Abstract
The search for effective therapies for orthopoxvirus infections has identified diverse classes of molecules with antiviral activity. Pyrimidine analogs, such as 5-iodo-2′-deoxyuridine (idoxuridine, IDU) were among the first compounds identified with antiviral activity against a number of orthopoxviruses and have been reported to be active both in vitro and in animal models of infection. More recently, additional analogs have been reported to have improved antiviral activity against orthopoxviruses including several derivatives of deoxyuridine with large substituents in the 5 position, as well as analogs with modifications in the deoxyribose moiety including (north)-methanocarbathymidine, and 5-iodo-4′-thio-2′-deoxyuridine (4′-thioIDU). The latter molecule has proven to have good antiviral activity against the orthopoxviruses both in vitro and in vivo and has the potential to be an effective therapy in humans.
Keywords: orthopoxvirus, antiviral, nucleoside, pyrimidine, idoxuridine, deoxyuridine, 4′-thioIDU
1. Introduction: Activity of Thymidine Analogs against the Orthopoxviruses
The efficacy of idoxuridine (IDU) against vaccinia virus replication both in vitro and in vivo was reported almost 50 years ago and helped set the stage for the development of effective antiviral therapies [1,2]. Descriptions of its efficacy in mice infected with this virus [3,4], coupled with reports of its incorporation into viral DNA [5], led to an appreciation of how the molecule might exert specific antiviral effects and thus control viral infections. But, subsequent studies with related analogs, such as 5-iodo-5′-amino-2′,5′,dideoxyuridine, showed that its phosphorylation induced by infection with herpes simplex virus (HSV) remarkably improved efficacy [6], and implicated the viral encoded thymidine kinase (TK) as the enzyme that mediated this effect [7]. While this strategy was used to enhance efficacy against HSV with E-5-(2-iodovinyl)-2′-deoxyuridine [8], which led to the development of (E)-5-(2-bromovinyl)-2′-deoxyuridine (BVDU) [9], and acyclovir [10], this approach was essentially ineffective against vaccinia virus because of the biological differences in the TK homologs encoded by the herpesviruses and the orthopoxviruses.
The resurgence of interest in therapies for orthopoxvirus infections prompted a reexamination of the antiviral activity of many agents against these viruses including IDU [11,12]. The in vitro efficacy of IDU was confirmed against vaccinia and cowpox viruses and was similar to that of many other 5-substituted 2′-deoxyuridine analogs [13]. Administration of IDU was also shown to delay mortality of severe combined immune deficiency (SCID) mice infected with vaccinia virus and reduced tail lesion severity in immunocompetent animals [14], which was consistent with previously published data obtained using the tail lesion model [15]. These results indicated that this class of molecule was indeed effective against the orthopoxviruses, albeit at doses much higher than used to inhibit the replication of HSV. This was not surprising since most analogs were selected for their efficacy against the herpesviruses, but it inspired the search for related molecules with the potential for improved efficacy against the orthopoxviruses.
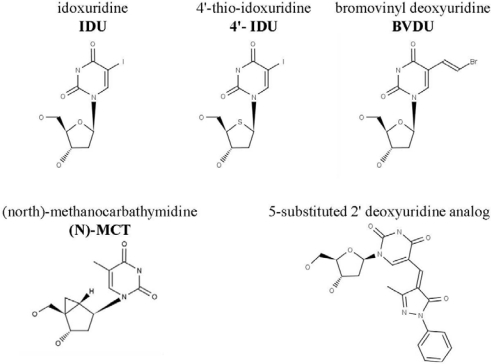
The activity of these analogs against the orthopoxviruses was reviewed recently [12], however recent efforts to identify compounds with antiviral activity against vaccinia virus have identified a number of additional analogs with good activity (Figure 1). The determination of the structure of the J2 TK [16], together with genetic and enzymatic studies confirmed that this enzyme expressed by vaccinia virus might be capable of phosphorylating a wider variety of thymidine analogs than previously thought, and was unanticipated [17]. N-methanocarbathymidine ((N)-MCT), which is active against some herpesviruses, also has good activity against the orthopoxviruses both in vitro and in vivo [18]. Several novel thymidine analogs from the laboratory of Paul Torrence (Northern Arizona University, Flagstaff, Arizona) also appeared to be good inhibitors of orthopoxvirus replication in cell culture [19–22]. The 4′-thio derivative of IDU (4′-thioIDU) has also been reported to have good antiviral activity against some herpesviruses [23,24], and recently has been shown to have excellent antiviral activity against the orthopoxviruses [25]. Related 6-azathymidine-4′-thionucleosides also exhibited activity against vaccinia virus, but did not appear to require TK for their mechanism of action [26]. These data were significant because it appeared that the modification of both the heterocycle as well as the deoxyribose sugar were tolerated and had the potential to enhance the antiviral activity against the orthopoxviruses. Thus, additional efforts exploring this series of molecules promise to identify compounds that interact with unique molecular targets of the orthopoxviruses and inhibit their replication. The complex phosphorylation pathways in orthopoxvirus infected cells offer opportunities to take advantage of the selective phosphorylation of these compounds and further reduce their toxicity. Recent developments with this class of compounds will be discussed to identify common themes and potential areas of research.
Figure 1.
Structure of selected thymidine analogs. Structures of thymidine analogs are shown with abbreviations in bold text. The specific example of a 5-substituted deoxyuridine analog is 1-(2-deoxypentofuranosyl)-5-(3-methyl-5-oxo-1-phenyl-4,5-dihydro-4Hpyrazol-4-ylidene)pyrimidine-2,4(1H,3H)-dione [17].
2. Molecular Targets of Thymidine Analogs in the Orthopoxviruses
Most compounds with antiviral activity against the orthopoxviruses are inhibitors of the DNA polymerase. Mutations that impart resistance to these compounds typically map to the E9L DNA polymerase gene and cluster in conserved domains [27]. Although mutations in this gene can confer resistance to compounds like cidofovir (CDV), they also inhibit viral replication in some cell lines and in animal models of infection [28–30]. Yet, large DNA viruses also encode a host of metabolic enzymes that alter the metabolism of nucleotides to promote the replication of the viral genome. The orthopoxviruses express several such enzymes and their role in viral replication and potential for targeting by antiviral drugs have been recently reviewed [31,32]. Four viral enzymes appear to be involved in phosphorylation and stability of deoxynucleoside and deoxynucleotide analogs.
Cells infected with vaccinia virus express a unique TK that can phosphorylate thymidine [33], and appears to influence the antiviral activity of a number of compounds [34]. This enzyme is encoded by the J2R gene and homologs are encoded by all the human orthopoxviruses [35]. These enzymes belong to the type II class of TKs and are closely related to the human cellular cytosolic TK (TK1) [36,37]. Like the host kinase, these homotetrameric enzymes phosphorylate a rather narrow range of substrates and are allosterically regulated by both thymidine diphosphate and thymidine triphosphate (dTTP) [38,39]. Genetic studies with TK negative mutants of vaccinia and cowpox viruses have identified a number of inhibitors with reduced efficacy in the absence of this gene, suggesting that they are substrates for the enzyme [34,40,41]. Additionally, mutations that confer resistance to 4′-thioIDU map to this gene [42]. Enzymatic studies also confirmed that the substrate specificity of the viral enzyme is reduced compared with TK1 and many more nucleoside analogs are efficiently phosphorylated by this enzyme [16,17].
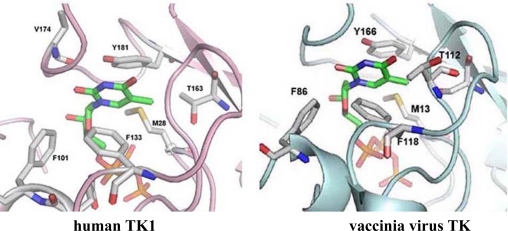
The structure of vaccinia virus TK in a complex with dTTP has been determined, and its substrate binding pocket appears to be slightly larger than that of the cellular enzyme such that it might accommodate slightly larger molecules [16]. Although this would be consistent with the current pharmacologic, genetic and enzymatic evidence, the crystallization of the allosteric effector in the active site of both the host and viral enzyme make it exceedingly difficult to utilize structural information in the design of new molecules (Figure 2). However, if the viral enzyme could be co-crystallized with other substrates it might provide valuable data to help understand differences in substrate specificity.
Figure 2.
Structure of the active sites of human TK1 (left) and vaccinia virus TK (right). Both the human and vaccinia virus enzymes co-purified with the allosteric effector, dTTP, which was resolved in the published three–dimensional structures of the enzymes. Shown is dTTP bound to the active site of both the human [43], and the viral enzyme [16]. (Figure provided by Debasish Chattopadhyay, University of Alabama at Birmingham).
The human orthopoxviruses also encode a thymidylate kinase (TMPK) that further phosphorylates thymidine monophosphate to the level of the diphosphate [44]. The structure of this enzyme has also been determined and is similar to that of the host homolog, although the association of the dimers results in a somewhat larger active site, which permits larger substrates such as BVDU monophosphate, which has been co-crystallized in the active site [45]. Enzymatic studies in this report also confirm the phosphorylation of this substrate by the enzyme and show that it can also play a role in the phosphorylation of antiviral drugs in infected cells. The enzyme also appears to phosphorylate deoxyguanosine monophosphate and related analogs, which are not substrates of the cellular TMPK [46,47]. Vaccinia virus also encodes DNA sequences that share homology with the cellular dGMP kinase gene and it has been hypothesized to possess this enzymatic activity [48]. However, the viral gene appears to be disrupted in all isolates of vaccinia virus examined to date and thus probably does not represent a legitimate viral gene.
The fourth gene of interest is the deoxyuridine triphosphatase (dUTPase) gene (F2L) that presumably minimizes the incorporation of dUTP into viral DNA [49–50]. This enzyme is not required for viral replication in vitro or in mice and its potential function in viral replication is unclear, except that it is predicted to be involved in the modulation of pyrimidine metabolites [51]. Recombinant viruses that do not express this protein do not appear to have altered susceptibility to IDU, although they do appear to be modestly hypersensitive to (N)-MCT. The significance of the latter observation is unclear given uncertainties of the effect of the enzyme on nucleotide pools and does not appear to shed light on the mechanism of action of the compound.
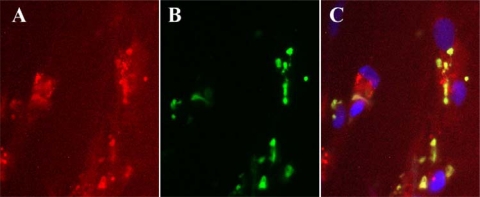
All of these enzymes have the potential to impact the antiviral activity of pyrimidine analogs in infected cells by influencing the formation of triphosphate metabolites that inhibit the viral DNA polymerase. Although it is possible to utilize this altered pathway of metabolism for the purposes of improving the selectivity of pyrimidine analogs, the pathways are incompletely understood. Understanding the impact of these viral enzymes on the antiviral activity on pyrimidine analogs is further complicated by host enzymes with partially overlapping substrate specificities, such as TK1 and the mitochondrial TK, which also phosphorylate IDU analogs. Since they are differentially expressed in the cell cycle, their impact on antiviral activity varies depending on the replication state of cell substrates [52], and perhaps the species from which the cells were derived [53]. Additionally, since viral DNA is synthesized in the cytoplasm, it is also possible that the formation of metabolites occurs within virus factories resulting in their sequestration in this compartment limiting their availability to the viral DNA polymerase in the synthesis of viral DNA. In fact, the specific incorporation of 5-bromodeoxyuridine (BrdU) into viral DNA was observed in infected cells utilizing a monoclonal antibody specific for the analog in the context of its incorporation into DNA (Figure 3). Complexities resulting from each of these factors, as well as specific binding of metabolites to the DNA polymerase can confound efforts to identify the best analogs in a series. For example, BVDU is a substrate of the vaccinia virus TK [39], and its monophosphate is further phosphorylated by the thymidylate kinase [45], yet the molecule is not particularly active in vitro [11,54]. Additional studies will be required to improve our understanding of this system and results from new active analogs promises to further this process.
Figure 3.
BrdU is incorporated into viral DNA within virus factories. (A) Cells infected with vaccinia virus were labeled with a virus-specific monoclonal antibody (red staining); (B) BrdU incorporated into viral DNA was visualized with a monoclonal antibody and localized to virus factories (green staining); (C) A merged image shown with nuclei labeled with DAPI show that the compound appears to be incorporated preferentially into viral DNA rather than in host DNA in the nuclei.
3. Antiviral Activity and Mechanism of Action of (N)-MCT
The thymidine analog, (N)-MCT, is a conformationally locked nucleoside analog with an (N)-methanocarba modification in the deoxyribose portion of the molecule and its synthesis and biological activity was reviewed recently [18]. This interesting molecule serves to illustrate the complexities of metabolism and interactions with molecular targets that result in antiviral activity. This compound exhibits antiviral activity against the alphaherpesviruses and appears to derive some of its specificity through selective phosphorylation by the TK homologs encoded by this group of viruses [55]. Although both (N)-MCT and the (South)-methanocarbathymidine analog, (S)-MCT, are substrates for herpes simplex virus type 1 (HSV-1) TK, (S)-MCT appears to be a better substrate for the enzyme, which was confirmed in co-crystallization studies that showed that the north conformation of the nucleobase in (N)-MCT induces a shift in isoleucine 197 of the enzyme relative to that of thymidine. A specific inhibitor of the HSV-1 TK (R0-32-2313) [56], was also used in further studies that suggested the phosphorylation pathways for (N)-MCT were complicated and also likely involved other cellular kinases [57]. Specific inhibition of the HSV-1 TK inhibited the formation of (N)-MCT diphosphate indicating that the thymidylate kinase activity associated with this enzyme was involved with the formation of this metabolite [58]. However, levels of the monophosphate were unaffected by the TK inhibitor suggesting that cellular kinases can also phosphorylate (N)-MCT. It is unclear which cellular enzymes might also participate in its phosphorylation however, since the compound is a poor substrate for the human cytosolic TK1 [59].
It is interesting that (N)-MCT is an inhibitor of HSV-1 and HSV-2 replication, while (S)-MCT is essentially inactive [60]. This result is consistent with studies in murine MC38 cells expressing the HSV-1 TK, where (N)-MCT is incorporated into cellular DNA by the cellular DNA polymerases at much higher levels than (S)-MCT, notwithstanding the much higher levels of (S)-MCT triphosphate [61]. Thus, the specificity of the HSV TK, unidentified host kinases and presumably the viral DNA polymerase all contribute to the antiviral activity of the compound.
The orthopoxviruses are also susceptible to the action of (N)-MCT, which inhibited the in vitro replication of both vaccinia and cowpox viruses at concentrations less than 2 μM (Table 1) [40]. However, the antiviral activity also appeared to be cell line dependent, with the best antiviral activity seen in murine cells, with less activity in other species including cells derived from the rabbit, monkey, and humans [53]. The compound is also effective in reducing mortality in mice infected with cowpox virus and vaccinia viruses (Table 2) [40,53,62].
Table 1.
Activity of thymidine analogs against vaccinia virus and cowpox virusa.
| Compound | Vaccinia virus (EC50, μM)b | Cowpox virus (EC50, μM) | Cytotoxicity (CC50, μM) |
|---|---|---|---|
| cidofovir | 19 ± 11 | 29 ± 6.1 | >317 ± 0 |
| idoxuridine | 8.4 ± 3.3 | 3.7 ± 2.7 | >100 ± 0 |
| fialuridine | 1.5 ± 0.4 | 0.2 ± 0.1 | >100 ± 0 |
| (N)-MCT | 0.6 ± 0.1 | 1.5 ± 1.2 | >100 ± 0 |
| 5-iodo-4′-thio-2′-deoxyuridine (4′-thioIDU) | 0.5 ± 0.2 | 0.1 ± 0.04 | >100 ± 0 |
| 1-(2-deoxy, 4′thio-β-D-ribofuranosyl)-thymidine | 0.03 ± 0.01 | 0.02 ± 0.01 | 29 ± 4.0 |
| 4-thio-β-D-arabinofuranosyl)-cytidine | 0.3 ± 0.2 | 1.6 ± 0.8 | 53 ± 6.4 |
| 1-(4-thio-β-D-arabinofuranosyl)-5-fluoro cytidine | 0.1 ± 0.01 | 0.4 ± 0.1 | 4.6 ± 1.1 |
| 5-iodo-4-thio-3′,5′-di-O-acetyl-2′-deoxyuridine | 0.9 ± 0.3 | 0.3 ± 0.2 | >80 ± 28 |
| 5-bromo-4′-thio-2′-deoxyuridine | 0.1 ± 0.02 | 0.05 ± 0.04 | >100 ± 0 |
| 5-trifluoromethyl-2′-deoxy-4′-thiouridine | 0.1 ± 0.004 | 0.1 ± 0.01 | >100 ± 0 |
Adapted from [25]
Concentration of compound sufficient to reduce viral replication by 50% (EC50).
Table 2.
Efficacy of (N)-MCT in BALB/c mice infected intranasally with vaccinia or cowpox virusa.
| Treatmentb | Mortality | P-value | MDDc | P-value | |
|---|---|---|---|---|---|
| Number | Percent | ||||
| Vaccinia virus | |||||
| vehicle | 15/15 | 100 | - | 7.9 | - |
| CDV | |||||
| 15 mg/kg | 0/15 | 0 | <0.001 | - | - |
| (N)-MCT | |||||
| 50 mg/kg | 0/15 | 0 | <0.001 | - | - |
| 16.7 mg/kg | 2/15 | 13 | <0.001 | 7.5 | NSd |
| 5.6 mg/kg | 12/15 | 80 | NS | 8.2 | NS |
| Cowpox virus | |||||
| vehicle | 15/15 | 100 | - | 9.6 | - |
| CDV | |||||
| 15 mg/kg | 0/15 | 0 | <0.001 | - | - |
| (N)-MCT | |||||
| 50 mg/kg | 2/15 | 13 | <0.001 | 7.5 | NS |
| 16.7 mg/kg | 3/15 | 20 | <0.001 | 13.3 | 0.01 |
| 5.6 mg/kg | 6/15 | 40 | <0.001 | 14.0 | 0.05 |
Adapted from [40];
(N)-MCT was prepared in 0.4% carboxymethylcellulose and delivered i.p. twice daily in 0.1 ml doses. CDV was prepared in sterile saline and given i.p. once daily in 0.1 ml doses. All animals were treated for 5 days beginning 24 h post infection;
Mean Day of Death;
Not significant.
The mechanism of action of this compound against the orthopoxviruses has not been examined in detail but preliminary work has provided a few insights. Like IDU, the compound exhibited reduced efficacy against a TK negative isolate of cowpox virus, which suggested that the viral kinase could phosphorylate the compound [40]. These data were confirmed with enzymatic studies that indicated the molecule is a substrate for the vaccinia virus TK with a Km similar to that of thymidine. In infected cells, vaccinia virus TK appeared to significantly increase the levels of the monophosphate metabolite in infected cells although no differences were observed in the levels of the triphosphate [41]. While these data are consistent with the viral TK phosphorylating (N)-MCT, it is complicated by cellular enzymes that also appear to phosphorylate the compound. The formation of the monophosphate metabolite also appears to occur in uninfected cells such the initial phosphorylation step by either the HSV or vaccinia virus TK homologs should not be absolutely required for antiviral activity [57]. In fact, in many cell lines it has not been possible to demonstrate that the vaccinia virus TK is significantly involved in the efficacy of the compound [53]. These data clearly indicate that cellular enzymes can play a significant role in the activation of the compound. While an obvious candidate cellular enzyme is the cytosolic TK (TK1), (N)-MCT has been shown to be a poor substrate for this enzyme and its contribution toward to the conversion of the monophosphate is thought to be negligible [59]. It is also possible that the mitochondrial enzyme (TK2) might phosphorylate the compound but at this time evidence for this is lacking. The phosphorylation of the compound in uninfected cells could potentially result in toxicity, which will need to be considered as the molecule undergoes further development.
The active metabolite of (N)-MCT that inhibits orthopoxvirus replication is presumed to be the triphosphate, but there is no direct evidence that it is a substrate for the viral DNA polymerase. Significant quantities of the triphosphate are observed in infected cells, although that quantity of this metabolite does not appear to correlate either with levels of the monophosphate, or the differential efficacy observed for cell lines derived from other species [41]. A recombinant virus that does not express the dUTPase appears to be modestly hypersensitive to (N)-MCT, but this does not necessarily imply that the active form is the triphosphate since specific effects of this enzyme on pyrimidine metabolism are poorly understood [51]. While this compound inhibits viral DNA replication with an efficacy comparable to that of its EC50 and is likely responsible for the inhibition of viral replication, it remains possible that this is an indirect effect and it may actually inhibit enzymes other than the DNA polymerase that are required for DNA replication.
4. Thymidine Analogs with Large Substituents at the 5 Position
Thymidine analogs with modifications at the 5 position have also been shown to be active against replication of vaccinia virus and have been reviewed recently [12]. Most substituents in this position have been small, notably halogens, amino, nitro and vinyl groups and some of these analogs are inhibitors of thymidylate synthetase [63]. More recent studies have investigated compounds with larger moieties that appear to retain antiviral activity against vaccinia virus [19]. Several publications have resulted from the evaluation of antiviral activity of these compounds synthesized in the laboratory of Dr. Torrence [17,19–22]. Most of the compounds in this series contain large substituents, including heterocyclic moieties, and inhibited the in vitro replication of vaccinia virus with EC50 values in the low micromolar range (Table 1) [20,22]. Subsequent studies indicated that the antiviral activity of the compounds was largely dependent on the orthopoxvirus TK [17]. All of the compounds tested proved to be good substrates for vaccinia virus TK with Km values comparable to or below that for thymidine and many were poor substrates for TK1 [17]. Thus, the larger binding pocket observed in vaccinia virus TK appears to accommodate the large substituents at the 5 position of deoxyuridine. The ultimate molecular target of these molecules is presumed to be the viral DNA polymerase, but has not been investigated.
Although this series of compounds exhibited good antiviral activity in vitro, it did not translate to in vivo activity since it was unable to reduce mortality in mice infected with vaccinia virus [64]. One of the compounds was shown to be degraded rapidly by porcine liver esterase [20], raising the possibility that poor pharmacokinetics are related to its lack of antiviral activity in animals.
5. Inhibition of Orthopoxvirus Replication with 4′-Thio Pyrimidine Analogs
Successes with (N)-MCT prompted an evaluation of additional thymidine analogs with modifications in the deoxyribose sugar. A series of 4′-thiopyrimidine analogs was synthesized and their antiviral activity against the alphaherpesviruses was reported previously [24]. Further studies evaluated a similar series against the alphaherpesviruses as well as cytomegalovirus (CMV) and some analogs, including 4′-thioIDU exhibited some activity against all the viruses [23]. The activity of this compound was subsequently evaluated against all the human herpesviruses including TK negative isolates of HSV and UL97 deficient isolates of CMV and showed that antiviral activity was dependent on the HSV TK homolog, but not the UL97 kinase in CMV [65]. Since the compound retains antiviral activity against CMV, which has no TK homolog, it appears that cellular enzymes are also capable of phosphorylating it to some degree. Consistent with this idea, EC50 values against TK negative strains of HSV are the same as those against CMV in primary human fibroblast cells. This report also utilized a monoclonal antibody to show that compound was incorporated into the DNA of HSV-2 and CMV and confirmed that they were substrates of the DNA polymerase. Furthermore, the incorporation into the host genome was noted in a subset of cells and confirmed that when cells were actively dividing, the compound was phosphorylated by cellular kinases and the triphosphate metabolite was a substrate for a host DNA polymerase. These results were expected and are similar to those observed with the related analogs BrdU and IDU [66].
The activity of 4′-thioIDU against the orthopoxviruses proved to be much greater than against the herpesviruses and was superior to that of IDU [25]. Several related 4′ pyrimidine analogs also had good antiviral activity (Table 1), but 4′-thioIDU proved to have the best combination of antiviral activity, low toxicity, and spectrum of antiviral activity and was subsequently selected by us for additional studies. This compound also was very active in animals infected with cowpox virus. Parenteral or oral administration of 5 mg/kg to infected mice significantly reduced mortality even if therapy was initiated 4 days after infection (Table 3), [25]. Subsequent studies also indicated that the compound was more potent than CDV and significantly reduced mortality with concentrations as low as 0.3 mg/kg [64]. This level of activity of 4′-thioIDU in mice is superior to that previously reported for IDU and (N)-MCT [14,40,67], and indicates that additional studies with this compound are warranted to determine its potential for use in treatment of orthopoxvirus infection of humans.
Table 3.
Effect of oral treatment with 4′-thioIDU on mortality of BALB/c mice inoculated intranasally with Cowpox Virus a.
| Treatmentb | Mortality | P-value | MDD + SDc | P-value | |
|---|---|---|---|---|---|
| Number | Percent | ||||
| Vehicle + 3 days | 14/15 | 93 | 11.9 ± 2.2 | ||
| CDV + 3 days | |||||
| 15 mg/kg | 0/14 | 0 | <0.001 | ||
| 4′-thioIDU + 3 days | |||||
| 15 mg/kg | 1/15 | 7 | <0.001 | 14.0 | NSc |
| 5 mg/kg | 0/15 | 0 | <0.001 | ||
| 1.5 mg/kg | 2/15 | 13 | <0.001 | 14.0 ± 5.7 | NSc |
| Vehicle + 4 days | 15/15 | 100 | 11.7 ± 2.3 | NS | |
| CDV + 4 days | |||||
| 15 mg/kg | 0/15 | 0 | <0.001 | ||
| 4′-thioIDU + 4 days | |||||
| 5 mg/kg | 4/15 | 27 | <0.001 | 13.3 ± 1.3 | <0.05 |
| Vehicle + 5 days | 12/15 | 80 | 14.6 ± 3.9 | ||
| CDV + 5 days | |||||
| 15 mg/kg | 4/15 | 27 | 0.01 | 10.5 ± 1.9 | 0.07 |
| 4′-thioIDU + 5 days | |||||
| 5 mg/kg | 13/15 | 87 | NSc | 9.8 ± 1.3 | <0.001 |
Adapted from [25];
4′-thioIDU was suspended in vehicle (10% DMSO in 0.4% CMC) and given orally in 0.2 ml doses. CDV was prepared in sterile saline and given i.p. in 0.1 ml doses. Animals were treated twice daily with vehicle or 4′-thioIDU for five days, except for CDV which was dosed once daily, beginning 3, 4 or 5 days post viral inoculation;
MDD = mean day of death; SD = standard deviation; NS = not significant when compared to the placebo control.
Further development of any molecule for the therapy of orthopoxvirus infections is dependent on its ability to inhibit the replication of virus isolates resistant to either CDV (or its prodrug, CMX001) or ST-246. Virus isolates that were resistant both drugs proved to be fully susceptible to 4′-thioIDU and confirmed that the compound possessed a mechanism of action distinct from either CDV, its lipophilic conjugated prodrug CMX001, or ST-246 (Table 4) [25]. Combinations of 4′-thioIDU together with either CMX001 or ST-246 synergistically inhibited viral replication in vitro, which is also consistent with each having a different mechanism of action [68]. A recombinant virus lacking the TK (VVTK::luc) also exhibited reduced susceptibility to 4′-thioIDU suggesting that it was involved in the activation of the compound [25]. This result was confirmed against TK positive and TK negative isolates of cowpox virus, and other studies in vaccinia virus that showed that 4′-thioIDU was a good inhibitor of viral DNA synthesis. Subsequent studies reported the selection of a 4′-thioIDU-resistant vaccinia virus isolate that acquired a 5 nucleotide deletion in the TK gene, resulting in a frameshift and a premature truncation following the first 51 amino acids of the protein but no mutations were observed in the DNA polymerase [42]. These data confirmed that the TK played a significant role in the mechanism of action of the compound. The resistant isolate also remained fully susceptible to both ST-246 as well as CMX001, consistent with the absence of cross resistance observed previously.
Table 4.
Activity of 4′-thioIDU Against Resistant Mutants of Vaccinia Virusa.
| Compound | WR (EC50, μM)b | CDVR 15 (EC50, μM) | VV911 (ST-246R) (EC50, μM) | VVTK::luc TK deficient (EC50, μM) |
|---|---|---|---|---|
| 4′-thioIDU | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.02 ± 0.003 | 0.3 ± 0.01 |
| CDV | 11 ± 1.5 | 62 ± 34 | 33 ± 5.0 | 9.4 ± 0.1 |
| ST-246 | 0.07 ± 0.01 | NDc | >20 ± 0 | NDb |
| IDU | 2.8 ± 0.3 | 1.0 ± 0.1 | 2.4 ± 0.4 | 7.1 ± 4.3 |
Adapted from [25];
Concentration required to reduce plaque formation by 50%. Values presented are the average of duplicate determinations with the standard deviations shown;
Not determined.
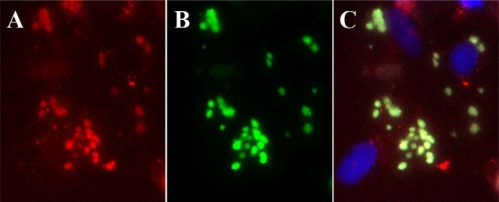
The use of monoclonal antibodies that are specific for IDU and BrdU molecules that have been incorporated into DNA also provided evidence to indicate that 4′-thioIDU is incorporated into DNA and provided a useful tool to study the mechanism of action of the compound [65]. Cells infected with vaccinia virus were exposed to 4′-thioIDU for 10 min prior to fixation, and its incorporation into DNA was visualized by immunofluorescence (Figure 4). The compound was incorporated into viral DNA within virus factories and indicated it was a substrate for the viral DNA polymerase and that its mechanism of action was likely similar to that of IDU. Furthermore, it appeared to be incorporated exclusively in viral DNA and no detectable staining of host DNA in the nucleus was observed, suggesting that it specifically targeted viral DNA synthesis and is similar to results shown with BrdU (Figure 2). However, some nuclear staining was identified in nuclei of uninfected cells which is also consistent with the phosphorylation and incorporation of the compound into host DNA during the S phase of the cell cycle (data not shown), as has been observed with BrdU and IDU [66]. It is unclear if the specific incorporation in viral DNA is a result of the selective phosphorylation and sequestration of the compound in virus factories or the inhibition of host DNA synthesis by the virus, but it illustrates the selective activity of this compound against vaccinia virus replication and is consistent with the antiviral data.
Figure 4.
4′-thioIDU is incorporated in virus factories. (A) Cells infected with vaccinia virus were labeled with a monoclonal antibody (red staining); (B) 4′-thioIDU incorporated in viral DNA was visualized with a monoclonal antibody and localized to virus factories (green staining); (C) a merged image shown with nuclei labeled with DAPI show that most of the compound appears to be incorporated in viral DNA rather than in host DNA in the nuclei.
Another report also documented the modest antiviral activity of another analog, dideoxy-6-azathymidine 4′-thionucleoside [26]. This compound did not appear to exhibit TK dependence and suggested that its mechanism of action was distinct from that of 4′-thioIDU.
6. Conclusions
The efficacy of pyrimidine analogs against the orthopoxviruses has been known for many years but recent concerns regarding the orthopoxviruses as weapons of bioterror have resulted in renewed interest in these compounds. Such analogs have many attractive qualities, particularly, their spectrum of antiviral activity that includes many DNA viruses including both the herpesviruses and the orthopoxviruses. Although the clinical benefit from this broader activity is modest, it could provide a viable development path for the development of a compound with antiviral against the orthopoxviruses. This will be critical for any candidate compounds proceeding to the clinic since it is impossible to conduct pivotal clinical studies against the orthopoxviruses, which would be required for their approval. However, if such a compound were also active against another DNA virus, such as a herpesvirus, it would provide an alternative mechanism for conducting clinical studies for determining efficacy and toxicity in humans and might prove to be economically viable. Once approved, the emergency use of the agent could then be approved for use against variola virus or monkeypox virus infections. Additional studies with these and other analogs may also serve to identify other molecules with desirable properties that might become available to treat orthopoxvirus infections in humans.
Acknowledgments
The authors thank Kathy Keith for help in preparation of the manuscript and the NIAID, NIH for funding some of the research reported here (N01-AI-30049).
References and Notes
- 1.Kaufman HE, Nesburn AB, Maloney ED. Cure of vaccinia infection by 5-iodo-2′-deoxyuridine. Virology. 1962;18:567–569. doi: 10.1016/0042-6822(62)90058-2. [DOI] [PubMed] [Google Scholar]
- 2.Prusoff WH. Synthesis and biological activities of iododeoxyuridine, an analog of thymidine. Biochim Biophys Acta. 1959;32:295–296. doi: 10.1016/0006-3002(59)90597-9. [DOI] [PubMed] [Google Scholar]
- 3.Calabresi P, Mc CR, Welch AD. Suppression of infections resulting from a deoxyribonucleic acid virus (vaccinia) by systemic adminstration of 5-iodo-2′-deoxyuridine. Nature. 1963;197:767–769. doi: 10.1038/197767a0. [DOI] [PubMed] [Google Scholar]
- 4.Loddo B, Muntoni S, Ferrari W. Effect of 5-iodo-2′-deoxyuridine on vaccinia virus in vitro. Nature. 1963;198:510. doi: 10.1038/198510a0. [DOI] [PubMed] [Google Scholar]
- 5.Prusoff WH, Bakhle YS, McCrea JF. Incorporation of 5-Iodo-2′-Deoxyuridine into the deoxyribonucleic acid of vaccinia virus. Nature. 1963;199:1310–1311. doi: 10.1038/1991310a0. [DOI] [PubMed] [Google Scholar]
- 6.Chen MS, Ward DC, Prusoff WH. Specific herpes simplex virus-induced incorporation of 5-iodo-5′-amino-2′,5′-dideoxyuridine into deoxyribonucleic acid. J Biol Chem. 1976;251:4833–4838. [PubMed] [Google Scholar]
- 7.Chen MS, Prusoff WH. Phosphorylation of 5-iodo-5′-amino-2′,5′,dideoxyuridine by herpes simplex virus type 1 encoded thymidine kinase. J Biol Chem. 1979;254:10449–10452. [PubMed] [Google Scholar]
- 8.Descamps J, De Clercq E. Specific phosphorylation of E-5-(2-iodovinyl)-2′-deoxyuridine by herpes simplex virus-infected cells. J Biol Chem. 1981;256:5973–5976. [PubMed] [Google Scholar]
- 9.De Clercq E. (E)-5-(2-bromovinyl)-2′-deoxyuridine (BVDU) Med Res Rev. 2005;25:1–20. doi: 10.1002/med.20011. [DOI] [PubMed] [Google Scholar]
- 10.Furman PA, De Miranda P, St Clair MH, Elion GB. Metabolism of acyclovir in virus-infected and uninfected cells. Antimicrob Agents Chemother. 1981;20:518–524. doi: 10.1128/aac.20.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kern ER. In vitro activity of potential anti-poxvirus agents. Antivir Res. 2003;57:35–40. doi: 10.1016/S0166-3542(02)00198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Clercq E. Vaccinia virus inhibitors as a paradigm for the chemotherapy of poxvirus infections. Clin Microbiol Rev. 2001;14:382–397. doi: 10.1128/CMR.14.2.382-397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Clercq E. Antiviral and antitumor activities of 5-substituted 2′-deoxyuridines. Methods Find Exp Clin Pharmacol. 1980;2:253–267. [PubMed] [Google Scholar]
- 14.Neyts J, Verbeken E, De Clercq E. Effect of 5-iodo-2′-deoxyuridine on vaccinia virus (orthopoxvirus) infections in mice. Antimicrob Agents Chemother. 2002;46:2842–2847. doi: 10.1128/AAC.46.9.2842-2847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Clercq E, Luczak M, Shugar D, Torrence PF, Waters JA, Witkop B. Effect of cytosine, arabinoside, iododeoxyuridine, ethyldeoxyuridine, thiocyanatodeoxyuridine, and ribavirin on tail lesion formation in mice infected with vaccinia virus. Proc Soc Exp Biol Med. 1976;151:487–490. doi: 10.3181/00379727-151-39241. [DOI] [PubMed] [Google Scholar]
- 16.El Omari K, Solaroli N, Karlsson A, Balzarini J, Stammers DK. Structure of vaccinia virus thymidine kinase in complex with dTTP: Insights for drug design. BMC Struct Biol. 2006;6:22. doi: 10.1186/1472-6807-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prichard MN, Keith KA, Johnson MP, Harden EA, McBrayer A, Luo M, Qiu S, Chattopadhyay D, Fan X, Torrence PF, Kern ER. Selective phosphorylation of antiviral drugs by vaccinia virus thymidine kinase. Antimicrob Agents Chemother. 2007;51:1795–1803. doi: 10.1128/AAC.01447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marquez VE, Hughes SH, Sei S, Agbaria R. The history of N-methanocarbathymidine: The investigation of a conformational concept leads to the discovery of a potent and selective nucleoside antiviral agent. Antivir Res. 2006;71:268–275. doi: 10.1016/j.antiviral.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Fan X, Zhang X, Zhou L, Keith KA, Kern ER, Torrence PF. Assembling a smallpox biodefense by interrogating 5-substituted pyrimidine nucleoside chemical space. Antivir Res. 2006;71:201–205. doi: 10.1016/j.antiviral.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Fan X, Zhang X, Zhou L, Keith KA, Kern ER, Torrence PF. 5-(Dimethoxymethyl)-2′-deoxyuridine: A novel gem diether nucleoside with anti-orthopoxvirus activity. J Med Chem. 2006;49:3377–3382. doi: 10.1021/jm0601710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan X, Zhang X, Zhou L, Keith KA, Kern ER, Torrence PF. A pyrimidine-pyrazolone nucleoside chimera with potent in vitro anti-orthopoxvirus activity. Bioorg Medicinal Chem Letter. 2006;16:3224–3228. doi: 10.1016/j.bmcl.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 22.Fan X, Zhang X, Zhou L, Keith KA, Prichard MN, Kern ER, Torrence PF. Toward orthopoxvirus countermeasures: A novel heteromorphic nucleoside of unusual structure. J Med Chem. 2006;49:4052–4054. doi: 10.1021/jm060404n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahim SG, Trivedi N, Bogunovic-Batchelor MV, Hardy GW, Mills G, Selway JW, Snowden W, Littler E, Coe PL, Basnak I, Whale RF, Walker RT. Synthesis and anti-herpes virus activity of 2′-deoxy-4′-thiopyrimidine nucleosides. J Med Chem. 1996;39:789–795. doi: 10.1021/jm950029r. [DOI] [PubMed] [Google Scholar]
- 24.Secrist JA, 3rd, Tiwari KN, Riordan JM, Montgomery JA. Synthesis and biological activity of 2′-deoxy-4′-thio pyrimidine nucleosides. J Med Chem. 1991;34:2361–2366. doi: 10.1021/jm00112a007. [DOI] [PubMed] [Google Scholar]
- 25.Kern ER, Prichard MN, Quenelle DC, Keith KA, Tiwari KN, Maddry JA, Secrist JA., 3rd Activities of certain 5-substituted 4′-thiopyrimidine nucleosides against orthopoxvirus infections. Antimicrob Agents Chemother. 2009;53:572–579. doi: 10.1128/AAC.01257-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jasamai M, Balzarini J, Simons C. 6-Azathymidine-4′-thionucleosides: Synthesis and antiviral evaluation. J Enzyme Inhib Med Chem. 2008;23:56–61. doi: 10.1080/14756360701442340. [DOI] [PubMed] [Google Scholar]
- 27.Gammon DB, Snoeck R, Fiten P, Krecmerova M, Holy A, De Clercq E, Opdenakker G, Evans DH, Andrei G. Mechanism of antiviral drug resistance of vaccinia virus: Identification of residues in the viral DNA polymerase conferring differential resistance to antipoxvirus drugs. J Virol. 2008;82:12520–12534. doi: 10.1128/JVI.01528-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrei G, Gammon DB, Fiten P, De Clercq E, Opdenakker G, Snoeck R, Evans DH. Cidofovir resistance in vaccinia virus is linked to diminished virulence in mice. J Virol. 2006;80:9391–9401. doi: 10.1128/JVI.00605-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smee DF, Wandersee MK, Bailey KW, Hostetler KY, Holy A, Sidwell RW. Characterization and treatment of cidofovir-resistant vaccinia (WR strain) virus infections in cell culture and in mice. Antivir Chem Chemother. 2005;16:203–211. doi: 10.1177/095632020501600306. [DOI] [PubMed] [Google Scholar]
- 30.Becker MN, Obraztsova M, Kern ER, Quenelle DC, Keith KA, Prichard MN, Luo M, Moyer RW. Isolation and characterization of cidofovir resistant vaccinia viruses. Virol J. 2008;5:58. doi: 10.1186/1743-422X-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prichard MN, Kern ER. Orthopoxvirus targets for the development of antiviral therapies. Curr Drug Targets Infect Disord. 2005;5:17–28. doi: 10.2174/1568005053174627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prichard MN, Kern ER. Antiviral targets in orthopoxviruses. In: LaFemina RL, editor. Antiviral Research: Strategies in Antiviral Drug Discovery. ASM Press; Washington, DC, USA: 2009. pp. 167–186. [Google Scholar]
- 33.Kit S, Piekarski LJ, Dubbs DR. Induction of thymidine kinase by vaccinia-infected mouse fibroblasts. J Mol Biol. 1963;6:22–33. doi: 10.1016/s0022-2836(63)80078-9. [DOI] [PubMed] [Google Scholar]
- 34.Prichard MN, Williams AD, Keith KA, Harden EA, Kern ER. Distinct thymidine kinases encoded by cowpox virus and herpes simplex virus contribute significantly to the differential antiviral activity of nucleoside analogs. Antivir Res. 2006;71:1–6. doi: 10.1016/j.antiviral.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Lefkowitz EJ, Wang C, Upton C. Poxviruses: Past, present and future. Virus Res. 2006;117:105–118. doi: 10.1016/j.virusres.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Black ME, Hruby DE. Site-directed mutagenesis of a conserved domain in vaccinia virus thymidine kinase. Evidence for a potential role in magnesium binding. J Biol Chem. 1992;267:6801–6806. [PubMed] [Google Scholar]
- 37.Hruby DE, Maki RA, Miller DB, Ball LA. Fine structure analysis and nucleotide sequence of the vaccinia virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1983;80:3411–3415. doi: 10.1073/pnas.80.11.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Black ME, Hruby DE. A single amino acid substitution abolishes feedback inhibition of vaccinia virus thymidine kinase. J Biol Chem. 1992;267:9743–9748. [PubMed] [Google Scholar]
- 39.Solaroli N, Johansson M, Balzarini J, Karlsson A. Substrate specificity of three viral thymidine kinases (TK): Vaccinia virus TK, feline herpesvirus TK, and canine herpesvirus TK. Nucleosides Nucleotides Nucleic Acids. 2006;25:1189–1192. doi: 10.1080/15257770600894451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prichard MN, Keith KA, Quenelle DC, Kern ER. Activity and mechanism of action of N-methanocarbathymidine against herpesvirus and orthopoxvirus infections. Antimicrob Agents Chemother. 2006;50:1336–1341. doi: 10.1128/AAC.50.4.1336-1341.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smee DF, Humphreys DE, Hurst BL, Barnard DL. Antiviral activities and phosphorylation of 5-halo-2′-deoxyuridines and N-methanocarbathymidine in cells infected with vaccinia virus. Antivir Chem Chemother. 2008;19:15–24. doi: 10.1177/095632020801900103. [DOI] [PubMed] [Google Scholar]
- 42.Harden EA, Keith KA, Daily S, Tiwari K, Maddry J, Secrist J, Kern ER, Prichard MN. Mutation of the thymidine kinases encoded by herpes simplex virus or vaccinia virus can confer resistance to 5-iodo-4′-thio-2′-deoxyuridine. Antivir Res. 2009;82:A48. [Google Scholar]
- 43.Birringer MS, Claus MT, Folkers G, Kloer DP, Schulz GE, Scapozza L. Structure of a type II thymidine kinase with bound dTTP. FEBS Lett. 2005;579:1376–1382. doi: 10.1016/j.febslet.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 44.Hughes SJ, Johnston LH, De Carlos A, Smith GL. Vaccinia virus encodes an active thymidylate kinase that complements a cdc8 mutant of Saccharomyces cerevisiae. J Biol Chem. 1991;266:20103–20109. [PubMed] [Google Scholar]
- 45.Caillat C, Topalis D, Agrofoglio LA, Pochet S, Balzarini J, Deville-Bonne D, Meyer P. Crystal structure of poxvirus thymidylate kinase: An unexpected dimerization has implications for antiviral therapy. Proc Natl Acad Sci U S A. 2008;105:16900–16905. doi: 10.1073/pnas.0804525105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Topalis D, Collinet B, Gasse C, Dugue L, Balzarini J, Pochet S, Deville-Bonne D. Substrate specificity of vaccinia virus thymidylate kinase. FEBS J. 2005;272:6254–6265. doi: 10.1111/j.1742-4658.2005.05006.x. [DOI] [PubMed] [Google Scholar]
- 47.Auvynet C, Topalis D, Caillat C, Munier-Lehmann H, Seclaman E, Balzarini J, Agrofoglio LA, Kaminski PA, Meyer P, Deville-Bonne D, El Amri C. Phosphorylation of dGMP analogs by vaccinia virus TMP kinase and human GMP kinase. Biochem Biophys Res Commun. 2009;388:6–11. doi: 10.1016/j.bbrc.2009.07.089. [DOI] [PubMed] [Google Scholar]
- 48.Goebel SJ, Johnson GP, Perkus ME, Davis SW, Winslow JP, Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990;179:247–266. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- 49.Broyles SS. Vaccinia virus encodes a functional dUTPase. Virology. 1993;195:863–865. doi: 10.1006/viro.1993.1446. [DOI] [PubMed] [Google Scholar]
- 50.Roseman NA, Evans RK, Mayer EL, Rossi MA, Slabaugh MB. Purification and characterization of the vaccinia virus deoxyuridine triphosphatase expressed in Escherichia coli. J Biol Chem. 1996;271:23506–23511. doi: 10.1074/jbc.271.38.23506. [DOI] [PubMed] [Google Scholar]
- 51.Prichard MN, Kern ER, Quenelle DC, Keith KA, Moyer RW, Turner PC. Vaccinia virus lacking the deoxyuridine triphosphatase gene (F2L) replicates well in vitro and in vivo, but is hypersensitive to the antiviral drug (N)-methanocarbathymidine. Virol J. 2008;5:39. doi: 10.1186/1743-422X-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keith KA, Harden EA, Gill R, Marquez VE, Kern ER, Prichard MN. Efficacy of N-methanocarbathymidine against herpes simplex virus is cell cycle dependent. Antivir Res. 2010;86:A58. [Google Scholar]
- 53.Smee DF, Wandersee MK, Bailey KW, Wong MH, Chu CK, Gadthula S, Sidwell RW. Cell line dependency for antiviral activity and in vivo efficacy of N-methanocarbathymidine against orthopoxvirus infections in mice. Antivir Res. 2007;73:69–77. doi: 10.1016/j.antiviral.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 54.Sauerbrei A, Meier C, Meerbach A, Schiel M, Helbig B, Wutzler P. In vitro activity of cycloSal-nucleoside monophosphates and polyhydroxycarboxylates against orthopoxviruses. Antivir Res. 2005;67:147–154. doi: 10.1016/j.antiviral.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Schelling P, Claus MT, Johner R, Marquez VE, Schulz GE, Scapozza L. Biochemical and structural characterization of (South)-methanocarbathymidine that specifically inhibits growth of herpes simplex virus type 1 thymidine kinase-transduced osteosarcoma cells. J Biol Chem. 2004;279:32832–32838. doi: 10.1074/jbc.M313343200. [DOI] [PubMed] [Google Scholar]
- 56.Martin JA, Thomas GJ, Merrett JH, Lambert RW, Bushnell DJ, Dunsdon SJ, Freeman AC, Hopkins RA, Johns IR, Keech E, Simmonite H, Kai-In PW, Holland M. The design, synthesis and properties of highly potent and selective inhibitors of herpes simplex virus types 1 and 2 thymidine kinase. Antivir Chem Chemother. 1998;9:1–8. [PubMed] [Google Scholar]
- 57.Zalah L, Huleihel M, Manor E, Konson A, Ford H, Jr, Marquez VE, Johns DG, Agbaria R. Metabolic pathways of N-methanocarbathymidine, a novel antiviral agent, in native and herpes simplex virus type 1 infected Vero cells. Antivir Res. 2002;55:63–75. doi: 10.1016/s0166-3542(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 58.Chen MS, Walker J, Prusoff WH. Kinetic studies of herpes simplex virus type 1-encoded thymidine and thymidylate kinase, a multifunctional enzyme. J Biol Chem. 1979;254:10747–10753. [PubMed] [Google Scholar]
- 59.Prota A, Vogt J, Pilger B, Perozzo R, Wurth C, Marquez VE, Russ P, Schulz GE, Folkers G, Scapozza L. Kinetics and crystal structure of the wild-type and the engineered Y101F mutant of Herpes simplex virus type 1 thymidine kinase interacting with (North)-methanocarba-thymidine. Biochemistry. 2000;39:9597–9603. doi: 10.1021/bi000668q. [DOI] [PubMed] [Google Scholar]
- 60.Marquez VE, Siddiqui MA, Ezzitouni A, Russ P, Wang J, Wagner RW, Matteucci MD. Nucleosides with a twist. Can fixed forms of sugar ring pucker influence biological activity in nucleosides and oligonucleotides. J Med Chem. 1996;39:3739–3747. doi: 10.1021/jm960306+. [DOI] [PubMed] [Google Scholar]
- 61.Marquez VE, Ben-Kasus T, Barchi JJ, Jr, Green KM, Nicklaus MC, Agbaria R. Experimental and structural evidence that herpes 1 kinase and cellular DNA polymerase(s) discriminate on the basis of sugar pucker. J Am Chem Soc. 2004;126:543–549. doi: 10.1021/ja037929e. [DOI] [PubMed] [Google Scholar]
- 62.Smee DF, Hurst BL, Wong MH, Glazer RI, Rahman A, Sidwell RW. Efficacy of N-methanocarbathymidine in treating mice infected intranasally with the IHD and WR strains of vaccinia virus. Antivir Res. 2007;76:124–129. doi: 10.1016/j.antiviral.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Clercq E, Descamps J, Huang GF, Torrence PF. 5-Nitro-2′-deoxyuridine and 5-nitro-2′-deoxyuridine 5′-monophosphate: Antiviral activity and inhibition of thymidylate synthetase in vivo. Mol Pharmacol. 1978;14:422–430. [PubMed] [Google Scholar]
- 64.Quenelle DC. 2010. University of Alabama at Birmingham, Birmingham, AL, USA. Personal Communication.
- 65.Prichard MN, Quenelle DC, Hartline CB, Harden EA, Jefferson G, Frederick SL, Daily SL, Whitley RJ, Tiwari KN, Maddry JA, Secrist JA, 3rd, Kern ER. Inhibition of herpesvirus replication by 5-substituted 4′-thiopyrimidine nucleosides. Antimicrob Agents Chemother. 2009;53:5251–5258. doi: 10.1128/AAC.00417-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 67.Smee DF, Sidwell RW. Anti-cowpox virus activities of certain adenosine analogs, arabinofuranosyl nucleosides, and 2′-fluoro-arabinofuranosyl nucleosides. Nucleosides Nucleotides Nucleic Acids. 2004;23:375–383. doi: 10.1081/ncn-120028334. [DOI] [PubMed] [Google Scholar]
- 68.Keith KA, Sanders S, Tiwari K, Maddry J, Secrist J, Jordan R, Hruby D, Lanier R, Painter G, Kern ER, Prichard MN. Combinations of 5-iodo-4′-thio-2′-deoxyuridine and ST-246 or CMX001 synergistically inhibit orthopoxvirus replication in vitro. Antivir Res. 2009;82:A33–A34. [Google Scholar]