Abstract
Jaagsiekte sheep retrovirus (JSRV) is the causative agent of ovine pulmonary carcinoma, a unique animal model for human bronchioalveolar carcinoma. We previously isolated a JSRV proviral clone and showed that it was both infectious and oncogenic. Thus JSRV is necessary and sufficient for the development of ovine pulmonary carcinoma, but no data are available on the mechanisms of transformation. Inspection of the JSRV genome reveals standard retroviral genes, but no evidence for a viral oncogene. However, an alternate ORF in pol (orf-x) might be a candidate for a transforming gene. We tested whether the JSRV genome might encode a transforming gene by transfecting an expression plasmid for JSRV [pCMVJS21, driven by the cytomegalovirus (CMV) immediate early promoter] into mouse NIH 3T3 cells. Foci of transformed cells appeared in the transfected cultures 2–3 weeks posttransfection; cloned transformants showed anchorage independence for growth, and they expressed JSRV RNA. These results indicate that the JRSV genome contains information with direct transforming potential for NIH 3T3 cells. Transfection of a mutated version of pCMVJS21 in which the orf-x protein was terminated by two stop codons also gave transformed foci. Thus, orf-x was eliminated as the candidate transforming gene. In addition, another derivative of pCMVJS21 (pCMVJS21ΔGP) in which the gag, pol (and orf-x) coding sequences were deleted also gave transformed foci. These results indicate that the envelope gene carries the transforming potential. This is an unusual example of a native retroviral structural protein with transformation potential.
Animal retrovirus-induced cancers have played fundamental roles in understanding the molecular basis of cancer (1). Jaagsiekte sheep retrovirus (JSRV) is the causative agent of ovine pulmonary carcinoma (OPC), a contagious lung cancer of sheep (also known as sheep pulmonary adenomatosis or SPA) (2–5). JSRV-induced OPC consists of transformed secretory epithelial cells of the lungs: type II pneumocytes and Clara cells. A characteristic feature of OPC tumors is the production of large amounts of fluid secreted from the tumor cells (containing infectious virus). OPC closely resembles human bronchiolo alveolar carcinoma (BAC), an adenocarcinoma not associated with cigarette smoking and whose etiology is currently unknown (6). Thus OPC is an important model for understanding human BAC pathogenesis.
Oncogenic retroviruses induce tumors by two mechanisms. Acutely transforming retroviruses (typically replication-defective) have captured a normal cell gene (a protooncogene) and converted it into a viral oncogene. Acutely transforming retroviruses typically induce rapid neoplasms in vivo, and they frequently can transform cells in culture (7, 8). Retroviruses that lack oncogenes also can induce tumors (nonacute retroviruses), although they typically require longer incubation periods and multiple rounds of infection in vivo (9). An important molecular mechanism for these viruses is insertional activation of protooncogenes: insertion of a provirus in the vicinity of a cellular protooncogene. This results in overexpression of the protooncogenes due to highly active transcriptional regulatory elements in the retroviral long terminal repeat (LTR).
We recently obtained an infectious molecular clone of JSRV (JSRV 21) and used virus recovered from it to demonstrate that JSRV is necessary and sufficient to induce OPC in sheep (2). However the mechanism of oncogenesis by JSRV has not been clear. Examination of the nucleotide sequence of the JSRV genome indicated the presence of typical structural genes for a retrovirus (gag, pro, pol, and env), but there were no additional genes with obvious characteristics of a viral oncogene (e.g., homology to a cellular protooncogene). However, JSRV does contain an additional alternate ORF (orf-x) overlapping pol whose significance is unknown (2, 5, 10, 11). Thus JSRV has the genome organization more typical of a nonacute retrovirus. On the other hand, when JSRV is used to experimentally induce OPC in newborn lambs, multifocal tumor lesions appear quite rapidly (4–6 weeks) (12, 13). This disease pattern is more typical of acute transforming retroviruses.
In this study, we tested the hypothesis that JSRV contains a gene with oncogenic potential. We report that JSRV DNA can induce the foci of transformation when transfected into murine NIH 3T3 cells. Additional experiments localized the transforming activity to the viral env gene.
Materials and Methods
Plasmids.
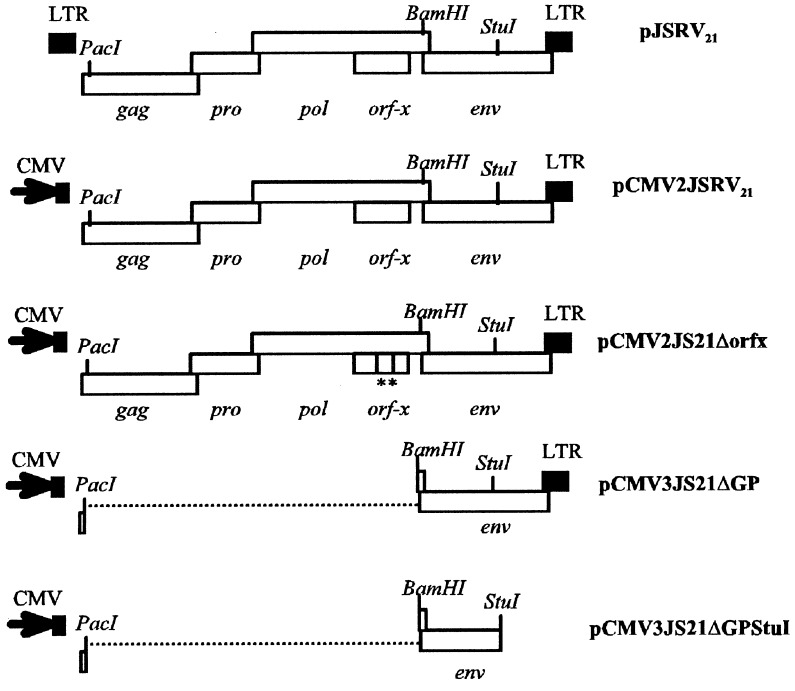
The schematic organization of the plasmids used in this study is shown in Fig. 1. pJSRV21 and pCMV2JS21 have been described (2). Briefly, pJSRV21 is an infectious and oncogenic molecular clone of JSRV; in pCMV2JS21 the U3 region in the upstream LTR has been replaced by the cytomegalovirus (CMV) immediate early promoter. pCMV2JS21Δorfx was obtained by the introduction of two stop codons in the orf-x reading frame of pCMV2JS21 (TTA to TAA at position 4821 and TTA to TAA at position 4952 with respect to the JSRV21 sequence; ref. 2). These mutations leave the amino acids in the overlapping pol reading frame unaltered. Plasmid pCMV3JS21ΔGP was obtained by digesting pCMV3JS21 with PacI and BamHI and religating the plasmid after a standard filling-in reaction (14). PacI is immediately downstream from the splice donor for env whereas BamHI is immediately upstream from the env splice acceptor; thus pCMV3JS21ΔGP expresses the env gene. pCMV3JS21 is identical to pCMV2JS21 with the exception of the removal of some restriction sites in the multiple cloning site of the backbone to facilitate cloning. pCMV3JS21ΔGPΔStuI was obtained from pCMV3JS21ΔGP by excision of the StuI fragment encompassing the transmembrane region of the JSRV envelope and the downstream LTR. pcDNA3.1(−) (Invitrogen) was used as a negative control in the transformation assays (see below). The plasmid pHR-IR-MuLV contained a Moloney murine leukemia virus (M-MuLV)-based vector expressing an activated human H-ras gene (from the T24 bladder carcinoma). The H-ras gene was downstream from an encephalomyocarditis virus internal ribosome entry site, and expression was under control of the M-MuLV LTR (W. Hsiao and H.F., unpublished work).
Figure 1.
Plasmids used in this study. They are described in Materials and Methods. The restriction sites used for cloning are indicated. The two stop codons in the orf-x reading frame of pCMV2JS21Δorfx are shown as vertical bars underlined by asterisks. The broken lines in pCMV3JS21ΔGP and pCMV3JS21ΔGPΔStuI indicate deletions.
Cell Culture.
Human 293T cells (15) were grown in DMEM-10% FBS. Mouse NIH 3T3 cells (obtained by R. Weinberg, Massachusetts Institute of Technology, Cambridge) were grown in DMEM-10% calf serum. Both cell lines were grown in an incubator at 37°C with 5% CO2. The OHH1 cell line (derived from deer lung) was obtained from the ATCC (CRL-6195) and was grown in DMEM-10% FBS.
RNA Extraction and Northern Blotting.
To analyze the pattern of expression of the plasmid constructs used in this study, 293T cells (≈1 × 106 cells per 10-cm plate) were transfected with 28 μg of plasmid DNA and the CalPhos mammalian transfection kit (CLONTECH) as recommended by the manufacturer. Forty-eight hours after transfection, total RNA was extracted by using a RNAqueous-Midi kit (Ambion, Austin, TX). RNA was extracted in the same way from JSRV-infected OHH1 cells, 3T3 transformed clones (see below), or lung tumors of OPC-affected animals (provided by J. M. Sharp, Moredun Research Institute, Penicuik, Scotland). RNA preparations were treated with RNase-free DNase (Qiagen, Chatsworth, CA). Six to 10 micrograms of total RNA was denatured with glyoxal/DMSO and run in a 1% agarose gel in 10 mM sodium phosphate and blotted to nylon membranes (Hybond-N Plus, Amersham Pharmacia) by using established methods (14). Membranes were hybridized to 32P-labeled JSRV env probes corresponding to the U3 region or to nucleotides 5347–5530 of the JSRV21 sequence (env2) or to nucleotides 6329–6641 (env-up) and subjected to autoradiography by exposure to x-ray film.
Transformation Assays.
NIH 3T3 cells (3 × 105 per 10-cm tissue culture plate) were transfected with 28 μg of plasmid DNA as above. Independent experiments were performed with at least two different plasmid preps for each construct. Approximately 12 h after transfection, cells were washed three times with PBS, and fresh medium was added. Cells were maintained in culture for 4–5 weeks with the media replaced every 3 days and monitored microscopically. Foci of transformed cells were counted 1 month after transfection. In parallel experiments, transfected or mock-transfected cells were passaged every 3 days and checked for changes in their morphology.
Colony Formation Assays.
Cultures of JSRV-transformed NIH 3T3 cells that had been serially passaged were seeded in agar suspension (104 cells per 6cm dish) as described (16). NIH 3T3 nontransformed cells were seeded in parallel. The cultures were incubated for 4 weeks, and colonies were counted in a microscope at low magnification. The diameters of 100 colonies with a diameter greater than 100 μm were determined with a measuring reticle in the microscope.
Establishment of NIH 3T3 Transformed Cell Lines.
Single cell clones of transformed NIH 3T3 cells were obtained by dilution of pCMV2JS21 transfected NIH 3T3 cells and growth into colonies on monolayers followed by selective trypsinization of transformed colonies. Alternatively, single colonies were picked from agar colony formation assays. DNA from 11 clones was extracted by using a Dneasy Tissue kit (Qiagen). The presence of JSRV DNA and RNA was confirmed by PCR for the JSRV LTR (17), Southern or Northern blotting analysis using JSRV-specific probes.
Infection of OHH1 with JSRV21 and Reverse Transcription (RT)–PCR.
A total of 2 × 105 OHH1 cells were plated in 5-cm tissue culture dishes and infected with JSRV21 virus as described (18). Infectious JSRV was obtained by transfecting 293T cells with pCMV2JS21 as described (2). Two micrograms of total RNA from OHH1 cells at the 11th passage postinfection, control OHH1 cells infected with heat-inactivated virus, or 293T cells transfected with pCMV2JS21 were subjected to a RT reaction primed with oligo(dT) using Omniscript (Quiagen) as recommended by the manufacturer. Three microliters of this reaction was amplified by using an oligo(dT) primer as the reverse primer and a forward primer designed in the JSRV untranslated gag region before the splice donor site (TTCTCTAGAGGGCTCGAGCTCGACAGTTTTC). The resulting PCR products were run in a 1% agarose gel and visualized under a UV transilluminator. The bands of interest were excised from the agarose gel, and the DNA was purified by using QIAquick Gel Extraction kit (Qiagen) and cloned into pBlueScript (Stratagene) as recommended by the manufacturer. Plasmids corresponding to PCR bands from the OHH1-infected cells and 293T-transfected cells were sequenced on an Applied Biosystems Prism 310 Genetic Analyzer (Perkin–Elmer), using a BigDye Terminator DNA cycle sequencing kit (Perkin–Elmer Applied Biosystems) as recommended by the manufacturer.
Results
JSRV DNA Transforms NIH 3T3 Cells.
To test the hypothesis that JSRV might carry genetic information with direct oncogenic potential, we performed a series of in vitro transformation assays on mouse NIH 3T3 cells using plasmid DNA corresponding to the full-length JSRV proviral genome. Initially, we used two constructs: pJSRV21 and pCMV2JS21 (2) (Fig. 1). They both contain the entire JSRV21 coding sequences and differ in the promoter/enhancer regions. In pJSRV21, expression is driven by the JSRV LTR whereas in pCMV2JS21 expression is driven by the CMV immediate-early promoter (2). NIH 3T3 cells were transfected with pJSRV21 and pCMV2JS21 and maintained in culture for 4 weeks. Negative controls included NIH 3T3 cells transfected with pcDNA3.1(−) or mock-transfected cells. pcDNA3.1(−) was chosen as negative control because it contains the same CMV immediate early promoter as in pCMV2JS21, but it does not contain JSRV sequences. Experiments were done independently five times with at least two different plasmid preps.
In seven of seven experiments (see experiments 1–7 in Table 1) pCMV2JS21 was able to induce foci of cell transformation (Fig. 2 A and B). The transformed foci became visible 2 weeks after transfection. Transformed cells were recognizable by their fusiform shape and the loss of contact inhibition, resulting in the formation of foci of cells piling on top of each other. By 4 weeks after transfection, foci assumed a characteristic morphology with the transformed cells radiating from the center of the focus (Fig. 2B); foci were generally visible to the naked eye at this stage. Transfection of 3 × 105 NIH 3T3 cells with 28 μg pCMV2JS21 DNA resulted in the formation of an average of 16.2 foci per experiment (SD = ±3.7) counted at 28 days posttransfection. No foci were observed in the mock-transfected 3T3 cells nor in the 3T3 cells transfected with pcDNA3.1(−). pJSRV21 induced 0–4 foci in five experiments, indicative of significantly lower transformation efficiency. This finding was not surprising, because we previously showed that the JSRV LTR is a weak promoter/enhancer in NIH 3T3 cells (19).
Table 1.
Transformation assays performed in this study
| Experiment | DNA(−) | pcDNA3.1(−) | pJSRV21 | pCMV2JS21 | pCMV2JS21Δorfx | pCMV3JS21ΔGP | PCMV3JS21ΔGPΔStuI | pHR-IR-MuLV (H-ras) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 15 | 14 | N.T. | N.T. | N.T. |
| 2 | 0 | 0 | 4 | 11 | 12 | N.T. | N.T. | N.T. |
| 3 | 0 | 0 | 1 | 16 | 12 | N.T. | N.T. | N.T. |
| 4 | 0 | 0 | 0 | 21 | 22 | N.T. | N.T. | N.T. |
| 5 | 0 | N.T. | 1 | 18 | 14 | N.T. | N.T. | N.T. |
| 6 | 0 | 0 | N.T. | 22 | N.T. | 31 | 0 | N.T. |
| 7 | 0 | 0 | N.T. | 11 | N.T. | 23 | 0 | N.T. |
| 8 | 0 | 0 | N.T. | N.T. | N.T. | 15 | N.T. | 25 |
| 9 | 0 | 0 | N.T. | N.T. | N.T. | 13 | N.T. | 28 |
| 10 | 0 | 0 | N.T. | N.T. | N.T. | 18 | N.T. | 44 |
NIH-3T3 were transfected with the above indicated plasmids as described in Materials and Methods. Numbers indicate the number of foci counted at 28 days posttransfection. N.T. = not tested.
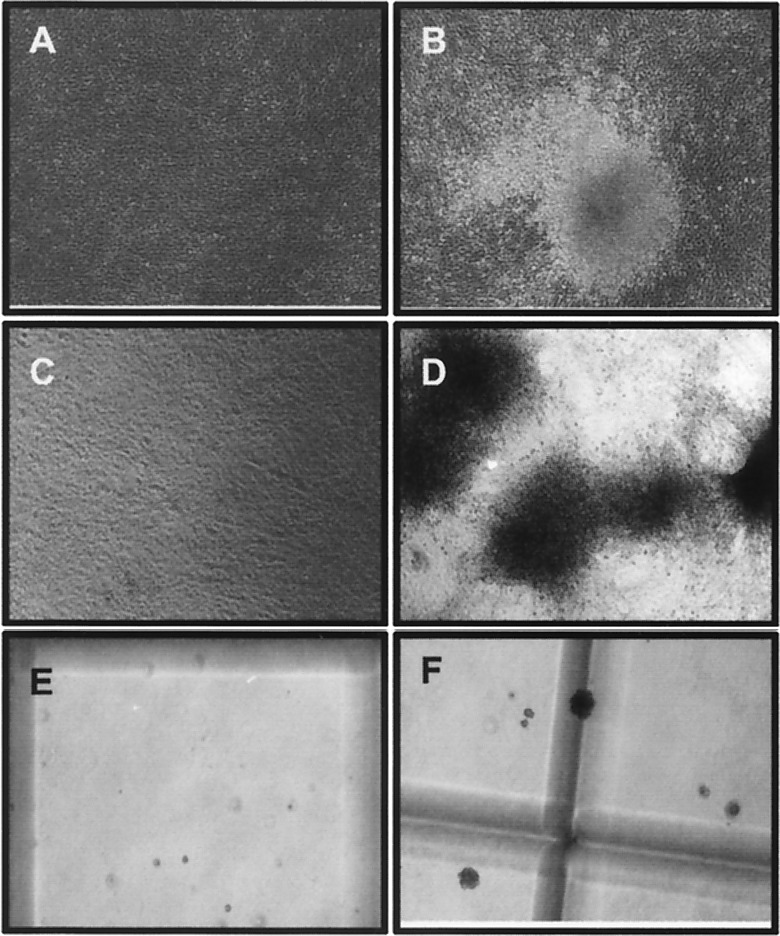
Figure 2.
Transformation of NIH 3T3 cells. NIH 3T3 cells were transfected with 28 μg of pCDNA3.1(−) (A) or pCMV2JS21 (B) and maintained in culture for 28 days. A typical focus of transformed cells is shown in B. In parallel experiments, transfected cells were passaged every 3–4 days. After 6 weeks, the cells transfected with pCDNA3.1(−) (C) had the typical appearance of NIH 3T3 cells, whereas the cells transfected with pCMV2JS21 (D) were morphologically altered, with loss of contact inhibition. pCDNA3.1(−)-transfected NIH 3T3 cells did not form large colonies in soft agar (E) whereas NIH 3T3 cells transformed by pCMV2JS21 showed anchorage independent colonies (F). (Magnification: ×40.)
In parallel experiments, pCMV2JS21-transfected cells were passaged every 3–4 days after transfection. In these cases, the transformed cells were gradually selected and large foci of transformed cells eventually occupied most of the plate by ≈6 weeks posttransfection (Fig. 2 C and D). These cells showed anchorage-independent properties as witnessed by their capacity to grow in soft agar, forming colonies with an average diameter of 440 μm at 4 weeks. The parental untransformed NIH 3T3 cells did not show any large colonies over 150 μm (Fig. 2 E and F). JSRV DNA also transformed the Rat 6 cell line (16) at low efficiency; in agar colony assays, a cloned Rat 6 transformant formed large colonies (>200 μm) with an efficiency of 23%, whereas parental Rat 6 cells remained as single cells (not shown).
We established 11 transformed cell lines from pCMV2JS21-transfected cultures by single-cell cloning from colonies grown in soft agar or by limiting dilution and picking individual transformed colonies. All 11 clones contained JSRV plasmid DNA as established by JSRV LTR PCR (17) or Southern blotting (data not shown), and they contained JSRV RNA as assessed by Northern blotting (see below). The results obtained indicated that one of the JSRV genes is able to transform NIH 3T3 cells in vitro.
The JSRV orf-x Is Not Responsible for Transformation.
We next investigated JSRV orf-x as the possible transforming gene. Orf-x is an ORF overlapping pol that is conserved among different JSRV isolates (10, 11) but has no obvious homologues in other related type D and B retroviruses. To test the role of this gene in transformation, we mutated pCMV2JS21 by the insertion of two stop codons in the orf-x ORF; these stop codons do not mutate the overlapping pol amino acid sequences. The resulting plasmid, called pCMV2JS21Δorf-x (Fig. 1), was used in transformation assays as above. pCMV2JS21Δorf-x was able to transform NIH 3T3 cells with the same efficiency as pCMV2JS21; the average number of foci obtained in five experiments was 14.4 (±4.1) (Table 1). Thus we concluded that the JSRV orf-x is not directly involved in cell transformation.
Expression of the JSRV Envelope Is Sufficient to Induce Cell Transformation.
Because the JSRV orf-x was not responsible for transformation, we next tested whether the env gene was responsible. We constructed a plasmid derived from pCMV2JS21 where gag, pro, pol (and consequently orf-x) were deleted. The only intact gene of pCMV2JS21 was env; both the env splice donor and splice acceptor sites were left intact. The resulting plasmid, pCMV3JS21ΔGP, was able to induce transformation of NIH 3T3 cells. In two independent experiments (Table 1, experiments 6 and 7), pCMV3JS21ΔGP induced the formation of 31 and 23 foci, respectively. Thus, the expression of the JSRV envelope gene is sufficient to induce transformation of NIH 3T3 cells. When compared with a plasmid expressing the activated c-ras protooncogene from human T24 bladder carcinoma cells driven by a murine leukemia virus LTR (pHR-IR-MuLV), pCMV3JS21ΔGP induced foci with similar (ca. 2-fold less) efficiency (Table 1, experiments 8–10).
Analysis of env Transcripts.
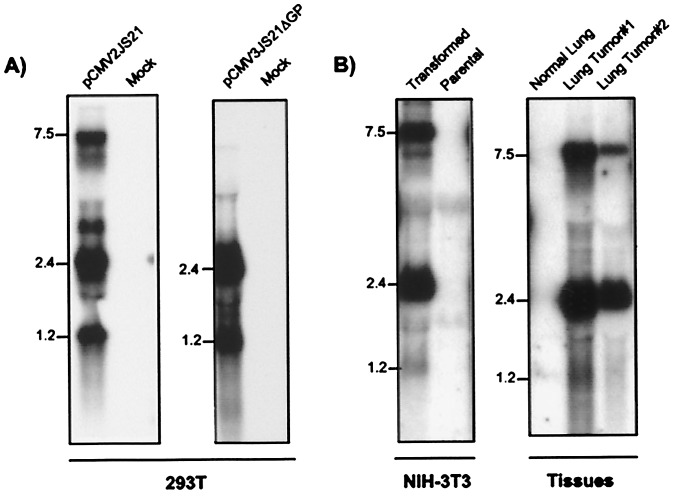
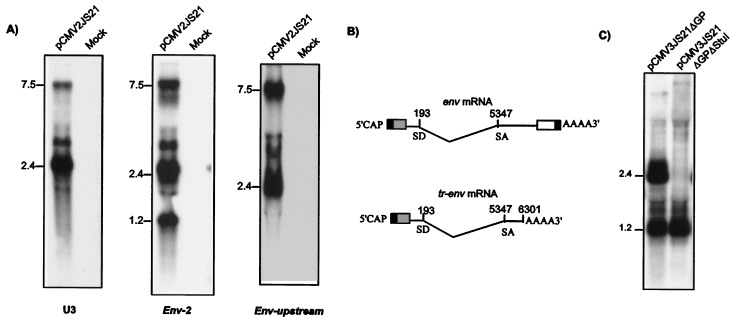
To characterize the JSRV env gene, we performed Northern blotting of RNA from human 293T cells transiently transfected with either pCMV2JS21 or pCMV3JS21Δorf-x, NIH 3T3 cell lines transformed by JSRV, and tumors of OPC-affected sheep. We previously used transfected 293T cells to produce large amounts of infectious JSRV (2) because they very efficiently express transiently transfected plasmid DNA. Filters were hybridized with a probe corresponding to the first 183 bp of the envelope gene (env2). Three major bands were detected in 293T cells transfected with pCMV2JS21, in 3T3 transformed clones, and in OPC tumors (Fig. 3). As expected, we detected JSRV-specific RNA of about 7.5 kb corresponding to the full-length JSRV viral genome and a transcript of about 2.5 kb corresponding to the spliced env mRNA. A third band of ≈1.2 kb also was observed. In 293T cells transfected with pCMV3JS21ΔGP, the 2.5- and the 1.2-kb bands were present. The 1.2-kb transcript was very abundant (approximately the same intensity of the 2.4-kb env transcript) in transfected 293T cells, while it was present at lower but detectable levels in 3T3 transformed clones and in OPC tumors. To identify the structure of the 1.2-kb band (tr-env for truncated env) Northern blots were hybridized in parallel with the env2 probe, a JSRV U3 probe, and another env probe corresponding to nucleotides 6329–6641 (env-upstream) (Fig 4A). The 7.5-kb and 2.5-kb bands were observed with all three probes. However the tr-env band was observed only with the env2 probe. It was particularly surprising that the JSRV U3 probe did not hybridize with the tr-env RNA, because U3 is typically present in all of the retroviral transcripts. These data suggested that tr-env RNA might be prematurely polyadenylated.
Figure 3.
JSRV env transcripts. (A) Northern blotting of total RNA collected from 293T cells transfected with pCMV2JS21, pCMV3JS21ΔGP, and mock-transfected 293T cells. Filters were hybridized with the env-2 probe as described in Materials and Methods. 293T cells transfected with pCMV3JS21ΔGP lack the 7.5-kb band (corresponding to the full-length JSRV genome) because the gag and pol sequences have been deleted. (B) Northern blotting of total RNA from NIH 3T3 cells transformed by pCMV2JS21, parental NIH 3T3 cells, lung from a healthy sheep, and lung from tumor lesions of two sheep affected by OPC. Both the 7.5- and 2.4-kb transcripts are readily visible whereas the 1.2-kb transcript is present at a low level.
Figure 4.
Identification of JSRV env transcripts. (A) Northern blotting on 293T cells transfected with pCMV2JS21 vs. mock-transfected cells was carried out to identify the structure of the 1.2-kb env transcript. Blot hybridization was carried out in parallel with a U3 probe and with the env-2 and env-upstream probes. The 1.2-kb transcript is apparent only with the env-2 probe. (B) RT-PCR cloning mapped the 1.2-kb transcript (tr-env) as a prematurely polyadenylated mRNA terminating at position 6301. (C) Transfection of plasmid pCMV3JS21ΔGPΔStuI that expresses only the 1.2-kb tr-env mRNA in 293T cells and Northern blotting.
To fully characterize the tr-env mRNA we performed RT-PCR on total RNA from 293T cells transfected with pCMV2JS21 and from deer OHH1 cells productively infected by JSRV21 virions. The cDNAs obtained from these samples were amplified by PCR using a forward primer in the untranslated gag region before the splice donor site and oligo(dT) as reverse primer. The use of human 293T cells and deer OHH1 allowed us to use PCR primers that would otherwise cross-react with sheep endogenous JSRV (enJSRVs) transcripts in sheep tissues (20, 21). A prominent PCR product of a size comparable to the tr-env RNA was obtained from both cells (not shown). The RT-PCR products were cloned and sequenced. The sequences of both products revealed that JSRV tr-env RNA uses the splice donor and splice acceptor of the canonical JSRV env mRNA (M.P., C. Murgia, N.M., and H.F., unpublished work) but it is prematurely terminated and polyadenylated at nucleotide 6301 (Fig. 4B). Thirteen nucleotides before the start of the poly(A) tail there is a putative polyadenylation site in the JSRV env sequence (ATTAAA). These results were consistent with the results obtained by the Northern blot analysis.
tr-env Is Not Sufficient for Transformation.
To test whether the full-length env mRNA and/or the tr-env mRNA are responsible for transformation we repeated the transformation assays with pCMV3JS21ΔGPΔStuI. This construct is derived from pCMV3JS21ΔGP by excision of a StuI fragment, which results in a construct that should express only the 1.2-kb tr-env transcript (the StuI site in env is 192 nt downstream from the cryptic polyadenylation site of tr-env, at the boundary between surface and the transmembrane region).
By Northern blotting, we confirmed that this plasmid expressed the tr-env mRNA in large quantities (Fig. 4C). However, NIH 3T3 transformed with pCMV3JS21ΔGPΔStuI did not show any sign of transformation (Table 1, experiments 6 and 7). Thus, the expression of the JSRV tr-env is not sufficient for transformation of NIH 3T3 cells.
Discussion
In these study, we have shown that transfection of JSRV DNA into NIH 3T3 cells resulted in consistent induction of foci of transformed cells with an efficiency similar to that observed for known oncogenes. Rodent cells do not express a functional receptor for JSRV (22), which indicates that the transformation did not result from cell-to-cell spread of infectious virus. Thus some viral gene product could directly induce transformation. This was further supported by the fact that it was necessary to drive expression of the JSRV sequences from a promoter/enhancer that is highly active in NIH 3T3 cells (the CMV immediate early promoter), and that transformed cells contained JSRV DNA and expressed viral RNA.
We investigated which JSRV gene was responsible for the transformation capacity. Mutation of the orf-x reading frame did not decrease the transformation activity, eliminating orf-x as the transforming gene. Further studies indicated that a deleted version of pCMVJS21 lacking all coding regions except env retained the ability to transform NIH 3T3 cells. Thus, an env gene product(s) is apparently responsible for the transformation. Analysis of env-containing transcripts indicated that in addition to the expected spliced env mRNA, JSRV also encodes a novel truncated form of env mRNA resulting from a cryptic cleavage/polyadenylation site in env. This mRNA would encode a truncated form of env polyprotein essentially containing only the surface domain. It is noteworthy that the cryptic cleavage/polyadenylation site appears to be conserved among three exogenous JSRV isolates, but it is missing from the env genes of several endogenous JSRV-related viruses present in the sheep genome (5, 11, 21). Transfection with a pCMVJS21 derivative that could express only the truncated form of env did not yield transformed foci. Thus, it appears that a full-length env gene is necessary for transformation, although the results do not rule out the possibility that expression of truncated env protein also is required.
The finding that JSRV Env protein is able to transform NIH 3T3 cells is striking, because this protein is a structural component of virions, and productive infection of cells by most retroviruses (even oncogenic ones) does not generally result in transformation. These results also suggest that JSRV might be considered an acute transforming virus, with the capacity to directly initiate oncogenesis. The results also raise questions about the mechanism of transformation. Because the Env protein is normally expressed on the surface of the virion (and of infected cells), it seems most likely that transformation results from interaction of Env protein with some other cell surface protein. Three possibilities are particularly interesting. First, it is possible that JSRV Env protein interacts with the murine homologue of the JSRV receptor, and in doing so interferes with a negative growth regulatory activity of the JSRV receptor. In this regard, the JSRV receptor has been cloned from human cells and shown to be the hyaluronidase-2 (HYAL-2) gene (A. D. Miller, personal communication). It is striking that chromosomal deletions of the region containing the HYAL-2 gene have been observed in cell lines derived from human lung cancer (23), suggestive of a tumor suppressor function for this protein. A second possibility is that JSRV Env protein interacts with the murine JSRV receptor, which results in a positive growth signal through the JSRV receptor. Murine cells do not have functional JSRV receptors, because these cells cannot propagate viral infection (18) and they cannot be efficiently transduced with retroviral vectors containing JSRV Env protein (22). However, low level transduction of murine cells was actually observed (22). Thus, JSRV Env can apparently interact with the murine HYAL-2; this interaction is inefficient for viral entry, but it is possible that it is sufficient for cell transformation. A third possibility is that the JSRV Env protein can interact with a distinct cell surface protein when it is causing transformation. Finally, it is possible that some form or domain of Env protein interacts with an intracellular protein to cause transformation.
At least one other retroviral Env protein has been described as a viral oncogene. The spleen focus-forming (SFFV) component of the Friend erythroleukemia virus complex can cause rapid proliferation of erythroid cells. SFFV encodes a deleted version of a recombinant envelope protein (gp55) that is responsible for proliferation of erythroid cells, and it has been further shown that gp55 protein binds to the erythropoietin receptor on erythroid cells (24, 25). This binding leads to constitutive activation of signal transduction pathways downstream from the erythropoietin receptor (26–29). Recently, it has been reported that the transforming gene of an avian hemangiosarcoma-inducing retrovirus is also env (30).
These results suggest that the JSRV Env protein may have direct oncogenic potential during OPC tumorigenesis in sheep. This suggestion would be consistent with experimentally induced disease (intratracheal inoculation of virus in newborn lambs), where there is rapid onset of disease and multiple tumor foci in the lungs (12, 31). However in the field, JSRV-induced OPC occurs much more slowly, with considerable delay between evidence of infection and appearance of the tumors (32). This finding suggests that in that setting (and perhaps infection of older animals) additional factors or events besides the intrinsic transforming potential of JSRV env are required for development of disease. In the future, it will be interesting to evaluate the relative roles of the oncogenic effects of Env protein and other factors in OPC pathogenesis.
Acknowledgments
We thank Dr. J. M. Sharp (Moredun Research Institute, Penicuik, Scotland) for providing tissues of animals affected by OPC and Dr. A. Dusty Miller for communicating results before publication. We thank L. Laimins for initially suggesting the pCMV2JS21 transfection experiment. M.P. was a recipient of an American Cancer Society Ray and Estelle Spehar Fellowship. This work is supported by National Institutes of Health Grant RO1CA82564. Support from the University of California Irvine Cancer Research Institute and the DNA sequencing core of the Chao Family Comprehensive Cancer Center is acknowledged.
Abbreviations
- JSRV
jaagsiekte sheep retrovirus
- OPC
ovine pulmonary carcinoma
- CMV
cytomegalovirus
- LTR
long terminal repeat
- RT
reverse transcription
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 4285.
References
- 1.Vogt P K. In: Retroviruses. Coffin J M, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. pp. 1–25. [PubMed] [Google Scholar]
- 2.Palmarini M, Sharp J M, De las Heras M, Fan H. J Virol. 1999;73:6964–6972. doi: 10.1128/jvi.73.8.6964-6972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmarini M, Fan H, Sharp J M. Trends Microbiol. 1997;5:478–483. doi: 10.1016/S0966-842X(97)01162-1. [DOI] [PubMed] [Google Scholar]
- 4.DeMartini J C, York D F. Vet Clin North Am Food Anim Pract. 1997;13:55–70. doi: 10.1016/s0749-0720(15)30364-9. [DOI] [PubMed] [Google Scholar]
- 5.York D F, Vigne R, Verwoerd D W, Querat G. J Virol. 1992;66:4930–4939. doi: 10.1128/jvi.66.8.4930-4939.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ives J C, Buffler P A, Greenberg S D. Am Rev Respir Dis. 1983;128:195–209. doi: 10.1164/arrd.1983.128.1.195. [DOI] [PubMed] [Google Scholar]
- 7.Bishop J M, Varmus H E. In: RNA, Tumor, Viruses. Weiss R, Teich N, Varmus H, Coffin J M, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1984. pp. 999–1108. [Google Scholar]
- 8.Rasheed S. In: The Retroviridae. Levy J A, editor. Vol. 4. New York: Plenum; 1995. pp. 293–408. [Google Scholar]
- 9.Rosenberg N, Jolicoeur P. In: Retroviruses. Coffin J M, Hughes S, Varmus H E, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. pp. 475–585. [PubMed] [Google Scholar]
- 10.Rosati S, Pittau M, Alberti A, Pozzi S, York D F, Sharp J M, Palmarini M. Virus Res. 2000;66:109–116. doi: 10.1016/s0168-1702(99)00118-5. [DOI] [PubMed] [Google Scholar]
- 11.Bai J, Bishop J V, Carlson J O, DeMartini J C. Virology. 1999;258:333–343. doi: 10.1006/viro.1999.9728. [DOI] [PubMed] [Google Scholar]
- 12.Sharp J M, Angus K W, Gray E W, Scott F M. Arch Virol. 1983;78:89–95. doi: 10.1007/BF01310861. [DOI] [PubMed] [Google Scholar]
- 13.Verwoerd D W, De Villiers E M, Tustin R C. Onderstepoort J Vet Res. 1980;47:13–18. [PubMed] [Google Scholar]
- 14.Ausbel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Protocols in Molecular Biology. New York: Wiley; 2000. [Google Scholar]
- 15.Lebkowsky J S, Clancy S, Calos M P. Nature (London) 1985;317:169–171. doi: 10.1038/317169a0. [DOI] [PubMed] [Google Scholar]
- 16.Hsiao W L, Castro M, Giezentanner J, Fan H, Hanecak R. Mol Carcinogen. 1992;5:140–154. doi: 10.1002/mc.2940050210. [DOI] [PubMed] [Google Scholar]
- 17.Palmarini M, Holland M J, Cousens C, Dalziel R G, Sharp J M. J Gen Virol. 1996;77:2991–2998. doi: 10.1099/0022-1317-77-12-2991. [DOI] [PubMed] [Google Scholar]
- 18.Palmarini M, Sharp J M, Lee C, Fan C. J Virol. 1999;73:10070–10078. doi: 10.1128/jvi.73.12.10070-10078.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmarini M, Datta S, Omid R, Murgia C, Fan H. J Virol. 2000;74:5776–5787. doi: 10.1128/jvi.74.13.5776-5787.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmarini M, Cousens C, Dalziel R G, Bai J, Stedman K, DeMartini J C, Sharp J M. J Virol. 1996;70:1618–1623. doi: 10.1128/jvi.70.3.1618-1623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmarini M, Hallwirth C, York D, Murgia C, de Oliveira T, Spencer T, Fan H. J Virol. 2000;74:8065–8076. doi: 10.1128/jvi.74.17.8065-8076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rai S K, DeMartini J C, Miller A D. J Virol. 2000;74:4698–4704. doi: 10.1128/jvi.74.10.4698-4704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daly M C, Xiang R H, Buchhagen D, Hensel C H, Garcia D K, Killary A M, Minna J D, Naylor S L. Oncogene. 1993;8:1721–1729. [PubMed] [Google Scholar]
- 24.Sitbon M, Sola B, Evans L, Nishio J, Hayes S F, Nathanson K, Garon C F, Chesebro B. Cell. 1986;47:851–859. doi: 10.1016/0092-8674(86)90800-7. [DOI] [PubMed] [Google Scholar]
- 25.Li J P, D'Andrea A D, Lodish H F, Baltimore D. Nature (London) 1990;343:762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- 26.Muszynski K W, Thompson D, Hanson C, Lyons R, Spadaccini A, Ruscetti S K. J Virol. 2000;74:8444–8451. doi: 10.1128/jvi.74.18.8444-8451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruscetti S K. Int J Biochem Cell Biol. 1999;31:1089–1109. doi: 10.1016/s1357-2725(99)00074-6. [DOI] [PubMed] [Google Scholar]
- 28.Nishigaki K, Hanson C, Ohashi T, Thompson D, Muszynski K, Ruscetti S. J Virol. 2000;74:3037–3045. doi: 10.1128/jvi.74.7.3037-3045.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamura Y, Senda H, Noda M, Ikawa Y. Leukemia. 1997;11, Suppl. 3:432–434. [PubMed] [Google Scholar]
- 30.Alian A, Sela-Donenfeld D, Panet A, Eldor A. Virology. 2000;276:161–168. doi: 10.1006/viro.2000.0550. [DOI] [PubMed] [Google Scholar]
- 31.Verwoerd D W, Williamson A L, De Villiers E M. Onderstepoort J Vet Res. 1980;47:275–280. [PubMed] [Google Scholar]
- 32.Sigurdsson B. Br Vet J. 1954;110:341–354. [Google Scholar]