Abstract
The use of bacterial artificial chromosomes (BACs) provides a consistent and high targeting efficiency of homologous recombination in embryonic stem (ES) cells, facilitated by long stretches of sequence homology. Here, we introduce a BAC targeting method which employs restriction fragment length polymorphisms (RFLPs) in targeted polymorphic C57BL/6/Cast/Ei F1 mouse ES cell lines to identify properly targeted ES cell clones. We demonstrate that knockout alleles can be generated either by targeting of an RFLP located in the open reading frame thereby disrupting the RFLP and ablating gene function, or by introduction of a transcription stop cassette that prematurely stops transcription of an RFLP located downstream of the stop cassette. With both methods we have generated Rnf12 heterozygous knockout ES cells, which were identified by allele specific PCR using genomic DNA or cDNA as a template. Our results indicate that this novel strategy is efficient and precise, by combining a high targeting efficiency with a convenient PCR based readout and reliable detection of correct targeting events.
INTRODUCTION
The discovery of mouse embryonic stem (ES) cells and the possibility to manipulate the ES cell genome through homologous recombination has provided a powerful methodology to study gene function in vitro and in vivo (1–3). Initial studies indicated that key factors important for efficient gene targeting include the length of the targeting arms, which positively correlates with the targeting efficiency (4,5), and the use of isogenic DNA for the generation of targeting constructs, as the presence of SNPs in a targeting vector would reduce the targeting efficiency (4,6). Increased targeting efficiency was obtained by targeting of mouse ES cells with a bacterial artificial chromosomes (BAC) strategy. Several annotated BAC libraries are now available for different mouse laboratory strains, to target a variety of different ES cell lines with isogenic targeting vectors (http://www.ncbi.nlm.nih.gov/clone/). Correct targeting with BAC targeting vectors is generally verified by quantitative real-time PCR (Q-PCR) amplifying a fragment spanning the projected deletion/insertion, together with a Q-PCR amplifying a fragment located in one of the arms (7). Also DNA–FISH has been applied to determine a correct genetic modification (8). However, because conventional Southern blotting techniques cannot be applied, these techniques are prone to detect false positive and negative clones. To avoid this problem, BAC targeting vectors are used that have both short and long targeting arms, allowing detection and/or confirmation of positive clones by Southern blotting using an external probe (9). This requires a BAC that is properly positioned around the insertion site, or is modified by trimming one of the arms through homologous recombination in bacteria. Together, the current strategy still is associated with several problems. In view of this, we have developed a new BAC targeting strategy which makes use of RFLPs present in genetically polymorphic ES hybrid cell lines, generated by crossing C57BL/6 female mice with Cast/Ei male mice, providing a convenient readout for proper gene targeting. In the present study, the proof of principle target gene was Rnf12, which encodes a nuclear factor involved in X chromosome inactivation (XCI) (10). For this gene, our results indicate that the new strategy can be used to efficiently introduce genetic modifications in ES cells using BAC targeting cassettes combined with a reliable readout based on allele specific PCR.
MATERIALS AND METHODS
ES cell derivation and cell culture
Female C57BL/6 mice were crossed to male Cast/Ei mice, and blastocysts were seeded onto irradiated mouse embryonic fibroblasts (MEFs) in DMEM, 15% v/v knockout serum replacement (Invitrogen), 100 U ml−1 penicillin, 100 mg ml−1 streptomycin, non-essential amino acids, 0.1 mM ß-mercaptoethanol, 5000 U ml−1 leukemia inhibitory factor (LIF) and 50 µM MEK1 inhibitor (New England Biolabs). The dissociated inner cell mass outgrowth was seeded on new feeders and after one passage grown in standard ES medium containing DMEM, 15% v/v fetal calf serum, 100 U ml−1 penicillin, 100 mg ml−1 streptomycin, non-essential amino acids, 0.1 mM ß-mercaptoethanol and 1000 U ml−1 LIF. To induce differentiation, ES cells were split, and pre-plated on non-gelatinized cell culture dishes for 60 min. ES cells were then seeded in non-gelatinized bacterial culture dishes containing differentiation medium to induce embryoid body (EB) formation. EB-medium consisted of IMDM-glutamax, 15% v/v fetal calf serum, 100 U ml−1 penicillin, 100 mg ml−1 streptomycin, non-essential amino acids, 37.8 μl l−1 monothioglycerol and 50 μg ml−1 ascorbic acid. To generate chimeras, C57BL/6 mice where superovulated and mated, and Day 3, 5 blastocysts were isolated. ES cells were injected, and the embryos were transfered to pseudopregnant foster mothers. Chimeras were crossed to C57BL/6 mice, and germ line transmission was judged by coat color. All animal experiments were in accordance with the regulations of the Erasmus MC Animal Experimental commission.
RFLP analysis and genotyping
To confirm the parental origin of the derived C57BL/6/Cast/Ei hybrid mouse ES cells, RFLP analysis on genomic DNA was performed by PCR followed by restriction digestion using the following primers and enzymes: Xist CAGTGGTAGCTCGAGCCTTT and CCAGAAGAGGGAGTCAGACG, BsrGI; Cdyl: ACAGGCAGAAGGAGCTGTGT, and CCCAGCTGTAAAGGCTTCAG, ZraI. Sry was amplified using ATTTATGGTGTGGTCCCGTGGT and TATGTGATGGCATGTGGGTTCC.
Karyotyping, RNA–FISH and DNA–FISH
For karyotyping, cells were blocked in metaphase using colcemid, and metaphase spreads were prepared by hypotonic treatment, followed by fixation in methanol acetic acid (3:1 v/v), according to standard procedures. Xist RNA–FISH and DNA–FISH were performed as described (10). For DNA–FISH, a mouse BAC probe (RP24-240J16) covering the Rnf12 gene was digoxigenin-labeled and used to determine the number of integration sites of the Rnf12 targeting constructs. A cocktail containing biotin-labeled BAC sequences covering Xist (CT7-474E4, CT7-45N16, CT7-155J2 and CT7-211B4) was used as a probe to determine the number of X chromosomes.
RT–PCR
RNA was isolated from undifferentiated ES cells using Trizol reagent (Invitrogen), according to manufacturers’ instruction, and cDNA was prepared using the SuperScript TM III First-Strand Synthesis System (Invitrogen). RT–PCR for pluripotency markers was performed using the following gene specific primers: Oct4: CCCCAATGCCGTGAAGTTG, TCAGCAGCTTGGCAAACTGTT; Nanog: AGGATGAAGTGCAAGCGGTG, TGCTGAGCCCTTCTGAATCAG; Sox2: CACAGATGCAACCGATGCA, GGTGCCCTGCTGCGAGTA; ß-Actin: CAACGAGCGGTTCCGATG, GCCACAGGATTCCATACCCA.
Construction of targeting constructs
The Rnf12 targeting construct has been described (10). To generate the SA-tpA stop constructs, a cassette containing a floxed splice acceptor and polyadenylation sequence and a Frt site flanked neomycin/kanamycin fusion gene was generated, starting with a pEGFP-NI vector (Clontech). A linker containing a Lox66 and EcoRV, BglII and BamHI sites together with a linker containing a Lox71 and Frt sites flanking a ScaI site were cloned BglII-NotI into pEGFP-N1, releasing EGFP (complete sequence: GATCTAATATAACTTCGTATAGCATACATTATACGAACGGTAGATATCAGATCTGGATCCTATTGAAGCATATTACATACGATATGCTTGCCATTTAATTCCGGAGAATCCGGGAAGTTCCTATTCTCTAGAAAGTATAGGAACTTCAGTACTGAAGTTCCTATTCTCTAGAAAGTATAGGAACTTCTAGG). The ScaI site was used to insert a DraIII-AseI kanamycin/neomycin fragment. The SA-tpA sequence was a BamHI fragment from pSStpA (11), inserted in the BamHI site of the linker. Three unique restriction sites in introns 2–4 of Rnf12 were PCR amplified, with 500 bp of flanking region, and cloned into pPCR-Topo-bluntII, using the following primers and unique restriction sites: intron 2 BglII GGGCTACACAGAGAAAGAAACC, AGCCATGCATGCTTGTGTTA; intron 3 NheI GAAACAGCTTGTTTTATAATGTTTCTT, TTGAACATGTGTTGCAAAATTAC; intron 4 AvrII ACATTTTGTTTGGGGAGGTG, GAATTGTGCAACTCGGAACA. The SA-tpA kanamycin/neomycin cassette was NheI-AflII released and inserted into the unique restriction sites of the intronic targeting constructs. The final constructs were used for homologous recombination in bacteria of a C57BL/6 BAC RP24-240J16 (12). Positive clones were screened by PCR for the correct recombination event using primers flanking the homologous recombination arms of the targeting constructs and insert specific primers: Rnf12 intron 2 AAAGGTTTTGGCTGGATGGT, rev TGTGCCATAATGCTTGGCTA, Rnf12 intron 3 CCCAGGTAAGCTGCATGTAA, rev TGTAGTCTTCTGAGCAACTCTTCC, Rnf12 intron 4 ACAGAGCCCCGATGAAAAT, rev ACACGATTAGGACACTCATGG.
Targeting of ES cells
Targeting constructs were linearized by PI-SceI digestion. For each electroporation, ~40 μg of DNA and 1.0 × 106 ES cells were used. Cells were seeded on drug resistant male MEFs, and selection with neomycin (270 μg ml−1, active) was started 24 h posttransfection and continued for 7–12 days. Drug resistant clones were picked and expanded.
RFLP analysis of targeted ES clones
Genomic DNA (gDNA) of ES clones was isolated, and RFLP analysis was performed using the following primers and enzymes: Rnf12 GCCTTCGAACATCTCTGAGC, GAGCCGGACTAATCCAAACA, NheI; Xist CAGTGGTAGCTCGAGCCTTT and CCAGAAGAGGGAGTCAGACG, BsrGI. For analysis of cell lines targeted with the stop constructs, RNA was isolated, cDNA prepared and expression of Rnf12 was analyzed by RFLP analysis using primers TAAAGAGGGTCCACCACCAC and GGCAGAGAGCCACTTTCATC followed by NheI restriction digestion.
Q-PCR copy number analysis
Copy number of genomic sequences was determined by real time Q-PCR with genomic DNA using the following primers: Rnf12 (NheI site): GTTCGTCCTGGAGAATACCG, GGAAAAGGTACGCCTAAAACC; Rnf12: AGCCCCGATGAAAATAGAGG, GGCATTTCTGGATAATCTTTGG; Zfp42: GCACCCATATCCGCATCCAC, GCATTTCTTCCCGGCCTTTG.
Southern blotting
An amount of 5–10 μg of genomic DNA was digested overnight with NheI, and separated on a 0.7% agarose gel. DNA was blotted to Hybond membranes using standard procedures, and the blot was hybridized with a PCR amplified probe (primers: GGCAGAGAGCCACTTTCATC, GCCAAAGACCTCCAACCATA)
RESULTS
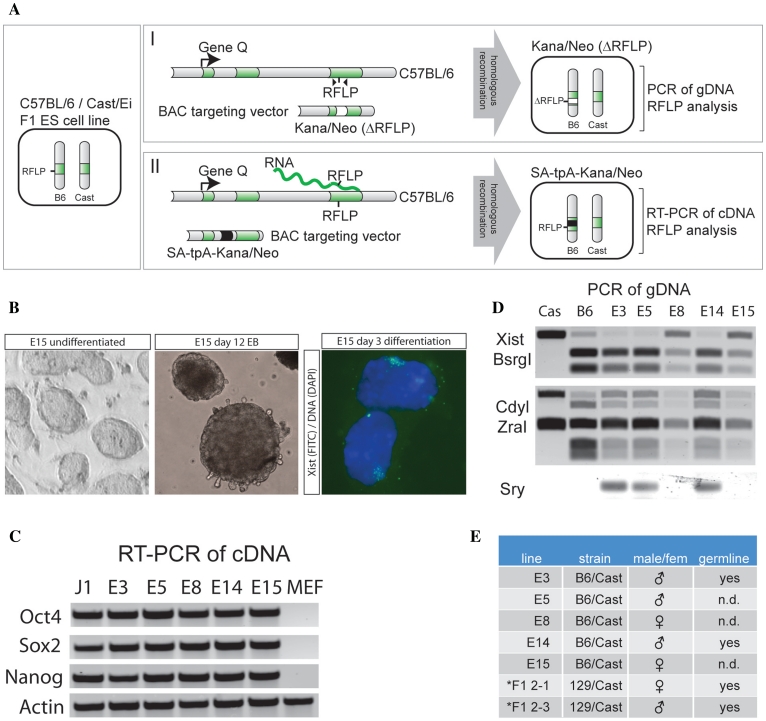
Existing methodology for screening positive clones after homologous recombination with BAC targeting cassettes involves Q-PCR or DNA–FISH. These methods are prone to detect false positive and negative clones, and we therefore set out to develop a method to screen targeting events by using RFLPs in F1 hybrid cell lines. In the first approach the BAC targeting vector destroys the RFLP and inserts a kanamycin/neomycin resistance cassette in the open reading frame of the gene of interest, thereby ablating gene function [Figure 1A(I)]. Removal of the RFLP can be screened by PCR using genomic DNA as a template and subsequent digestion of the PCR product with the restriction enzyme recognizing the targeted RFLP. In another approach, we introduce a splice acceptor poly-adenylation transcription stop cassette (SA-tpA) in an intron of the gene of interest, prematurely abrogating gene transcription resulting in a non-functional protein. For this second approach, positive clones are identified by RT–PCR amplification of a cDNA sequence, which contains an RFLP that is located downstream of the transcription stop cassette [Figure 1A(II)].
Figure 1.
Two approaches of a new strategy for manipulation of hybrid mouse ES cell lines. (A) Schematic overview of RFLP mediated BAC targeting of hybrid C57BL/6/Cast/Ei ES cells. Chromosomes from C57BL/6 are indicated with B6 and from Cast/Ei with Cast. Exons of the gene of interest are indicated in green, the kanamycin/neomycin resistance cassette in white, and the transcription stop cassette in black. (B) C57BL/6/Cast/Ei hybrid ES cells show proper ES cell morphology (left panel), are able to differentiate in vitro in EBs (middle panel), and female lines can initiate XCI upon differentiation (right panel, showing Xist RNA labeled in FITC, and DNA stained with DAPI). (C) RT–PCR of pluripotency genes. The generated C57BL/6/Cast/Ei ES cells (E3–E15) express the pluripotency factors Oct4, Sox2 and Nanog. J1 is a male control ES cell line, MEFs were used as negative control, and Actin is a control mRNA. (D) PCR amplification of gDNA of different C57BL/6/Cast/Ei ES cell lines (E3–E15) and control gDNA (Cas and B6), and digestion with restriction enzymes identifying allele specific products for Xist (X-encoded, BsrGI, top panel), Cdyl (autosomal, ZraI, middle panel) and PCR amplification of Sry (Y-encoded, bottom panel). (E) Table summarizing the characteristics of the C57BL/6/Cast/Ei ES cell lines analyzed in this study, and two other polymorphic 129/Sv/Cast/Ei cell lines (Asterisk) that have been used in other gene targeting studies (10).
Generation of F1 ES hybrid cell lines
To obtain genetically polymorphic ES cell lines with a high number of RFLPs that could be used for gene targeting, we generated F1 hybrid ES cell lines by crossing C57BL/6 female mice with Cast/Ei male mice. The C57BL/6 classical inbred Mus musculus mouse strain is among the most widely used and best characterized mouse strains. The C57BL/6 mouse genome has been sequenced, and several well-annotated BAC libraries have been generated (13). The Cast/Ei inbred strain has been derived from a wild population of the subspecies Mus musculus castaneus, is more difficult to breed, but offers advantages related to its variant genetic background (14). Intercrosses between Cast/Ei and other strains have been extensively used for SNP based distinction of the maternal and paternal genome, for instance to study genomic imprinting and XCI. The C57BL/6 and Cast/Ei mouse strains are highly polymorphic, with an estimate of one SNP per 311 bp, providing a sufficient number of RFLPs to allow targeting of almost every gene (14). The Cast/Ei Mus m. subspecies is currently being sequenced, a BAC library is available, and a SNP database is publically accessible (www.perlegen.com).
We generated five different F1 ES cell lines, with the proper karyotype and ES cell morphology, which were successfully differentiated into EBs (Figure 1B). RT–PCR expression analysis confirmed expression of the pluripotency markers Oct4, Sox2 and Nanog (Figure 1C). Karyotyping and PCR analysis of genomic DNA with a primer set specific for Sry showed that three ES cell lines were male and two female (Figure 1D). We confirmed the C57BL/6/Cast/Ei F1 genotype by PCR amplification of the autosomal gene Cdyl and the X-linked gene Xist, and digestion with restriction enzymes specific for RFLPs that discriminate between a C57BL/6 or Cast/Ei PCR product (ZraI for Cdyl, and BsrGI for Xist). Digestion of the Xist PCR products from genomic DNA (gDNA) of the male ES cell lines revealed only a C57BL/6 product, as expected, because the single X chromosome in male cells is inherited from the C57BL/6 mother. Two male ES cell lines, E3 and E14, were injected in C57BL/6 blastocysts and several founders (five for E3, two for E14) were retrieved, all showing high coat color contribution (representative animals are shown in Supplementary Figure S1). Different founders were crossed with C57BL/6 females and all tested animals showed germline transmission (Figure 1E). Taken together, we generated three male and two female C57BL/6/Cast/Ei F1 ES cell lines. Because of our interest in the female specific XCI process female ES line E15 was used for further targeting studies.
Targeted disruption of the Rnf12 open reading frame
For BAC mediated targeting of an RFLP we selected the X-chromosomal Rnf12 gene as a target (Figure 2A). We have recently shown that the encoded RNF12 acts as a dose-dependent activator of XCI in female ES cells (10,15,16). RNF12 is an E3 ubiquitin ligase that regulates XCI through activation of the X-linked gene Xist (16). The transcribed Xist RNA coats the inactive X chromosome in cis (Figure 1B), thereby attracting chromatin modifying enzymes involved in establishing inactive chromatin (17).
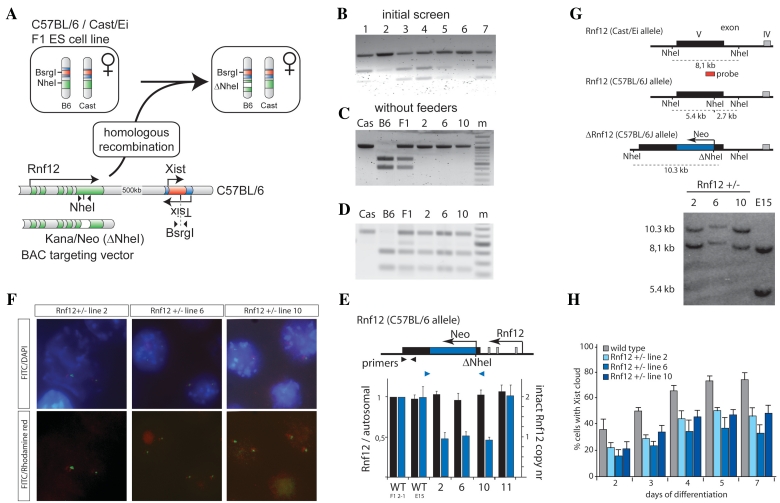
Figure 2.
Generation of Rnf12 knockout ES cell lines by disruption of the Rnf12 open reading frame. (A) Targeting of Rnf12 by insertion of a kanamycin/neomycin resistance cassette into the exon containing a NheI RFLP destroys the RFLP (present in the C57BL/6 allele). (B) PCR analysis with primers indicated in Figure 2A amplifying the NheI RFLP located in Rnf12 using genomic DNA of targeted clones 1–7. PCR fragments were digested with NheI. (C) Same as in Figure 2B, but with gDNA isolated from clones 2, 6 and 10, grown without feeder cells. Cast/Ei (Cas), C57BL/6 (B6) and Cast/Ei / C57BL/6 (F1) DNA was used as a control (m = marker). (D) Same as in Figure 2C, but PCR analysis amplifying the BsrGI RFLP located in Xist using gDNA of the targeted clones. PCR fragments were digested with BsrGI. (E) Q-PCR analysis with primers indicated using gDNA of different targeted clones. Values were normalized to values obtained with a primer set amplifying the autosomal Zfp42 gene. (F) DNA–FISH analysis with a BAC probe covering the targeting cassette (FITC), and a mix of BAC probes detecting Xist and flanking regions (Rhodamine red) (DNA stained with DAPI). (G) Southern blotting analysis, using a probe that distinguishes between the Cast/Ei (8.1 kb) and C57BL/6 (5.4 kb) alleles. A neo insertion event destroying the NheI RFLP present on the C57BL/6 allele results in a 10.3-kb fragment (clones 2, 6 and 10). (H) Three different Rnf12+/− ES cell lines and a wild-type female ES cell line were differentiated, and subjected to Xist RNA FISH. The relative number of cells showing an Xist cloud, indicative for initiation of XCI, is shown at different time points of differentiation (N > 100 per time point, error bars represent 95% confidence intervals).
Rnf12 consists of five exons, spanning 24 kb. In the SNP database we identified NheI as an RFLP located in exon 5 of the C57BL/6 Rnf12 allele, and confirmed the RFLP by sequencing analysis of gDNA isolated from C57BL/6 and Cast/Ei mice. Disruption of RNF12 activity by insertion of a neomycin/kanamycin resistance cassette in this NheI RFLP would lead to a premature translation stop of RNF12, resulting in a 331 amino acid protein lacking the RING finger that is crucial for RNF12 activity. Based on this, we generated a targeting construct to disrupt Rnf12, by PCR amplification of the NheI RFLP and 500 bp of flanking sequence, and subsequent insertion of a kanamycin/neomycin resistance cassette in the NheI site. The C57BL/6 BAC RP24-240J16, covering the Rnf12 gene, was targeted through homologous recombination in bacteria (12), and correct targeting was confirmed by PCR amplification using primers inside the resistance cassette and outside the targeting arms (data not shown). The targeting efficiency in bacteria was >80%. The modified BAC sequence was linearized with SceI and targeted to female ES cell line E15, and subsequent to neomycin selection clones were picked and expanded for further analysis. Genomic DNA of these clones was subjected to PCR using primers spanning the targeted NheI site, and the PCR product was digested with NheI. Correctly targeted clones are expected to have an undigested Cast/Ei product only, although contamination by feeder cells (C57BL/6) might result in the presence of some digested material (Figure 2B). We therefore also grew the targeted clones without feeders, which indicated that 12% of the picked clones showed a loss of the C57BL/6 specific PCR product (Figures 2C and 3C).
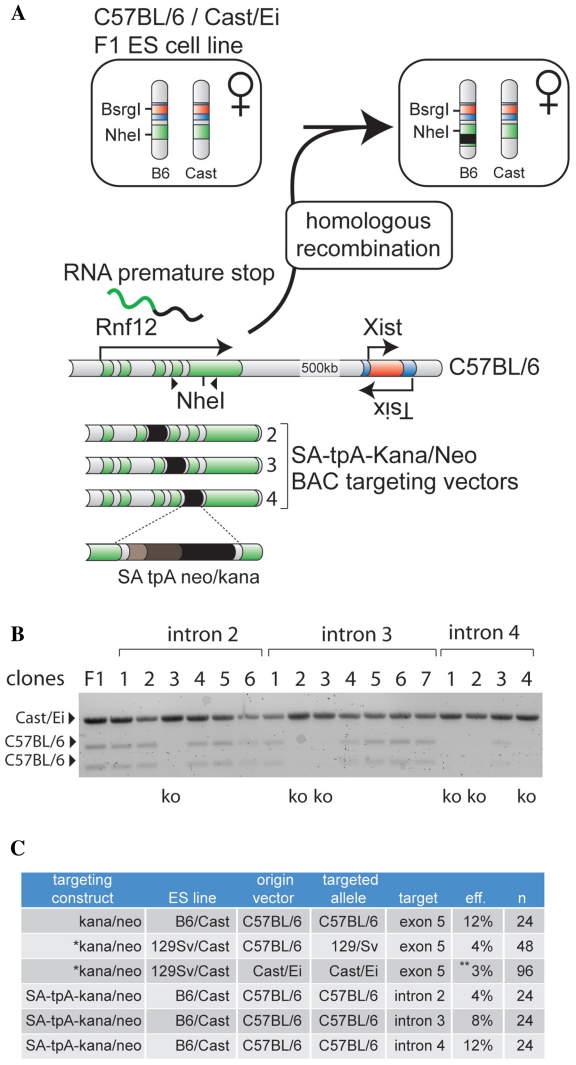
Figure 3.
Generation of Rnf12 knockout ES cell lines by insertion of a transcription stop cassette. (A) Generation of Rnf12 knockout ES cell lines by integration of a splice acceptor triple poly-adenylation sequence (SA-tpA) together with a kanamycin/neomycin resistance cassette. Different targeting constructs were generated to target the stop cassette to one of the introns 2, 3 or 4. (B) RT–PCR analysis with cDNA form clones targeted with cassettes targeting different introns, using primers amplifying the NheI RFLP located in exon 5. RT–PCR products were digested with NheI to reveal allele specific PCR products. Correctly targeted clones are indicated (ko). (C) Table summarizing the targeting efficiency of the constructs described for targeting the C57BL/6 allele (n = number of clones picked). (Asterisk) Targeting efficiencies of previously described experiments involving C57BL/6, and Cast/Ei exon 5 targeting constructs used to target the 129/Sv and Cast/Ei alleles in F1 2-1 129/Sv/Cast/Ei female ES cells (10,16). (Double asterisk) The Cast/Ei allele was targeted in Rnf12+/− (129/Sv/Cast/Ei) ES cells creating a Rnf12 null ES cell, which may affect the targeting efficiency.
Our results were confirmed by Q-PCR analysis with primers spanning the NheI site, which indicated a reduction in copy number from two to one, and primers amplifying a region proximal to the NheI site, indicating no change in the copy number (Figure 2E). The loss of one NheI site could be attributed to a correct targeting event, or loss of an X chromosome. Although the Q-PCR results indicated that both X chromosomes were present, we also performed a PCR amplification of a BsrGI polymorphism in the Xist gene. Digestion of the PCR products with BsrGI, which only digests the C57BL/6 PCR product, indicated that both X chromosomes were present in all clones that showed a loss of the C57BL/6 Rnf12 PCR product (Figure 2D). We confirmed this finding by DNA–FISH with two different probes, one covering the BAC used for targeting the ES cells, and the other covering Xist and flanking sequences. We found that clones that lost the RFLP had retained the expected 40, XX karyotype, providing evidence for a correct targeting event, and precluding the presence of randomly integrated BACs (Figure 2F). Finally, genomic DNA of targeted clones was subjected to Southern blotting analysis, which indicated the correct targeting event; loss of the C57BL/6 specific 5.4-kb band in knockout clones that were selected based on the RFLP PCR assay and no loss in control clones (Figure 2G).
Analysis of all our targeted clones indicated that targeting with a C57BL/6 construct, was specific for the C57BL/6 allele. However, our previous studies also indicated that the same construct can be used to target the 129/Sv allele in F1 2-1 129/Sv/Cast/Ei female ES cells (10). The targeting efficiency for this experiment was lower, probably due to the presence of SNPs in the targeting construct (Figure 3C). Using a Cast/Ei targeting construct in which kanamycin/neomycin was replaced by a ampicilin/puromycin resistance cassette, we were also able to target the Cast/Ei allele, resulting in a homozygous Rnf12−/– ES cell line (16). In this experiment the efficiency was lower (3%) than found for the C57BL/6 construct targeting the C57BL/6 allele, possibly due to selection against cells deficient for Rnf12 (Figure 3C).
To demonstrate the value of the strategy described herein for studying a specific cellular process, we tested the effect of the heterozygous Rnf12 deletion on XCI. We analyzed the percentage of cells that initiated XCI, by Xist RNA FISH at different time points of EB differentiation, for three Rnf12+/– clones. Previously, we found that a heterozygous deletion of Rnf12 in female cells results in a significant reduction of XCI, as part of the evidence that RNF12 is an important activator of XCI (10). In agreement with this, analysis of the present C57BL/6/Cast/Ei Rnf12 +/− female ES lines also showed a reduction in the number of cells that initiated XCI (Figure 2H). These results indicate that homologous recombination of RFLPs with BAC targeting constructs provides an efficient and precise method to generate knockout ES cell lines.
Premature abrogation of Rnf12 transcription
Even in the highly polymorphic ES cells used for the present study, a relatively small percentage of all genes will not contain suitable RFLPs to allow targeting of a resistance cassette abolishing expression of a functional transcript. For such genes, a splice acceptor triple poly-adenylation (SA-tpA) cassette could be used to insert into an intronic RFLP, thereby leading to a premature stop of transcription. Alternatively, a SA-tpA cassette could be inserted into an intron of the gene of interest, using a transcribed RFLP located downstream of the insertion site to screen for proper integration. Both modifications of the present method provide many more possible targeting sequences. As proof of principle, we generated targeting vectors aimed to prematurely stop transcription in one of the introns 2, 3 or 4 of Rnf12 (Figure 3A). BAC targeting cassettes were generated through homologous recombination of a SA-tpA cassette flanked by a kanamycin/neomycin resistance cassette in different introns of Rnf12 in C57BL/6 BAC RP24-240J16. Proper integration of the SA-tpA cassette was verified by PCR (data not shown), and the targeting vectors were electroporated into female E15 C57BL/6/Cast/Ei ES cells. Neomycin resistant clones were expanded without feeder cells for RNA isolation. To determine the clones with a proper integration of the SA-tpA cassette abolishing Rnf12 expression from the targeted C57BL/6 allele, we generated cDNA and performed RT–PCR amplifying the exons 4 and 5 junction of Rnf12 including the previously described NheI RFLP. Allele specific expression was determined by digestion of the RT–PCR product with NheI, which only digests the C57BL/6 PCR product. For each targeting vector we obtained positive clones which showed expression of the Cast/Ei allele only, indicating proper integration of the transcription stop cassette (Figure 3B). Loss of an X chromosome was ruled out by PCR amplification of a BsrGI RFLP located in the Xist gene, using gDNA and subsequent digestion with BsrGI (data not shown). The targeting efficiencies were 4, 8 and 12%, for targeting of introns 2–4, respectively, which is in the range of previously reported targeting efficiencies of BAC targeting constructs (8). These results indicate that different targeting methods, either removing an RFLP or ablating expression of an RFLP combined with an allele specific PCR to detect a correct targeting event, can be applied to introduce genetic modifications in ES cells.
DISCUSSION
Previous attempts to generate genetically modified ES cell lines by homologous recombination of BAC targeting vectors have been challenging, mainly because a clear readout for proper integration of the targeting cassette was missing. Here, we have generated and targeted F1 C57BL/6/Cast/Ei hybrid ES cell lines, which are genetically polymorphic, carrying a high density of RFLPs.
We have opted for two different approaches for BAC mediated gene targeting, both using RFLPs as a readout for properly targeted clones. In one strategy a resistance cassette is integrated into the open reading frame of a gene, ablating gene function and disrupting the RFLP. In another approach, a splice acceptor transcription stop cassette is inserted in a transcribed RFLP, or in a genomic location upstream of an RFLP, and subsequently the RFLP is used to detect loss of expression of the targeted allele. The last strategy can even be used to generate conditional knockout or rescue alleles, by using inverted asymmetric lox sites. The combination of these approaches provides an opportunity to disrupt a wide selection of candidate target genes. Nonetheless, even in the F1 C57BL/6/Cast/Ei ES cell lines for some genes no RFLPs will be available. Especially, single exon genes or genes covering a relative small genomic region may not entail enough SNPs for the design of a proper targeting approach. Also, the targeting strategy based on screening of expressed RFLPs requires the targeted gene to be expressed in ES cells. Fortunately, this is true for many genes and even genes which only play a role in embryonic development and differentiation processes appear to be expressed at a base level in ES cells, sufficient to allow application of the second approach.
The targeted F1 C57BL/6/Cast/Ei ES cell lines can directly be used to study the effect of the mutation introduced in vitro, or can be used for the generation of mice, either through the generation of chimeric mice by injection of ES cells in a diploid blastocyst or by the tetraploid complementation technology (18). For several studies, the analysis of mutant mice generated with these hybrid ES cell lines requires extensive back crossing. However, mice generated by tetraploid complementation could avoid this problem. Also, the effect of many mutations can be studied in hybrid mice, and for studies involving genomic imprinting and X inactivation a hybrid back ground is even the preferred back ground for studying the consequence of an introduced mutation.
Regarding efficiency of the new strategy, for targeting of the Rnf12 locus, we find that analysis of less than a hundred clones results in a sufficient number of properly targeted cell lines (Figure 3C), in agreement with previous findings using homologous recombination with BAC targeting vectors (8). Although there is neither a gain or loss in efficiency, the use of RFLPs to perform an allele specific PCR profoundly facilitates the detection of positive clones, and omits less reliable and more laborious techniques such as DNA–FISH and Q–PCR. Therefore, our new strategy for gene targeting combines the high efficiency of BAC targeting technology with a convenient readout to screen for positive targeting events. The highly polymorphic C57BL/6/Cast/Ei ES cell lines we generated, in combination with the different approaches described here, for targeting a gene of interest, provides many options for efficient and precise BAC targeting of almost every gene in the mouse genome.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Dutch Research Council (NWO-TOP and -VICI to J.G.). Funding for open access charge: Erasmus MC.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Maureen Eijpe and all laboratory members for helpful discussions.
REFERENCES
- 1.Capecchi MR. Altering the genome by homologous recombination. Science. 1989;244:1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- 2.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 3.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng C, Capecchi MR. Reexamination of gene targeting frequency as a function of the extent of homology between the targeting vector and the target locus. Mol. Cell. Biol. 1992;12:3365–3371. doi: 10.1128/mcb.12.8.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasty P, Rivera-Perez J, Chang C, Bradley A. Target frequency and integration pattern for insertion and replacement vectors in embryonic stem cells. Mol. Cell. Biol. 1991;11:4509–4517. doi: 10.1128/mcb.11.9.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.te Riele H, Maandag ER, Berns A. Highly efficient gene targeting in embryonic stem cells through homologous recombination with isogenic DNA constructs. Proc. Natl Acad. Sci. USA. 1992;89:5128–5132. doi: 10.1073/pnas.89.11.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valenzuela DM, Murphy AJ, Frendewey D, Gale NW, Economides AN, Auerbach W, Poueymirou WT, Adams NC, Rojas J, Yasenchak J, et al. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat. Biotechnol. 2003;21:652–659. doi: 10.1038/nbt822. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Seed B. Site-specific gene targeting in mouse embryonic stem cells with intact bacterial artificial chromosomes. Nat. Biotechnol. 2003;21:447–451. doi: 10.1038/nbt803. [DOI] [PubMed] [Google Scholar]
- 9.Song H, Chung SK, Xu Y. Modeling disease in human ESCs using an efficient BAC-based homologous recombination system. Cell Stem Cell. 2010;6:80–89. doi: 10.1016/j.stem.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Jonkers I, Barakat TS, Achame EM, Monkhorst K, Kenter A, Rentmeester E, Grosveld F, Grootegoed JA, Gribnau J. RNF12 is an X-Encoded dose-dependent activator of X chromosome inactivation. Cell. 2009;139:999–1011. doi: 10.1016/j.cell.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 11.Luikenhuis S, Wutz A, Jaenisch R. Antisense transcription through the Xist locus mediates Tsix function in embryonic stem cells. Mol. Cell. Biol. 2001;21:8512–8520. doi: 10.1128/MCB.21.24.8512-8520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- 13.Osoegawa K, Tateno M, Woon PY, Frengen E, Mammoser AG, Catanese JJ, Hayashizaki Y, de Jong PJ. Bacterial artificial chromosome libraries for mouse sequencing and functional analysis. Genome Res. 2000;10:116–128. [PMC free article] [PubMed] [Google Scholar]
- 14.Frazer KA, Eskin E, Kang HM, Bogue MA, Hinds DA, Beilharz EJ, Gupta RV, Montgomery J, Morenzoni MM, Nilsen GB, et al. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature. 2007;448:1050–1053. doi: 10.1038/nature06067. [DOI] [PubMed] [Google Scholar]
- 15.Barakat TS, Jonkers I, Monkhorst K, Gribnau J. X-changing information on X inactivation. Exp. Cell Res. 2010;316:679–687. doi: 10.1016/j.yexcr.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Barakat TS, Gunhanlar N, Pardo CG, Achame EM, Ghazvini M, Boers R, Kenter A, Rentmeester E, Grootegoed JA, Gribnau J. RNF12 activates Xist and is essential for X chromosome inactivation. PLoS Genet. 2011;7:e1002001. doi: 10.1371/journal.pgen.1002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wutz A, Gribnau J. X inactivation Xplained. Curr. Opin. Genet. Dev. 2007;17:387–393. doi: 10.1016/j.gde.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Eggan K, Akutsu H, Loring J, Jackson-Grusby L, Klemm M, Rideout WM, 3rd, Yanagimachi R, Jaenisch R. Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc. Natl Acad. Sci. USA. 2001;98:6209–6214. doi: 10.1073/pnas.101118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.