Abstract
In the Quadrupole Magnetic Sorter (QMS) magnetic particles enter a vertical flow annulus and are separated from non-magnetic particles by radial deflection into an outer annulus where the purified magnetic particles are collected via a flow splitter. The purity of magnetically isolated particles in QMS is affected by the migration of nonmagnetic particles across transport lamina in the annular flow channel. Computational Fluid Dynamics (CFD) simulations were used to predict the flow patterns, pressure drop and nonspecific crossover in QMS flow channel for the isolation of pancreatic islets of Langerhans. Simulation results were compared with the experimental results to validate the CFD model. Results of the simulations were used to show that one design gives up to 10% less nonspecific crossover than another and this model can be used to optimise the flow channel design to achieve maximum purity of magnetic particles.
Keywords: Quadrupole Magnetic Sorter, Computational Fluid Dynamics, Annulus Flow, Nonspecific Crossover
INTRODUCTION
Insulin independence can be achieved in type 1 diabetes patients by transplanting purified pancreatic islets of Langerhans (Sakuma et al., 2008). Isolation of the islets is the important step in preparing the islets for the transplantation. To achieve high yields with required purities, various islet purification techniques have been developed for isolation of islets from exocrine pancreas tissue either by targeting islets or by targeting exocrine tissue (Soon-Shiong et al., 1990; Winoto-Morbach et al., 1989), e.g. hand picking of islets, fluorescence-activated sorting by staining islets with neutral red (Gray et al., 1989; Jindal et al., 1994), or destruction of non-islet tissue by laser energy (Brunicardi et al., 1994). All of these techniques were successfully employed to purify rat islets but failed to scale up to the human tissue digest volume. Present human islet isolation processes rely on the density gradient centrifugation method which depends on small density difference between islets and acinar tissue. The density gradient centrifugation process, however, has been identified as a potential source of islet mass loss and islet damage due to mechanical stress associated with centrifugation and prolonged exposure to proteolytic enzymes (Samejima et al., 1998; Pinkse et al., 2004).
Quadrupole magnetic sorting (QMS) is a well-designed and tested technology for magnetic separations of single cells, e.g., stem cells (Moore et al., 2001; Nakamura et al., 2001; Lara et al., 2002) and has been successfully applied to several cell separations and purifications (Sun et al., 1998; Tong et al., 2007; Jing et al., 2007). When QMS, developed for single cell separation, was used for isolation of islets, very low purity was achieved with good yields. Low purity is due to the size difference between single cells and islets (Shenkman et al., 2009). Islet diameters are distributed between 50 to 500 microns whereas single cell size is around 1 to 10 microns. It has been observed that the mechanical stress applied in QMS does not affect islets functionality after isolation (Shenkman et al., 2009). To overcome limitations associated with the commonly used density gradient method and to develop a technique that yields sufficient number of islets to reduce the donor to recipient ratio to 1:1, we redesigned (Kennedy et al., 2007) and tested high capacity QMS to isolate porcine islets of Langerhans.
In QMS isolation procedures particles of interest labeled with magnetic micro particles are isolated from unlabeled particles in a magnetic field while flowing through an annular flow channel. Labeled particles migrate radially outward in a quadrupole magnetic field and leave the channel on its outer periphery as a purified fraction. The presence of any unlabeled particles in isolated magnetically labeled particles is known as nonspecific crossover. The occurrence of nonspecific crossover is of particular importance in the isolation of islets as the islets constitute only one to two percent of the entire pancreas. Thus, even one percent of nonspecific crossover could reduce the purity of islets by 50 percent. There are many factors that contribute to nonspecific crossover in QMS, such as hydrodynamic forces, particle concentration in the sample, total flow rate, flow ratios, splitter imperfections (Williams et al., 2003; Williams et al., 2003a; Williams et al., 2008; Contado et al., 2007) and splitter position. The effects of splitter position, sample concentration, total flow rate and flow ratios were tested in this study. Computational fluid dynamics software was used to predict the fluid flow pattern throughout the flow channel and nonspecific crossover. The availability of the commercial CFD software made it easier to numerically study the fluid flow as well as the particle movements in the QMS flow channel (Middleman, 1998; Tsuji et al., 1993; Fletcher, 1991; Mikami et al., 1998). CFD was previously used to study the fluid and particle behaviour in the split-flow thin channel fractionation as well as to study the effect of the splitter imperfections on the nonspecific crossover (Zhang et al., 2005; Nguyen et al., 2002; Zhang et al., 2004; Fuh et al., 1995). CFD has also proved useful to study the deviation of the flow from laminar behaviour near the edges of the splitters. In this study we used the CFD simulations to find out the flow behaviour in the QMS flow channel and the effect of the splitter thickness on the nonspecific crossover. After proving the simulations results were consistent with the experimental results with the current geometry, new geometry was proposed and simulations studies were made to compare the nonspecific crossover between two geometry models.
MATERIALS AND METHODS
QMS and Separation Theory
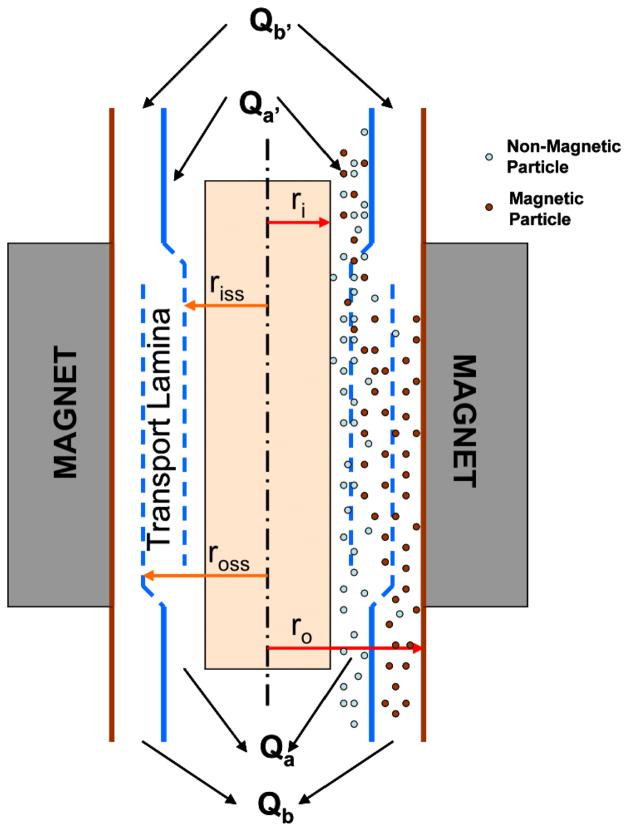
The QMS technology is based on a process known as split-flow lateral transport thin separation, which is a subset of Field Flow Fractionation (FFF) technology that separates immunomagnetically labeled islets based on their magnetophoretic mobility (m). Magnetophoretic mobility is defined as the velocity of a particle per unit magnetic energy gradient. A QMS system consists of three essential components: flow channel, magnet and pumps. Separation takes place in the flow channel which consists of a cylindrical core that is concentric with an external cylindrical shell (Figure 1). The sample, consisting of magnetically labeled islets along with unlabelled tissue particles, enters at a’ close to the core through a specially designed inlet: carrier fluid, enters the channel at b’ adjacent to the outer wall. Both flows merge at the end of the inlet splitter and continue to flow at each side of a virtual surface, known as inlet splitting surface (ISS) at distance riss from the center of the core.
Figure 1.
Schematic diagram of the quadrupole magnetic cell sorter (QMS).
ISS can be seen in the Figure 1 close to the core and remaining at the same distance along the fully developed laminar flow in the annulus region of the channel. The outer flow rate Qb’ is maintained higher than the inner flow rate Qa’ to keep the sample flow near the core. A second virtual surface known as the outer splitting surface (OSS) at a distance ross from the center of the core separates the two outlets which are separated by the outlet splitter. The distance between ISS and OSS, ross-riss, is known as the transport lamina. Magnetically labeled particles in the sample migrate radially and cross the transport lamina and leave in the positive fraction b. The unlabeled and weakly labeled particles which cannot cross the OSS will exit in the negative fraction a.
For an ideal separation in QMS with laminar flow, the transport lamina thickness should be zero as the unlabeled particles would not be expected to cross the transport lamina. However, magnetically labeled islets are generally not uniform in magnetisation. A range of magnetisation is exhibited due to variation in the number of magnetic particles that enter the vascular structure of islets. To completely isolate pure labeled particles, it is necessary to create conditions that allow the least mobile particles to migrate across the thickness of the transport lamina. The thickness of the transport lamina changes with changes in inlet and outlet flow rate ratios and change in splitter position
The particle trajectory in the annulus is described by the integral equation:
| (3) |
where v(k) is the velocity profile of the fluid in the annulus is, vs is the Stokes sedimentation velocity of the particle in the fluid, um(k) is the radial magnetophoretic velocity of the particle and k is defined as:
| (4) |
Here r is defined as the radial coordinate from the center of the flow channel core and r0 is the radius from the center to the wall. Upon the integration of eq. (3) and simplification we can get:
| (5) |
where mm is called the magnetophoretic mobility of the particle, Sm0 is the magnetic force at the inner surface of the outside wall of the flow channel and vs is sedimentation velocity of the particle. A1, A2 and I1 (k,k1) are functions used to simplify the calculations and are given by:
| (6) |
| (7) |
| (8) |
The radial position of the ISS, KISS is calculated using the following integration factor (Kennedy et al., 2007):
| (9) |
Similarly, the position of the OSS can be calculated using eq. (9) by replacing kISS with kOSS.
Experimental Measurements
The flow channel used for the experiments was called “prototype I”. Nonspecific crossover experiments were performed with nonmagnetic particles, Cultisphers®, macro-porous gelatin-coated micro-carrier beads with diameter range of 200 to 380 μm, similar in size and density to islets and fragments of exocrine tissue in pancreas digests. The sample consisting of cultisphers was introduced into the a’ inlet flow stream and carrier buffer was introduced into the b’ inlet flow stream by dual head Watson-Marlow peristaltic pumps, and the positive fraction outlet was controlled by a peristaltic pump while leaving the negative fraction outlet to exit at atmospheric pressure to maintain equilibrium of flow in the flow channel.
Experiments were carried out at total flow rates of 250, 300 and 400 ml/min with inlet flow ratio (Qa’/Q) of 0.25 and at different outlet flow ratios (Qa/Q) 0.25, 0.3, 0.5 and 0.7. Experiments were conducted at different sample concentrations to study the effect of particle concentration in the sample on nonspecific crossover. Turbidity sensors are connected to positive and negative fraction outlets to detect absorbance. Nonspecific crossover was calculated from the areas of the absorbance peaks.
Computational Fluid Dynamics
The commercial CFD code, FLUENT, was used for the simulations, and FLUENT’s preprocessor, GAMBIT, was used to generate flow-field meshes. An unstructured mesh consisting of tetrahedral volumetric elements in 3D is used in the entire domain with the domain divided into 1,671,756 tetrahedral shaped discrete control volumes that comprise the computational grid. Velocity inlet boundary condition with velocities normal to the plane of the channel inlets with fluid properties corresponding to the water at 298K are set at the inlets, ‘outflow’ boundary conditions were used at the outlets and the no-slip condition was applied to all walls.
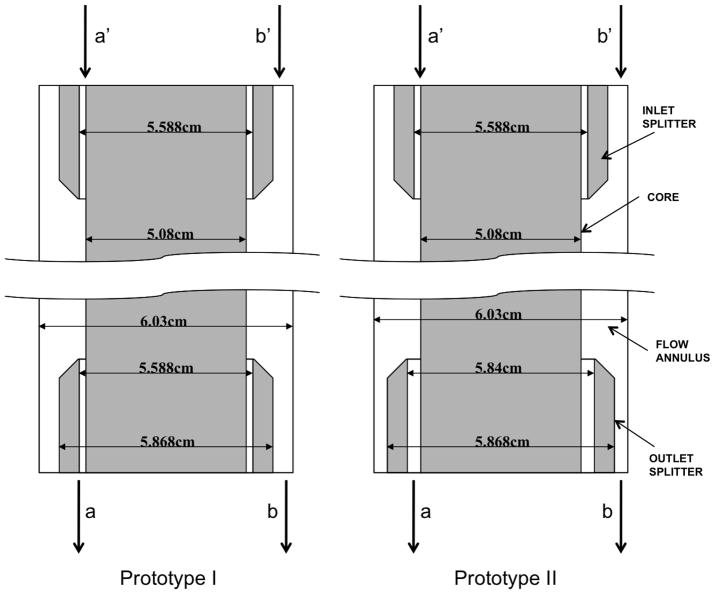
The two QMS separation flow channels analysed in the study are shown in Figure 2. The geometry used for the first case was based on the quadrupole magnetic separation channel for islets, “prototype I”. The length of this flow channel is 32.52cm with inner diameter of the outer wall of 6.03cm and core diameter is 5.08cm. Splitters with diameter 5.588cm and thickness 0.14cm were used at inlet and outlet. A specially designed flow distributor with diameter 5.96cm is placed in the b inlet. For the second case (prototype II), the channel length and outer wall diameter are same as for Prototype I, but the core diameter is 5.08cm and inlet splitter diameter 5.58cm with thickness 0.14cm and outlet splitter diameter 5.84cm with thickness 0.14cm.
Figure 2.
Geometry drawings of Prototype I and Prototype II flow channels.
RESULTS AND DISCUSSION
CFD Simulations: Flow Analysis and Pressure Drop Predictions
Laminar flow should be maintained in the annulus flow region to achieve the separations of the desired cells with high purity and yields with minimum nonspecific crossover of nonmagnetic cells. The differences in the inlet and outlet flow ratios cause some disturbances in the flow at the splitter tips and the angle of fluid inlets to the splitter affects the flow mixing at the inlet splitter. The primary objective of this study is to modify the design of the splitter in a way that will favourably alter the flow pattern at the outlet splitter tip to reduce the nonspecific crossover. Uniform vertical flow should be maintained at the sample and buffer inlets. Sample enters at 45° angles onto the splitter to reach laminar vertical flow at the splitter edge and a flow distributor placed in the buffer inlet helps to achieve smooth flow of the buffer.
Flow channel was fabricated by injection molding using medical grade plastics. Initial simulations were performed only with liquid flow to observe the flow patterns and pressure drops in the flow channel. One important feature of the flow channel is the design of the flow distributor placed at the carrier buffer inlet (b’) to obtain circumferentially uniform flow in the flow channel. Uniform flow around the core is important to maintain laminar flow in the annulus to yield minimum crossover and maximum isolation. Improvements made to the flow distributor design were verified by CFD analysis. Incoming fluid enters the distributor from two branches. Direct flow through the distributor from these branches is prevented by the lack of notches at the two inflow locations. The simulations showed the uniform flow developed by the time fluid reaches the splitter end point.
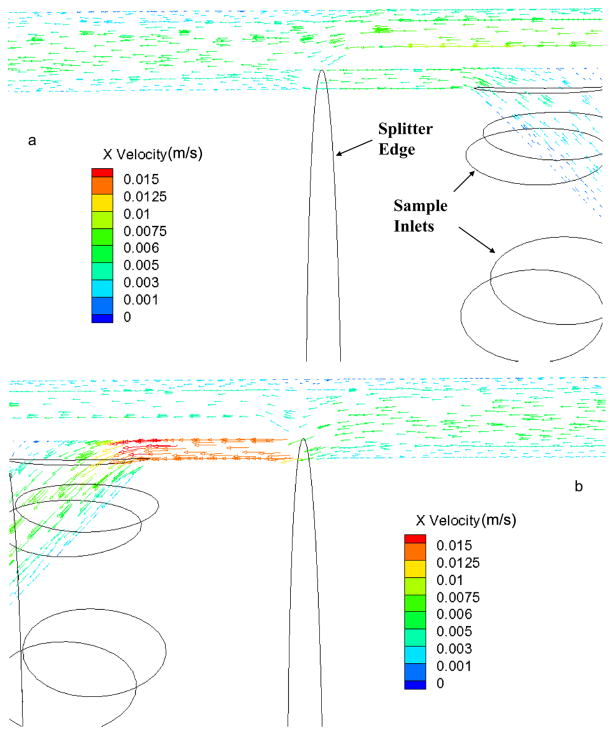
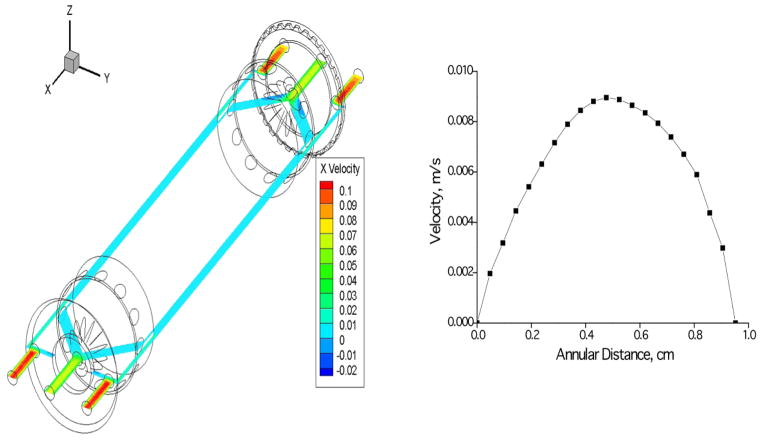
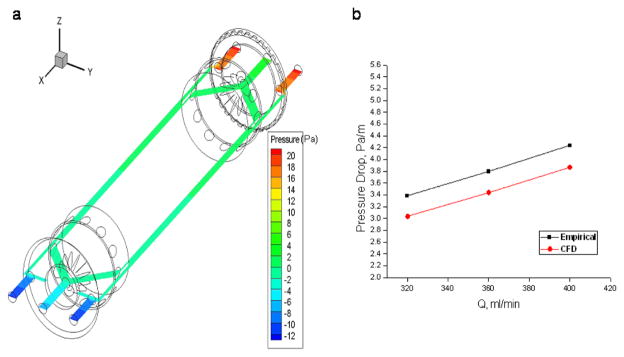
CFD simulations are performed using the generated flow-field meshes in order to predict the pressure drop and analyse the details of the flow in flow channel. Figure 3 shows the velocity vectors in the annulus region of the flow channel at an inlet flow ratio of 0.25 and outlet flow ratio of 0.4. Figure 4a shows the velocity profile vectors at the inlet splitter edge and figure 4b shows the velocity vectors at the outlet splitter. The virtual ISS and OSS can be observed in these figures and it can be observed the laminar flow development immediately crossing the splitter. A plane is extracted at the center of the domain (i.e., Z=3.01625 mm) and velocity contours were displayed as a coloured ring for the case with total flow rate 400 ml/min with inlet and outlet flow ratio 0.25. The velocity magnitude contours are shown in Figure 4a for sample (a’) velocity 0.05 m/s and buffer (b’) velocity 0.15 m/s. Careful examination of the calculated fluid velocity profiles revealed that the flow was fully developed within a relatively short distance of the splitter edge for every ratio of inlet and outlet flows. Figure 4b shows the velocity profile at the middle of the channel. Pressure magnitude contours are shown in Figure 5 along with the pressure gradient found from CFD simulations plotted against total flow rates compared with the theoretically calculated pressure drop using the Haigen-Poiseuille equation for annular pipes. Figure 5b shows that the pressure drop in annulus region between two splitters predicted using CFD is in good agreement with theoretical calculations with pressure drop increasing with the increase in total flow rate.
Figure 3.
(a) Velocity vectors at the inlet splitter. (b) Velocity vectors at the outlet splitter.
Figure 4.
a) Velocity (m/s) magnitude contours in the flow channel at z = 1.1875” (flow is in positive x direction. b) Velocity profile of the fluid at the middle point of the channel. X axis is the annular distance from core to the outer wall of the flow channel and Y axis is the velocity.
Figure 5.
a) Pressure (Pa) contours in the flow channel at z = 1.1875” b) Comparison of the pressure drop from CFD simulations and empirical correlations measured between splitters.
Nonspecific Crossover
The new design of the flow channel is tested for nonspecific crossover at different total flow rates ranging from 250 ml/min to 400 ml/min, input flow ratios (Qa’/Q), outlet flow ratios (Qa/Q) ranging from 0.25 to 0.7. When flow rates less than 250ml/min were used, cultisphers settled in the tubing and clogging of the inlet was observed. The effect of the particle concentrations in the sample and total flow rate on the crossover was examined. Some of these experiments were also conducted with pig pancreas (results not presented) and the same results as with cultisphers were observed. CFD simulations were conducted for all of these cases using the discrete phase model and compared with the experimental results. Nonspecific crossover (Sb) is calculated as the ratio of non-magnetic particles leaving in positive collection (Nb) to the particles leaving in both negative (Na) and positive collection.
| (10) |
Figure 6 shows the nonspecific crossover of cultisphers at different outlet flow ratios and at fixed inlet flow ratio of 0.25 and total flow rate of 400ml/min. These experiments were conducted with the flow channel design prototype I, in which the inlet and outlet splitters have the same 5.58mm diameter. A clear decrease in nonspecific crossover with increase in outlet flow ratio is observed because the increase in outlet flow ratio increases the transport lamina thickness. Figure 6 also presents the comparison of nonspecific crossover obtained from experiments with prototype I and CFD simulation-predicted crossover for prototype II. Good agreement is found between experimental results and CFD simulation-predicted values with same prototype flow channel with less than 10% deviation. This deviation might be due to the negligence of particle-particle interaction in CFD modelling. CFD-predicted crossover for prototype II is compared with that of CFD predictions and experimental values with Prototype I. Crossover is slightly less for prototype II when compared to prototype I. The diameter of the outlet splitter is increased in prototype II which increases the transport lamina thickness and reduces the number of nonmagnetic particle that leave in the positive fraction. Straight line in the figure represents the ideal flow of the fluid without solid particles.
Figure 6.
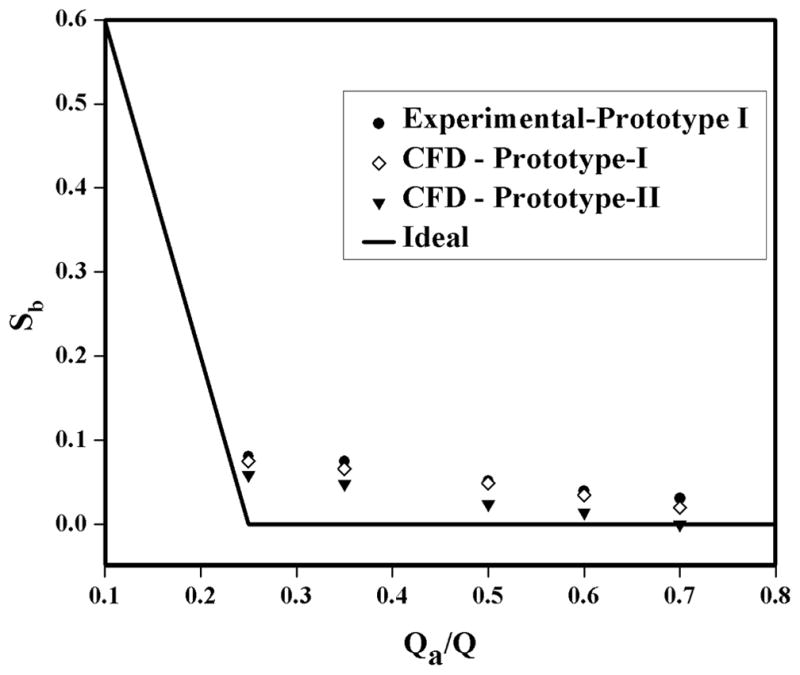
Comparison of calculated and observed nonspecific crossover as a function of outlet flow ratio at a total flow rate of 400 ml/min and inlet flow ratio of 0.25.
Figure 7 shows the crossover of nonmagnetic particles at different total flow rates in the Prototype I channel at a fixed inlet and outlet flow ratio of 0.25. Experimental crossover values increase with decreasing total flow rates as the hydrodynamic lift forces move the particles away from wall at low flow rates which helps particles to cross the transport lamina and leave in the positive fraction. Crossover obtained from CFD simulations for different total flow rates are under predicted by 5 to 10 percent for prototype I. Comparison of calculated crossover values at different total flow rates for the two prototypes shows a decrease in crossover for prototype II due to the increase in transport lamina thickness resulting from the outlet splitter position. Figure 8 shows crossover values at different sample concentrations at a fixed total flow rate of 400ml/min. Outlet flow ratio is maintained equal to inlet flow ratio at 0.25. Increasing cultisphers concentration increases the particle-particle interaction which results in more nonspecific crossover. Higher concentrations of particles during experiments also clogged the flow paths in the sample inlets. CFD simulations predict decreased crossover with the new design prototype II when compared with the prototype I.
Figure 7.
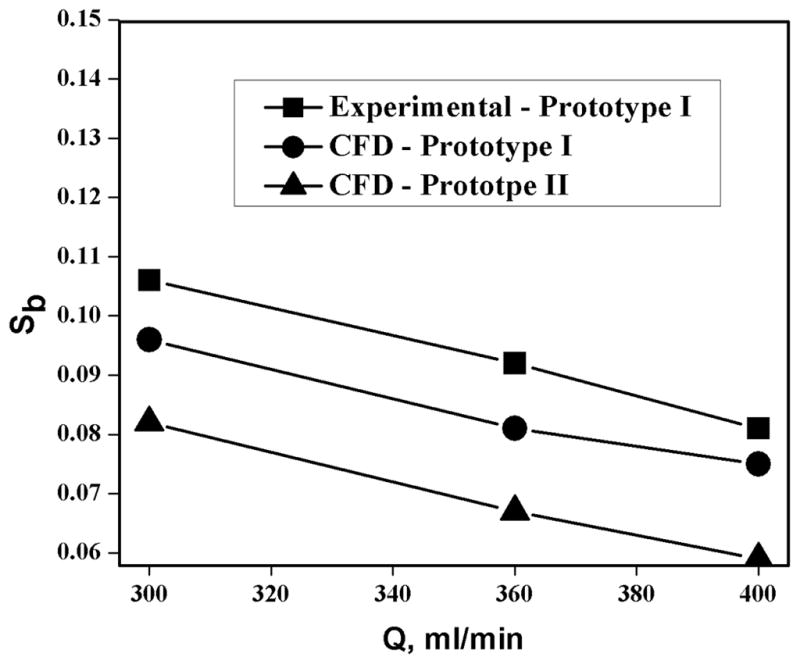
Comparison of the nonspecific crossover as a function of total flow rate at an inlet and outlet flow ratio of 0.25.
Figure 8.
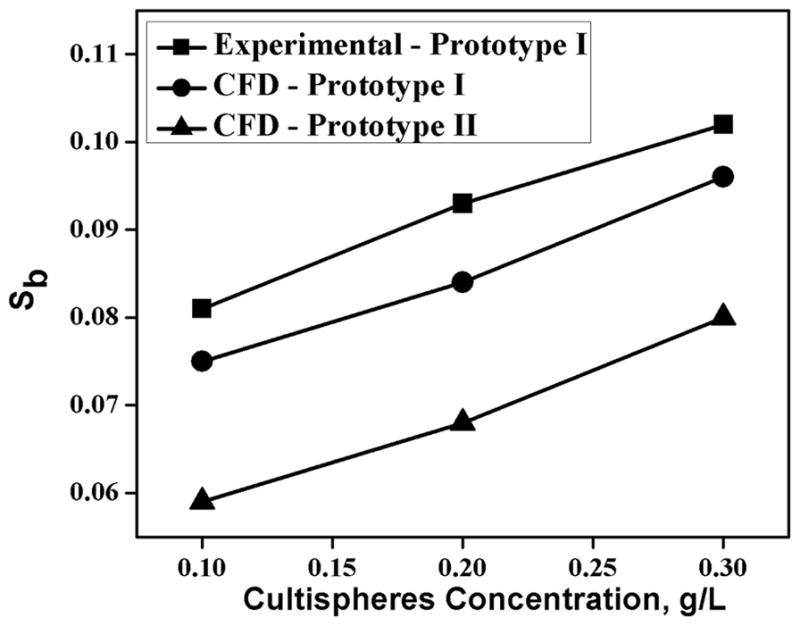
Nonspecific crossover as a function of particle concentration in the sample at a total flow rate of 400ml/min, inlet and outlet flow ratio of 0.25.
CONCLUSIONS
CFD simulations of flow pattern performed on the new design of QMS flow channel confirm circumferentially uniform flow development around the annular channel. Quantitative agreement between experimental measurements of nonspecific crossover and prediction based on CFD modelling of the fluid flow was shown. For all flow conditions, crossover predicted by CFD simulations was found to be slightly lower than experimentally observed results. This difference may be due to the contribution of crossover from other factors such as particle lift and particle interactions. Diffusion was not considered in the CFD modelling. The good agreement between experimental and CFD predicted results allowed the performance of simulations with different channel prototype models to develop a design to minimise nonspecific crossover. Though increasing the diameter of the outlet splitter decreases nonspecific crossover the diameter needs to be optimised based on the yield of magnetically labeled particles with change in splitter diameter. Therefore future CFD simulations must include the migration of the magnetic particles.
Acknowledgments
This research was funded in part by the U.S. Department of Health and Human Services under SBIR grant 5R44DK072647-03 from the National Institute and Digestive and Kidney Research (NIDDK) awarded to Techshot, Inc., Greenville, Indiana, USA.
References
- Brunicardi FC, Oh Y, Shevlin L, Suh E, Kleinman R, Stein E, Lipaz G, Plant DV, Imagawa D, Fetterman HR. Laser destruction of human nonislet pancreatic tissue. Transplant Proc. 1994;26(6):3354–3355. [PubMed] [Google Scholar]
- Contado C, Hoyos M. SPLITT cell analytical separation of silica particles. Non-specific crossover effects: does the shear-induced diffusion play a role? Chromatographia. 2007;65(7–8):453–462. [Google Scholar]
- Fletcher CAJ. Computational Techniques for Fluid Dynamics. 2. II. Springer-Verlag; New York: 1991. [Google Scholar]
- Fuh CB, Trujillo EM, Giddings JC. Hydrodynamic characterisation of SPLITT fractionation cells. Sep Sci Technol. 1995;30(20):3861–3876. [Google Scholar]
- Gray DWR, Gohde W, Carter N, Heiden T, Morris PJ. Separation of Pancreatic islets by Fluorescence-Activated Sorting. Diabetes. 1989;38(suppl 1):133–135. doi: 10.2337/diab.38.1.s133. [DOI] [PubMed] [Google Scholar]
- Jindal RM, McShane P, Gray DWR, Morris PJ. Isolation and Purification of Pancreatic Islets by Fluorescence Activates Islet Sorter. Transplant Proc. 1994;26(2):653–657. [PubMed] [Google Scholar]
- Jing Y, Chalmers JJ, Zborowski M. Blood progenitor cell separation from clinical leukapheresis product by magnetic nanoparticle binding and magnetophoresis. Biotechnol Bioeng. 2007;96(6):1139–1154. doi: 10.1002/bit.21202. [DOI] [PubMed] [Google Scholar]
- Kennedy DJ, Todd P, Logan S, Becker M, Papas KK, Moore LR. Engineering quadrupole magnetic flow sorting for the isolation of pancreatic islets. J of Magnetism and Magnetic Materials. 2007;311:388–395. [Google Scholar]
- Lara O, Nakamura M, Zborowski M, Chalmers JJ. Negative depletion cell sorting using a quadrupole magnetic cell sorter. Eur Cells Mater. 2002;3:62–64. [Google Scholar]
- Middleman S. An Introduction to Fluid Dynamics: Principles of Analysis and Design. 2. Wiley; New York: 1998. pp. 411–421. [Google Scholar]
- Mikami T, Kamiya H, Horio M. Numerical simulation of cohesive powder behaviour in a fluidized bed. Chem Eng Sci. 1998;53:1927–1940. [Google Scholar]
- Moore LR, Rodriguez AR, Williams PS, McCloskey K, Bolwell BJ, Nakamura M, Chalmers JJ, Zborowski M. Progenitor cell isolation with a high capacity quadrupole magnetic flow sorter. J Magn Magn Mater. 2001;225:277–284. [Google Scholar]
- Nakamura M, Decker K, Chosy J, Comella K, Melnik K, Moore LR, Lasky LC, Zborowski M, Chalmers JJ. Separation of Breast Cancel Cell Line from Human Blood Using a Quadrupole Magnetic Flow Sorter. Biotechnol Prog. 2001;17:1145–1155. doi: 10.1021/bp010109q. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Liffman K, Rudman M, McKinnon I, Beckett R. Computational Modelling and Simulation of Fluid Flows and Particle Transport in a SPLITT Fractionation Channel. A-24, the 10th International Symposium on Field-Flow Fractionation; Amsterdam, The Netherlands. 2002. [Google Scholar]
- Pinkse G, Steenvoorde E, Hogendoorn S, Noteborn M, Terpstra OT, Bruijn JA, De Heer E. Stable transplantation results of magnetically retracted islets: a novel method. Diabetologia. 2004;47:55–61. doi: 10.1007/s00125-003-1268-4. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Ricordi C, Miki A, Yamamoto T, Pileggi A, Khan A, Alejandro R, Inverardi L, Ichii H. Factors That Affect Human Islet Isolation. Transplant Proc. 2008;40:343–345. doi: 10.1016/j.transproceed.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima T, Yamaguchi K, Iwata H, Morkawa N, Ikada Y. Gelatin density gradient for isolation of islets of Langerhans. Cell Transplant. 1998;7(1):37–45. doi: 10.1177/096368979800700106. [DOI] [PubMed] [Google Scholar]
- Shenkman RM, Chalmers JJ, Hering BJ, Kirchhof N, Papas KK. Quadrupole Magnetic Sorting of Porcine Islets of langerhans. Tissue Eng: Part C. 2009;15(2):147–156. doi: 10.1089/ten.tec.2008.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkman RM, Godoy-Silva R, Papas KK, Chalmers JJ. Effects of Energy Dissipation Rate on Islets of Langerhans: Implications for isolation and Transplantation. Biotechnol and Bioeng. 2009;103(2):413–423. doi: 10.1002/bit.22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soon-Shiong P, Fujioka T, Terasaki P, Heintz R, Lanza RP. Islet purification by a novel immunomicrosphere cell depletion technique. Transplant Proc. 1990;22(2):780–781. [PubMed] [Google Scholar]
- Sun L, Zborowski M, Moore LR, Chalmers JJ. Continuous, Flow-Through Immunomagnetic Cell Separation in a Quadrupole Field. Cytometry. 1998;33:469–475. doi: 10.1002/(sici)1097-0320(19981201)33:4<469::aid-cyto11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Tong X, Xiong Y, Zborowski M, Farag SS, Chalmers JJ. A Novel High Throughput Immunomagnetic Cell Sorting System for Potential Clinical Scale Depletion of T Cells for Allogeneic Stem Cell Transplantation. Exp Hematol. 2007;35(10):1613–1622. doi: 10.1016/j.exphem.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji Y, Kawaguchi T, Tanaka T. Discrete particle simulation of two-dimensional fluidized bed. Powder Technol. 1993;77:79–87. [Google Scholar]
- Williams PS, Moore LR, Chalmers JJ, Zborowski M. Splitter imperfections in annular Split-flow Thin separation channels: effects on non-specific crossover. Anal Chem. 2003;75(6):1365–1373. doi: 10.1021/ac020649h. [DOI] [PubMed] [Google Scholar]
- Williams PS, Decker K, Nakamura M, Chalmers JJ, Moore LR, Zborowski M. Splitter Imperfections in Annular Split-Flow Thin Separation Channels: Experimental Study of Nonspecific Crossover. Anal Chem. 2003a;75:6687–6695. doi: 10.1021/ac030152n. [DOI] [PubMed] [Google Scholar]
- Williams PS, Hoyos M, Kurowski P, Salhi D, Moore LR, Zborowski M. Characterisation of Nonspecific Crossover in Split-Flow Thin Channel fractionation. Anal Chem. 2008;80:7105–7115. doi: 10.1021/ac800841q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winoto-Morbach S, Ulrichs K, Leyhausen G, Muller-Ruchholtz W. New principle for large-scale preparation of purified human pancreas islets. Diabetes. 1989;38(Supp 1):146–149. doi: 10.2337/diab.38.1.s146. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Barber RW, Emerson DR. Particle separation in microfluidic Devices SPLITT fractionation and microfluidics. Curr Anal Chem. 2005;1(3):345–354. [Google Scholar]
- Zhang Y, Emerson DR. Effect of flow development region and fringing magnetic force field on annular split-flow thin fractionation. J Chromatogr A. 2004;1042:137–145. doi: 10.1016/j.chroma.2004.05.018. [DOI] [PubMed] [Google Scholar]