Abstract
Although retrograde transsynaptic degeneration in the visual pathway of monkeys has been described since 1963, data in humans are sparse. The authors present a 24-year-old female referred to a neuro-ophthalmology consult for assessment before neurosurgery for a right occipital ependymoma. Clinical examination was unremarkable, including visual fields evaluated by computerised static perimetry. Four years after tumour extraction, the patient showed a left homonymous haemianopia documented by computerised static perimetry and a bow-tie like atrophy on the left on funduscopy. MRI revealed right occipital cortex lobectomy and optic tract atrophy. The presence of left homonymous haemianopia, the characteristic pattern of optic disc atrophy and right optic tract atrophy 4 years after right occipital tumour excision, strongly suggest the presence of retrograde transsynaptic degeneration. To our knowledge, this is the first time that retrograde transsynaptic degeneration-associated optic tract atrophy is clearly demonstrated by MRI.
Background
Retrograde transsynaptic degeneration in the visual pathway of monkeys has been described since 1963, with evidence of retinal ganglion cell atrophy across the lateral geniculate body after occipital lesions.1 Moreover, it has been postulated that retrograde transsynaptic degeneration is inversely proportional to the subject’s age at time of injury and directly proportional to the time elapsed since the injury.2 However, data in humans are sparse and the question as to whether retinal degeneration is transneuronal still remains controversial. With recent advances in neuroimaging of the optic disc, non-invasive and efficient techniques such as optical coherence tomography allow retinal nerve fibre layer thickness measurements around the optic nerve head, with the possibility of a quantitative assessment of retinal ganglion cell atrophy.3
It is well known that any process affecting the visual pathway anterior to the lateral geniculate body may cause secondary optic atrophy. If the lesion is at the level of the optic tract, a characteristic pattern of atrophy known as band atrophy or bow-tie atrophy develops, as the decussating fibres of the contralateral eye and the non-decussating fibres of the ipsilateral eye are affected. The patient will develop a contralateral homonymous haemianopia. It has also been shown that the decrease in retinal nerve fibre layer thickness measured by optical coherence tomography corresponds to the characteristic pattern of axonal damage seen in eyes with bow-tie atrophy.3 In the eye with the nasal field defect, the arcuate fibres of the temporal retina will be damaged and optical coherence tomography will show a thinning of the retinal nerve fibre layer in all the evaluated sectors, while in the eye with the temporal field defect and involvement of the nasal retina, thinning will be at the nasal and temporal sectors (bow-tie atrophy). In patients with acquired unilateral occipital lesions and retrograde transsynaptic degeneration, a contralateral homonymous haemianopia develops and the subsequent retinal nerve fibre layer thinning can be documented by optical coherence tomography.4
Case presentation
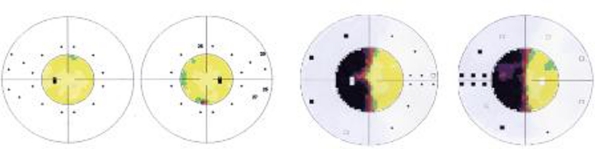
In July 2003, a 24-year-old female with no ophthalmologic complaints, was referred to a neuro-ophthalmology consult for preoperative assessment before right occipital ependymoma excision. Clinical examination was unremarkable, including visual acuity, colour vision, pupillary reflexes, fundus and visual fields evaluated by computerised static perimetry (figure 1).
Figure 1.
Computerised static perimetry (Octopus), normal visual fields before tumour extraction (on the left) and left homonymous haemianopia, 1 year after right occipital lobectomy (on the right).
In June 2004, after tumour extraction, the patient was re-evaluated and showed normal visual acuity and colour vision in both eyes, no pupillary defect, normal bilateral funduscopy and a left homonymous haemianopia documented by computerised static perimetry (figure 1).
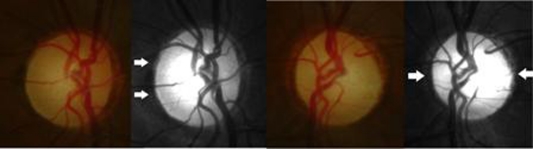
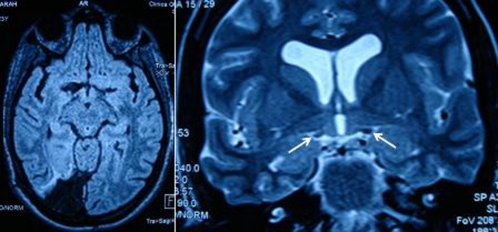
In April 2008, the patient maintained left homonymous haemianopia and at funduscopy, showed a temporal optic pallor on the right and a horizontal band pallor on the left (figure 2). MRI revealed right occipital cortex lobectomy and asymmetric optic tracts with right optic tract atrophy (figure 3). Optical coherence tomography showed a generalised thinning of the retinal nerve fibre layer on the right and of the nasal and temporal sectors on the left, corresponding to the left bow-tie atrophy pattern.
Figure 2.
Fundus and red free photograph, 4 years after tumour extraction. Temporal pallor of the right optic disc (on the left) and bow-tie atrophy of the left optic disc (on the right).
Figure 3.
MRI, axial view, right occipital cortex lobectomy after right occipital ependymoma extraction (on the left) and right optic tract atrophy (see arrows) on coronal view (on the right).
Investigations
In July 2003, normal visual fields were evaluated by computerised static perimetry (figure 1).
In June 2004, left homonymous haemianopia was documented by computerised static perimetry (figure 1).
In April 2008, MRI revealed right occipital cortex lobectomy and asymmetric optic tracts with right optic tract atrophy (figure 3). Optical coherence tomography showed a generalised thinning of the retinal nerve fibre layer on the right and of the nasal and temporal sectors on the left, corresponding to the left bow-tie atrophy pattern.
Discussion
Retrograde transsynaptic degeneration has been described in primates since 19631, with the demonstration of lateral geniculate nucleus atrophy following occipital lobectomy. It has also been shown that retrograde transsynaptic degeneration can occur in the human central nervous system after congenital or acquired retrogeniculate lesions. Bow-tie atrophy on the contralateral eye and temporal atrophy on the ipsilateral eye of patients with congenital lesions of a cerebral hemisphere5 or occipital cortex,6 have been previously described. The pattern of bow-tie atrophy results from Wallerian anterograde degeneration after any optic chiasm or optic tract lesions. In the latter, the crossing fibres of the contralateral eye and non-crossing fibres of the ipsilateral eye are affected. This ophthalmoscopic pattern can also occur after acquired occipital cortex lesions through retrograde transsynaptic degeneration, and the characteristic thinning of retinal nerve fibre layer can be documented by optical coherence tomography which has been proven to be a sensitive and reliable technique for measuring retinal nerve fibre layer thickness after occipital lobe lesions in patients with homonymous haemianopia.4
One year after surgery for a right occipital ependymoma, our patient showed a left homonymous haemianopia, and 4 years later, a right temporal atrophy and a left bow-tie atrophy developed with thinning of the retinal nerve fibre layer documented by optical coherence tomography. The presence of normal visual fields prior to surgery allows us to exclude a pre-existing visual pathway lesion and the right optic tract atrophy was also visible on MRI, which strongly suggests the presence of retrograde transsynaptic degeneration since the posterior surgical approach used in this case (occipital lobectomy) excludes the possibility of a direct lateral geniculate body iatrogenic lesion.
A previous study suggested that retrograde transsynaptic degeneration is inversely proportional to the subject’s age at time of injury.2 This may explain the development of optic atrophy over the course of 4 years, since our patient was only 24-years-old. Moreover, retinal nerve fibre layer thinning as measured by optical coherence tomography was also shown in a group of patients with acquired unilateral occipital lobe lesions after 3, 5 years of median duration of disease.4
In this case, optical coherence tomography helped to document in vivo the thinning of the retinal nerve fibre layer following an acquired lesion of the retrogeniculate visual pathway, which confirms the presence of retrograde transsynaptic degeneration and to our knowledge, this is the first time that the associated optic tract atrophy is clearly demonstrated by MRI.
Learning points.
-
▶
Retrograde transsynaptic degeneration can occur in the human central nervous system after congenital or acquired retrogeniculate lesions.
-
▶
Optical coherence tomography is a sensitive and reliable technique for measuring retinal nerve fibre layer thickness after occipital lobe lesions in patients with homonymous haemianopia.
-
▶
The retrograde transsynaptic degeneration-associated optic tract atrophy can be demonstrated by MRI.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Van Buren JM. Trans-synaptic retrograde degeneration in the visual system of primates. J Neurol Neurosurg Psychiatry 1963;26:402–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beatty RM, Sadun AA, Smith L, et al. Direct demonstration of transsynaptic degeneration in the human visual system: a comparison of retrograde and anterograde changes. J Neurol Neurosurg Psychiatr 1982;45:143–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanamori A, Nakamura M, Matsui N, et al. Optical coherence tomography detects characteristic retinal nerve fiber layer thickness corresponding to band atrophy of the optic discs. Ophthalmology 2004;111:2278–83 [DOI] [PubMed] [Google Scholar]
- 4.Jindahra P, Petrie A, Plant GT. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain 2009;132(Pt 3):628–34 [DOI] [PubMed] [Google Scholar]
- 5.Hoyt WF, Rios-Montenegro EN, Behrens MM, et al. Homonymous hemioptic hypoplasia. Fundoscopic features in standard and red-free illumination in three patients with congenital hemiplegia. Br J Ophthalmol 1972;56:537–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fletcher WA, Hoyt WF, Narahara MH. Congenital quadrantanopia with occipital lobe ganglioglioma. Neurology 1988;38:1892–4 [DOI] [PubMed] [Google Scholar]