Abstract
The importance of using primary cells, rather than cancer cell lines, for biological studies is becoming widely recognized. Primary cells are preferred in studies of cell cycle control, apoptosis, and DNA repair, as cancer cells carry mutations in genes involved in these processes. Primary cells cannot be cultured indefinitely due to the onset of replicative senescence or aneuploidization. Hence, new cultures need to be established regularly. The procedure for isolation of rodent embryonic fibroblasts is well established, but isolating adult fibroblast cultures often presents a challenge. Adult rodent fibroblasts isolated from mouse models of human disease may be a preferred control when comparing them to fibroblasts from human patients. Furthermore, adult fibroblasts are the only available material when working with wild rodents where pregnant females cannot be easily obtained. Here we provide a protocol for isolation and culture of adult fibroblasts from rodent skin and lungs. We used this procedure successfully to isolate fibroblasts from over twenty rodent species from laboratory mice and rats to wild rodents such as beaver, porcupine, and squirrel.
Protocol
1. Before Starting
Sterilize scissors and forceps with 70% ethanol.
Place small magnetic stirrer inside a 30 mL beaker, cover with two layers of foil, and autoclave.
Prepare a 28 Wunsch units/mL stock solution of Liberase Blendzyme 3 in sterile water. Make 0.5 mL aliquots and freeze at -20°C. Thaw a new aliquot before every use. The solution may appear cloudy after thawing. Vortex the solution until it becomes clear.
Warm up the cell culture media.
2. Animal Sample Preparation
Caution: wild animals may contain pathogens such as a rabies virus. Always be aware of open sharps.
Euthanize the animal and place the carcass at 4°C. It is best to use the carcass immediately, however, if this is not practical such as if the animals are collected in the field, the carcasses may be used within 24 hours. After 24 hours the cell yield declines.
Dissect the animal in the surgical suite or in chemical hood (not in tissue culture hood).
Underarm area is a convenient site to collect skin samples as underarm skin is thinner, contains less fat, and the fur is less dense. Clean the incision site with 70% ethanol. Make sure the fur is soaked with ethanol.
Shave the fur around the incision site with a sharp scalpel. Shave a larger area than the desired incision site. Try to minimize cuts to the skin. Spray the area with 70% ethanol and let it dry.
To collect a skin sample excise a fragment of approximately 1 cm2. Pinch the skin with tissue forceps, and cut with scissors. Try not to cut the fat layer with the skin, as fat interferes with collagenase digestion. To collect the lung samples, wash the chest area with 70% ethanol, and open the skin on the chest with scissors by making a T-shape incision and pulling apart the flaps of the skin. Wash the opened muscle area with 70% ethanol and let dry. Cut the rib cage using sterile forceps and scissors (use bone cutters for larger animals). Use sterile techniques to avoid contaminating internal organs. Do not touch the internal organs with instruments that touched the animal fur. Cut lung fragments of approximately 1 cm2 using sterile forceps and scissors.
Place tissue fragments in 50 mL tubes with sterile PBS to avoid drying.
Wash the outside of the 50 mL tubes with tissue fragments with ethanol, and take them to the tissue culture hood.
Properly dispose of animal carcass.
3. Extracting Cells
Transfer the tissue fragments into a 10 cm tissue culture dish using sterile scalpel. Do not transfer too much PBS with the sample.
Cut the tissue into ~1 mm pieces using two scalpels. Use two blades using a scissor action starting from the center and pulling apart. Keep the tissue balled up, do not cut piece by piece. When the cutting is sufficient the skin resembles putty, it will not separate into pieces but will stretch thin. The lung tissue is easier to cut, and it will separate into tiny pieces.
Using a scalpel, transfer the cut tissue into sterile 30 mL beaker with sterile stir bar. Wash the plate used for cutting tissue with 10 mL of DMEM/F12 media with 0.14 Wunsch units/mL Liberase Blendzyme 3, and 1X antibiotic/antimycotic, and add the solution to the 30 mL beaker with tissue fragments.
Cover the beaker with sterile foil, and incubate at 37°C, stirring slowly, for 30 to 90 minutes. The length of the incubation depends on the species and tissue type. Take care not to over-digest the tissue. Best yields are obtained when tissue fragments are still present at the end of the digestion. Skin takes longer to digest than the lung. Skin from large animals takes longer to digest. Check the digestion after 30 min, and then every 10 min. When the skin digestion is complete, the media becomes cloudy and skin fragments separate from each other, and the edges of the pieces become "fuzzy". Lung digestion should be stopped when lung fragments change color from red to white and start forming sticky fibers.
Pipet the solution containing tissue fragments up and down to break the clumps. Transfer the solution to sterile 50 mL tube. If the fragments move easily through the 10 mL pipette cutting and digestion was done well. Rinse the beaker 3 times with 10 mL of warm DMEM/F12 media with 15% FBS, 1X antibiotic/antimycotic and add the media to the 50 mL tube with tissue fragments. Close the 50 mL tube and mix by inversion a few times. The FBS in the media will stop Liberase digestion.
Spin at 524 g in a swinging bucket tissue culture centrifuge for 5 min. Remove the supernatant. Resuspend the pellet in 10 mL of warm DMEM/F12 media with 15% FBS, 1X antibiotic/antimycotic. Pipet the suspension with maximum force to break the tissue pieces.
Add another 30 mL of DMEM/F12 media with 15% FBS, 1X antibiotic/antimycotic, mix and centrifuge at 524 g in a swinging bucket tissue culture centrifuge for 5 min. Repeat one more time to remove the traces of Liberase.
Resuspend the pellet in 10 mL of DMEM/F12 media with 15% FBS, 1X antibiotic/antimycotic and transfer to a 10 cm tissue culture dish and place in a tissue culture incubator at 37°C, 5%CO2, 3%O2.
Check the plates every day for fibroblasts and media color. If the isolation was successful, fibroblasts crawl out of the tissue fragments and attach to the plate (Figure 1). The fibroblast start to exit tissue fragments within 2-5 days.
If the media changes color to yellow, this indicates potential contamination or overcrowding of cells. Examine the plates under the microscope at high magnification. If bacteria, fungi, or worms are present discard the plates (this is rarely an issue with laboratory animals, however may occur when the samples are collected from the wild). If no contamination is present, but the media changed color, this is caused by either too many cells or too many tissue fragments placed in the same dish. If more than 60% of the plate is covered by attached fibroblasts, change the media on the plate and transfer the tissue fragments to a new plate with the new media. If not many fibroblasts attached to the plate, change the media and split the tissue pieces to a 2-4 plates.
After 7 days, if media had not been changed earlier, change the media and transfer the tissue fragments to a new plate with new media.
Incubate the cells and tissue fragments for an additional 7 days. By day 14 all viable fibroblasts have exited the tissue fragments.
After 14 days from the beginning of the cell isolation, discard the old media and tissue fragments, harvest the cells and plate them on a new plate at 5x105 cells/plate EMEM with 15% FBS, 1X Penicillin/Streptomycin, non essential amino acids, and sodium pyruvate. EMEM media will support growth of fibroblasts only and other cell types will die or stop proliferating.
After the cells reach 80-90% confluence, freeze an aliquot of cells for future use.
Continue culturing the cells by splitting them at 5x105 cells/plate when the cells reach 80-90% confluence.
4. Representative Results
Normal fibroblasts are large cells with prominent protrusions (lamellipodia) (Figure 2). Fibroblasts grow in a monolayer. A healthy growing culture contains 1-10% cells in M stage, recognized as rounded up cells elevated over the surface of the plate, but not detached from the plate (Figure 3). Typically, a 10 cm dish seeded with 5x105 cells becomes confluent in 3-4 days. The doubling time varies greatly between species, and may be greater for some of the long-lived rodent species1. When cells fill a plate they arrest proliferation in G1 stage. Typical confluent plate of fibroblasts contains a tightly packed layer of cells (Figure 4). When the cells reach 90% confluence they are ready for splitting. If desired, the cells can be maintained on an arrested confluent plate for extended periods of time with regular media changes (once or twice a week).
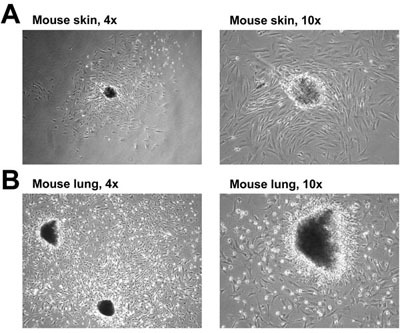 Figure 1. Fibroblast isolation from mouse skin (A) and lung (B). The media was changed to remove unattached cells and debris prior to taking the pictures on Day 7.
Figure 1. Fibroblast isolation from mouse skin (A) and lung (B). The media was changed to remove unattached cells and debris prior to taking the pictures on Day 7.
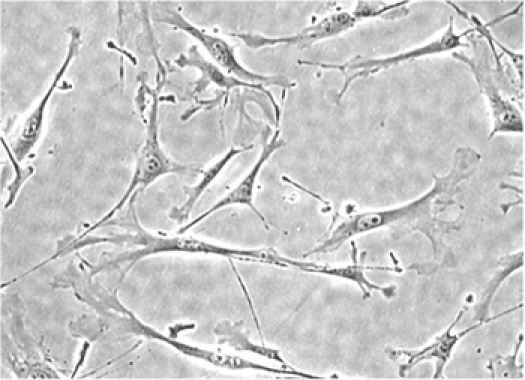 Figure 2. Mouse lung fibroblasts.
Figure 2. Mouse lung fibroblasts.
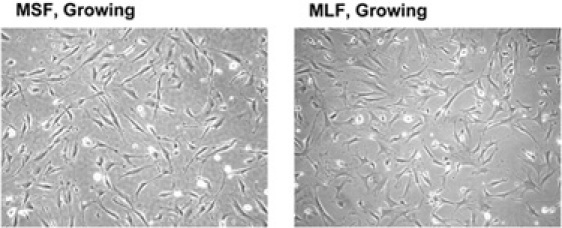 Figure 3. A field of mouse fibroblasts containing cells in M-stage, photographed at 10X magnification.
Figure 3. A field of mouse fibroblasts containing cells in M-stage, photographed at 10X magnification.
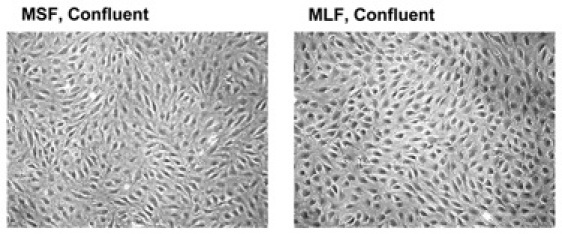 Figure 4. A confluent plate of mouse fibroblasts, photographed at 10X magnification.
Figure 4. A confluent plate of mouse fibroblasts, photographed at 10X magnification.
Discussion
Normal primary fibroblasts provide an excellent alternative to established cancer cell lines in biological research. An important advantage of fibroblasts is that they do not carry mutations in oncogenes and tumor suppressors and maintain intact cell cycle checkpoints. This makes normal fibroblasts a preferred system for the studies of cell cycle regulation, DNA repair, and apoptosis. The protocol described here provides a simple recipe for isolation and maintenance of primary fibroblasts. This protocol was used to successfully isolate fibroblasts from over 20 species of rodents.
It is important to maintain sterility at all times when working with cell cultures to avoid contamination. If the laboratory also cultures aggressive cancer cell lines such as HeLa cells, fibroblasts should be handled separately from cancer cells. It is preferred to have a designated hood and incubator for primary cultures.
It is preferable to maintain primary cell cultures at physiological oxygen concentration of 3%. Atmospheric (20%) oxygen shortens lifespan of cultures and increases oxidative stress. Mouse fibroblasts are sensitive to oxidative stress and will senesce or enter crisis within ~14 population doublings when maintained at 20% (atmospheric) oxygen. Mouse fibroblasts can be maintained indefinitely at 3% oxygen2.
Always keep record of the number of population doubling of the cultures. Large species (body mass above 8,000 g) are likely to exhibit replicative senescence, and will have a limited lifespan in culture, even at 3% oxygen1. Cells from smaller species, such as mice and rats, will proliferate indefinitely at 3% oxygen, but may eventually become aneuploid. If possible, begin the experiments using cells at low population doubling.
Disclosures
No conflicts of interest declared.
Acknowledgments
We thank Dr. Steven Austad for providing us with the first version of this protocol. This work was supported by grants from the NIH and the Ellison Medical Foundation to V.G. and A.S.
References
- Seluanov A. Distinct tumor suppressor mechanisms evolve in rodent species that differ in size and lifespan. Aging . Cell. 2008;7:813–823. doi: 10.1111/j.1474-9726.2008.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello S. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]


