Abstract
The precise role of adaptive immune responses in the clinical outcome of HCV infection is still only partially defined. Recent studies suggest that viral-host cell interactions during the acute phase of infection are essential for viral clearance or progression into chronic HCV infection. This review focuses on different aspects of the adaptive immune responses as determinants of the different outcomes of HCV infection, clearance or persistent infection, and outlines current concepts of HCV evasion strategies. Unravelling these important mechanisms of virus-host interaction will contribute to the development of novel strategies to prevent and control HCV infection.
Keywords: viral clearance, neutralizing antibodies, T cells, escape, vaccine development
1. Introduction
Hepatitis C virus (HCV), a member of the Flaviridae family, infects 3% of the population resulting in chronic infection in the majority of cases. HCV chronic hepatitis frequently results in progressive fibrosis, cirrhosis with an increased risk of hepatocellular carcinoma [1]. These latter complications have become leading indications for liver transplantation in developed countries. There is no vaccine and the standard of care treatment, a combination of pegylated interferon and ribavirin, is limited by resistance in a large fraction of patients, toxicity and high costs. After exposure to HCV, 60 to 80% of infected persons develop persistent viremia despite the generation of HCV-specific antibodies and HCV-specific cellular immune responses [2,3]. Persistent viremia - detected by polymerase chain reaction - remains positive after more than 6 months. Studies of host responses in the course of HCV infection have been hampered by the fact that acute HCV infection is asymptomatic in most individuals and thus frequently not recognized. Moreover, the chimpanzee is the only immunocompetent animal susceptible to HCV infection and there are major differences between HCV infection in chimpanzees and in humans. Studies of the host’s immune responses in humans thus rely on patient cohorts. Through the availability of serial samples from acute and chronic HCV infected patients, insights into the humoral and cellular immune responses in the course of HCV infection could be gained in the past years.
The present review focuses on different aspects of the adaptive immune responses as determinants of the different outcomes of HCV infection, clearance or persistent infection, and outlines current concepts of HCV evasion strategies.
2. The humoral responses to HCV infection
Neutralizing antibodies are generally an important mechanism for control of initial viremia and protection from re-infection in viral infections. However, the role of the humoral immune response in the clearance of HCV infection has been questioned for a long time. While anti-HCV antibodies can easily be detected in the course of HCV infection by commercially available antibody assays approximately 50 to 60 days after HCV infection [4], these tests only attest a humoral immune response to HCV proteins but they do not evaluate the neutralizing ability of these antibodies. The ability of antibodies to neutralize HCV can solely be evaluated using relevant model systems.
Determining the relative role of antibodies in the course of HCV infection has long been hampered by the absence of a convenient model system for evaluating the neutralizing activity of anti-HCV antibodies. HCV infects only humans and chimpanzees and for a long time the chimpanzee represented the only validated animal model for the study of HCV (reviewed in [5]). Over the past years, the development of sensitive and robust in vitro neutralization assays based on human hepatoma cell lines and HCV pseudotyped particles[6–8], HCV-like particles [9–11] and recombinant cell culture-derived HCV (HCVcc) [12–19] then allowed to conveniently study the role of neutralizing antibodies in acute and chronic HCV infection. Moreover, the recent development of an in vivo model based on immunodeficient mice repopulated with human livers, the uPA-SCID mice [20], enabled investigators for the first time to determine the role of antibodies in HCV infection in a small animal model [21,22].
Early studies investigating immune responses in chimpanzees and humans suggested that HCV clearance could occur in the absence of neutralizing antibodies or that antibody responses alone are not sufficient to eradicate HCV in the majority of cases [23–27]. Moreover, individuals who cleared HCV are not protected against re-infection, although chimpanzees and individuals who have cleared HCV seem to be less likely to develop chronic infection after re-exposure [28–30]. Since the development of novel model systems for the study of HCV infection and neutralization in vitro, the availability of sequential serum samples from homogenous patient cohorts, well-defined viral inoculum and viral surrogate ligands used for neutralization assays, isolate-specific neutralizing antibodies have been detected in acutely HCV infected individuals who subsequently cleared viral infection. In contrast, the humoral immune responses seem to be delayed in patients developing chronic HCV infection, thereby allowing the virus to escape the host’s immune surveillance.
2.1. Neutralizing antibodies and control of viral infection
Since the availability of several in vitro HCV model systems [8,9,12–14], considerable progress has been made in understanding how HCV enters into host cells and how antibodies may neutralize this process. Binding and entry of HCV is believed to be a complex process involving both viral and cellular factors. The essential viral factors are the HCV envelope glycoproteins E1 and E2 which have been demonstrated to directly interact with cellular factors and to trigger conformational changes necessary to initiate infection. Several cellular factors have been identified to mediate viral attachment and entry, such as CD81, scavenger receptor class B type I (SR-BI), members of the claudin family and occludin [31–41]. HCV envelope glycoprotein E2 has been demonstrated to directly interact with CD81 and SR-BI [31,32] but the interaction of HCV envelope glycoproteins with the other host entry factors is still elusive [36,38]. As the HCV envelope glycoprotein E1 and E2 interaction with host cell factors is mandatory to initiate productive infection, it is an important target for virus neutralization.
Using retroviral pseudoparticles bearing HCV envelope glycoproteins (HCVpp), neutralizing antibodies have been detected in patients with acute and chronic HCV infection. The association between the induction of neutralizing antibodies for resolution of infection during acute HCV infection has been demonstrated using well defined viral inoculum and autologous surrogate ligands [42–44]. Lavillette et al. and Pestka et al. have shown that neutralizing antibodies are induced in the early phase of infection by patients who subsequently control [42] or resolve [43] viral infection. In hemodialysis patients with nosocomial acquired HCV infection, strong neutralizing responses correlated with decrease in viremia and control of HCV replication whereas absent neutralizing response associated with persistent high viremia and failure to control HCV infection [42]. Moreover, in an accidental single-source outbreak of hepatitis C in pregnant women, viral clearance was associated with the rapid induction of high-titer and cross-neutralizing antibodies in the acute phase of infection while chronic HCV infection was characterized by a complete absence or reduced capacity to neutralize the transmitted virus as well as heterologous viruses in the early phase of infection [43]. These results suggest that a strong early broad neutralizing antibody response may contribute to resolution of HCV in the acute phase of infection while delayed induction of neutralizing antibodies may contribute to development of chronic HCV infection (Figure 1).
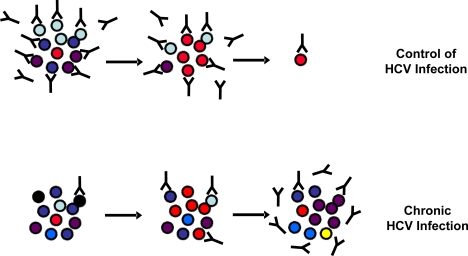
Figure 1.
Viral escape from neutralizing antibody responses. In infected individuals, HCV exists as a quasispecies, i.e., a pool of constantly changing, distinct but related genomic variants. Resolving HCV infection is associated with a relatively stable pool of viral variants and the early induction of high-titer cross-neutralizing antibodies. Chronic HCV infection is correlated with diversification of the quasispecies population associated with a delayed induction of cross-neutralizing antibodies allowing viral escape from the host humoral responses.
2.2. Viral escape from neutralizing antibodies
As HCV has evolved several mechanisms to escape from the host immune responses (reviewed in [45]), neutralizing antibodies and HCV co-exist during chronic infection in patients who did not mount efficient immune responses able to clear the virus during acute infection (Figure 1). Viral escape from antibody-mediated neutralization has been shown to occur on several levels and in line to reports of other viruses, a combination of different mechanisms may also apply to HCV. These include (1) the high variability of the HCV genome and limited induction of cross-neutralization antibodies, (2) induction of antibodies interfering with neutralizing antibodies, (3) the association of HCV with serum factors such as low-density lipoproteins (LDL) and very low density lipoproteins (VLDL), (4) the interplay of HCV glycoproteins with high-density liporoteins (HDL), (5) the shielding of neutralizing epitopes by glycosylation of defined amino acids of envelope glycoproteins, and (6) direct cell-to-cell transfer of the virus. As these mechanisms have been reviewed elsewhere [45], this review will focus on recent studies demonstrating both in vitro and in vivo viral adaptations leading to escape from neutralizing antibodies.
Using the state-of-the-art HCV cell culture model, Zhong et al. investigated adaptation of HCV in vitro [46]. The authors demonstrated that HCV can establish persistent infection in vitro, which lead to the selection of viral and cellular variants that favour the survival of both the virus and the host [46]. The virus acquired increased specific infectivity whereas the host cell became resistant to HCV infection. This resistance may be due to down-regulation of HCV entry factor expression or a defect in HCV replication or a combination of these mechanisms [46]. While substantial progress in understanding the HCV life cycle has been made, the interplay between host cell entry factors, HCV envelope glycoproteins and neutralizing antibodies is only about to be investigated. Recent evidence suggests that neutralizing antibodies isolated from chronic HCV patients interfere with entry steps that are closely linked to the interaction of HCV with SR-BI and CD81 [15,47]. Evasion from antibody-mediated neutralization through decreased receptor binding has been reported for viruses such as HIV-1 [48]. This mechanism seems also to apply to HCV. The cell culture-adapted mutation G451R initially described by Zhong et al. [46] has been shown to be less dependent on SR-BI and CD81 on the entry level [16]. Moreover, this mutant demonstrated an increased binding to CD81 and CD81 mimics while being more sensitive to neutralizing antibodies [16].
In chronic HCV infected patients, HCV coexists with anti-HCV antibodies. It is thus most interesting to understand how HCV evolves in the presence of neutralizing antibodies. A recent study addressed this important question by investigating in vitro HCV escape mutants through multiple rounds of selection by the well-described anti-E2 monoclonal antibody AP33 [49]. The authors described an in vitro escape mutation HCV N415Y that lowered viral fitness probably by affecting viral entry but without affecting binding to CD81 [49] suggesting that mutations modulating interaction with host cell factors other than CD81 may contribute to escape of HCV from neutralizing antibodies. Taken together, these studies show that in cell culture, mutations within the HCV envelope glycoproteins arise that modulate viral entry and neutralization by anti-HCV antibodies.
As described above, resolution of infection appears to require rapid, vigourous and multi-specific antiviral host immune responses [43,45,50,51]. Patients who subsequently develop chronic infection have been shown to develop a delayed and inefficient neutralizing antibody response [43] allowing HCV infection to persist for lifetime despite the presence of neutralizing antibodies. It is believed that the adaptive immune system exerts constant pressure on the virus thereby leading to the emergence of HCV escape mutants. However, little is known about the role of neutralizing antibodies in driving HCV sequence evolution in the course of infection. A recent study addressed this important question and provided insights into the time-course of induction of neutralizing antibodies and viral escape from neutralizing responses in a cohort of young intravenous drug users [44]. Studying autologous humoral immune responses in individual subjects, the authors demonstrate that during acute HCV infection, earlier HCV variants were neutralized by autologous plasma samples prior to neutralization of later HCV variants, similar to what has been shown in a chronic HCV patient [52], suggesting that neutralizing antibodies are responsible for envelope sequence changes over time [44]. In line with previous results obtained in a cohort of patients from a single-source HCV outbreak [43], this study demonstrated an association of high-titer neutralizing antibodies and spontaneous viral clearance whereas persistent HCV infection was associated with low-titer or absent neutralizing antibodies during the acute phase [44]. These data suggest that humoral immune response pressure drives HCV envelope glycoprotein sequence evolution resulting either in effective clearance of circulating viral variants and resolution of infection or emergence of viral escape variants and progression into chronic infection. Analysis of sequence substitutions that occurred in HCV envelope glycoproteins during acute infection were monitored throughout E1E2 but most of them were located in the HVR1 region [44]. Mapping of amino acid substitutions involved in escape from neutralizing antibodies showed that significant loss of sensitivity to neutralizing antibodies could be attributed to 3 HVR1 mutations (K384T, K408R and S405P) [44]. A similar time-course study had previously been conducted in the well-characterized chronic HCV patient H [52]. Consistent with the results obtained during the acute phase study described above [44], von Hahn et al. demonstrated that throughout the course of this chronic HCV infection, the patient’s antibodies lagged behind the rapidly evolving viral variants, i.e. they were able to neutralize HCV strains that had been circulating several months or years before but not the present or future viral variants of the patient [47,52]. This raised the question of the mechanisms underlying escape of such quasispecies from neutralizing antibodies. By investigating the interaction of these neutralizing antibody-escape variants and HCV host cell factors, Keck et al. described a single viral variant from this patient that was characterized by reduced infectivity, diminished CD81 binding and resistance to a panel of anti-E2 antibodies (domain B antibodies and AP33). Thus by escaping from neutralizing antibodies, HCV seems to loose in infectivity due to lower binding to CD81. It is worth noting that several mutations within E2 but outside the anti-E2 epitopes as well as the CD81 binding regions may account for escape from these neutralizing antibodies as site-directed mutations were able to restore sensitivity to neutralizing antibodies and CD81 dependency [47]. The most important mutations responsible for reduced infectivity and binding to CD81 were S501N and V506A, suggesting that mutation of theses amino acids affect the conformation of E2 necessary for interaction with CD81 [47]. However, these mutations did not account for escape from humoral responses. Interestingly, an additional mutation at residue 444 is necessary in order to lead to complete escape from neutralizing antibodies – this additional mutation at position 444 seems to negatively modulate antibody-mediated neutralization in concert with the mutations at residues 501 and 506 [47].
Recently, Zhang et al. described an additional escape mechanism whereby the presence of non neutralizing antibodies interferes with the function of neutralizing antibodies, resulting in the reduction or blockage of their effect [53,54]. Two epitopes within a short segment of E2 were mapped: epitope I, at amino acids 412–419, and epitope II, at amino acids 434–446. Epitope I has been recognized as an important neutralization site, while epitope II interfered with antibody to epitope I inhibiting neutralization of the virus [53]. Epitope I- and epitope II- specific antibodies were detected in plasma from chronically HCV-infected patients. Kinetic studies in patient H revealed that antibody to epitope II appeared within 51 days of infection, while antibody to epitope I was not detectable until day 643. Interestingly, by absorbing out antibody to epitope II, neutralizing activity of plasma was enhanced and broadened to include additional genotypes of HCV [54].
3. T cell responses to HCV infection
The majority of primary infections are asymptomatic and often unrecognized. Thus, studies of T cell immune responses during acute HCV infection have only been possible in experimentally infected chimpanzees or individuals with occupational needle stick exposure or IVDU involved in epidemiological follow-up for which the time of contamination is documented. A large body of evidence suggests that a strong, multispecific and long-lasting T-cell immune response appears to be important for control of viral infection (reviewed in [27,55]).
Three types of T cell-mediated responses can be raised against HCV [27,55]. First, an efficient primary immune response during the acute phase, leading to a resolved HCV infection and maintenance of an efficient CD4 and CD8 memory. This immune response is sustained and targets multiple viral proteins, especially during the acute phase of the response. Second an efficient but transient primary immune response, leading to partial control of the infection, but ultimately CD4 memory cells are absent while CD8 memory cells are present at a variable level, leading to chronic infection. Third, a lack of efficient primary immune response, leading to chronic infection. Memory CD4 and CD8 memory cells are less frequent, functionally impaired and target less viral proteins than in patients with resolved infection.
This review will focus on cellular and viral factors that may influence the efficiency and maintenance of primary T cell mediated immune responses including incomplete differentiation of effector and memory T cell populations, immune exhaustion resulting from persistent high viral loads mediated by programmed death-1 (PD-1) protein signalling, suppression by regulatory T (Treg) cells and immune escape mutations.
3.1. T cell immune responses and control of viral infection
In humans and chimpanzees a self-limited course of acute hepatitis C is associated with vigorous CD4+ and CD8+ T cell responses targeting multiple HCV regions and with intrahepatic production of IFN-γ [23,24,51,56,57]. In a study of five healthcare workers the only subject able to clear acute HCV infection mounted an early, vigorous and sustained CD4+and CD8+ T cell response [51]. A study using IFN-γ enzyme-linked immmunospot (ELISPOT) and human histocompatibility leukocyte antigen (HLA) peptide tetramer assays, revealed that at the earliest time points of acute infection highly activated CTL populations are observed that temporarily fail to secrete IFN- γ, a “stunned” phenotype, from which they recovered as viremia declined [24]. Resolution of acute infection was associated with T cell recovery of an activated phenotype and the ability to produce IFN-γ [24].
The non structural proteins have been described to be preferentially targeted by the CD4 +T cell responses in those who clear infection [58–61]. One of the most recent cross-sectional study of proliferative responses in 22 subjects with resolved infection and 23 with chronic infection showed that at least three of the six non-structural proteins were targeted by all subjects who had cleared HCV infection, with less frequent responses against the core protein and the variable regions of the envelope protein [58]. In all studies, one or more epitopes on NS3 were targeted, suggesting that epitopes in this protein may be immunodominant [50,59,62,63]. This observation is supported by a study examining IFN-γ ELISpot responses to three peptide pools spanning NS3 which showed that all subjects who had recovered from infection mounted a strong CD4+ response to all three pools [62]. Similar to CD4+ T cell responses against HCV, it has been suggested that the breadth of the CD8+ T cell response is associated with clearance in both humans and chimpanzees [23,24,64]. In a recent study of 17 patients with acute HCV leading to persistence and 14 with primary infections resulting in clearance [65] this notion was corroborated: total HCV-specific specific CD4+ and CD8+ T-cell responses were examined and functional T-cell thresholds that predict recovery identified. The likelihood of recovery was considerably greater in individual subjects exceeding these thresholds ; for example if five or more HCV peptides pools (or ∼15% of the HCV genome) are targeted by CD4+ T cells early after infection the chance of recovery was more than seven times higher than if this threshold was not achieved. Similarly it has been shown by logistic regression analysis that patients demonstrating HCV-specific IFN- γ-producing CTL responses to at least two HCV peptides pools were statistically more likely to contain HCV infection than patients demonstrating responses to only one or none of the HCV peptide pools.
The kinetics of onset and the durability of the cellular immune responses may also be an important determinant of outcome. Both human and chimpanzee studies have demonstrated a CD4+ response that is initially effective with a subsequent rebound in viremia and progression to chronic infection [23,51]. A prospective study of 20 subjects with acute infection showed that the number of Th1 cytokine–producing CD4+ cells was higher in the first 12 weeks after disease onset in the subjects with rapid viral clearance compared to those with only transient or no control of viremia [63]. The strongest CD4+ T cell response to HCV infection has been shown to occur within the first six months after infection regardless of outcome [60,63,64,66]. Thus, it appears that a successful CD4+ T cell response needs to develop early and also to be sustained to achieve viral clearance [67].
Smyk-Pearson et al. studied the relative importance of CD4 help in spontaneous recovery in acute HCV infection and demonstrated that [65] the presence of HCV-specific cytotoxic T lymphocytes – able to proliferate, exhibit cytotoxicity and produce IFN – γ - did not ensure recovery, but whether these CTLs were primed in the presence or absence of T-cell help (HCV-specific IL-2 production) was a critical determinant. This is also strongly supported by CD4-depletion studies in the chimpanzee model of infection [68]. Helper CD4+ T cells are important through the maintenance of the effector functions of cytotoxic CD8+ T cells. This is mediated both by activation of co-stimulatory pathways and via the production of cytokines notably IL-2 and IFN – γ [69]. Only patients able to finally control infection show maturation of CD8 memory sustained by progressive expansion of CD127+ CD8 cells [67].
The cellular immunity appears to persist for many years after resolution of infection in chimpanzees and humans [62,66,70]. After viral clearance, memory T cells maintain over decades and can mediate protective immunity in spontaneously HCV-recovered chimpanzees following re-challenge with homologous and heterologous HCV [29,30,71]. The rapid control of HCV viremia following re-challenge was found to be associated with early anamnestic HCV-specific CD4+ and CD8+ T cell responses, including memory CD4+ T cell responses [68,72–74]. However, recent studies in chimpanzees contradict the early studies [75]. Thus, a chimpanzee that had previously demonstrated protective immunity following multiple re-challenges with heterologous viruses became chronically infected when re-exposed to the virus originally inoculated into the animal [75]. These findings are supported by evidence that chimpanzees tend to mount weak humoral responses to HCV envelope glycoprotein E2. Indeed, a lower percentage of HCV-inoculated chimpanzees develop detectable antibodies to envelope glycoproteins E1 (22%) and E2 (15%) as compared to humans [76].
3.2. Mechanisms of T cell failure
In contrast to acute resolving HCV infection, persisting acute HCV infection is associated with a weak and only monospecific CD4+ T cell responses [77]. Regarding the role of CD8+ T cells, recent studies in humans demonstrated that even strong CD8+ T cell responses in the acute phase of infection may not be adequate to prevent progression to chronicity [64,67,78]. Urbani et al. showed that at clinical onset, CD8 responses are similarly weak and narrowly focused in both self-limited and chronically evolving infections [67]. At this stage, CD4 responses are deeply impaired in patients with a chronic outcome as they are weak and of narrow specificity, unlike the strong, broad and T helper 1-oriented CD4 responses associated with resolving infections.
An important issue is to determine what signals allow to sustain memory cells. In murine models of viral infections, an acute viral infection is generally associated with a high expansion of effector cells that differentiate from naïve cells [79]. This expansion phase is followed by a contraction phase leading to the elimination of ∼90% of effector cells, while the remaining effector cells differentiate into long-lived protective memory cells. In human, the differentiation pathways of effector and memory cells may not be similar, and effector cells may be replenished from memory cells. Therefore, it is of crucial importance to identify the mechanisms that allow some patients to maintain HCV-specific memory, while some other are inefficient in controlling the infection. Some key factors may be IL-7 and IL-15, that have been demonstrated to be involved in the induction and homeostasis of CD8 memory cells [80]. IL-7Rα expression is decreased upon T-cell activation: during acute viral infections, the expression of IL-7Rα by viral antigen-specific T cells is transiently decreased [69,81,82] and recovers at late time points after infection when an efficient memory response is obtained [69,83] while IL-7Rα expression remains at a low level in the setting of inefficient memory responses in chronically infected subjects [81]. IL-7Rαhigh expression may therefore allow identifying cells that will give rise to memory cells, at least in the setting of infections that lead to an inflammatory response [69], but not when antigen is presented in a non inflammatory context [84]. Indeed, IL-7Rα expression follows an IL-7-independent program of expression [85] that may be controlled by the level of inflammation [84,85] or the strength of TCR signalling or viral load at time of antigen presentation [82]. IL-15 is also involved in survival of memory cells, especially when IL-7 signalling is present in limiting conditions [69,80]. Indeed, IL-15 is critical for memory cell survival in normal animals, where IL-7 may be limiting due to competition with naïve cells, which use IL-7, but not IL-15, signalling for homeostatic proliferation [86–90]. The CD4-mediated production of another cytokine of the same family, IL-21, has been shown recently to be of crucial importance in avoiding deletion and maintaining memory responses of CD8 T cells in the murine model of LCMV infection [91–93]. Whether IL-21 is also critically involved in maintaining memory HCV-specific memory in humans remains to be determined.
In the context of HCV infection, expression of IL-7Rα by total CD4 and CD8 T cells as well as by HCV-specific cells has been reported to be reduced in the blood of patients with chronic infection as compared with patients with resolved infection [94], although such decreased IL-7Rα expression by HCV-specific memory cells remains controversial [95,96]. IL-7Rα expression is even more decreased in liver CD8+ lymphocytes than in blood lymphocytes from chronic patients [95]. Interestingly, patients with acute infection who subsequently resolved the infection had higher baseline values of IL-7Rα expression (i.e. at time of acute infection) than patients with acute infection who subsequently evolved toward chronic infection [94]. However, this picture may be even more complex, as two profiles of IL-7Rα expression have been observed in chronic HCV patients: most patients have exhausted HCV-specific CD8+ T cells, with low IL-7Rα expression, low proliferative and IFN-γ secretion potential, but some patients have HCV-specific T cells that express high levels of IL-7Rα expression and maintain an efficient proliferative and IFN-γ secretion potential, similar to HCV-specific T cells from patients who resolved their infection [96].
In the chronic phase, virus specific CD4+ and CD8+ T cell responses are also detectable. However HCV-specific CD4+ and CD8+ T cells isolated from chronically infected patients usually display functional and maturation defects including reduced cytotoxic potential, reduced secretion of Th1-type cytokines and a reduced proliferative capacity in response to ex vivo antigenic stimulation [57,97,98]. CD8+ T cell exhaustion such as observed during chronic HCV infection is described in different murine models of persistent infection with highly replicative viruses and may result from deficient CD4+ T cell help (reviewed in [99]). Ulsenheimer et al. have described functionally altered HCV specific CD4+ T cells in acute and chronic hepatitis C [100]. CD8+T cell exhaustion and persistent infection are more likely to develop when CD4+ T cells help is lacking or lost (Figure 2). Helper CD4+ T cells activate or license dendritic cells to optimally prime CD8+ T cells, recognition of antigen on the same antigen-presenting cell by CD4+ and CD8+ T cells is likely to be a key feature of antigen-specific T cell help. Thus the failure of CD4+HCV specific T cells may limit CD8+ T cells opportunities of priming by fully activated HCV antigen- loaded DC [27].
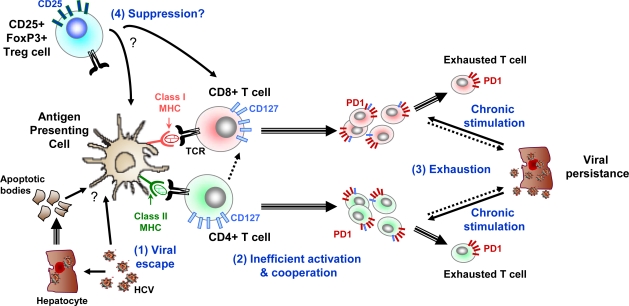
Figure 2.
Examples of mechanisms resulting in impairment of T cell responses leading to chronic HCV infection. Chronic HCV infection is associated with impaired CD8+ T cell responses including reduced cytotoxic potential, reduced secretion of Th1 type cytokines and reduced proliferative capacity in response to ex vivo antigenic stimulation. Four possible mechanisms of T cell response failure are shown here: (1) viral escape with mutations in HLA restricted epitopes impairing antigen recognition, (2) loss of functional CD4+ T cell responses, (3) overexpression of PD1 in CD8+ T cells; when PD1 binds to its ligand PD-ligand 1 (PD-L1), which is preferentially expressed by virus-infected cells, an inhibitory signal is transmitted to CD8+ T cells, resulting in blocking of the T cell receptor-mediated activation signal, (4) induction of regulatory T cells. Arrows with single line indicate functional interactions while arrows with double lines indicate cell differentiation.
Another mechanism that may be involved in secondary T cell failure of HCV-specific CD4+ and CD8+ T cells in chronic HCV infection is signalling through programmed death 1 receptor (PD-1) (Figure 2). Down regulation of virus –specific T-cell responses via signalling through PD-1 on T cells has been linked with virus-specific T-cell deficiency during chronic viral infections in a murine model and in humans [101,102]. Several recent studies have demonstrated high expression levels of PD-1 in HCV-specific CD8+ T cells in patients with persistent HCV infection [55,95,103,104]. HCV-specific T cells that demonstrated increased expression of PD-1 on their surface exhibited impaired IFN-γ production, cytotoxic activity and proliferative potential in response to ex vivo HCV antigen stimulation [55,95,103]. Such impaired functional properties could be reversed by in vitro blockade of PD-1 interaction with its ligand PD-ligand 1 (PD-L1), demonstrating a causal relationship between PD-1 expression and exhaustion [95,103].
Different T-cell subsets with suppressive functions have been described (Figure 2). Among these, CD4+ CD25+ FoxP3+ regulatory T (Treg) cells have been involved in the control of auto-immunity and immune responses, through various mechanisms including the inhibition of APC maturation and T-cell activation (reviewed in [105]). An increased frequency of Treg cells has been observed in patients with chronic HCV infection compared to individuals who spontaneously resolved HCV infection [106–109]. However, a recent study in chimpanzees showed no difference in the frequency of Treg cells and the extent of suppression irrespective of the outcome of the infection [110]. Evidence against a role for Treg in promoting the development of chronic infection was recently reported in a prospective study of 27 acutely infected subjects. This study showed that there was no significant difference in the proportion of CD4+CD25high T cells in the peripheral blood at baseline between the 15 subjects who developed chronic infection and the 12 subjects that subsequently cleared the infection [65]. The frequency for both groups was higher than in healthy controls and did not vary over time. Further studies are thus required to define the potential role of Treg in the outcome of primary HCV infection.
Viral escape from CD8+T cells is another important mechanism of T cell response failure in patients developing persistent infection [111–115] (Figure 2). Studies in humans and chimpanzees have shown that mutations in HLA class I restricted epitopes targeted by CD8+ T cells, occur early in HCV infection and are associated with persistence [116,117]. The role of HLA alleles in determining the outcome of HCV infection has been recently studied in an Irish cohort of women accidentally infected with HCV [118]. The HLA class I alleles A3, B27 and Cw*01 were associated with viral clearance whereas B8 was associated with viral persistence indicating that the host genetic background is an important variable that can influence infection outcome [118]. Interestingly stable cytotoxic T cell escape mutations have been linked to maintenance of viral fitness [119]. According to these authors, these observations elucidate potential mechanism by which viral persistence is established. Whereas consequences of stable integration of escape mutations into viral genomes are not clear, it is possible that epitopes presented by the most prevalent MHC class I molecules in human population will eventually be lost or become less dominant [120].
4. Conclusions and perspectives
In the last few years, considerable progress has been made in studying humoral and cellular responses in the course of HCV infection. While the role of neutralizing antibodies in outcome of HCV infection has long been questioned, the development of novel and convenient model systems for HCV infection showed an association between strong and early neutralizing responses and viral clearance. A self-limited course of acute hepatitis C is associated with a vigorous CD4+ and CD8+ T cell response targeting multiple HCV regions and with intrahepatic production of IFN-γ [23,24,51,56,57]. Clearance of HCV is thus probably mediated by a coordinated action of cellular and neutralizing immune responses. Only rare studies analyzed in parallel both humoral and cellular immune responses in the course of HCV infection [52]. Von Hahn et al. demonstrated that during chronic HCV infection in patient H, HCV is subjected to selection pressure from humoral and cellular immune responses resulting in the continuous generation of escape variants [52]. These data underscore that neutralizing antibody responses and cellular antiviral immunity are frequently impaired due to both viral and host factors leading to viral escape from the host’s immune surveillance and development of chronic infection.
Novel insights into the mechanisms underlying successful immune responses against HCV in individuals spontaneously clearing infection and elucidation of escape mechanisms from adaptive immune responses in chronic HCV patients will be essential for an improved understanding of HCV pathogenesis. Unravelling these important mechanisms of virus-host interactions will contribute to the development of novel strategies to prevent and control HCV infection.
Acknowledgments
The authors thank Dr. Heidi Barth, Inserm U748, Strasbourg for helpful discussions. The authors’ work is supported by Inserm, France, the European Union (ERC-2008-AdG-233130-HEPCENT), the Chair of Excellence Program of the Agence Nationale de la Recherche (ANR-05-CEXC-008), France, the Agence Nationale de la Recherche sur le SIDA et les Hépatites Virales (ANRS-06221 and 2008/354), France, the CONECTUS programme of the University of Strasbourg, France, the Ligue contre le Cancer, France, and the Else-Kröner-Fresenius Stiftung (P17/07//A83/06), Bad Homburg, Germany.
References and Notes
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, Schraut WW, Schirren CA, Waechtler M, Backmund M, Pape GR. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–88. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 3.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 4.Colin C, Lanoir D, Touzet S, Meyaud-Kraemer L, Bailly F, Trepo C. Sensitivity and specificity of third-generation hepatitis C virus antibody detection assays: an analysis of the literature. J Viral Hepat. 2001;8:87–95. doi: 10.1046/j.1365-2893.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 5.Bukh J. A critical role for the chimpanzee model in the study of hepatitis C. Hepatology. 2004;39:1469–1475. doi: 10.1002/hep.20268. [DOI] [PubMed] [Google Scholar]
- 6.Bartosch B, Bukh J, Meunier JC, Granier C, Engle RE, Blackwelder WC, Emerson SU, Cosset FL, Purcell RH. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc Natl Acad Sci U S A. 2003;100:14199–14204. doi: 10.1073/pnas.2335981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu M, Zhang J, Flint M, Logvinoff C, Cheng-Mayer C, Rice CM, McKeating JA. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc Natl Acad Sci U S A. 2003;100:7271–7276. doi: 10.1073/pnas.0832180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartosch B, Dubuisson J, Cosset FL. Infectious hepatitis C virus pseudo-particles containing functional E1–E2 envelope protein complexes. J Exp Med. 2003;197:633–642. doi: 10.1084/jem.20021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumert TF, Ito S, Wong DT, Liang TJ. Hepatitis C virus structural proteins assemble into viruslike particles in insect cells. J Virol. 1998;72:3827–3836. doi: 10.1128/jvi.72.5.3827-3836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumert TF, Wellnitz S, Aono S, Satoi J, Herion D, Tilman Gerlach J, Pape GR, Lau JY, Hoofnagle JH, Blum HE, Liang TJ. Antibodies against hepatitis C virus-like particles and viral clearance in acute and chronic hepatitis C. Hepatology. 2000;32:610–617. doi: 10.1053/jhep.2000.9876. [DOI] [PubMed] [Google Scholar]
- 11.Steinmann D, Barth H, Gissler B, Schürmann P, Adah MI, Gerlach JT, Pape GR, Depla E, Jacobs D, Maertens G, Patel AH, Inchauspé G, Liang TJ, Blum HE, Baumert TF. Inhibition of hepatitis C virus-like particle binding to target cells by antiviral antibodies in acute and chronic hepatitis C. J Virol. 2004;78:9030–9040. doi: 10.1128/JVI.78.17.9030-9040.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Krausslich HG, Mizokami M, Bartenschlager R, Liang TJ. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci U S A. 2005;102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA, Rice CM. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 15.Haberstroh A, Schnober EK, Zeisel MB, Carolla P, Barth H, Blum HE, Cosset FL, Koutsoudakis G, Bartenschlager R, Union A, Depla E, Owsianka A, Patel AH, Schuster C, Stoll-Keller F, Doffoel M, Dreux M, Baumert TF. Neutralizing host responses in hepatitis C virus infection target viral entry at postbinding steps and membrane fusion. Gastroenterology. 2008;135:1719–1728 e1711. doi: 10.1053/j.gastro.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Grove J, Nielsen S, Zhong J, Bassendine MF, Drummer HE, Balfe P, McKeating JA. Identification of a residue in hepatitis C virus E2 glycoprotein that determines scavenger receptor BI and CD81 receptor dependency and sensitivity to neutralizing antibodies. J Virol. 2008;82:12020–12029. doi: 10.1128/JVI.01569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meunier JC, Russell RS, Goossens V, Priem S, Walter H, Depla E, Union A, Faulk KN, Bukh J, Emerson SU, Purcell RH. Isolation and characterization of broadly neutralizing human monoclonal antibodies to the e1 glycoprotein of hepatitis C virus. J Virol. 2008;82:966–973. doi: 10.1128/JVI.01872-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keck ZY, Li TK, Xia J, Gal-Tanamy M, Olson O, Li SH, Patel AH, Ball JK, Lemon SM, Foung SK. Definition of a conserved immunodominant domain on hepatitis C virus E2 glycoprotein by neutralizing human monoclonal antibodies. J Virol. 2008;82:6061–6066. doi: 10.1128/JVI.02475-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owsianka AM, Tarr AW, Keck ZY, Li TK, Witteveldt J, Adair R, Foung SK, Ball JK, Patel AH. Broadly neutralizing human monoclonal antibodies to the hepatitis C virus E2 glycoprotein. J Gen Virol. 2008;89:653–659. doi: 10.1099/vir.0.83386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meuleman P, Libbrecht L, De Vos R, de Hemptinne B, Gevaert K, Vandekerckhove J, Roskams T, Leroux-Roels G. Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera. Hepatology. 2005;41:847–856. doi: 10.1002/hep.20657. [DOI] [PubMed] [Google Scholar]
- 21.Law M, Maruyama T, Lewis J, Giang E, Tarr AW, Stamataki Z, Gastaminza P, Chisari FV, Jones IM, Fox RI, Ball JK, McKeating JA, Kneteman NM, Burton DR. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 22.Vanwolleghem T, Bukh J, Meuleman P, Desombere I, Meunier JC, Alter H, Purcell RH, Leroux-Roels G. Polyclonal immunoglobulins from a chronic hepatitis C virus patient protect human liver-chimeric mice from infection with a homologous hepatitis C virus strain. Hepatology. 2008;47:1846–1855. doi: 10.1002/hep.22244. [DOI] [PubMed] [Google Scholar]
- 23.Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, Purcell RH, Chisari FV. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A. 2002;99:15661–15668. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lechner F, Gruener NH, Urbani S, Uggeri J, Santantonio T, Kammer AR, Cerny A, Phillips R, Ferrari C, Pape GR, Klenerman P. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000;30:2479–2487. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 26.Logvinoff C, Major ME, Oldach D, Heyward S, Talal A, Balfe P, Feinstone SM, Alter H, Rice CM, McKeating JA. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci U S A. 2004;101:10149–10154. doi: 10.1073/pnas.0403519101. Epub 12004 Jun 10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dustin LB, Rice CM. Flying under the radar: the immunobiology of hepatitis C. Annu Rev Immunol. 2007;25:71–99. doi: 10.1146/annurev.immunol.25.022106.141602. [DOI] [PubMed] [Google Scholar]
- 28.Mehta SH, Cox A, Hoover DR, Wang XH, Mao Q, Ray S, Strathdee SA, Vlahov D, Thomas DL. Protection against persistence of hepatitis C. Lancet. 2002;359:1478–1483. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- 29.Bassett SE, Guerra B, Brasky K, Miskovsky E, Houghton M, Klimpel GR, Lanford RE. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology. 2001;33:1479–1487. doi: 10.1053/jhep.2001.24371. [DOI] [PubMed] [Google Scholar]
- 30.Major ME, Mihalik K, Puig M, Rehermann B, Nascimbeni M, Rice CM, Feinstone SM. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J Virol. 2002;76:6586–6595. doi: 10.1128/JVI.76.13.6586-6595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G, Abrignani S. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 32.Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, Filocamo G, Traboni C, Nicosia A, Cortese R, Vitelli A. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21:5017–5025. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akazawa D, Date T, Morikawa K, Murayama A, Miyamoto M, Kaga M, Barth H, Baumert TF, Dubuisson J, Wakita T. Cd81 Expression Is Important For Heterogeneous Hcv Permissiveness Of Huh7 Cell Clones. J Virol. 2007;81:5036–5045. doi: 10.1128/JVI.01573-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koutsoudakis G, Kaul A, Steinmann E, Kallis S, Lohmann V, Pietschmann T, Bartenschlager R. Characterization of the early steps of hepatitis C virus infection by using luciferase reporter viruses. J Virol. 2006;80:5308–5320. doi: 10.1128/JVI.02460-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeisel MB, Koutsoudakis G, Schnober EK, Haberstroh A, Blum HE, Cosset F-L, Wakita T, Jaeck D, Doffoel M, Royer C, Soulier E, Schvoerer E, Schuster C, Stoll-Keller F, Bartenschlager R, Pietschmann T, Barth H, Baumert TF. Scavenger receptor BI is a key host factor for Hepatitis C virus infection required for an entry step closely linked to CD81. Hepatology. 2007;46:1722–1731. doi: 10.1002/hep.21994. [DOI] [PubMed] [Google Scholar]
- 36.Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wolk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 37.Meertens L, Bertaux C, Cukierman L, Cormier E, Lavillette D, Cosset FL, Dragic T. The tight junction proteins claudin-1, -6, and -9 are entry cofactors for hepatitis C virus. J Virol. 2008;82:3555–3560. doi: 10.1128/JVI.01977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, de Jong YP, Rice CM. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dreux M, Dao Thi VL, Fresquet J, Guerin M, Julia Z, Verney G, Durantel D, Zoulim F, Lavillette D, Cosset FL, Bartosch B. Receptor complementation and mutagenesis reveal SR-BI as an essential HCV entry factor and functionally imply its intra-and extra-cellular domains. PLoS Pathog. 2009;5:e1000310. doi: 10.1371/journal.ppat.1000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helle F, Dubuisson J. Hepatitis C virus entry into host cells. Cell Mol Life Sci. 2008;65:100–112. doi: 10.1007/s00018-007-7291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Hahn T, Rice CM. Hepatitis C virus entry. J Biol Chem. 2008;283:3689–3693. doi: 10.1074/jbc.R700024200. [DOI] [PubMed] [Google Scholar]
- 42.Lavillette D, Morice Y, Germanidis G, Donot P, Soulier A, Pagkalos E, Sakellariou G, Intrator L, Bartosch B, Pawlotsky JM, Cosset FL. Human serum facilitates hepatitis C virus infection, and neutralizing responses inversely correlate with viral replication kinetics at the acute phase of hepatitis C virus infection. J Virol. 2005;79:6023–6034. doi: 10.1128/JVI.79.10.6023-6034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pestka JM, Zeisel MB, Blaser E, Schurmann P, Bartosch B, Cosset FL, Patel AH, Meisel H, Baumert J, Viazov S, Rispeter K, Blum HE, Roggendorf M, Baumert TF. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci U S A. 2007;104:6025–6030. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dowd KA, Netski DM, Wang XH, Cox AL, Ray SC. Selection Pressure From Neutralizing Antibodies Drives Sequence Evolution During Acute Infection With Hepatitis C Virus. Gastroenterology. 2009;136:2377–2386. doi: 10.1053/j.gastro.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeisel MB, Cosset FL, Baumert TF. Host neutralizing responses and pathogenesis of hepatitis C virus infection. Hepatology. 2008;48:299–307. doi: 10.1002/hep.22307. [DOI] [PubMed] [Google Scholar]
- 46.Zhong J, Gastaminza P, Chung J, Stamataki Z, Isogawa M, Cheng G, McKeating JA, Chisari FV. Persistent hepatitis C virus infection in vitro: coevolution of virus and host. J Virol. 2006;80:11082–11093. doi: 10.1128/JVI.01307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keck ZY, Li SH, Xia J, von Hahn T, Balfe P, McKeating JA, Witteveldt J, Patel AH, Alter H, Rice CM, Foung SK. Mutations in HCV E2 located outside the CD81 binding sites lead to escape from broadly neutralizing antibodies but compromise virus infectivity. J Virol. 2009;83:6149–6160. doi: 10.1128/JVI.00248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, Steenbeke TD, Venturi M, Chaiken I, Fung M, Katinger H, Parren PW, Robinson J, Van Ryk D, Wang L, Burton DR, Freire E, Wyatt R, Sodroski J, Hendrickson WA, Arthos J. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 49.Gal-Tanamy M, Keck ZY, Yi M, McKeating JA, Patel AH, Foung SK, Lemon SM. In vitro selection of a neutralization-resistant hepatitis C virus escape mutant. Proc Natl Acad Sci U S A. 2008;105:19450–19455. doi: 10.1073/pnas.0809879105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diepolder HM, Zachoval R, Hoffmann RM, Wierenga EA, Santantonio T, Jung MC, Eichenlaub D, Pape GR. Possible mechanism involving T-lymphocyte response to nonstructural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006–1007. doi: 10.1016/s0140-6736(95)91691-1. [DOI] [PubMed] [Google Scholar]
- 51.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Hahn T, Yoon JC, Alter H, Rice CM, Rehermann B, Balfe P, McKeating JA. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132:667–678. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 53.Zhang P, Wu CG, Mihalik K, Virata-Theimer ML, Yu MY, Alter HJ, Feinstone SM. Hepatitis C virus epitope-specific neutralizing antibodies in Igs prepared from human plasma. Proc Natl Acad Sci U S A. 2007;104:8449–8454. doi: 10.1073/pnas.0703039104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang P, Zhong L, Struble EB, Watanabe H, Kachko A, Mihalik K, Virata-Theimer ML, Alter HJ, Feinstone S, Major M. Depletion of interfering antibodies in chronic hepatitis C patients and vaccinated chimpanzees reveals broad cross-genotype neutralizing activity. Proc Natl Acad Sci U S A. 2009;106:7537–7541. doi: 10.1073/pnas.0902749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bowen DG, Shoukry NH, Grakoui A, Fuller MJ, Cawthon AG, Dong C, Hasselschwert DL, Brasky KM, Freeman GJ, Seth NP, Wucherpfennig KW, Houghton M, Walker CM. Variable patterns of programmed death-1 expression on fully functional memory T cells after spontaneous resolution of hepatitis C virus infection. J Virol. 2008;82:5109–5114. doi: 10.1128/JVI.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 57.Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 58.Schulze zur Wiesch J, Lauer GM, Day CL, Kim AY, Ouchi K, Duncan JE, Wurcel AG, Timm J, Jones AM, Mothe B, Allen TM, McGovern B, Lewis-Ximenez L, Sidney J, Sette A, Chung RT, Walker BD. Broad repertoire of the CD4+ Th cell response in spontaneously controlled hepatitis C virus infection includes dominant and highly promiscuous epitopes. J Immunol. 2005;175:3603–3613. doi: 10.4049/jimmunol.175.6.3603. [DOI] [PubMed] [Google Scholar]
- 59.Smyk-Pearson S, Tester IA, Lezotte D, Sasaki AW, Lewinsohn DM, Rosen HR. Differential antigenic hierarchy associated with spontaneous recovery from hepatitis C virus infection: implications for vaccine design. J Infect Dis. 2006;194:454–463. doi: 10.1086/505714. [DOI] [PubMed] [Google Scholar]
- 60.Gerlach JT, Diepolder HM, Jung MC, Gruener NH, Schraut WW, Zachoval R, Hoffmann R, Schirren CA, Santantonio T, Pape GR. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933–941. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 61.Ferrari C, Valli A, Galati L, Penna A, Scaccaglia P, Giuberti T, Schianchi C, Missale G, Marin MG, Fiaccadori F. T-cell response to structural and nonstructural hepatitis C virus antigens in persistent and self-limited hepatitis C virus infections. Hepatology. 1994;19:286–295. [PubMed] [Google Scholar]
- 62.Wertheimer AM, Miner C, Lewinsohn DM, Sasaki AW, Kaufman E, Rosen HR. Novel CD4+ and CD8+ T-cell determinants within the NS3 protein in subjects with spontaneously resolved HCV infection. Hepatology. 2003;37:577–589. doi: 10.1053/jhep.2003.50115. [DOI] [PubMed] [Google Scholar]
- 63.Aberle JH, Formann E, Steindl-Munda P, Weseslindtner L, Gurguta C, Perstinger G, Grilnberger E, Laferl H, Dienes HP, Popow-Kraupp T, Ferenci P, Holzmann H. Prospective study of viral clearance and CD4(+) T-cell response in acute hepatitis C primary infection and reinfection. J Clin Virol. 2006;36:24–31. doi: 10.1016/j.jcv.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 64.Cox AL, Mosbruger T, Lauer GM, Pardoll D, Thomas DL, Ray SC. Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology. 2005;42:104–112. doi: 10.1002/hep.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smyk-Pearson S, Tester IA, Klarquist J, Palmer BE, Pawlotsky JM, Golden-Mason L, Rosen HR. Spontaneous recovery in acute human hepatitis C virus infection: functional T-cell thresholds and relative importance of CD4 help. J Virol. 2008;82:1827–1837. doi: 10.1128/JVI.01581-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Folgori A, Spada E, Pezzanera M, Ruggeri L, Mele A, Garbuglia AR, Perrone MP, Del Porto P, Piccolella E, Cortese R, Nicosia A, Vitelli A. Early impairment of hepatitis C virus specific T cell proliferation during acute infection leads to failure of viral clearance. Gut. 2006;55:1012–1019. doi: 10.1136/gut.2005.080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Urbani S, Amadei B, Fisicaro P, Tola D, Orlandini A, Sacchelli L, Mori C, Missale G, Ferrari C. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006;44:126–139. doi: 10.1002/hep.21242. [DOI] [PubMed] [Google Scholar]
- 68.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 69.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 70.Chang KM, Thimme R, Melpolder JJ, Oldach D, Pemberton J, Moorhead-Loudis J, McHutchison JG, Alter HJ, Chisari FV. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267–276. doi: 10.1053/jhep.2001.21162. [DOI] [PubMed] [Google Scholar]
- 71.Weiner AJ, Paliard X, Selby MJ, Medina-Selby A, Coit D, Nguyen S, Kansopon J, Arian CL, Ng P, Tucker J, Lee CT, Polakos NK, Han J, Wong S, Lu HH, Rosenberg S, Brasky KM, Chien D, Kuo G, Houghton M. Intrahepatic genetic inoculation of hepatitis C virus RNA confers cross-protective immunity. J Virol. 2001;75:7142–7148. doi: 10.1128/JVI.75.15.7142-7148.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nascimbeni M, Mizukoshi E, Bosmann M, Major ME, Mihalik K, Rice CM, Feinstone SM, Rehermann B. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J Virol. 2003;77:4781–4793. doi: 10.1128/JVI.77.8.4781-4793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lanford RE, Guerra B, Chavez D, Bigger C, Brasky KM, Wang XH, Ray SC, Thomas DL. Cross-genotype immunity to hepatitis C virus. J Virol. 2004;78:1575–1581. doi: 10.1128/JVI.78.3.1575-1581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bukh J, Thimme R, Meunier JC, Faulk K, Spangenberg HC, Chang KM, Satterfield W, Chisari FV, Purcell RH. Previously infected chimpanzees are not consistently protected against reinfection or persistent infection after reexposure to the identical hepatitis C virus strain. J Virol. 2008;82:8183–8195. doi: 10.1128/JVI.00142-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bassett SE, Thomas DL, Brasky KM, Lanford RE. Viral persistence, antibody to E1 and E2, and hypervariable region 1 sequence stability in hepatitis C virus-inoculated chimpanzees. J Virol. 1999;73:1118–1126. doi: 10.1128/jvi.73.2.1118-1126.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thimme R, Lohmann V, Weber F. A target on the move: innate and adaptive immune escape strategies of hepatitis C virus. Antiviral Res. 2006;69:129–141. doi: 10.1016/j.antiviral.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 78.Kaplan DE, Sugimoto K, Newton K, Valiga ME, Ikeda F, Aytaman A, Nunes FA, Lucey MR, Vance BA, Vonderheide RH, Reddy KR, McKeating JA, Chang KM. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology. 2007;132:654–666. doi: 10.1053/j.gastro.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 79.Welsh RM, Kim SK, Cornberg M, Clute SC, Selin LK, Naumov YN. The privacy of T cell memory to viruses. Curr Top Microbiol Immunol. 2006;311:117–153. doi: 10.1007/3-540-32636-7_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prlic M, Lefrancois L, Jameson SC. Multiple choices: regulation of memory CD8 T cell generation and homeostasis by interleukin (IL)-7 and IL-15. J Exp Med. 2002;195:F49–52. doi: 10.1084/jem.20020767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Leeuwen EM, de Bree GJ, Remmerswaal EB, Yong SL, Tesselaar K, ten Berge IJ, van Lier RA. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood. 2005;106:2091–2098. doi: 10.1182/blood-2005-02-0449. [DOI] [PubMed] [Google Scholar]
- 83.Boettler T, Panther E, Bengsch B, Nazarova N, Spangenberg HC, Blum HE, Thimme R. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J Virol. 2006;80:3532–3540. doi: 10.1128/JVI.80.7.3532-3540.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lacombe MH, Hardy MP, Rooney J, Labrecque N. IL-7 receptor expression levels do not identify CD8+ memory T lymphocyte precursors following peptide immunization. J Immunol. 2005;175:4400–4407. doi: 10.4049/jimmunol.175.7.4400. [DOI] [PubMed] [Google Scholar]
- 85.Klonowski KD, Williams KJ, Marzo AL, Lefrancois L. Cutting edge: IL-7-independent regulation of IL-7 receptor alpha expression and memory CD8 T cell development. J Immunol. 2006;177:4247–4251. doi: 10.4049/jimmunol.177.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kieper WC, Tan JT, Bondi-Boyd B, Gapin L, Sprent J, Ceredig R, Surh CD. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J Exp Med. 2002;195:1533–1539. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 91.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 92.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Golden-Mason L, Burton JR, Jr, Castelblanco N, Klarquist J, Benlloch S, Wang C, Rosen HR. Loss of IL-7 receptor alpha-chain (CD127) expression in acute HCV infection associated with viral persistence. Hepatology. 2006;44:1098–1109. doi: 10.1002/hep.21365. [DOI] [PubMed] [Google Scholar]
- 95.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, Altman JD, Rouse BT, Freeman GJ, Ahmed R, Grakoui A. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bengsch B, Spangenberg HC, Kersting N, Neumann-Haefelin C, Panther E, von Weizsacker F, Blum HE, Pircher H, Thimme R. Analysis of CD127 and KLRG1 expression on hepatitis C virus-specific CD8+ T cells reveals the existence of different memory T-cell subsets in the peripheral blood and liver. J Virol. 2007;81:945–953. doi: 10.1128/JVI.01354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Urbani S, Boni C, Missale G, Elia G, Cavallo C, Massari M, Raimondo G, Ferrari C. Virus-specific CD8+ lymphocytes share the same effector-memory phenotype but exhibit functional differences in acute hepatitis B and C. J Virol. 2002;76:12423–12434. doi: 10.1128/JVI.76.24.12423-12434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Spangenberg HC, Viazov S, Kersting N, Neumann-Haefelin C, McKinney D, Roggendorf M, von Weizsacker F, Blum HE, Thimme R. Intrahepatic CD8+ T-cell failure during chronic hepatitis C virus infection. Hepatology. 2005;42:828–837. doi: 10.1002/hep.20856. [DOI] [PubMed] [Google Scholar]
- 99.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 100.Ulsenheimer A, Gerlach JT, Gruener NH, Jung MC, Schirren CA, Schraut W, Zachoval R, Pape GR, Diepolder HM. Detection of functionally altered hepatitis C virus-specific CD4 T cells in acute and chronic hepatitis C. Hepatology. 2003;37:1189–1198. doi: 10.1053/jhep.2003.50194. [DOI] [PubMed] [Google Scholar]
- 101.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 102.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 103.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rutebemberwa A, Ray SC, Astemborski J, Levine J, Liu L, Dowd KA, Clute S, Wang C, Korman A, Sette A, Sidney J, Pardoll DM, Cox AL. High-programmed death-1 levels on hepatitis C virus-specific T cells during acute infection are associated with viral persistence and require preservation of cognate antigen during chronic infection. J Immunol. 2008;181:8215–8225. doi: 10.4049/jimmunol.181.12.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 106.Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437–1448. doi: 10.1016/j.hep.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 107.Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–1071. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 108.Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, Blum HE, von Weizsacker F, Thimme R. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860–7867. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rushbrook SM, Ward SM, Unitt E, Vowler SL, Lucas M, Klenerman P, Alexander GJ. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852–7859. doi: 10.1128/JVI.79.12.7852-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Manigold T, Shin EC, Mizukoshi E, Mihalik K, Murthy KK, Rice CM, Piccirillo CA, Rehermann B. Foxp3+CD4+CD25+ T cells control virus-specific memory T cells in chimpanzees that recovered from hepatitis C. Blood. 2006;107:4424–4432. doi: 10.1182/blood-2005-09-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chang KM, Rehermann B, McHutchison JG, Pasquinelli C, Southwood S, Sette A, Chisari FV. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J Clin Invest. 1997;100:2376–2385. doi: 10.1172/JCI119778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weiner A, Erickson AL, Kansopon J, Crawford K, Muchmore E, Hughes AL, Houghton M, Walker CM. Persistent hepatitis C virus infection in a chimpanzee is associated with emergence of a cytotoxic T lymphocyte escape variant. Proc Natl Acad Sci U S A. 1995;92:2755–2759. doi: 10.1073/pnas.92.7.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Erickson AL, Kimura Y, Igarashi S, Eichelberger J, Houghton M, Sidney J, McKinney D, Sette A, Hughes AL, Walker CM. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity. 2001;15:883–895. doi: 10.1016/s1074-7613(01)00245-x. [DOI] [PubMed] [Google Scholar]
- 114.Cox AL, Mosbruger T, Mao Q, Liu Z, Wang XH, Yang HC, Sidney J, Sette A, Pardoll D, Thomas DL, Ray SC. Cellular immune selection with hepatitis C virus persistence in humans. J Exp Med. 2005;201:1741–1752. doi: 10.1084/jem.20050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tester I, Smyk-Pearson S, Wang P, Wertheimer A, Yao E, Lewinsohn DM, Tavis JE, Rosen HR. Immune evasion versus recovery after acute hepatitis C virus infection from a shared source. J Exp Med. 2005;201:1725–1731. doi: 10.1084/jem.20042284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Timm J, Lauer GM, Kavanagh DG, Sheridan I, Kim AY, Lucas M, Pillay T, Ouchi K, Reyor LL, Schulze zur Wiesch J, Gandhi RT, Chung RT, Bhardwaj N, Klenerman P, Walker BD, Allen TM. CD8 epitope escape and reversion in acute HCV infection. J Exp Med. 2004;200:1593–1604. doi: 10.1084/jem.20041006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ray SC, Fanning L, Wang XH, Netski DM, Kenny-Walsh E, Thomas DL. Divergent and convergent evolution after a common-source outbreak of hepatitis C virus. J Exp Med. 2005;201:1753–1759. doi: 10.1084/jem.20050122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Neumann-Haefelin C, McKiernan S, Ward S, Viazov S, Spangenberg HC, Killinger T, Baumert TF, Nazarova N, Sheridan I, Pybus O, von Weizsacker F, Roggendorf M, Kelleher D, Klenerman P, Blum HE, Thimme R. Dominant influence of an HLA-B27 restricted CD8+ T cell response in mediating HCV clearance and evolution. Hepatology. 2006;43:563–572. doi: 10.1002/hep.21049. [DOI] [PubMed] [Google Scholar]
- 119.Uebelhoer L, Han JH, Callendret B, Mateu G, Shoukry NH, Hanson HL, Rice CM, Walker CM, Grakoui A. Stable cytotoxic T cell escape mutation in hepatitis C virus is linked to maintenance of viral fitness. PLoS Pathog. 2008;4:e1000143. doi: 10.1371/journal.ppat.1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Timm J, Li B, Daniels MG, Bhattacharya T, Reyor LL, Allgaier R, Kuntzen T, Fischer W, Nolan BE, Duncan J, Schulze zur Wiesch J, Kim AY, Frahm N, Brander C, Chung RT, Lauer GM, Korber BT, Allen TM. Human leukocyte antigen-associated sequence polymorphisms in hepatitis C virus reveal reproducible immune responses and constraints on viral evolution. Hepatology. 2007;46:339–349. doi: 10.1002/hep.21702. [DOI] [PubMed] [Google Scholar]