Abstract
Understanding the relationships between genetic and microenvironmental factors that drive normal and malformed embryonic development is fundamental for discovering new therapeutic strategies. Advancements in imaging technology have enabled quantitative investigation of the organization and maturing of the body plan, but later stage embryonic morphogenesis is less clear. Chicken embryos are an attractive vertebrate animal model system for this application because of its ease of culture and surgical manipulation. Early embryos can be cultured for a short time on filter paper rings, which enables complete optical access for cell patterning and fate studies1,2. Studying advanced developmental processes such as cardiac morphogenesis are traditionally performed through a window of the eggshell3-5, but this technique limits optical access due to window size. We previously developed a simple method to culture whole embryos ex-ovo on hexagonal weigh boats for up to 10 days, which enabled high resolution imaging via ultrasonography6,7. These cultures were difficult to transport, limiting the types of imaging tools available for live experiments. We here present an improved shell-less culture system with a cost-effective, portable environmental chamber. Eggs were cracked onto a hammock created by a polyurethane membrane (cling wrap) affixed circumferentially to a plastic cup partially filled with sterile water. The dimensions of the circumference and depth of the hammock were both critical to maintain surface tension, while the mechanics of the hammock and water beneath helped dampen vibrations induced by transportation. A small footprint circulating water bath was also developed to enable continuous temperature control during experimentation. We demonstrate the ability to culture embryos in this way for at least 14 days without morphogenic defect or delay and employ this system in several microsurgical and imaging applications.
Protocol
1. Ex-ovo Culture Protocol:
- Incubate fertilized chicken eggs
- Fertilized chicken eggs can be stored at 13°C up to 5 days before incubation without initiating development. A red wine cooler can maintain this temperature.
- Incubate eggs blunt side up in a 60% constant humidity incubator with continuous rocking at 37.5°C for 72 hours.
- Prepare hammocks (Figure 1a)
- Fill ¾ of 9 oz plastic cups with warm sterile water. A regular 9 oz cup has a top diameter of 8 cm. We found that this size was most suitable for long time culture application presented here.
- Cut about 20 cm cling wrap. Affix circumferentially on top of the cup by placing a rubber band around. Plastic should be spread on top of the water. Cut the excess plastic around the band. We found cling wrap to be most appropriate for this application since it prevents adhesion of the yolk, resulting in the yolk splitting during transportation.
- Wipe the plastic with kimwipes (Kimberly-Clark, Inc.) wetted with 70% ethanol.
Remove eggs from incubator after 72 hours of incubation.
Sterilize eggs by wiping surface with 70% ethanol wetted kimwipes.
Lay the eggs horizontally for 1-2 minutes for proper positioning of the embryo (Figure 1b). An egg carton can be used for laying eggs horizontally. Embryos will rotate to the top after 1-2 minutes.
- Transfer embryos onto hammocks aseptically in a laminar flow hood.
- Using a sharp edge (like the edge of a metal bucket or a glass beaker), tap the egg gently until there is a small dent on the underside of the egg (Figure 1c). Remember, the embryo is positioned on the upperside.
- Put thumbs in opposite sides of the dent and pull the shells apart horizontally (Figure 1d).
- Place the yolk with embryo as well as albumin onto the hammock (figure 1e). Albumin will provide a dampening environment around the yolk to absorb shocks induced by moving the system as well as it provides nutrition to the embryo for long culture practices. Water underneath the hammock will ensure the embryo will stay parallel to the ground during transport and serve as a conductor of heat during imaging if a water circulation heater is used.
- The embryo should already be positioned on top if the transfer is performed properly. If not, use an unsharp, sterile object (ie. Blunt tip of closed curved scissors) and directionally caress the yolk such that the embryo rotates to the top.
- Place a 10 cm diameter Petri dish on top of the cup to seal the embryo.
Place cup culture into the incubator set at 37.5°C. If a portable incubator is used, placing 1-2 9 oz cups filled with distilled water keeps the humidity at ~60% (Figure 1f).
If culturing embryos beyond embryonic day 7, crushed eggshell pieces should be scattered around the periphery of the embryo as a calcium source for bone development and maturation.
Accessory equipment for outside incubator imaging/ manipulation:
A circulating water bath was built in house to maintain incubation temperatures while imaging or manipulation (Figure 3). A water bath that can fit the cup is connected via appropriate size tubing to a water reservoir. A resistance heater heats the water in the reservoir which is regulated manually by following the water temperature. A water pump circulates the water. Pictoral representation depicts imaging ease via ultrasongraphy/florescent microscopy (Figure 3).
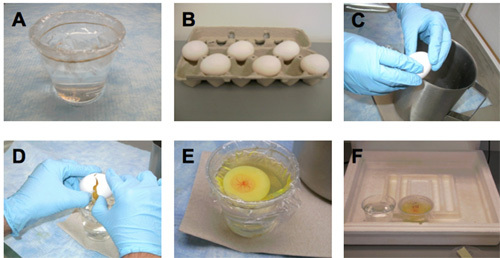 Figure 1. Preparation of hammock and transfer of chicken embryos to hammocks.
Figure 1. Preparation of hammock and transfer of chicken embryos to hammocks.
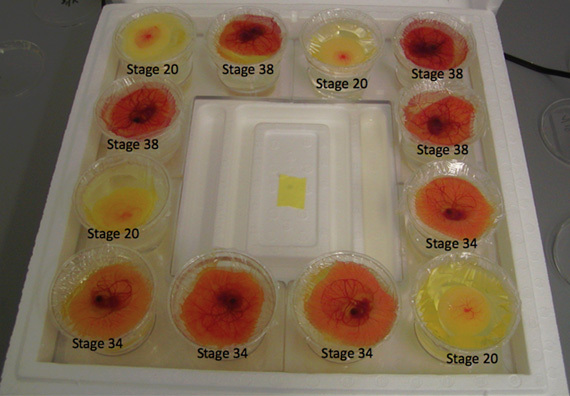 Figure 2. Representative results - different stage chicken embryos grown via ex ovo culture technique. Embryos from HH 17 onwards can be grown via this technique.
Figure 2. Representative results - different stage chicken embryos grown via ex ovo culture technique. Embryos from HH 17 onwards can be grown via this technique.
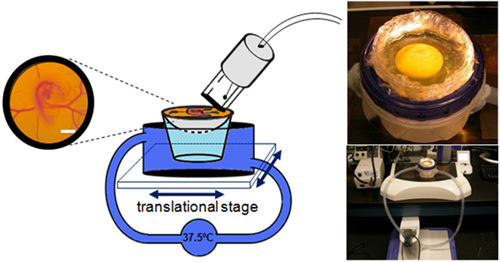 Figure 3. Schematic and representative pictures of the water circulation system used in conjunction with the ex-ovo culture system to keep embryos at ideal development temperature.
Figure 3. Schematic and representative pictures of the water circulation system used in conjunction with the ex-ovo culture system to keep embryos at ideal development temperature.
2. Selected Applications:
I. Microinjection
This embryo culture is suitable for microinjection applications where solutions need to be injected to vasculature for contrast based imaging (ie. fluorescent microscopy, micro-computed topography etc.) (Figure 4). Below is the microinjection protocol:
- Prepare the injection apparatus
- Fashion pulled 0.75 ID glass capillary tubes into beveled tip microneedles using a microforge. Size of the tip required depends on target vein diameter/flow rate desired.
- Connect the syringe to 0.03 inch ID silicone tubing.
- Load the tubing with the solution to be injected and connect the microneedle to the tubing.
- Place the microneedle onto the micromanipulator and syringe to syringe holder or syringe pump.
- Add sterile water in the cup thereby raise the embryo. We experienced that for proper penetration of the needle into a blood vessel (ie. vitelline vessel), horizontal penetration is necessary. This requires the embryo to be on the same plane as the edges of the cup.
- Take out the rubber band holding the plastic under the embryo.
- Lift one side of the plastic gently and add some sterile water to the cup.
- Secure the rubber band onto the plastic again.
- Inject the solution to an embryonic vessel. In our experience, when solution is injected to a vitelline vessel, microneedles needed to be sized approximately 1/10th of the vessel diameter to maintain efficient perfusion without causing bleeding.
- Pump the solution to the microneedle ensuring no formation of air bubbles.
- Align the tip of the microneedle onto the selected vessel. For vitelline vessels, selecting a fork area will give maximum area to penetrate the vessel.
- Push the needle towards the vessel. The vessel will first retract. Once the needle penetrates, start perfusion. The color of the flowing blood should change with perfusion. If color change is not seen, the needle may have penetrated out of the vessel. In that case, slowly retract the microneedle while simultaneously pumping solution till color change in the vessel is seen. Once the perfusion is complete, retract the microneedle out of the vessel.
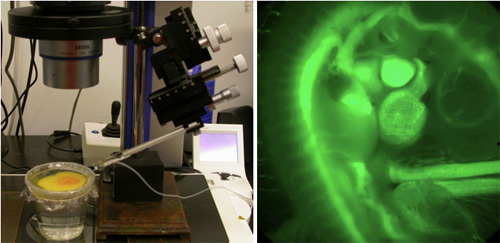 Figure 4: Microinjection of a fluorescent dye into the vasculature of an ex-ovo cultured chick embryo.
Figure 4: Microinjection of a fluorescent dye into the vasculature of an ex-ovo cultured chick embryo.
II. Microsurgical Procedure - Left Atrial Ligation
This ex-ovo culture setup is ideal for performing surgical applications to chick embryos since it provides full accessibility to the embryo. Below is the protocol for an example technique, left atrial ligation (LAL) (Figure 5). The procedure is performed approximately 24 hours into the culture period (HH24):
Prepare overhand knots ~0.5 mm diameter using 10-0 nylon surgical suture.
With fine forceps, locally remove the chorionic and allantoic membranes over the embryo and lift the embryo from the back to rotate it vertically such that the left side of the embryo is visible.
Using fine forceps, remove the pericardium over the left atrium.
Align the knot over the left atrium and tighten it. The knot should be tied so as to prohibit ~75% of the original blood flow to the left side. Cut the edges of the knot with microscissors and carefully remove and discard excess suture.
Rotate the embryo back to its original orientation (right side on top).
Sham controls will involve the same procedure but the suture will just be passed through and not tied. Effective LAL treatment (75% constriction) will be confirmed at 24 hours post-surgery, and disqualified embryos will be excluded.
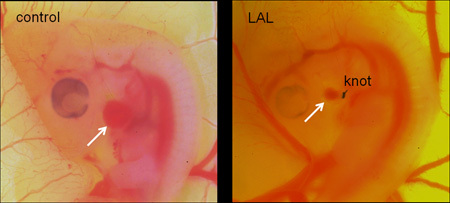 Figure 5: A control and a left atrial ligated chick embryo. Arrow shows the left atria, which is about 75% smaller in the ligated embryo.
Figure 5: A control and a left atrial ligated chick embryo. Arrow shows the left atria, which is about 75% smaller in the ligated embryo.
Discussion
Optical access and experimentation in avian embryos is challenging due to constraints of the egg shell. Windowing significantly limits the number of microvessels accessible for injection and microsurgical approaches8. As a result only early embryos can be manipulated and continuous observation is not possible. Early ex-ovo cultures using Petri dishes were of limited use because of inadequate control of the surface tension on the embryo prevented long term culture9. We recently improved this technique using a hexagonal weigh boat that maintains acceptable surface tenstion7, but these cultures were difficult to transport. The technique presented here solves this problem and significantly expands the type of experimental methods and imaging techniques that can be employed including microinjection and surgical procedures. As research focuses on understanding later morphogenic events, this technique will enable monitoring and modulation of development events that are currently impossible to experimentally study continuously.
Disclosures
No conflicts of interest declared.
References
- Zamir EA, Czirok A, Cui C, Little CD, Rongish BJ. Mesodermal cell displacements during avian gastrulation are due to both individual cell-autonomous and convective tissue movements. Proc Natl Acad Sci U S A. 1980;103:19806–19811. doi: 10.1073/pnas.0606100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman LA, Yutzey KE. Lack of regulation in the heart forming region of avian embryos. Dev Biol. 1999;207:163–175. doi: 10.1006/dbio.1998.9167. [DOI] [PubMed] [Google Scholar]
- Clark EB, Hu N, Rosenquist GC. Effect of conotruncal constriction on aortic-mitral valve continuity in the stage 18, 21 and 24 chick embryo. Am J Cardiol. 1984;53:324–327. doi: 10.1016/0002-9149(84)90447-8. [DOI] [PubMed] [Google Scholar]
- deAlmeida A, McQuinn T, Sedmera D. Increased ventricular preload is compensated by myocyte proliferation in normal and hypoplastic fetal chick left ventricle. Circ Res. 2007;100:1363–1370. doi: 10.1161/01.RES.0000266606.88463.cb. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Funahashi J. Introduction of DNA into chick embryos by in ovo electroporation. Methods. 2001;24:43–48. doi: 10.1006/meth.2001.1155. [DOI] [PubMed] [Google Scholar]
- Butcher JT, McQuinn TC, Sedmera D, Turner D, Markwald RR. Transitions in early embryonic atrioventricular valvular function correspond with changes in cushion biomechanics that are predictable by tissue composition. Circ Res. 2007;100:1503–1511. doi: 10.1161/CIRCRESAHA.107.148684. [DOI] [PubMed] [Google Scholar]
- McQuinn TC. High-frequency ultrasonographic imaging of avian cardiovascular development. Dev. Dyn. 2007;236:3503–3513. doi: 10.1002/dvdy.21357. [DOI] [PubMed] [Google Scholar]
- Korn MJ, Cramer KS. Windowing chicken eggs for developmental studies. J Vis Exp. 2007 doi: 10.3791/306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach R, Kubai L, Knighton D, Folkman J. A simple procedure for the long-term cultivation of chicken embryos. Dev Biol. 1974;41:391–394. doi: 10.1016/0012-1606(74)90316-9. [DOI] [PubMed] [Google Scholar]


