Abstract
A naturally graded interface due to functional demands can deviate toward a discontinuous interface, eventually decreasing the functional efficiency of a dynamic joint. It is this characteristic feature in a human fibrous joint i.e. bone-tooth complex that will be discussed through histochemistry, and site-specific high resolution microscopy, micro tomography, X-ray fluorescence imaging and wet nanoindentation techniques. Results demonstrated two causes for the occurrence of 5-50 μm narrowed PDL-space: 1) microscopic scalloped regions at the PDL-insertion sites and macroscale stratified layers of bone with rich basophilic lines, and 2) macroscopic bony protrusions. Narrowed PDL-complexes illustrated patchy appearance of asporin, and when imaged under wet conditions using an atomic force microscope (AFM), demonstrated structural reorganization of the PDL, collagen periodicity, organic-dominant areas at the PDL-cementum and PDL-bone entheses and within cementum and bone. Scanning electron microscopy (SEM) results confirmed AFM results. Despite the narrowed PDL, continuity between PDL and vasculature in endosteal spaces of bone was demonstrated using a Micro XCT™. The higher levels of Ca and P X-ray fluorescence using a microprobe were correlated with higher elastic modulus values of 0.1-1.4 and 0.1-1.2 GPa for PDL-bone and PDL-cementum using wet nanoindentation. The ranges in elastic modulus values for PDL-bone and PDL-cementum entheses in 150-380 μm wide PDL-complex were 0.1-1.0 and 0.1-0.6 GPa. Based on these results we propose that strain amplification at the entheses could be minimized with a gradual change in modulus profile, a characteristic of 150-380 μm wide functional PDL-space. However, a discontinuity in modulus profile, a characteristic of 5-50 μm narrowed PDL-space would cause compromised mechanotransduction. The constrictions or narrowed sites within the bone-tooth fibrous joint will become the new “load bearing sites” that eventually could cause direct local fusion of bone with cementum.
1. Introduction
The hallmark of a natural interface is that it binds two dissimilar materials with gradually varying physical and chemical properties [1]. The gradients in physical and chemical properties allow optimum distribution of functional loads by eliminating discontinuities [1]. Discontinuities are most commonly observed in engineered systems and are abrupt transitions between two dissimilar materials leading to a local stress concentration and a shorter functional life [1]. Local stress concentrations between dissimilar materials are intelligently eliminated by Nature through the development of functionally graded interfaces (FGI) [1-3]. In natural systems the inherent biochemical, structural, elemental composition and mechanical properties of matrices that form the FGI change gradually in all three spatial dimensions [1, 2]. In fact, in most natural systems the combination of genetic and epigenetic factors [4] could control and maintain the functionally graded characteristics specifically between two dissimilar materials throughout the life of an organism. Thus the fundamental concept of FGI has permeated life sciences and is consistently being used as a biomimetic template to engineer interfaces. FGI is one of the pivotal biomimetic parameters for tissue regeneration of the soft-hard tissue attachments i.e. tendon-bone, or ligament-bone interfaces [5, 6].
Amongst other natural systems, several FGIs within the musculoskeletal and, oral and craniofacial systems were identified including ligament-bone, tendon-bone, tendon-muscle, enamel-dentin, cementum-dentin, periodontal ligament (PDL)-bone and PDL-cementum [3, 7-16]. Such FGIs accommodate and attach hard-hard, hard-soft and soft-soft tissue interfaces permitting function. The bone-tooth fibrous joint is an intricate and a dynamic system with a continuous cross-talk between several mineralized and soft tissues. The joint is subjected to a variety of loads, in particular compressive loads due to chewing. Functional loads on the bone-tooth joint lead to stress induced deformation in the mineralized tissues and strain-induced deformation in the vascular PDL [17]. The stress and strain fields vary within the three dimensional complex as the tooth forms an association with the bony complex during loading. Functional loads also regulate fluid flow and hydrostatic pressure within tissues and the complex, stimulating the cells in interstitial spaces and revitalizing the organ [18, 19].
Functional homeostasis of the PDL-space is maintained by continuous PDL-turnover, remodeling of bone, and deposition of lamellar cementum. Previous studies from our laboratory on healthy teeth and bone-tooth fibrous joint with a PDL-space of 250 μm identified a gradual variation in the intrinsic parameter i.e. elastic modulus at the PDL-bone and PDL-cementum interfaces [16]. The gradual variation at the soft-hard tissue interfaces was another necessary criterion for the bone-tooth fibrous joint to be a functionally efficient system. Additionally, the integration of PDL with bone and cementum provided insights into local functional modeling at the bone-PDL and cementum-PDL attachment sites since adaptive changes resulted from higher strains at these sites [17]. Hence it is proposed that in a healthy bone-tooth fibrous joint, the functional loads alter the relative motion between tooth and bone, which is most felt as tension, compression and/or shear strain at the soft-hard tissue interfaces PDL-bone and PDL-cementum. The local strains could be responsible for maintaining a normal periodontal ligament space of 150-380 μm with a decrease in PDL-space observed due to an increase in physiological age [20]. Contrastingly, the homeostasis of the complex could be altered by aberrations in strains at the bone-PDL and cementum-PDL attachment sites due to mechanobiological responses.
In the orofacial system, the FGI between the compliant, innervated and vascularized PDL and the mineralized hard tissue(s) (vascularized bone and avascularized cementum) [16] can be affected by two globally prevalent environmental factors. The factors are periodontal disease and/or nonphysiological loads which are known to decrease the biomechanical/functional efficiency of the bone-tooth fibrous joint [21-25]. We hypothesize that the bacterial- [26] and/or load-induced perturbations affect the PDL-bone and PDL-cementum attachment sites by altering the graded interface into a discontinuous interface or more step-like interface. Such an altered interface could affect the overall biomechanics of the bone-PDL-cementum complex [16]. In this study the stiffness graded properties of an undiseased fibrous joint with a 5 – 50 μm narrower PDL interfacing bone and tooth will be reported. Specifically, the physical and chemical properties of the narrowed bone-tooth complex will be discussed by comparing to the normal complex with 150-380 μm healthy i.e. functional PDL-space [20]. Macro-scale biomechanical models will be proposed to describe the observed micro-scale adaptations in the bone-PDL-tooth complex, i.e. narrowed PDL-space within the human periodontium. Structural integration, chemical composition and elastic modulus of various tissues and their interfaces put together could affect biomechanical function. Hence, detailed PDL-bone and PDL-cementum integration of the 5-50 μm wide complex will be discussed using various complementary high resolution characterization techniques, such as atomic force and scanning electron microscopy techniques (AFM, SEM), micro X-ray computed tomography (Micro XCT™), micro X-ray fluorescence imaging (μ-XRF), and AFM-based nanoindentation technique.
2. Materials and Methods
Permanent molars (N = 15) with attached bone were obtained from humans requiring extractions as a part of dental treatment following a protocol approved by the UCSF Committee on Human Research. All specimens were sterilized using 0.31 Mrad of γ-radiation [27]. Five specimens were used for immunohistochemistry and histology. Another five specimens were used for AFM, and the same five were characterized for site-specific mechanical properties using AFM-based nanoindentation and X-ray microprobe techniques. The remaining five specimens were cryofractured and imaged using SEM, and used for Micro XCT™ analyses. It should be noted that most specimens were taken from the first two-thirds of the root identified by using primary cementum (acellular cementum) as the marker [28].
2.1. Deparaffinized sections for conventional histology and immunohistochemistry
Extracted molars (N = 5) containing PDL and alveolar bone were prepared for histology as stated in our previous work [16]. The demineralized specimens were embedded in paraffin (Tissue Prep-II, Fisher Scientific, Fair Lawn, NJ) and 5-6 μm thick paraffin sections were mounted on Superfrost Plus microscope slides (Fisher Scientific, Fair Lawn, NJ). The sections were subsequently deparaffinized with xylene then stained with hematoxylin and eosin. The stained tissues were characterized for structural orientation and integration of the PDL with bone and cementum, using a light microscope (BX 51, Olympus America Inc., San Diego, CA) and analyzed using Image Pro Plus v6.0 software (Media Cybernetics Inc., Silver Springs, MD). Polarized light was used to enhance the birefringence of collagen in bone.
2.1.1. Preparation of sections for antibody tagging
Antibody tagging was performed using the procedure described in our previous work [16]. Following deparaffinization of sections, antigen retrieval was performed by trypsin digestion and glycosaminoglycan (GAG) removal enzymatically in 35 mM Tris-HCl pH 7.4, 35 mM sodium acetate, 15 mU/mL chondroitinase ABC (Seikagaku Biobusiness Corporation, Tokyo, Japan), 3 mU/mL keratanase (Seikagaku Biobusiness Corporation, Tokyo, Japan) for 1 hour at 37°C in a humidified chamber. A primary goat anti-asporin polyclonal antibody (Abcam, Cambridge, MA) concentration of 1:25 was used with the VECTASTAIN ABC Kit, Goat IgG (Vector Labs, Burlingame, CA) for staining asporin. The following modifications to the manufacturer's protocol were made: the secondary rabbit anti-goat antibody incubation included 1% BSA, and subsequent washing steps used PBS with 0.1% Tween-20 added. Specimens were blocked at room temperature for 20 minutes in 1% bovine serum albumin (Sigma, St. Louis, MO), 1.5% mouse serum (Sigma, St. Louis, MO) in PBS. Antibody incubation was then performed overnight (18 hours) at 4°C, with the appropriate antibody diluted in blocking solution. The next day, the slides were washed 3 times in PBS for 5 minutes each. Secondary antibody incubation of mouse anti-rabbit-IgG conjugated to HRP (Sigma, St. Louis, MO), diluted 1:100 in blocking solution, was performed at room temperature for 30 minutes, and then washed 3 times in PBS 10 minutes each.
2.1.2. Staining of antibody localization
3,3′-Diaminobenzidine (DAB) Enhanced Liquid Substrate System (Sigma, St. Louis, MO) was used per manufacturer's instructions with an incubation of 1 hour to provide a brown coloration of epitope locations. The specimens were then counterstained with Gill's III Hematoxylin (Sigma), dehydrated through serial solutions of 80% alcohol, 95% alcohol, 100% alcohol, and xylene, and mounted with Permount (Sigma). An Olympus BX51 light microscope was used for imaging with analyses using Image Pro software (Media Cybernetics Inc., Bethesda, MD).
2.2. Specimen preparation for AFM, AFM-based nanoindentation and μ-XRF characterization
Prior to micro- and nano-scale characterization using AFM and AFM-based nanoindentation, light microscopy (BX 51, Olympus America Inc., San Diego, CA) was performed on the surface of ultrasectioned blocks (N = 5), specifically to identify the narrowed PDL-space between bone and cementum. Specimens were mounted on AFM steel stubs (Ted Pella, Inc., Redding, CA) using epoxy and were ultrasectioned (MicroStar Technologies, Huntsville, TX) [29] and characterized using an AFM, AFM-based nanoindentation and microprobe for μ-XRF. The width of the PDL-space in ultrasectioned surface blocks was analyzed using Image Pro Plus v6.0 software (Media Cybernetics Inc., Silver Springs, MD) and narrowed PDL-spaces of 5-50 μm were identified within the sectioned bone-tooth fibrous joint.
2.3. AFM for structural analysis
Semi-qualitative data representative of PDL-space, collagen periodicity, hygroscopicity of PDL and PDL-inserts within bone and cementum was performed using contact mode AFM (Nanoscope III, Multimode; DI-Veeco Instruments Inc., Santa Barbara, CA) under dry and hydrated conditions [30] on ultrasectioned block specimens. AFM micrographs were analyzed with Nanoscope III version 5.12r3 software (Nanoscope III, Multimode; DI-Veeco Instruments Inc., Santa Barbara, CA).
2.4. Scanning electron microscopy (SEM)
Five specimens were cryofractured for SEM characterization. The specimen preparation for cryofracturing specimens was detailed in our previous work [29]. Topography of the cryofractured surfaces were characterized by mounting the specimens on SEM stubs with a carbon tape followed by a 20 - 50 Å sputtering of gold-palladium (Hummer VII, Anatech Ltd., VA, USA). Specimens were examined using an SEM (S4300, Hitachi, Tokyo, Japan) at an energy of 5-10 keV.
2.5. Specimen preparation for micro X-ray computed tomography (Micro XCT™)
2 mm – 3 mm thick bone-PDL-cementum complex were isolated from the remaining halves of the cryofractured teeth using a low-speed saw (Isomet, Buehler, Lake Bluff, IL) under wet conditions. Specimens were imaged using the Micro XCT™ (Xradia, Pleasanton, CA) at 10X and 40X at a tungsten anode setting of 40KVp and 8W, using the protocol described in our previous work [16]. Computed tomography (CT) was used to study the 3D structure of bone-PDL-cementum complex and allowed selection of virtual parallel slices spaced by 1 μm in different planes, thus illustrating the bulk structure of tissues.
2.6. Micro-XRF mapping of ultrasectioned specimens
In addition to performing electron microscopy on ultrasectioned surfaces of tissue block specimens, micro-X-ray fluorescence (μ-XRF) maps were used to identify elemental distribution and localization, and were collected at the Stanford Synchrotron Radiation Lightsource (SSRL) using beam line 2–3. Data were acquired using an incident x-ray energy of 12 keV, which was set by using a Si (111) double crystal monochromator with the storage ring Stanford Positron Electron Accelerating Ring (SPEAR) containing 150–200 mA at 3.0 GeV. The fluorescence lines of calcium (Ca) and phosphorus (P) within the narrowed complex and adjacent mineralized tissues were monitored using a silicon drift Vortex detector (SII NanoTechnology USA Inc.). The microfocused beam of 2×2μm was provided by a Pt-coated Kirkpatrick-Baez mirror pair (Xradia Inc., Pleasanton, CA). The incident and transmitted X-ray intensities were measured with nitrogen-filled ion chambers. Samples were mounted at 45° to the incident X-ray beam and were spatially rastered in the microbeam using a Newport VP-25XA-XYZ stage. Using a beam exposure of 100 ms per pixel, low resolution maps were generated with scanning step size of 5 μm, while high resolution maps were generated with scanning step size of 1 μm.
2.7. AFM-based nanoindentation for site-specific mechanical property evaluation
Nanoindentation [31] was performed on the ultrasectioned block specimens using an AFM attached to a load displacement transducer (Triboscope, Hysitron Incorporated, Minneapolis, MN). A sharp diamond Berkovich indenter with a conventional radius of curvature less than 100 nm (Triboscope, Hysitron Incorporated, Minneapolis, MN) was fitted to the transducer. Site-specific measurements of reduced elastic modulus (Er) were made under wet conditions using a displacement control mode and a penetration depth of 500 nm, with a load, hold, and unload for 3 seconds each. Fused silica was used to calibrate the transducer under dry and wet conditions [14, 15].
3. Results
In this study we evaluated structural, chemical composition and site-specific mechanical properties of a narrowed 5-50 μm bone-PDL-cementum complex. Structural characterization was achieved through complementary imaging techniques including atomic force microscopy (AFM), scanning electron microscopy (SEM), and X-ray computed tomography (Micro XCT™). Structural properties were correlated to biochemical, elemental composition, and site-specific mechanical properties using μ-XRF analysis and AFM based nano-indentation, respectively.
3.1. Structural and biochemical analyses using light microscopy, AFM, SEM, and Micro XCT™
Light microscopy images of sectioned specimens demonstrated structural heterogeneity of alveolar bone (Fig. 1). Figure 1 shows four representative images (Fig. 1a) in which, narrowed PDL sites illustrate presence of bundle bone (asterisks, Fig. 1a) adjacent to lamellar-like alveolar bone. Microscopy of the bundle bone regions using a polarized light illustrated thick radial fibers (straight double headed arrows, Fig. 1b). Bundle bone was attached to lamellar bone containing circumferential collagen (curved arrows, Fig. 1b).
Figure 1.
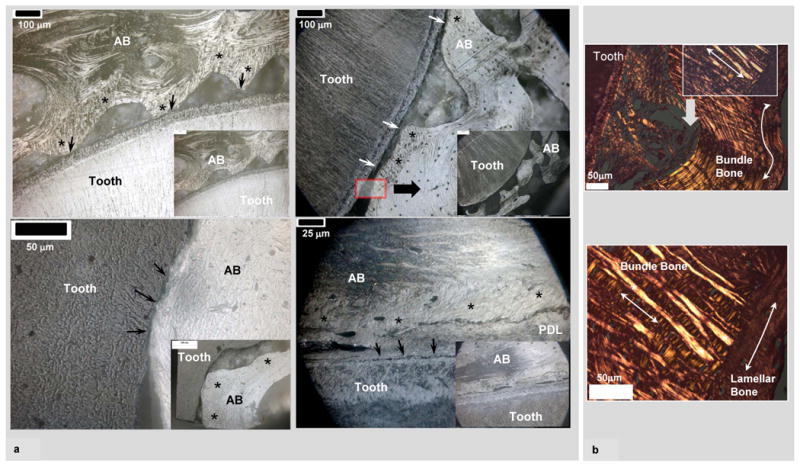
a) Low (insets) and high resolution light micrographs of ultrasectioned surface blocks illustrated narrowed PDL-space (arrows) between alveolar bone (AB) and tooth. All micrographs illustrate AB, which is a combination of bundle bone (asterisks) and lamellar bone. Bundle bone is significantly closer to cementum (C). b) Hematoxylin and eosin staining (H&E) of AB coupled with polarized light microscopy demonstrated dominant collagen fibers of different dimension and orientation within bundle bone (mainly radial fibers, straight arrows) compared to lamellar bone (mainly circumferential fibers, curved arrows). It should be noted that both types of bone within AB contain interwoven fabric-like structure.
The bone-PDL-cementum complex labeled for asporin illustrated asporin localization within PDL-space including vascular network (black arrow heads, Fig. 2a). Interestingly, patchy staining indicative of heterogeneous distribution of asporin within the complex was observed (asterisks). Additionally, several basophilic lines, between stratified layers of bone representative of bone advancement into PDL-space were observed (black block arrows, Figs. 2a and 2c). Directional bone growth as protrusions (star burst, Fig. 2c) into PDL-space were also observed. Higher resolution light micrographs of PDL-cementum and PDL-bone attachment sites detailed multiple micro protrusions, i.e. “scalloped” micro regions (white arrow heads, Figs. 2d and 2e).
Figure 2.
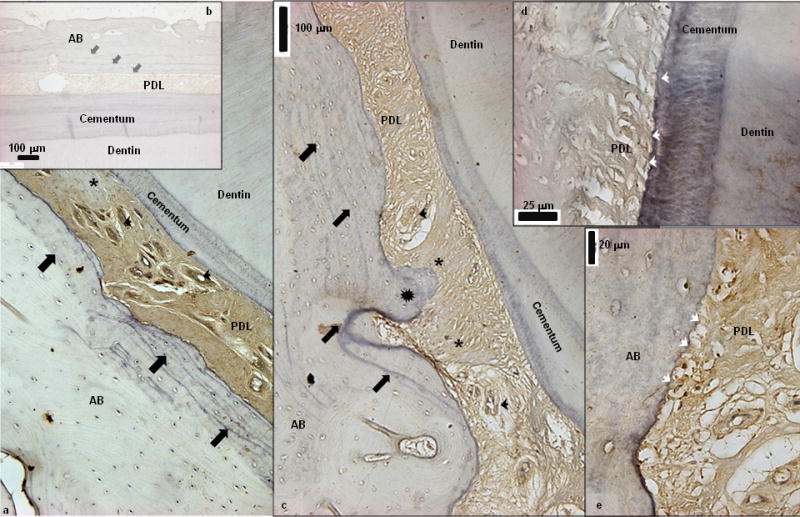
a) Immunohistochemistry illustrated localization of asporin within PDL-space including vascular network (black arrow heads). Interestingly patchy staining indicative of heterogeneous distribution of asporin within the complex was observed (asterisks). Additionally, several basophilic lines representative of bone advancement into PDL-space can be observed (black block arrows) b) Negative control is shown as an inset. c) Stratified basophilic lines (lamellae, block arrows) in addition to directional bone growth (protrusions indicated by star burst into PDL-space was observed. d, e) High resolution light micrographs of PDL-cementum and PDL-bone attachment sites illustrated multiple micro protrusions, i.e. “scalloped” micro regions (white arrow heads). AB: Alveolar bone, PDL: Periodontal ligament.
Higher resolution microscopy using an AFM (Fig. 3) and SEM (Fig. 4) allowed imaging of PDL fibrous tissue integration with respective mineralized tissues, and potentially less mineralized regions. AFM micrographs (Fig. 3) of the narrowed PDL-space, demonstrated collagen fibrils (arrows in Fig. 3b), identified by their periodicity with a parallel orientation adjacent to the surface of alveolar bone and cementum. As the PDL-fibers approached cementum and alveolar bone, a directional change from circumferential- (cir-PDL) to radial-PDL (rad-PDL) was observed in addition to lack of collagen periodicity; however, with a beaded-like fibrillar structure (Figs. 3b1 and 3b2) specifically in cementum. The observations in directional change of PDL and insertion of PDL were similar to normal bone-PDL-cementum complex reported by us [16] and is consistent with the commonly known 1-2 μm wide Sharpey's fibers or PDL-inserts in bone and cementum. Additionally, PDL-inserts of the narrowed bone-PDL-cementum complex under wet conditions demonstrated hygroscopicity which is indicated by an increase in topographical height due to swelling (curved arrows in Fig. 3a).
Figure 3.
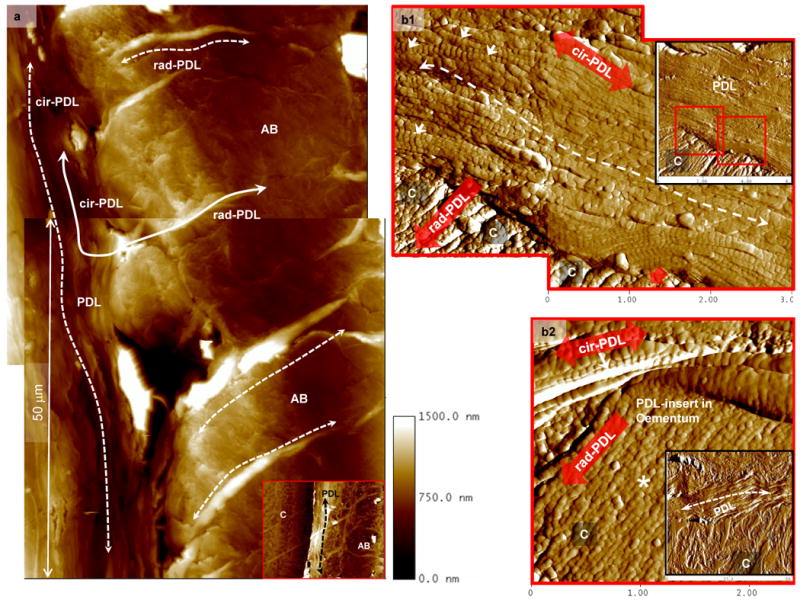
a). AFM scans of wet complex illustrated swelling of radial PDL-inserts within AB (rad-PDL, dashed curved arrows) in addition to parallel or circumferential orientation of periodontal ligament (cir-PDL) (dashed curved arrows) between cementum (C) and alveolar bone (AB) (inset in Fig. 3a). b). The 20 μm wide PDL (insets in Figs. 3b1 and 3b2) parallel to the cementum (C) surface (dashed curved arrow) contains collagen fibrils (arrows) identified by their characteristic periodicity. PDL is attached to cementum via rad-PDL. b1) Representative micrographs for cementum and bone illustrated presence (arrows) and absence of periodicity within PDL collagen fibrils and PDL-inserts. b2) Additionally, PDL-inserts in cementum contained collagen fibrils with beaded appearance and lacking periodicity (asterisk). In all images, the insets are lower magnification AFM micrographs containing the highlighted regions of interest at higher magnifications.
Figure 4.
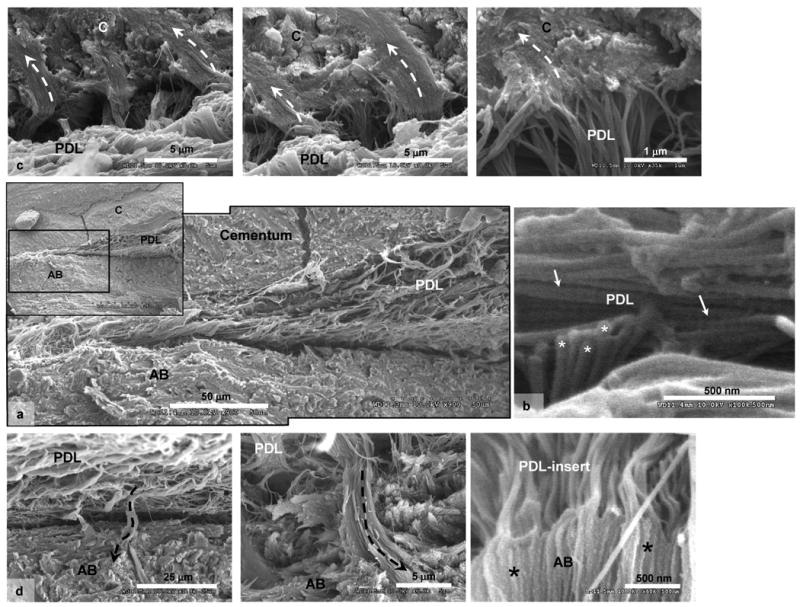
a) SEM micrographs of cryofractured surfaces illustrated narrowed PDL-space between cementum (C) and alveolar bone (AB). The collagen fibrils within the narrowed PDL are parallel to cementum surface. b) Characteristic periodicity of collagen fibrils within PDL can be noticed (straight arrows) in addition to out-of-plane collagen fibrils (asterisks). c) Top row of micrographs illustrate PDL-inserts into cementum (dashed-curved arrows), while d) the bottom row illustrates PDL-inserts (dashed-curved arrows) into alveolar bone (AB). Note that the collagen fibrils within the PDL-inserts lack periodicity.
Scanning of cryofractured specimens using an SEM confirmed the 5-10 μm organic-rich layer with a parallel orientation of collagen fibers (Fig 4) indicated by fibrils running in-plane (arrows, Fig. 4b) and out-of-plane (asterisks, Fig. 4b) at the narrowed PDL-space. Both SEM and AFM demonstrated intrinsic collagen periodicity in the PDL tissue which was obscured as the fibers approached respective mineralized tissues (Figs. 4c and 4d). Complementing AFM results and previous observations [16], the PDL with parallel collagen fiber orientation at the constricted sites is attached to respective mineralized tissues via rad-PDL inserts in cementum (Fig. 4c, dashed white arrows) and bone (Fig. 4d, dashed black arrows).
High resolution Micro XCT™ 3D images of narrowed PDL-space illustrated structural arrangement of PDL and the blood vessel spaces relative to bone and tooth (Fig. 5). The corresponding 2D virtual slices at magnifications of 10X (Figs. 5a and 5c) and 40X (Figs. 5b and 5d) revealed variations in the width of PDL-space demonstrating narrowed PDL-space (5-50 μm). Higher magnification (40X) 2D slice images (Figs. 5b and 5d) revealed the presence of bundle bone, defined by characteristic radial PDL-inserts (white arrows). Compared to normal conditions i.e. a PDL-space of 150 – 380 μm, the narrowed PDL had no rad-PDL and only cir-PDL fibers. Although vasculature in the circumferentially oriented PDL was not evident, it should be noted that the PDL between the two mineralized tissues was continuous with the fibrous tissue containing blood vessels in the alveolar bone (Figs. 2 and 5).
Figure 5.
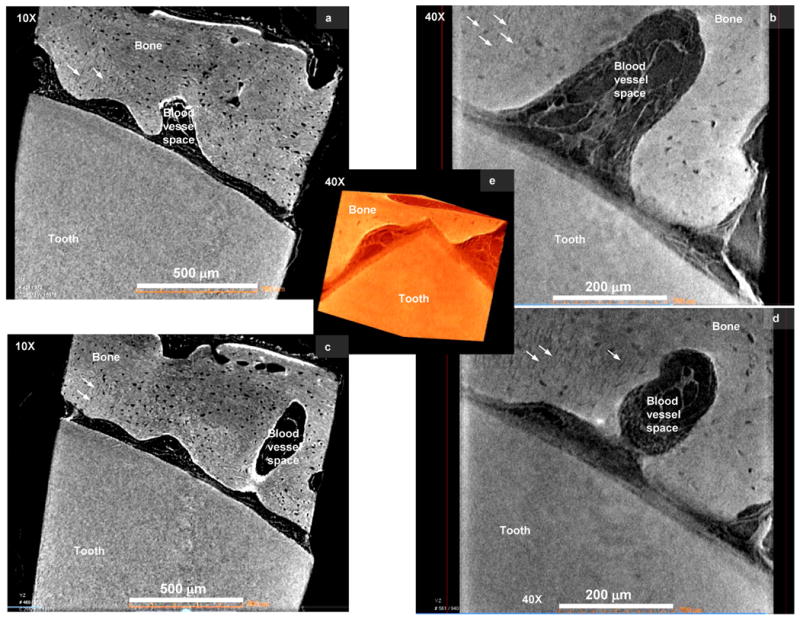
a) Micro XCT™ slices of the bone-tooth complex illustrated narrowed PDL-space, PDL-orientation and association with alveolar bone and tooth (a, c) at a lower magnifications of 10X, (b, d) higher magnification, 40X. All images including the 3D image illustrate network of the PDL continuous with the blood vessel space in the alveolar bone. b and d) Virtual sections taken from the 3D images illustrate the 2D network of the bone-PDL-tooth complex, in addition to bundle bone identified by radial PDL-inserts (arrows). Note: Movies demonstrating virtual sectioning of the narrowed PDL-complex taken at 10X and 40X are also included as supplementary data.
3.2. Elemental analysis using a microprobe
Calcium (Ca), phosphorus (P) X-ray fluorescence mapping using a microprobe demonstrated heterogeneous distribution of both elements in the mineralized tissues (Fig. 6). Ca and P concentrations were graded from the PDL-space into respective mineralized tissues. The gradients at the PDL-bone were significantly higher compared to PDL-cementum (Fig. 6a and 6b). At a higher resolution, the heterogeneity in bone and cementum was observed with the higher concentration in Ca and P near the PDL-space (Fig. 6).
Figure 6.
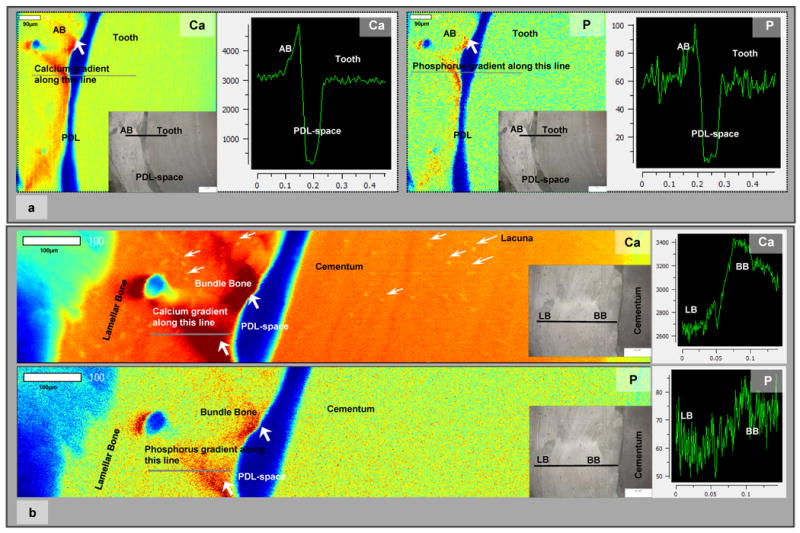
a) Low resolution μ-XRF area maps of bone-PDL-tooth complex illustrated regions with higher counts of Ca and P in bone and closer to the PDL-space. Line maps illustrated steep gradients in Ca and P at the bone-PDL attachment site/interface. White bar represents 90 μm. Insets in the area maps represent mapped regions. Images were taken using a light microscope. b) High resolution μ-XRF maps illustrated Ca and P dominant regions within bundle bone relative to lamellar bone. Additionally, several lacunae in cementum and bone were identified (white arrows). Line maps illustrated gradients in Ca and P between bundle bone and lamellar bone. Additionally heterogeneity in Ca and P can be observed in bone and cementum within the complex. White bar represents 100 μm. BB: Bundle bone, LB: Lamellar bone, Ca: Calcium, P: Phosphorus.
3.3. Modulus gradient profiles of compromised bone-PDL-cementum complexes
The line profiles of nanoindents (Fig 7) illustrated changes in site-specific nano-mechanical properties of wet alveolar bone, PDL-bone interface, PDL, PDL-cementum interface, and cementum. A sharp gradient in reduced elastic modulus “Er” was observed between the interface of the narrowed fibrous PDL and respective mineralized tissues. The decrease in Er values on the tooth side indicated hygroscopic CDJ with an increase in mechanical properties corresponding to mantle dentin and tubular dentin (Fig. 7, Table 1). Significant variations in Er between PDL-bone and PDL-cementum can be discerned. The insets in respective plots demonstrate gradients on a linear axis (Figs. 7b and 7d) and respective tomographs are representative blocks where the site-specific properties were measured. The reported ranges of Er under wet conditions illustrated heterogeneity of respective mineralized tissues and their attachment sites (Table 1) including higher hygroscopic effects of normal complex (Fig. 7a) compared to narrowed complex (Fig. 7c). It should be noted that the reported values of respective regions are relative comparisons between tissues and respective sites and should not be considered as absolute values.
Figure 7.
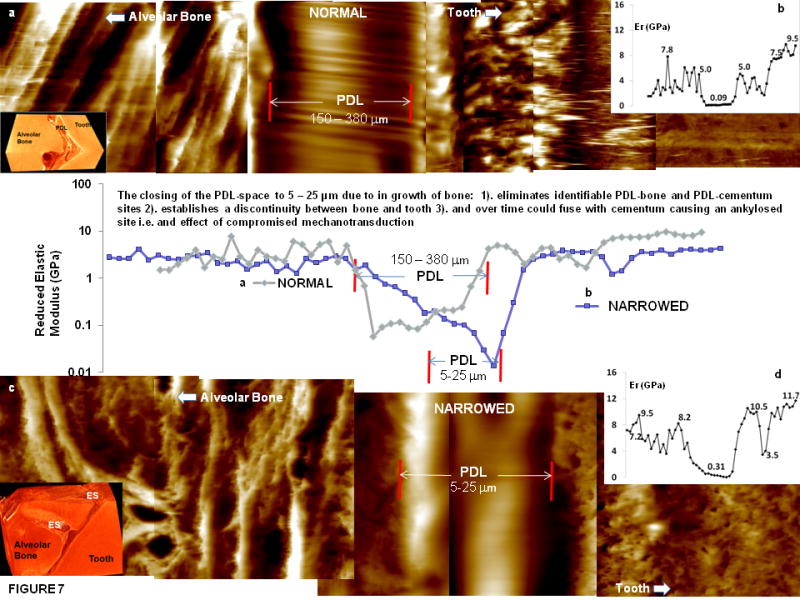
Line profiles of reduced elastic modulus values with significant gradients at bone-PDL and cementum-PDL attachment sites of normal (a) and narrowed (c) wet bone-PDL-tooth complex. b, d) Insets illustrate linear elastic modulus profiles of normal (b) and narrowed (d) bone-tooth complex. Insets in the left hand corner illustrate representative tomographies of a normal and narrowed bone-tooth complex. PDL: periodontal ligament, ES: endosteal space, Er: reduced elastic modulus (GPa).
Table 1.
Reduced elastic modulus values are representative of heterogeneity of respective mineralized tissues and their attachment sites within the normal (top row) and narrowed (bottom row) bone-PDL-cementum complexes.
| Bone-PDL-Cementum Complex | Reduced Elastic Modulus ‘Er’ (GPa) | ||||
|---|---|---|---|---|---|
| Bone | PDL-AB | PDL-C | Cementum | Tubular Dentin | |
| Nanoindentation (WET) (Normal) | 0.2 - 9.6 | 0.1 - 1.0 | 0.1 - 0.6 | 1.1 - 8.3 | 10.0 – 25.0 |
| Nanoindentation (WET) (Narrowed) | 0.1 - 11.2 | 0.1 - 1.4 | 0.1 - 1.2 | 0.4 - 8.4 | 8.0 – 22.1 |
4. Discussion
In this study, a macro-scale bone-tooth organ from a human was discretized to millimeter sized blocks to evaluate the site-specific properties of a narrow 5-50 μm bone-PDL-cementum complex. The site-specific adaptation was identified by mapping changes in physicochemical properties at the PDL-bone and PDL-cementum entheses and respective tissues. The properties were correlated to plausible root association with the bony socket as a result of functional loads. Ultimately, overall functional adaptation resulting in function efficiency of an organ can be understood by identifying changes in range of motion of the joint [32] and in this case the tooth within the bony socket.
The functional efficiency of the bone-tooth fibrous joint lies not only in the tissues of the tooth or the binding interfaces (dentin-enamel junction, cementum-dentin junction, bone-PDL or cementum-PDL), but also in the continuum with which all the tooth-related tissues interface with the vascularized bone and the vascularized/innervated PDL. As a result, functional efficiency of an organ can be impaired if any of the above mentioned sites or tissues adapt significantly. Parameters indicative of a loss in functional efficiency include a significant change in range of joint motion, and/or altered tissues mechanics i.e. tissue “quality”. Based on results from this study, another parameter would be a “compromised interface”, which is defined as a significant deviation from the original graded characteristics of a FGI. Naturally graded interface due to functional demands can deviate toward a discontinuous interface, eventually decreasing the functional efficiency of a dynamic joint. It is this characteristic feature that will be discussed in this study.
In musculoskeletal and dental orthopedics, macro-scale form-function behavior/adaptation at the organ level provides an insight to local behavior at the tissue level and resulting cellular responses and subsequent genetic and molecular expressions [33]. These hierarchical length-scale events provide a continuum-like effect to address functional homeostasis. During function, at an organ level, joints facilitate relative motions between members through a combination of soft and hard tissues and their interfaces. Tissues continue to adapt locally over time via principles of mechanobiology and mechanotransduction [34-36] under normal functional loads. However, significant deviation from functional loads in the form of magnitude and/or frequency, eccentric loads, which individually or combined can impair range of joint motion, i.e. a loss in overall functional efficiency of a joint.
A normal bone-tooth functional joint has a PDL-space ranging from 150-380 μm [20]. The lower value of 150 μm corresponds to a uniform PDL-space with a decrease in functional space as age increases [20]. However, apparently normally functioning teeth can also have localized regions of narrower PDL-space (Fig. 1). In this study such narrowed spaces were identified and their structure and properties were evaluated. Such specimens with narrow PDL-spaces of 5-50 μm had narrowing induced in two ways: 1) apposition of long stratified layers of bone with rich basophilic lines, 2) bony protrusions (Fig. 2). It is conceivable that as the bone grows more into the PDL-space (Fig. 1), tooth mobility in the bony socket is minimized because of the physical hindrance, thus compromising range of motion and reducing joint efficiency. These initial observations using a fundamental technique of light microscopy coupled with conventional histology raised subsequent questions. Is PDL attachment at the bone and cementum entheses similar to normal conditions i.e. 150-380 μm PDL-space? What is the chemical composition at the constricted sites? What is the elastic modulus of respective tissues and entheses at the constriction site? What are the biomechanical reasons that generated the observed constriction sites in certain regions of a bone-tooth fibrous joint extracted from a healthy human? In order to address these questions we furthered our investigation of the narrow PDL-complex using immunohistochemistry, site-specific higher resolution imaging for structural and chemical analyses, and wet nanoindentation techniques.
Based on the seminal and fundamental concept by Julius Wolff, the long-term effect of functional aberrations at a macro-scale could result in pathological deformations to maintain function [37]. Over time pathological deformations of mineralized tissues change the internal architecture, but will continue to function [37]. These spatiotemporal changes in physical and chemical properties of soft and mineralized tissues including their interfaces result in an overall change in form-function relationship, commonly termed as functional or biomechanical adaptation [9, 37-39]. In the bone-tooth fibrous joint, eccentric loads such as those imposed by parafunctional habits with varying magnitudes and frequencies [40] can cause functional adaptation. Other significant load-induced perturbations include traumatic and therapeutic loads from orthodontic braces [24] all of which can change the form-function relationship and decreased functional efficiency of the bone-tooth fibrous joint. Clinically, the narrowed PDL-space can be considered asymptomatic, but when superimposed with other clinical interventions can cause failure of the bone-tooth complex.
Analogous interfaces within the musculoskeletal system include bone-ligament, muscle-tendon, bone-cartilage. Interestingly in all these systems, adaptation in the form of movement of mineralized fronts into softer tissues due to cyclic loads at higher than normal functional loads have been observed. Repeated loading of the interfaces caused loss of joint mobility [7, 10, 39, 41, 42]. On the other hand, lack of loading (disuse) caused loss in skeletal bone density [18], or in case of cranial bone, premature fusion due to bone growth into softer tissue when the sutures (another representative fibrous joint) were not exercised [43, 44]. From these long established arguments, it is conceivable that every joint has its own functional limits and when exercised above or below its functional threshold limits for a prolonged time results in adaptive changes leading to decreased functional efficiency. Based on the results from this study, we propose that “higher strains at the soft-hard tissue interfaces act as sites of altered mechanotransduction due to activation of various mechanoreceptors between matrix to cells, and cell to cell. The bone-PDL and cementum-PDL entheses are “regulators” of cellular and extracellular temporal events; earlier events result in strain-induced local cell gene expression and later events that result in matrix biochemistry and physico-chemical properties”. This fundamental mechanism could explain tissue, interface development, regeneration, and/or adaptation in various biomechanically active organs.
Physiological remodeling and load-mediated modeling resulting adapted alveolar bone were identified as necessary anabolic and catabolic events [18, 45] to address functional demands on a tooth-bone fibrous joint. For example, resorption of alveolar bone occurs when left unloaded [46, 47]. Results of this study indicate adaptive form of bone and PDL with no detectable physical effects in cementum. Adaptive form of bone interfacing with PDL is termed bundle bone. Bundle bone is identified by dominant radial fibers with a woven fabric-like structure compared to the commonly known lamellar bone. Bundle bone, a term normally used in oral histology refers to 1-2 μm wide (mineralized) radial inserts of collagen fiber bundles from the PDL that forms a strong interface with lamellar bone (Fig. 1). Interestingly, natural mesial drift of teeth throughout the life span of a human mediated through functional loads can also cause bone modeling, resulting in bundle bone, but an uniform PDL-space [48, 49]. Hence, the observed bundle bone could be due to abnormal loads (nonphysiological loads) resulting in a non uniform PDL-space. This observation in the bone-tooth complex parallels a distinctive pattern of bone formation causing enthesophytes in osteotendinous and osteoligamentous regions, a pathological condition in the diarthroidal joints of the human musculoskeletal system [39]. Pathology in these studies was described as non-physiological mineralization and advancement of mineral fronts into softer tissue impeding range of joint motion [39]. Other, identifiable load-mediated changes within tissues include altered concentration of polyanionic molecules i.e. proteoglycan (PG) content which in turn could regulate mineral formation. In diarthroidal joints the relatively large range of motion is also impeded due to cartilage mineralization as a result of significant changes in PG concentration [50].
PG concentration controls many aspects of extracellular matrix properties including tissue structure, mineralization, and mechanical integrity resulting in load resisting and dampening properties of tissues. Under healthy conditions PGs along with collagen are promoters of functional mineralization which maintain the overall load bearing characteristics of joints. Changes in load can cause changes in glycosaminoglycans and respective tissue hydration characteristics resulting in altered load bearing conditions. Although several small and higher molecular weight PGs have been implicated toward tissue quality and joint efficiency, asporin was not correlated with loads. However, its existence in several load bearing tissues, such as cartilage and PDL was reported [50-52]. More importantly and quite relevant to this study is that this PDL-specific SLRP prevents non-physiological mineralization [50] and allows maintenance of the PDL-space, was also observed in the narrowed PDL-space (Fig. 2). Patchy localization of asporin compared to an uniform distribution in normal 150-380 μm wide PDL-complex [16] was observed with no evidence of a mineralized PDL or mineral nodules within the PDL. Moreover, the narrowed PDL retained its organic phenotype by consistent demonstration of characteristic collagen periodicity (Figs. 3 and 4). Though not shown, we also observed several other SLRPs within the PDL-complex including biglycan, decorin and fibromodulin. These consistently observed proteoglycans could be responsible for the hygroscopic nature of the PDL-inserts within bone and cementum (Fig. 3) and maintenance of the PDL-space. This is complemented by the lower X-ray attenuating PDL-inserts demonstrating lack of mineral as seen at a higher magnification using Micro XCT™ (Figs. 5b and 5d). It should also be noted, that the narrowed PDL between the two mineralized tissues demonstrates minimal hygroscopicity owing to higher packing density of the collagen fibers and not necessarily higher mineral content (Fig. 3). Along with the aforementioned proteoglycans [53], tenascin and fibronectin have been found by others [54] at the entheses, and it was suggested that they accommodate mechanical loads at the soft-hard tissue interfaces. We propose that the combination of local tensile strain along with polyanionic constituents could promote crystal growth and precipitation within or between collagenous structures of the PDL, more commonly known as intra- or extra-fibrillar mineral related to collagen fibrils [55, 56]. That is, the mineral growth process is controlled by organic cues that could change in molecular weight, molecular confirmation, and concentration with time so that the inorganic phase over prolonged time is directed along the mechanically strained fibers of the PDL. Supporting this argument is the presence of biglycan at the entheses and within PDL which continues to be reported as a PG with an osteogenic potential [57]. The presence of biglycan predominantly at the entheses along with the patchy appearance of asporin within the PDL-space could be the necessary chemical cues to make the locally stretched fibers more osteogenic in nature promoting a controlled and time-dependent vectorial bone growth [58]. The microscopic area over which the tensile loads occur at the bone-PDL tethered ends seen as scallops (white arrow heads, Figs. 2d and 2e) could either promote bone growth 1) over a macroscopic surface resulting in higher ordered structures seen as stratified bone with basophilic lines (black arrows, Figs. 2a and 2c), or 2) if load acted over a shorter surface area could promote bone growth seen as protrusion (star burst Fig. 2c, Fig. 5). This argument parallels the proposed theory in our previous work [16] that states that pulling forces exist at the tethered ends of the PDL that can induce biological activity promoting biomineralization. These mechanically prompted biomineralization events could cause increased levels of Ca and P in narrowed PDL-complex, compared to similar amounts in a normal 150-380 μm PDL-complex (Fig. 6). However, several studies related to type of mineral, the stochiometry of the developed crystal, crystal size and texture within the strained organic matrix should be identified to better elucidate events regulating site-specific biomineralization. The combined effects of physically narrowed 5-50 μm PDL and increased Ca and P elements indicate reduced mechanical damping of PDL to functional loads. It is only conceivable that the observed physical and chemical changes in PDL and bundle bone can significantly alter the matrix stiffness from gradual varying to an abrupt change i.e. a discontinuity as seen in this study (Fig. 7). Despite the presence of heterogeneous adaptive bone attached to commonly known lamellar bone (Table 1), and the subsequent changes in the stiffness gradients, the question that still remains to be answered is, what are the biomechanical reasons that generated the observed constriction sites in certain regions of a bone-tooth fibrous joint extracted from a healthy human (Fig. 8a)?
Figure 8.
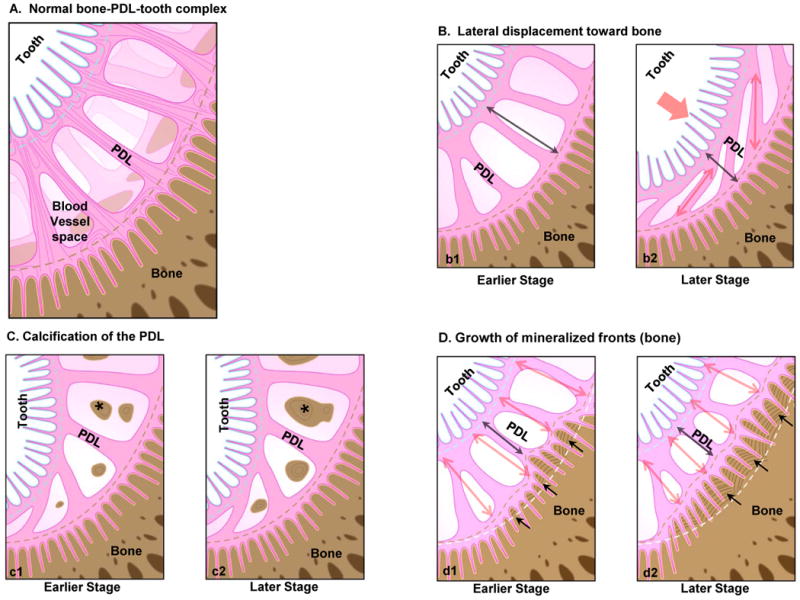
a) Schematic of a healthy bone-tooth complex illustrating a normal PDL-space. b-d) Schematics provide plausible scenarios that can be activated locally due to macroscale loads eventually narrowing PDL-space. Macroscale functional loads can cause the tooth to displace spatially toward the alveolar bone compressing the PDL-space (b) resulting in circumferential PDL fibers (b2). Functional loads can cause tilting of the root within the alveolar bone socket (c), altering hydrostatic pressure of PDL and blood vessels within PDL, thus stimulating cell differentiation, and expression of biomolecules as nucleators promoting PDL mineralization (asterisks). Functional loads can also cause local tension and compression sites that dominate events within PDL, and at the bone-PDL and cementum-PDL attachment sites. One such event is alveolar bone modeling due to pull-out forces at the tethered ends of the PDL causing vectorial growth of mineralization fronts into PDL-space (d1). Incremental layers represent stratified growth of bone and the scallop-like features at the PDL-bone attachment sites representing directional mineralization and advancement into PDL-space (d2). Although not show, similar events can occur in cementum at a slower rate, but can seen more in vascularized bone. Double headed red arrows represent tension within the PDL, while black arrows represent movement of the mineral front (red dashed line) into PDL-space. Note: Figures not drawn to scale.
It is known that functional loads on a tooth can cause tilting of the root in the bony socket [17, 59] and a lateral displacement of the root toward bone (Fig. 8b). Virtual dissection of higher resolution Micro XCT™ illustrated reorganized fibrillar structures of the PDL (Fig. 5), complementing light microscopy (Figs. 1, 2), AFM (Fig. 3) and SEM (Fig. 4) micrographs. However, the radial integration of the narrowed PDL with cementum and bone was similar to that observed in normal 150-380 μm wide bone-PDL-cementum complex [16]. Although the narrowed space illustrated pure circumferential-PDL using AFM (Figs. 3), radial fibrils (out of plane) from the PDL-space were also identified using SEM micrographs (Fig. 4). In the absence of the adaptive form of bone, i.e. bundle bone, the SEM results could support the theory that the PDL was physically constricted by lateral displacement of the root into the PDL-space. Under such circumstances calcification of degenerating PDL was observed in in vivo models [60] and schematically shown as one of the plausible biomechanical reasons to cause narrowed PDL-space (Fig. 8c). However, AFM and SEM results illustrated intact fibrils with characteristic periodicity (Figs. 3 and 4) demonstrating no mineralization, thus maintaining its organic phenotype despite the constriction. Hence, the observations in this study can also be a result of lateral translation due to shearing of the root against bone, promoting a combination of tensile and compressive strains at the entheses and within the PDL.
Based on fundamental biomechanics, the tooth is subjected to a variety of loads and when superimposed with higher compressive loads, the PDL tissue can undergo increasing shear (Fig. 8d), a combination of tension and compression, supplemented by flexural moments at the tethered ends of the ligament i.e. the radial PDL-inserts with bone and cementum (Fig. 8d). At the soft-hard tissue attachment sites it is known that the presence of hydrophilic molecules including various types of proteoglycans, helps modulate cell migration and adhesion, tissue/interface biomineralization, and other biochemical processes responsible for continuous remodeling of the mechanically strained PDL-cementum and PDL-bone attachment sites [53] making them into micro-scale dynamic sites i.e. “local regulators” (Fig. 8d1) eventually causing waves of mineral fronts toward PDL-space (Fig. 8d2). Therefore, any significant variation in macro-scale biomechanical load can alter the local strain levels in the PDL and at the PDL-bone and PDL-cementum attachment sites. Altered strains could lead to an altered mechanotransduction through integrin based cell-matrix response or cell to cell response identified through cell gene expressions causing detrimental downstream effects [25, 36, 53]. These cellular expressions at the strained attachment sites during function could manifest into local changes in biochemical composition permitting mineral nucleation subsequently resulting in higher concentrations of Ca, and P at the bone-PDL and cementum-PDL attachment sites (Fig. 5). These higher concentrations along with structural changes in the PDL-space are indicative of the steeper gradients in modulus observed at the bone-PDL and cementum-PDL attachment sites and can be used as indicators of attachment site/enthesis adaptation to macro-scale loading. Hence, it could be that the observed bone growth in this study is due to significantly altered mechanotransduction at the highly strained dynamic sites relative to adequately strained attachment sites within the same periodontal complex. These significant changes in PDL-space and shape of the outer surface of the bony socket can be identified as necessary modeling to address functional demands.
Following the primary event of bone ingrowth is the secondary event causing function impairment. With the narrowing of the PDL-space, the strain amplification at the PDL-bone and PDL-cementum could be minimized; however, a discontinuity in modulus profile between the tooth and bone now would permit prolonged compromised mechanotransduction. The compromised mechanotransduction will continue upon further loading, as the constriction sites will become the new “load bearing sites” that eventually cause direct local fusion of bone with cementum.
5. Conclusions
In this study, narrowed PDL-spaces were identified in a seemingly normal human bone-PDL-cementum complex. Narrowing was caused by several basophilic lines contained within long stratified layers and/or protrusions of bone into the PDL-space. The adapted regions of bone near the nonmineralized 5-50 μm PDL were chemically and mechanically heterogeneous. Adapted regions were also higher in calcium, phosphorus with increased elastic modulus values when compared to bone and cementum within a functional PDL-space of 150-380 μm. More importantly, a discontinuity in modulus profile at the narrowed region was observed, compared to the naturally graded interfaces of the bone-PDL and cementum-PDL entheses. These significant changes in the PDL-space due to the changes in outer surface of the bony socket are identified as modeling related events necessary to address functional demands. The adapted sites are also constriction sites impeding natural range of tooth motion within the bony socket. Constriction sites over time can become into new load bearing sites and could eventually cause direct local fusion of bone with cementum.
Supplementary Material
Acknowledgments
The authors thank Dr. Peter Sargent, Department of Cell and Tissue Biology for the use of the ultramicrotome, and Dr. Donald Curtis at UCSF for technical and insightful discussions. The authors also thank Lawrence Berkeley National Laboratory and support staff for the use of scanning electron microscope, and support staff of beam line 2-3 of the SSRL for assistance during data collection. SSRL is supported by the Department of Energy and by NIH. This work was supported by NIH/NIDCR R00 DE018212 (SPH), NIH/NCRR 1S10RR026645-01 (SPH); Departments of Preventive and Restorative Dental Sciences and Orofacial Sciences, UCSF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Suresh S. Graded materials for resistance to contact deformation and damage. Science. 2001;292(5526):2447–51. doi: 10.1126/science.1059716. [DOI] [PubMed] [Google Scholar]
- 2.Fratzl P. Biomimetic materials research: what can we really learn from nature's structural materials? J R Soc Interface. 2007;4:637–42. doi: 10.1098/rsif.2007.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson DAW. On Growth and Form. Cambridge University Press; 1961. [Google Scholar]
- 4.Carter DR, Beaupre GS. Skeletal Function and Form Mechanobiology of Skeletal Development, Aging, and Regeneration. Cambridge University Press; 2001. [Google Scholar]
- 5.Genin GM, Kent A, Birman V, Wopenka B, Pasteris JD, Marquez PJ, et al. Functional Grading of Mineral and Collagen in the Attachment of Tendon to Bone. Biophys J. 2009;97(4):976–85. doi: 10.1016/j.bpj.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J, II Choi W, Tae G, Kim YH, Kang SS, Kim SE, et al. Enhanced regeneration of the ligament-bone interface using a poly(L-lactide-co-epsilon-caprolactone) scaffold with local delivery of cells/BMP-2 using a heparin-based hydrogel. Acta Biomater. 2011;7(1):244–57. doi: 10.1016/j.actbio.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. The skeletal attachment of tendons--tendon “entheses”. Comp Biochem Physiol A Mol Integr Physiol. 2002;133(4):931–45. doi: 10.1016/s1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin M, McGonagle D. Entheses: tendon and ligament attachment sites. Scand J Med Sci Sports. 2009 doi: 10.1111/j.1600-0838.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin M, Ralphs JR. Entheses--the bony attachments of tendons and ligaments. Ital J Anat Embryol. 2001;106(2 Suppl 1):151–7. [PubMed] [Google Scholar]
- 10.Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat. 2006;208(4):471–90. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balooch G, Marshall GW, Marshall SJ, Warren OL, Asif SA, Balooch M. Evaluation of a new modulus mapping technique to investigate microstructural features of human teeth. J Biomech. 2004;37(8):1223–32. doi: 10.1016/j.jbiomech.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Habelitz S, Marshall SJ, Marshall GW, Jr, Balooch M. The functional width of the dentino-enamel junction determined by AFM-based nanoscratching. J Struct Biol. 2001;135(3):294–301. doi: 10.1006/jsbi.2001.4409. [DOI] [PubMed] [Google Scholar]
- 13.Marshall GW, Jr, Balooch M, Gallagher RR, Gansky SA, Marshall SJ. Mechanical properties of the dentinoenamel junction: AFM studies of nanohardness, elastic modulus, and fracture. J Biomed Mater Res. 2001;54(1):87–95. doi: 10.1002/1097-4636(200101)54:1<87::aid-jbm10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Ho SP, Balooch M, Goodis HE, Marshall GW, Marshall SJ. Ultrastructure and nanomechanical properties of cementum dentin junction. J Biomed Mater Res A. 2004;68(2):343–51. doi: 10.1002/jbm.a.20061. [DOI] [PubMed] [Google Scholar]
- 15.Ho SP, Balooch M, Marshall SJ, Marshall GW. Local properties of a functionally graded interphase between cementum and dentin. J Biomed Mater Res A. 2004;70(3):480–9. doi: 10.1002/jbm.a.30105. [DOI] [PubMed] [Google Scholar]
- 16.Ho SP, Kurylo MP, Fong TK, Lee SS, Wagner HD, Ryder MI, et al. The biomechanical characteristics of the bone-periodontal ligament-cementum complex. Biomaterials. 2010;31(25):6635–46. doi: 10.1016/j.biomaterials.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qian L, Todo M, Morita Y, Matsushita Y, Koyano K. Deformation analysis of the periodontium considering the viscoelasticity of the periodontal ligament. Dent Mater. 2009;25(10):1285–92. doi: 10.1016/j.dental.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–98. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 19.Robling AG, Turner CH. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr. 2009;19(4):319–38. doi: 10.1615/critreveukargeneexpr.v19.i4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nanci A. Ten Cate's Oral Histology Development, Structure, and Function. Mosby Elsievier Inc.; 2008. [Google Scholar]
- 21.Giannobile WV, Somerman MJ. Growth and amelogenin-like factors in periodontal wound healing. A systematic review. Ann Periodontol. 2003;8(1):193–204. doi: 10.1902/annals.2003.8.1.193. [DOI] [PubMed] [Google Scholar]
- 22.Somerman MJ, Ouyang HJ, Berry JE, Saygin NE, Strayhorn CL, D'Errico JA, et al. Evolution of periodontal regeneration: from the roots' point of view. J Periodontal Res. 1999;34(7):420–4. doi: 10.1111/j.1600-0765.1999.tb02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidovitch Z, Krishnan V. Adverse effects of orthodontics: a report of 2 cases. World J Orthod. 2008;9(3):e64–77. [PubMed] [Google Scholar]
- 24.Krishnan V, Davidovitch Z. On a path to unfolding the biological mechanisms of orthodontic tooth movement. J Dent Res. 2009;88(7):597–608. doi: 10.1177/0022034509338914. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129(4):469 e1–32. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Lin JL, Ryder MI, Webb S, Schuck PJ, Ho SP. Adaptation of cementum-dentin complex to periodontal disease. American Association for Dental Research; San Diego, California: 2011. [Google Scholar]
- 27.Brauer DS, Saeki K, Hilton JF, Marshall GW, Marshall SJ. Effect of sterilization by gamma radiation on nano-mechanical properties of teeth. Dent Mater. 2008;24(8):1137–40. doi: 10.1016/j.dental.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Nanci A. Ten Cate's Oral Histology. Mosby; 2007. [Google Scholar]
- 29.Ho SP, Goodis H, Balooch M, Nonomura G, Marshall SJ, Marshall G. The effect of sample preparation technique on determination of structure and nanomechanical properties of human cementum hard tissue. Biomaterials. 2004;25(19):4847–57. doi: 10.1016/j.biomaterials.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 30.Ho SP, Sulyanto RM, Marshall SJ, Marshall GW. The cementum-dentin junction also contains glycosaminoglycans and collagen fibrils. J Struct Biol. 2005;151(1):69–78. doi: 10.1016/j.jsb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Pharr GM, Oliver WC, Brotzen FR. On the generality of the relationship among contact stiffness, contact area, and elastic-modulus during indentation. J Mater Res. 1992;7(3):613–7. [Google Scholar]
- 32.Thompson DAW. On Growth and Form. 1992. Cambridge University Press; 1961. [Google Scholar]
- 33.Carter DR, Beaupre GS. Skeletal function and form. Cambridge University Press; 2001. [Google Scholar]
- 34.Ingber DE. Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Biol. 2008;97(2-3):163–79. doi: 10.1016/j.pbiomolbio.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingber DE. Tissue adaptation to mechanical forces in healthy, injured and aging tissues. Scand J Med Sci Sports. 2005;15(4):199–201. doi: 10.1111/j.1600-0838.2005.00481.x. [DOI] [PubMed] [Google Scholar]
- 36.Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35(8):564–77. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- 37.Wolff J. The law of bone remodelling. Berlin: Springer-Verlag Berlin Heidelberg; 1986. [Google Scholar]
- 38.Benjamin M, McGonagle D. Entheses: tendon and ligament attachment sites. Scandinavian Journal of Medicine & Science in Sports. 2009;19(4):520–7. doi: 10.1111/j.1600-0838.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- 39.Benjamin M, Toumi H, Suzuki D, Hayashi K, McGonagle D. Evidence for a distinctive pattern of bone formation in enthesophytes. Ann Rheum Dis. 2009;68(6):1003–10. doi: 10.1136/ard.2008.091074. [DOI] [PubMed] [Google Scholar]
- 40.Hartsfield JK, Jr, Everett ET, Al-Qawasmi RA. Genetic Factors in External Apical Root Resorption and Orthodontic Treatment. Crit Rev Oral Biol Med. 2004;15(2):115–22. doi: 10.1177/154411130401500205. [DOI] [PubMed] [Google Scholar]
- 41.Benjamin M, McGonagle D. Basic concepts of enthesis biology and immunology. J Rheumatol Suppl. 2009;83:12–3. doi: 10.3899/jrheum.090211. [DOI] [PubMed] [Google Scholar]
- 42.Benjamin M, McGonagle D. The Enthesis Organ Concept and Its Relevance to the Spondyloarthropathies. Molecular Mechanisms of Spondyloarthropathies. 2009:57–70. doi: 10.1007/978-1-4419-0298-6_4. [DOI] [PubMed] [Google Scholar]
- 43.Kawakami T, Takise S, Fuchimoto T, Kawata H. Effects of masticatory movement on cranial bone mass and micromorphology of osteocytes and osteoblasts in developing rats. Asia Pac J Clin Nutr. 2009;18(1):96–104. [PubMed] [Google Scholar]
- 44.Herring SW. Mechanical influences on suture development and patency. Front Oral Biol. 2008;12:41–56. doi: 10.1159/0000115031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts WE, Huja S, Roberts JA. Bone modeling: biomechanics, molecular mechanisms, and clinical perspectives. Semin Orthod. 2004;10(2):123–61. [Google Scholar]
- 46.Roberts WE. Bone dynamics of osseointegration, ankylosis, and tooth movement. J Indiana Dent Assoc. 1999;78(3):24–32. [PubMed] [Google Scholar]
- 47.Frost HM. Wolff's Law and bone's structural adaptations to mechanical usage: an overview for clinicians. Angle Orthod. 1994;64(3):175–88. doi: 10.1043/0003-3219(1994)064<0175:WLABSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 48.Weinmann JP. Bone formation and bone resorption. Oral Surg Oral Med Oral Pathol. 1955;8(10):1074–8. doi: 10.1016/0030-4220(55)90058-x. [DOI] [PubMed] [Google Scholar]
- 49.Weinmann JP. Bone changes related to eruption of the teeth. Angle Orthod. 1941;11(2):83. [Google Scholar]
- 50.Yamada S, Tomoeda M, Ozawa Y, Yoneda S, Terashima Y, Ikezawa K, et al. PLAP-1/asporin, a novel negative regulator of periodontal ligament mineralization. J Biol Chem. 2007;282(32):23070–80. doi: 10.1074/jbc.M611181200. [DOI] [PubMed] [Google Scholar]
- 51.Gruber HE, Ingram JA, Hoelscher GL, Zinchenko N, Hanley EN, Jr, Sun Y. Asporin, a susceptibility gene in osteoarthritis, is expressed at higher levels in the more degenerate human intervertebral disc. Arthritis Res Ther. 2009;11(2):R47. doi: 10.1186/ar2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikegawa S. Asporin, a susceptibility gene for osteoarthritis. Clin Calcium. 2006;16(9):1548–52. [PubMed] [Google Scholar]
- 53.McCulloch CAG, Lekic P, McKee MD. Role of physical forces in regulating the form and function of the periodontal ligament. Periodontology 2000. 2000;24:56–72. doi: 10.1034/j.1600-0757.2000.2240104.x. [DOI] [PubMed] [Google Scholar]
- 54.Lukinmaa PL, Mackie EJ, Thesleff I. Immunohistochemical localization of the matrix glycoproteins--tenascin and the ED-sequence-containing form of cellular fibronectin--in human permanent teeth and periodontal ligament. J Dent Res. 1991;70(1):19–26. doi: 10.1177/00220345910700010201. [DOI] [PubMed] [Google Scholar]
- 55.Su X, Sun K, Cui FZ, Landis WJ. Organization of apatite crystals in human woven bone. Bone. 2003;32(2):150–62. doi: 10.1016/s8756-3282(02)00945-6. [DOI] [PubMed] [Google Scholar]
- 56.Balooch M, Habelitz S, Kinney JH, Marshall SJ, Marshall GW. Mechanical properties of mineralized collagen fibrils as influenced by demineralization. J Struct Biol. 2008;162(3):404–10. doi: 10.1016/j.jsb.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Young MF, Bi Y, Ameye L, Chen XD. Biglycan knockout mice: new models for musculoskeletal diseases. Glycoconj J. 2002;19(4-5):257–62. doi: 10.1023/A:1025336114352. [DOI] [PubMed] [Google Scholar]
- 58.Mann S. In: Biomineralization Principles and Concepts in Bioinorganic Materials Chemistry. Compton RG, Davies SG, Evans J, editors. Oxford: Oxford University Press; 2001. [Google Scholar]
- 59.Chattah NL. Design strategy of minipig molars using electronic speckle pattern interferometry: comparison of deformation under load between the tooth-mandible complex and the isolated tooth. Adv Mater. 2008;20:1–6. [Google Scholar]
- 60.Nakamura Y, Tanaka T, Noda K, Shimpo S, Oikawa T, Hirashita A, et al. Calcification of degenerating tissues in the periodontal ligament during tooth movement. J Periodontal Res. 2003;38(3):343–50. doi: 10.1034/j.1600-0765.2003.00671.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


