Abstract
Studies in rodent models of breast cancer show that exposures to dietary/hormonal factors during the in utero and pubertal periods, when the mammary gland undergoes extensive modeling and re-modeling, alter susceptibility to carcinogen-induced mammary tumors. Similar findings have been described in humans: for example, high birthweight increases later risk of developing breast cancer, and dietary intake of soy during childhood decreases breast cancer risk. It is thought that these prenatal and postnatal dietary modifications induce persistent morphological changes in the mammary gland that in turn modify breast cancer risk later in life. These morphological changes likely reflect epigenetic modifications, such as changes in DNA methylation, histones and miRNA expression that then affect gene transcription . In this article we describe how changes in mammary gland morphology can predict mammary cancer risk in rats. Our protocol specifically describes how to dissect and remove the rat abdominal mammary gland and how to prepare mammary gland whole mounts. It also describes how to analyze mammary gland morphology according to three end-points (number of terminal end buds, epithelial elongation and differentiation) and to use the data to predict risk of developing mammary cancer.
Keywords: Medicine, Issue 44, mammary gland morphology, terminal end buds, mammary cancer, maternal dietary exposures, pregnancy, prepubertal dietay exposures
Protocol
1. Removal of the Abdominal Mammary Gland and Preparing a Whole Mount
Euthanize the animal according to the IACUC guidelines. Pin the animal on its back from its legs with needles, or tape the legs onto the surface board , and wipe the skin wet with ethanol (EtOH). Lift the skin a little bit with forceps and with sharp scissors make a midline incision into the skin starting between the pair #5 nipples and cut towards the neck . Make an inverted Y incision from the midline towards the hind legs . If thoracic mammary glands are also to be collected, make also an Y incision from the midline between the pair #2 nipples towards the front legs.
Dissect the skin open with scissors on one side of the inverted Y incision to expose the abdominal #4 mammary gland; the mammary glands are attached to the skin. Pin the skin with needles onto the surface board , to completely expose the gland. Work one side and gland at the time.
Dissect the mammary gland free from the skin either using sharp scissors and/or a scalpel, starting from the proximal area close to the nipple and working towards the distal end of the gland towards the spine of the animal . (This can take several minutes, especially in an older rat.)
Immediately spread the detached gland onto an appropriately labeled glass slide (use a proper permanent marker pen and/or 'a diamond pen'), and spread the gland carefully corresponding to its original size and shape in situ . The glass slide must be bigger than the gland. After spreading the gland onto the slide, let it sit on the table for a while, so that the gland sticks onto the slide, but don't let it dry.
Put the slide into a jar containing Carnoy's fixative (75% glacial acetic acid, 25% absolute ethanol=EtOH) , let it get fixed at room temperature (RT) in the fume hood for 2 days or longer. Slides can also be left in the fixative for a longer period of time.
Wash the slides in 70% EtOH for 1 hour at RT.
Rinse in distilled water for 30 min at RT.
Stain in Carmine Alum stain*) for 2 days or longer (until you see that the lymph nodes have stained through; look at the back side of the slide).
Wash in increasing series of EtOH, 1 hour in each: 70% -> 95% -> 100% . After the last wash in absolute EtOH put the glands in xylene . Let sit in the fume hood in RT at least for 2 days. This last step is clearing of the mammary gland meaning delipidation of the mammary fat pad, and subsequent increase in transparency. The fattier the gland is the longer clearing time is required.
Mount with cover-slips using a mounting media, such as Permount . Let the slides dry well (several days) before observing under a stereo microscope.*Carmine Alum Stain:Place 1 g carmine (Sigma C1022) and 2.5 g aluminum potassium sulfate (Sigma A7167) in 500 mL distilled water and boil for 20 min. Adjust final volume to 500 mL with water. Filter and refrigerate. Solution can be used for several months. Discard when color becomes weak.
2. Analysis of Mammary Gland Whole Mount Morphology
Mammary gland whole mounts morphology is analyzed according to the following end-points and the results correlated with mammary cancer risk.
- Mammary gland whole-mount at PND 21 (pre-pubertal age):
- Epithelial growth:
- Distance from the nipple to the end of epithelial tree (in millimeters), measured using a ruler
- Potential for malignant transformation:
- Number of TEBs (terminal end buds), counted under a light microscope . TEBs are the largest bulbous structures located only at the distal end of the mammary epithelial tree
- Differentiation:
- AB1 (alveolar bud) score (0-5). Alveolar buds are spread across the epithelium.
- AB2 score (0-5)
- Mammary gland whole-mount collected at PND 50 (post-pubertal age) :
- Epithelial growth:
- Distance from the lymph node to the end of epithelial tree (in millimeters), measured using a ruler.
- Distance from the tip of the epithelial tree to the end of fat pad, measured using a ruler.
- Potential for malignant transformation:
- Number of TEBs, counted under a light microscope
- Differentiation:
- AB1 score (0-5)
- AB2 score (0-5)
- Lobules score (0-5)
3. Palpation and Mammary Tumor Measurement in Rats and Correlation to Mammary Gland Morphology
To begin this procedure, hold the rat by grasping the whole body with the palm over the back, with forefinger behind the head and the thumb and second finger under the opposite axilla. Turn the rat so it is lying on its posterior, and palpate to detect any mammary tumors; palpable tumors should feel like a "lump".
Next use a caliper to measure the width, length, and height of the tumor. Calculate the tumor volume using the ellipsoid formula, Volume =1/6πabc, where 'a'= width, 'b' = length, and 'c' = height.
Manually record the location and size of the tumor in a notebook on a weekly basis. This data will be transferred to a spreadsheet later.
4. Representative Results
Careful dissection of the mammary fat pad and its processing to wholemount will allow assessment of developmental state of the mammary gland. When each step is done correctly, the whole mammary epithelial tree is clearly visible within the fat pad, and this allows easy determination of the number of TEBs and calculation of the density of alveolar buds and lobules. Missteps in preparing wholemounts may include failure to dissect the whole fat pad, insufficient fixing, and inadequate clearing.
Assessment of mammary gland morphology will provide information about the number of structures present that can give rise to mammary tumors (TEBs), degree of differentiation of the epithelial structures (alveolar buds and lobules), and measure of growth (ductal elongation). It is important to separate TEBs to terminal ends; the latter are located at the distal end of the epithelium, similarly to TEBs, but they are smaller than TEBs and do not give rise to malignant tumors. Terminal ends are also seen within the epithelial tree.
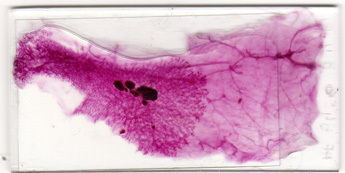 Figure 1: Representative result of well prepared mammary gland whole mount. All components of the gland have been properly dissected and carmine staining is optimal.
Figure 1: Representative result of well prepared mammary gland whole mount. All components of the gland have been properly dissected and carmine staining is optimal.
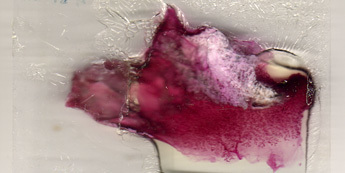 Figure 2: Representative result of a poorly prepared mammary gland whole mount. The gland has been properly dissected (the distal portion of the gland is missing), has not been properly stretched and delipidation has not completely occurred.
Figure 2: Representative result of a poorly prepared mammary gland whole mount. The gland has been properly dissected (the distal portion of the gland is missing), has not been properly stretched and delipidation has not completely occurred.
Discussion
Assessment of mammary gland morphological end-points and the growth of the epithelial tree can be used to predict whether early life dietary manipulations, or other manipulations which alter in utero or prepubertal hormonal environment, modify later mammary cancer risk. Since breast cancer in humans is initiated in mammary structures (terminal ductal lobular units, TDLUs) similar to TEBs in rats, this technique can be used to determine the potential of early life exposures to affect breast cancer risk. Further, in women high mammographic density increases the risk of breast cancer by 4-6 fold, and assessment of the growth of mammary epithelial tree can be used to identify factors which determine mammographic density and factors which reduce this density. To obtain this information, mammary gland should be fully dissected, properly stretched on a glass slide so that all its components can be visualized under a microscope. In addition, proper carmine staining and delipidation in xylene will provide whole mounts that are suitable for mammary cancer risk assessment.
Disclosures
No conflicts of interest declared.
Acknowledgments
NCI (U54 CA00100970), NCI (1 R03 CA150040-01), ACS(116602-PF-09-018-01-CNE)
References
- Rasmussen SB, Young LJT, Smith GH. Preparing mammary gland whole mounts form mice. In: Ip MM, Asch BB, editors. Methods in Mammary Gland Biology and Breast Cancer Research. New York: Kluwer Academic / Plenum Publishers; 2000. pp. 75–85. [Google Scholar]
- Consensus statements from Mammary Gland Whole Mount Round Robin meeting; Oakland, CA. 2009. [Google Scholar]
- Hilakivi-Clarke L, Clarke R, Onojafe I, Raygada M, Cho E, & Lippman, E M. A maternal diet high in n-6 polyunsaturated fats alters mammary gland development, puberty onset, and breast cancer risk among female rat offspring. Proc Natl Acad Sci. 1997;94:9372–9377. doi: 10.1073/pnas.94.17.9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo IH, Russo J. Mammary gland neoplasia in long-term rodent studies. Environ. Health Perspect. 1996;104(9):938–967. doi: 10.1289/ehp.96104938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, de Assis S. Fetal origins of breast cancer. TRENDS in Endocrinol and Metabol. 2006;17(9):340–348. doi: 10.1016/j.tem.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L. Nutritional modulation of terminal end buds: its relevance to breast cancer prevention. Curr Cancer Drug Targets. 2007;7(4):465–474. doi: 10.2174/156800907781386641. [DOI] [PubMed] [Google Scholar]
- Silva Idos S, De Stavola B, Mccormack V. Collaborative Group On Pre-Natal Risk Factors And Subsequent Risk Of Breast Cancer. Birth size and breast cancer risk: re-analysis of individual participant data from 32 studies. PloS Medicine. 2008;5:e193–e193. doi: 10.1371/journal.pmed.0050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warri A, Saarinen NM, Makela S, Hilakivi-Clarke L. The role of early life genistein exposures in modifying breast cancer risk. Br J Cancer. 2008;98:1485–1493. doi: 10.1038/sj.bjc.6604321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo J, Hu YF, Yang X, Russo IH. Developmental, cellular, and molecular basis of human breast cancer. J Natl Cancer Inst Monogr. 2000. pp. 17–37. [DOI] [PubMed]
- Oza AM, Boyd NF. Mammographic parenchymal patterns: A marker of breast cancer risk. Epidemiological Reviews. 1993;15:196–208. doi: 10.1093/oxfordjournals.epirev.a036105. [DOI] [PubMed] [Google Scholar]


