopus embryos is that its messenger RNA (mRNA) injection induces misgastrulation and spina bifida, due to exaggerated PCP signalling (Ohkawara et al, 2011; Fig 4A). Importantly, the spina bifida phenotype was rescued by coinjection with an antisense morpholino oligonucleotide (Mo1) targeting the 5′ untranslated region of LGR4 (Fig 4A,B). This indicates that LGR4 is required for Rspo3 signalling in vivo. LGR5 Mo1 failed to rescue the Rspo3 overexpression phenotype (not shown), probably because the gene is expressed predominantly in endoderm, while gastrulating cells are mesodermal.
Figure 4.
LGR4 and LGR5 are required for Wnt/PCP signalling in Xenopus. (A,B) Rspo3 signalling requires LGR4 to induce gastrulation defects. Embryos were injected equatorially at 4-cell stage with morpholinos and/or messenger RNA (mRNA) as indicated (250 pg xRspo3 mRNA, 10 ng (+) and 20 ng (++) of LGR4 Mo1). Scale bar indicates 500 μm. (C–E) ATF2-luciferase reporter activity is reduced by LGR4/5 Mos in Xenopus embryos. Embryos were injected equatorially with ATF2-luc reporter (100 pg) and Renilla reporter plasmids (25 pg) and the indicated morpholinos (20 ng LGR4 Mo1, 5 ng LGR5 Mo1 in E) and mRNAs. Luciferase reporter assays were performed from whole embryos collected at gastrula stage. Luciferase activity in embryos injected with either Co Mo or Co Mo plus mRNA of the indicated activators within each condition were set to 100%. RLA, relative luciferase activity. All experiments were performed at least twice with three replicates each. Co, control medium; Mo, morpholino oligonucleotide.
We designed two more antisense Mos each against LGR4 and LGR5 to validate Mo specificities, as the mRNA rescue of the LGR4/5 Mos proved technically difficult (not shown). Mos2 targeted different 5′ regions for translation inhibition and Mos3 each targeted a splice site of the LGR4 and LGR5 pre-mRNAs. We individually injected the six LGR4/5 Mos into Xenopus embryos and monitored Wnt/PCP signalling by analysing expression of ATF2-luciferase, which in early Xenopus embryos is a specific reporter for Wnt/PCP signalling (Ohkawara & Niehrs, 2011). When PCP signalling was stimulated by coinjection with Wnt5a mRNA, reporter activity was inhibited by Rspo3 Mo (Fig 4C), as recently described (Ohkawara et al, 2011). Likewise, all six LGR4 and LGR5 Mos inhibited ATF2-luciferase activity (Fig 4C). Similarly, PCP signalling stimulated by coinjection of Rspo3 and Fz7 mRNAs was inhibited by LGR4/5 Mos (Fig 4D). In contrast, neither LGR4 nor LGR5 Mo inhibited signalling by Nodal or BMP4 (supplementary Fig S5C,D online), further attesting specificity. Finally, low Mo doses were injected and endogenous PCP signalling from unstimulated embryos was monitored: reporter activity was inhibited when Rspo3 Mo was combined with either LGR4 or LGR5 Mo, indicating functional interaction, or when LGR4 and LGR5 Mo were coinjected, indicating functional redundancy (Fig 4E). This Mo synergy corroborates once again their specificity. We conclude that LGR4 and LGR5 are required for R-spondin signalling by the Wnt/PCP pathway in Xenopus embryos.
We provide independent lines of evidence that LGR4 and LGR5 function as Rspo3 receptors, including reporter assay epistasis experiments, cell surface binding, recombinant protein binding, coendocytosis and functional interaction in Xenopus. While this study was underway two reports came to the same conclusion (Carmon et al, 2011; de Lau et al, 2011). As a minor difference, inhibition of Wnt3a signalling by siLGR4 was not observed by de Lau et al (2011), but we used an siRNA pool, which might be more effective.
We provide further evidence that LGR4/5 not only mediate R-spondin signalling by the Wnt/β-catenin but also the Wnt/PCP pathway. SDC4 mediates PCP signalling and binds to the R-spondin TSP1 domain, while LGR4/5 bind to the Furin domains. This suggests that R-spondins bridge between syndecans and LGR4/5 in the case of PCP signalling. As LGR4/5 associate with Fz and LRP6 in β-catenin signalling (Carmon et al, 2011; de Lau et al, 2011), the specificity of R-spondin to signal either by β-catenin or the PCP pathway might be determined by the presence of available Wnt/Fz combinations as well as of syndecans.
LGR4 is cointernalized with Rspo3 by Clathrin-mediated endocytosis. Our finding that Clathrin is essential for Rspo3-triggered β-catenin signalling is consistent with the model that GSK3 bound to LRP6 signalosomes needs to be sequestered in multivesicular bodies to allow accumulation of β-catenin (Taelman et al, 2010). If internalization is a rate-limiting step in Wnt signalling, it is likely that the used endocytic route has an important role in determining the rate and/or effectiveness of GSK3 sequestration and hence downstream signalling. As we show, Rspo3 and Wnt3a have a differential requirement for Clathrin- and Caveolin-mediated endocytosis, respectively. This raises the possibility that R-spondin/LGR4/5 amplify low-level Wnt signalling because GSK3 is more effectively sequestered if LRP6 signalosomes internalize by Clathrin than by Caveolin. The fact that LRP6 phosphorylation at the GSK3 site is enhanced by R-spondin (Carmon et al, 2011) is not inconsistent with this possibility.
Methods
Constructs. Human LGR5 (BC096324) and Xenopus tropicalis LGR4 (BC158183) complementary DNAs were obtained from Source BioScience. Tagged LGR4/5 constructs were generated by inserting LGR4/5 into a pCS-based vector containing a V5 or Flag epitope after the signal peptide of mouse krm2.
Cell culture, conditioning of media, cell surface binding, Wnt reporter and Xenopus assays were carried out as described (Cruciat et al, 2010; Ohkawara et al, 2011). In Wnt reporter assays performed in HEK293T cells, if not indicated elsewhere, the amounts of transfected plasmids per 96-well were 1 ng Xenopus tropicalis LGR4; 5 ng Flag-LGR5; 1, 2, 5 ng Dkk1; 5, 10, 20 ng CK1γ1(K73R) and GSK3β and 1, 5 ng mouse Axin-HA. For stimulation with recombinant proteins, a limiting amount of Wnt3a, Rspo3-ΔC, Rspo3-ΔC+ΔTSP and Rspo3-ΔC+ΔFurin-conditioned medium was used. siRNAs targeting the open reading frame of LGR4 and LGR5 (supplementary Table S2 online) were from Thermo Scientific Dharmacon.
In vitro binding assay. N-V5-tagged LGR4/5 were transfected in HEK293T cells using Xtreme 9 (Roche). After 2 days, membrane fractions were prepared and extracted with buffer (150 mM NaCl, 20 mM Tris–HCl, pH 7.5, 2% NP40, proteinase inhibitor mix (Roche)). Binding, purification, blocking and washing steps were done as described (Ohkawara et al, 2011). In brief, for binding, 96-well white plates (Greiner) were coated overnight at 4°C with 100 μl of 2 μg/ml anti-V5 antibody (Invitrogen) in bicarbonate buffer (50 mM NaHCO3, pH 9.6). After blocking and washing steps, N-V5-LGR4/5-containing extracts were applied to a plate overnight at 4°C. Following extensive washing, partially purified hRspo3-ΔC–AP fusion protein of known concentration was applied for 2 h. After washes, AP activity was measured and analysed by Scatchard plot using Excel.
Rspo3 internalization assay, endocytic inhibitors and immunostaining. For immunofluorescence, cells were grown in 24-well plates on collagen-treated glass coverslips. siRNA transfection was carried out with DharmaFECT1 (Dharmacon) and either 500 nM (siClathrin and siCaveolin) or 1,000 nM (siLGR4+5) siRNAs were used. DNA transfection was performed by Fugene 6 (Roche) with the following amounts of DNA per well: 10 ng Caveolin–GFP, 10 ng Clathrin–GFP and 100 ng Xenopus tropicalis LGR4-EYFP. For endocytosis inhibition, cells were pretreated with MDC (100 μM, Sigma), filipin III (3 μg/ml, Sigma), nystatin (25 μg/ml, Sigma), or 2% dimethylsulphoxide for 1 h, and this treatment continued for 1 h in the presence of hRspo3-ΔC–HRP. For the Rspo3 internalization assay, hRspo3-ΔC–HRP (25 U/μl) was used and detected by tyramide signal amplification (TSA; Dubois et al, 2001; Speel et al, 2006). After 1 h incubation with Rspo3 at 37°C, cells were washed three times with cold Hank's buffer and fixed with 0.5 mM dithiobis[succinimidyl propionate] (DSP) in Hanks's 10 mM HEPES, pH 7.2, for 30 min and then were permeabilized with 0.1% saponin in TSA buffer (100 mM Tris, pH 8.8, 10 mM imidazole). The TSA reaction was carried out for 30 min in dark with the following development solution: 30 μM tyramide-Rhodamine and 0.003% H2O2 in TSA buffer. Cells were washed with TSA buffer, followed by immunofluorescence staining where indicated, stained with Hoechst and embedded in Mowiol. For immunostaining, anti-GFP (Invitrogen), anti-β-catenin (BD Bioscience) and Alexa-488-conjugated antibodies (Invitrogen) were used.
Xenopus mRNA and Morpholino injections. The mRNA doses for injections were as follows (per embryo): 250 pg Fz7, 250pg Rspo3, 500pg Wnt5a, 400pg BMP4 and 400pg xnr1. The antisense Mos (supplementary Table S2 online) were used as follows, if not described otherwise (per embryo): 40 ng LGR4 Mo1, 10 ng LGR4 Mo2/3, 10 ng LGR5 Mo1, 5 ng LGR5 Mo2, 2.5 ng LGR5 Mo3, 10 ng Rspo3 Mo (Ohkawara et al, 2011) and 2.5 ng LRP6 Mo (Hassler et al, 2007). Equal amounts of total Mo/RNA were injected by adjustment with the standard control Mo (Gene Tools) or PPL RNA, where necessary.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank P. Stannek and C. Reinhard for technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (DFG).
Author contributions: A.G., binding and endocytosis assays. C.D. and N.K., Xenopus experiments. C.-M.C., siRNA screens and reporter assays. Y.-L.H. and O.K., endocytosis assays. D.I. and M.B., siRNA screens cooperation. All authors, experimental planning. C.N., paper writing.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aoki M, Mieda M, Ikeda T, Hamada Y, Nakamura H, Okamoto H (2006) R-spondin3 is required for mouse placental development. Dev Biol 301: 218–226 [DOI] [PubMed] [Google Scholar]
- Barker N, Clevers H (2010) Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 138: 1681–1696 [DOI] [PubMed] [Google Scholar]
- Binnerts ME et al. (2007) R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci USA 104: 14700–14705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaydon DC et al. (2006) The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat Genet 38: 1245–1247 [DOI] [PubMed] [Google Scholar]
- Carmon KS, Gong X, Lin Q, Thomas A, Liu Q (2011) R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc Natl Acad Sci USA 108: 11452–11457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C (2010) Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327: 459–463 [DOI] [PubMed] [Google Scholar]
- de Lau W et al. (2011) Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476: 293–297 [DOI] [PubMed] [Google Scholar]
- Dubois L, Lecourtois M, Alexandre C, Hirst E, Vincent JP (2001) Regulated endocytic routing modulates wingless signaling in Drosophila embryos. Cell 105: 613–624 [DOI] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W (2008) Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev 22: 2308–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassler C, Cruciat CM, Huang YL, Kuriyama S, Mayor R, Niehrs C (2007) Kremen is required for neural crest induction in Xenopus and promotes LRP6-mediated Wnt signaling. Development 134: 4255–4263 [DOI] [PubMed] [Google Scholar]
- Kazanskaya O, Glinka A, Del Barco Barrantes I, Stannek P, Niehrs C, Wu W (2004) R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for xenopus myogenesis. Dev Cell 7: 525–534 [DOI] [PubMed] [Google Scholar]
- Kazanskaya O, Ohkawara B, Heroult M, Maltry N, Augustin HG, Niehrs C (2008) The Wnt signaling regulator R-spondin3 acts upstream of VEGF to control the switch between angioblastic and hematopoietic cell fate determination. Development 135: 3655–3664 [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H (2007) Regulation of Wnt signalling by receptor-mediated endocytosis. J Biochem 141: 443–451 [DOI] [PubMed] [Google Scholar]
- Kim KA et al. (2005) Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 309: 1256–1259 [DOI] [PubMed] [Google Scholar]
- Kim KA et al. (2008) R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol Biol Cell 19: 2588–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustata RC, Van Loy T, Lefort A, Libert F, Strollo S, Vassart G, Garcia MI (2011) Lgr4 is required for Paneth cell differentiation and maintenance of intestinal stem cells ex vivo. EMBO Rep 12: 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK (2006) Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J Biol Chem 281: 13247–13257 [DOI] [PubMed] [Google Scholar]
- Nusse R (2005) Wnt signaling in disease and in development. Cell Res 15: 28–32 [DOI] [PubMed] [Google Scholar]
- Ohkawara B, Niehrs C (2011) An ATF2-based luciferase reporter to monitor non-canonical Wnt signaling in Xenopus embryos. Dev Dyn 240: 188–194 [DOI] [PubMed] [Google Scholar]
- Ohkawara B, Glinka A, Niehrs C (2011) Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev Cell 20: 303–314 [DOI] [PubMed] [Google Scholar]
- Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, Guerra L, Schedl A, Camerino G (2006) R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet 38: 1304–1309 [DOI] [PubMed] [Google Scholar]
- Speel EJ, Hopman AH, Komminoth P (2006) Tyramide signal amplification for DNA and mRNA in situ hybridization. Methods Mol Biol 326: 33–60 [DOI] [PubMed] [Google Scholar]
- Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM (2010) Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143: 1136–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Yokota C, Semenov MV, Doble B, Woodgett J, He X (2007) R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. J Biol Chem 282: 15903–15911 [DOI] [PubMed] [Google Scholar]
- Zhao J, Kim KA, De Vera J, Palencia S, Wagle M, Abo A (2009) R-Spondin1 protects mice from chemotherapy or radiation-induced oral mucositis through the canonical Wnt/beta-catenin pathway. Proc Natl Acad Sci USA 106: 2331–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.