Abstract
The 3′ end of the human T-cell leukemia/lymphoma virus type-1 (HTLV-1) genome contains four overlapping open reading frames (ORF) that encode regulatory proteins. Here, we review current knowledge of HTLV-1 orf-I and orf-II protein products. Singly spliced mRNA from orf-I encodes p12, which can be proteolytically cleaved to generate p8, while differential splicing of mRNA from orf-II results in production of p13 and p30. These proteins have been demonstrated to modulate transcription, apoptosis, host cell activation and proliferation, virus infectivity and transmission, and host immune responses. Though these proteins are not essential for virus replication in vitro, p8, p12, p13, and p30 have an important role in the establishment and maintenance of HTLV-1 infection in vivo.
Keywords: human T-cell leukemia/lymphoma virus type-1, HTLV-1, ORF-I, ORF-II, p8, p12, p13, p30
1. Introduction
Human T-cell leukemia/lymphoma virus type-1 (HTLV-1) is an oncogenic retrovirus first discovered in 1980 in T-cells of a patient with cutaneous T-cell lymphoma [1,2]. HTLV-1 is the etiological agent of two major diseases: adult T-cell leukemia (ATL), a disease characterized by malignant proliferation of CD4+ T-lymphocytes, and tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM), a neurodegenerative condition [3,4]. HTLV-1 is also associated with other clinical disorders including HTLV-1-associated arthropathy, HTLV-1-associated uveitis, infective dermatitis, and polymyositis [5,6]. HTLV-1 primarily infects CD4+ T-cells and has been detected in ex vivo CD8+ T-cells, dendritic cells (DC), and B-cells from infected individuals. While cell-free virions have been shown to efficiently infect DCs in vitro, HTLV-1 is believed to be transmitted to T-cells and DCs mostly by cell-to-cell contact through a virological synapse, biofilm-like extracellular viral assemblies, or cellular conduits [7–10]. An estimated 10–20 million people worldwide are infected with HTLV-1 [11]. While the majority of HTLV-1-infected individuals remain asymptomatic, a low percentage of patients develop either ATL (3–5%) or TSP/HAM (0.3–2%) after a long period of clinical latency [12–19].
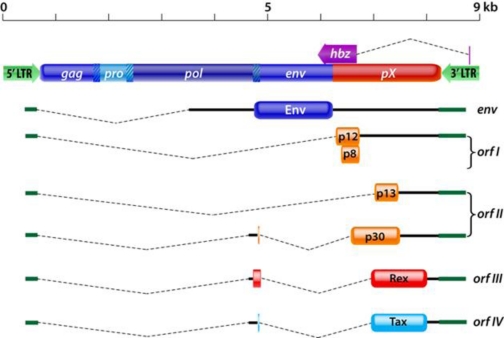
As shown in Figure 1, the HTLV-1 genome contains the typical retroviral structural and enzymatic genes gag, pro, pol, and env [13]. In addition, a region located between env and the 3′ long terminal repeat (LTR), contains four partially overlapping open reading frames (ORF) [13]. This unique region encodes several regulatory proteins through the use of alternative splicing and internal initiation codons [20–22]. Orf-I produces the p12 protein which can be proteolytically cleaved at the amino terminus to generate the p8 protein, while differential splicing of mRNA from orf-II results in production of the p13 and p30 proteins [20–23]. Orf-III and orf-IV encode for the Rex and Tax proteins, respectively, and an antisense mRNA transcribed from the 3′ LTR that generates the HTLV-1 basic leucine zipper (HBZ) protein [24–26].
Figure 1.
A scheme of the human T-cell leukemia/lymphoma virus type-1 (HTLV-1) genome. Spliced mRNAs and encoded proteins for orf-I and orf-II are shown. Orf-I encodes for the p12 protein which can be proteolytically cleaved at the amino terminus to generate the p8 protein. The p30 protein is translated from doubly spliced mRNA transcribed from orf-II and the 5′ end of env. The p13 protein is translated from singly spliced mRNA transcribed from orf-II and corresponds to the carboxyl terminus of p30.
Tax and Rex are required for viral replication. Tax is a potent transcriptional transactivator of viral gene expression. Tax also regulates the expression of several cellular genes, including those involved in cell proliferation, cell cycle progression, apoptosis, and DNA damage responses. Rex is a post-transcriptional regulator that facilitates nuclear export of unspliced and singly spliced viral mRNA. In addition, Rex inhibits splicing and transport of doubly spliced mRNA. HBZ is a negative regulator of Tax-mediated transactivation and thus suppresses viral expression. For further detailed information about Rex, Tax, and HBZ, the reader is referred to recent reviews [27–30]. In this review, we will focus on the current knowledge of the functions of the proteins encoded by orf-I and orf-II: p8, p12, p13, and p30.
In contrast to Tax and Rex, orf-I and orf-II are dispensable for viral replication in vitro yet are important for viral persistence in vivo [31]. Early work demonstrated that in the rabbit model, orf-I was required for viral infectivity while orf-II was required to maintain high viral load [32,33]. Further work in the rabbit model showed reversion of HTLV-1 clones lacking p30 to the wildtype p30-expressing virus, suggesting the importance of p30 to HTLV-1 viral persistence [34]. However, in these early studies the HTLV-1 clones that were used contained a frameshift that affected hbz, making it unclear as to whether these effects were due to the loss of hbz or orf-I and orf-II-encoded proteins. In a more recent study, the ablation of p12/p8, p30, or HBZ impaired the establishment of persistent infection in the macaque model [35]. Nevertheless, ablation of these proteins did not affect viral replication in the rabbit model [35]. The orf-I and orf-II-encoded proteins are able to modulate a diverse range of viral and cellular mechanisms including transcriptional regulation, mitochondrial function, cell cycle progression, host cell activation and proliferation, apoptosis, virus infectivity and transmission, and host immune responses. Though these proteins are not essential for virus replication in vitro, p8, 12, p13, and p30 have an important role in the establishment and maintenance of HTLV-1 infection in vivo.
2. HTLV-1 p12 and p8
HTLV-1 orf-I encodes the 99 amino acid p12 protein which can be proteolytically cleaved at the amino terminus to generate the p8 protein (Figure 1). Computational analysis of the amino acid sequence of p12 predict the existence of a noncanonical endoplasmic reticulum (ER) retention/retrieval signal between amino acids 1–5, two putative leucine zipper (LZ) motifs, two putative transmembrane domains between amino acids 12–30 and amino acids 48–67, a calcineurin-binding motif between amino acids 70–86, four putative proline-rich (PXXP) Src homology 3 (SH3)-binding domains, and a putative adaptin motif [23,36]. These structural features may contribute to protein localization, homodimerization, and protein-protein interactions. The p12 protein exhibits amino acid similarity with a portion of the bovine papillomavirus (BPV)-transforming E5 protein, except that E5 does not carry putative SH3 binding motifs [37,38]. The p12 protein undergoes complex post-translational modifications through proteolytic cleavage. The first cleavage occurs between amino acid positions 9 and 10 and is followed by a second cleavage between amino acids 29 and 30 [23]. The first proteolytic cleavage removes the ER retention/retrieval signal at the amino terminus of p12, while the second cleavage generates the p8 protein [23]. The p12 protein localizes to cellular endomembranes, particularly within the ER and Golgi apparatus, while p8 traffics to lipid rafts at the cell surface and is recruited to the immunological synapse upon T-cell receptor (TCR) ligation [23,39–41].
The singly spliced mRNA encoding p12/p8 has been detected in vitro and in ex vivo HTLV-1-infected T-cells and macrophages [42]. The p12 recombinant protein is recognized in serum from humans infected with HTLV-1 and rabbits experimentally infected with HTLV-1 [43]. In addition, a cytotoxic T-lymphocyte (CTL) response to orf-I products can be detected in HTLV-1-infected individuals [44]. Two natural variants of the p12 protein have been identified; one variant carries a lysine residue at position 88 and is commonly found in HTLV-1 strains from TSP/HAM patients while the second variant carries an arginine residue at position 88 and is found in HTLV-1 strains from all ATL patients and healthy carriers studied. The R88 variant protein has a much greater stability compared to the K88 variant, which is ubiquitinated and rapidly degraded by the proteasome [45].
2.1. T-Cell Signaling
2.1.1. Calcium Release
The p12 protein resides in the endoplasmic reticulum, which has a role in protein and lipid synthesis, carbohydrate metabolism, and calcium concentration regulation. Within the ER, p12 is able to mediate an increase in cytosolic calcium in T-cells by increasing calcium release from the ER through inositol trisphosphate receptors and from capacitative calcium entry through Ca2+ channels at the plasma membrane in response to the lower ER calcium content (Figure 2(1)) [46,47]. By depleting ER calcium stores and increasing cytosolic calcium, p12 is able to modulate a range of processes including T-cell proliferation, viral replication, and viral spread. Early studies on orf-I showed that p12 is able to activate nuclear factor of activated T-cells (NFAT), which is dependent on calcium-binding proteins for its dephosphorylation and nuclear import, to increase T-cell proliferation (Figure 2(2)) [46–48]. Furthermore, p12 can impact other calcium-regulated proteins, including the transcriptional coactivator p300, which can modulate transcription of viral genes from the HTLV-1 LTR [49,50]. Moreover, p12 can promote cell-to-cell viral spread by inducing lymphocyte function-associated antigen 1 (LFA-1) clustering on T-cells through a calcium-dependent mechanism (Figure 2(5)) [51].
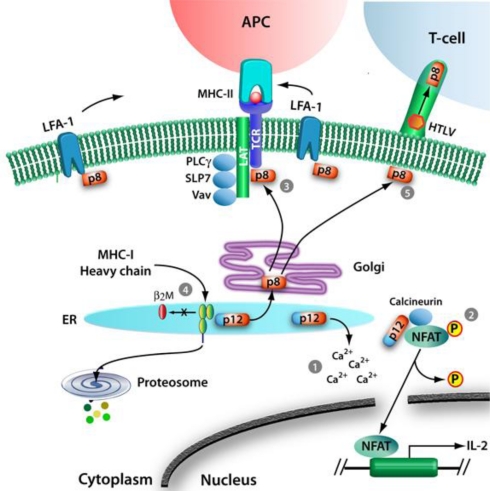
Figure 2.
Functions of p12 and p8. In the ER, p12 is proteolytically cleaved at the amino terminus to generate p8, which traffics to the cell surface through the secretory pathway. (1) In the ER, p12 mediates Ca2+ release, which enables (2) either calcineurin binding of NFAT and subsequent dephosphorylation, nuclear translocation, and upregulation of the IL-2 gene, or p12 binding to calcineurin and inhibition of NFAT activation. (3) Upon trafficking through the secretory pathway, p8 localizes at the immunological synapse where it interacts with LAT and inhibits proximal TCR signaling. (4) In the ER, p12 binds the immature heavy chains of the MHC-I and prevents their interactions with the β2-microglobulin, leading MHC-I degradation by the proteosome. (5) At the cell surface, p8 increases the clustering of LFA-1 and the formation of intracellular conduits and facilitates viral transmission to target cells.
2.1.2. NFAT Activation and Signaling
Prior to the discovery of p12 cleavage, expression of orf-I-encoded proteins (ORF-I) was shown to enhance T-cell proliferation [46,51,52]. Early studies found that ORF-I was able to mediate activation of NFAT, a transcription factor that regulates activation, proliferation, and differentiation of T-cells [46–48]. In uninfected cells, NFAT can be activated through a complex TCR signaling cascade. Following TCR engagement at the cell surface, the protein tyrosine kinases Lck and Fyn phosphorylate TCRζ and the CD3 subunits. These phosphorylated domains become the docking sites for ZAP70. Activated ZAP70 phosphorylates linker for activation of T-cells (LAT), which then binds and activates phospholipase C-γ-1 (PLCγ1), leading to the production of inositol-1,4,5-trisphosphate and release of Ca2+ from ER calcium stores. The increase in cytosolic calcium activates calmodulin and calcineurin, which dephosphorylate NFAT, allowing for NFAT nuclear import. As discussed in Section 2.1.1, by modulating the regulation of cytosolic calcium levels, p12 is able to mediate NFAT activation and does so independent of the proximal TCR signaling molecules, LAT and PLCγ1 (Figure 2(2)) [47]. Interestingly, p12 is able to bind calcineurin and the calcineurin-binding motif of p12 is homologous to the calcineurin-binding motif of NFAT (Figure 2(2)) [48]. Thus, p12 could both enhance and inhibit NFAT activation by competing with NFAT for calcineurin binding. Further studies found that p8, which localizes at the cell surface, is also able to downregulate NFAT activity, but in a LAT-dependent manner (Figure 2(3)) [52].
2.1.3. Proximal T-Cell Signaling and T-Cell Anergy
Recent studies indicate that p8 decreases T-cell activation by inhibiting proximal T-cell receptor signaling [52]. Upon ligation of the TCR to the major histocompatibility complex class II (MHC-II) of an antigen presenting cell, p8 localizes to the immunological synapse where it decreases phosphorylation of LAT, PLCγ1, and Vav by a LAT-dependent mechanism (Figure 2(3)) [23,52]. By dampening TCR signaling, p8 downregulates NFAT activation, a crucial pathway in T-cell activation [47,52]. Furthermore, the induction of T-cell anergy, a state in which T-cells become unresponsive to TCR stimulation, results in decreased Tax activity and HTLV-1 replication [52]. Lastly, since it has been recently shown that p8 transfers to neighboring cells, it is possible that p8-induced T-cell anergy allows a safe transfer of the virus to target cells [9]. These results may underlie the finding that HTLV-1-infected individuals experience some immune deficiency and are susceptible to opportunistic infections [53,54].
2.1.4. IL-2 Receptor Activation and STAT5 Signaling
HTLV-1-infected T-cells proliferate in the absence of IL-2 and this IL-2 independence correlates with constitutive activation of the Janus-associated kinase and signal transducer and activator of transcription (JAK-STAT) pathway, a transcription factor cascade that affects cell proliferation, differentiation, and apoptosis [55]. Early work showed that ORF-I did not have a role in IL-2 independence since it did not affect expression of the interleukin-2 receptor (IL-2R) or IL-2 responsiveness [56]. Also, expression of ORF-I did not affect phosphorylation of JAK-STAT proteins [56]. However, more recent studies have demonstrated that ORF-I binds the β and γc chains of the immature IL-2R [57]. This interaction stabilizes the IL-2R β and γc chains in a pre-Golgi compartment and prevents their trafficking to the plasma membrane, leading to a decrease in IL-2R at the cell surface [57]. Specifically, ORF-I binds the 20 amino acid region proximal to amino acid 350 of the IL-2R β chain that is critical for JAK1 and JAK3 recruitment, which occurs after IL-2 signaling [58]. The interaction of ORF-I and IL-2R leads to an increase in STAT5 phosphorylation and DNA binding activity in the absence of IL-2 [58]. This effect is dependent on the presence of the β and γc chains and JAK3 [58]. By binding the IL-2R, ORF-I decreases the requirement of IL-2 for proliferation in T-cells in the presence of suboptimal antigen stimulation [58].
2.2. MHC-I Degradation
HTLV-1 modulates T-cell activation and, in addition, has evolved mechanisms to avoid immune recognition of infected cells. On the cell surface, MHC-I complexes present peptides to TCRs of cytotoxic T-lymphocyte. In the case of virus-infected cells, this interaction leads to recognition of viral peptides and destruction of infected cells. Prevention of MHC-I expression is useful to a number of viruses to maintain the balance between the host and pathogen. Adenovirus E19 protein can retain MHC-I in the ER by interacting with the α1 and α2 regions of class I heavy chains through a dilysine motif [59]. HCMV type I membrane glycoproteins US2 and US11 target MHC-I heavy chains for degradation by the proteasome [60]. Additionally, HIV Nef and Vpu proteins accelerate endocytosis of MHC-I complexes and bind to and destabilize newly synthesized MHC-I, respectively [61–63]. In the ER, p12 binds to newly synthesized MHC-I heavy chains and prevents them from associating with the β2-microglobulin, a component of the mature MHC-I complex (Figure 2(4)) [40]. Since improperly assembled proteins are removed from the ER for degradation, the p12-mediated inhibition of MHC-I heavy chain association with the β2-microglobulin leads to its degradation by the proteasome and results in decreased MHC-I cell surface expression. By decreasing antigen presentation through degradation of the MHC-I, p12 may diminish presentation of viral peptides and decrease recognition by cytotoxic T-lymphocytes.
2.3. Modulation of ICAM
Natural killer (NK) cells recognize and destroy cells that express low levels of MHC-I at the cell surface. ORF-I decreases MHC-I expression to inhibit presentation of viral proteins to cytotoxic T-lymphocytes, which could make HTLV-1-infected cells susceptible to NK cell cytotoxicity [40,64]. In contrast, HTLV-1-infected T-cells are resistant to NK cell-mediated killing [64]. This resistance can be moderately ameliorated by pretreatment of NK cells with IL-2 [64]. Early data demonstrated that several ATL cells lines had altered expression of intercellular cell adhesion molecule 1 (ICAM-1), a glycoprotein that facilitates the interaction between NK cells and T-cells [65]. Furthermore, the majority of HTLV-1-infected primary CD4+ T-cells do not express ligands for the NK cell activating receptors, natural cytotoxicity receptors, and NKG2D [64]. Recent work has elucidated these findings as it is now known that ORF-I decreases expression of ICAM-1 and ICAM-2, but not ICAM-3, in T-cells. Thus, ORF-I inhibits NK cell adhesion to T-cells and prevents virus-infected cells from being recognized in the presence of low levels of MHC-I [64].
2.4. V-ATPase
The BPV E5 oncoprotein interacts with the 16 kDa subunit of the H+ vacuolar ATPase (V-ATPase), resulting in alkalization of the Golgi apparatus [66,67]. The sequence homology between HTLV-1 ORF-I and BPV E5 led to the demonstration that p12 interacts with 16 kDa subunit of the V-ATPase [68,69]. The transmembrane domains of ORF-I appear to be dispensable for binding to the V-ATPase, while conservation of the proline-rich domains between amino acids 36 and 48 contributes to the strength of this interaction [37,69]. The 16 kDa protein is a membrane component of the V-ATPase, which is also found in clathrin coated vesicles, lysosomes, endosomes, Golgi vesicles, endoplasmic reticulum, and synaptic vesicles. This proton pump is responsible for the acidification of these intracellular vesicles [70]. The atypical function of the proton pump through binding of viral proteins such as HTLV-I p12 and BPV E5 proteins may interfere in functions like the dissociation of receptor-ligand complexes and trafficking within the endosomal/lysosomal compartment. In addition, the acidification is essential for the formation of endosome carrier vesicles, which are intermediates between early and late endosomes [71,72]. HTLV-1 is known to infect dendritic cells and the acidification of lysosomes could play an important role in virus entry [7,73,74]. Indeed, the ablation of ORF-I expression impairs HTLV-1 replication in dendritic cells [35].
2.5. Modulation of Virus Transmission in vitro and in vivo
HTLV-1 requires orf-I in vivo to establish a persistent viral infection [33,35]. Early studies reported that orf-I expression was necessary for HTLV-1 infection in the rabbit model [33]. However, these early studies used HTLV-1 clones that, in addition to deleting orf-I, produced a frameshift affecting the gene encoding HBZ. Therefore, it is unclear whether these results are due to deletion of hbz, orf-I, or both. More recently, HTLV-1 molecular clones with nucleotide mutations were used to selectively disrupt orf-I expression. This study shows orf-I is essential for infectivity in the macaque model but not in the rabbit model [35]. Orf-I expression in HTLV-1-infected T-cells enhances virus transmission to target cells [75]. Independent of IL-2, ORF-I increases chemotaxis to facilitate the migration of infected cells toward target cells [75]. Importantly, HTLV-1 infection requires cell-cell contact for efficient transmission through a virological synapse, biofilm-like extracellular viral assemblies, or cellular conduits [8–10,76]. Transfer of virus between cells at the virological synapse requires polarization of cytoskeletal proteins and adhesion molecules toward the site of cellular contact [8]. Recent evidence suggests that p8, one of the two orf-I products, modulates the clustering of the adhesion molecule LFA-1 to increase the formation of cell-cell contacts and facilitate virus transfer (Figure 2(5)) [9,51]. In addition, p8 promotes the formation of thin membranous cellular conduits, which allows intracellular communication between several cell types [9,77,78]. Through these conduits, the HTLV-1 proteins p8, Gag, and Env are transferred to target T-cells [9]. Altogether, p8 promotes cellular contacts to favor HTLV-1 transmission.
3. HTLV-1 p30
Initially identified in 1992, the p30 protein is translated from doubly spliced monocistronic mRNA, containing exons 1, 2, and B, transcribed from HTLV-1 orf-II (Figure 1) [20,21,79]. p30 is a highly basic protein with a net positive charge that contains three nuclear localization signals (NLS1, NLS2, and NLS3) located between amino acids 66–73, 91–98, and 200–241 and an arginine-rich nucleolar localization/retention (NoRS) domain between amino acids 73–78 [80]. p30 also contains a Rex-binding domain (RexBD) between amino acids 131–164, a p300-binding domain between amino acids 1–132, and a DNA-binding domain between amino acids 100–179 [81]. The DNA-binding domain has been shown to repress LTR-mediated transcription [82]. Notably, HTLV-1 p30 has low genetic variability and is similar to HTLV-2 p28, suggesting a conserved mechanism for negative modulation of virus replication [79,83,84]. p30 shares distant similarities with some human serine-rich transcriptional activators such as Oct-1, Oct-2, Pit-1, and POU-M1 [22]. The p30 protein localizes within the nucleus and nucleolus. p30 shows high mobility within the nucleus, yet it is strongly retained in the nucleolus. Specifically, p30 is located in a granular component where ribosome subunits are assembled and de novo mRNA is produced. This localization is consistent with the ability of p30 to bind the ribosomal subunit L18a and to retain in the nucleus the newly transcribed tax/rex mRNA (Figure 3(1)) [80]. Since L18a and the eukaryotic initiation factor 3 facilitate re-initiation of translation in the cytoplasm, it is possible that p30 translocates from the nucleoli to the cytoplasm [80]. Similarly, p30 is specifically delocalized from the nucleoli to the nucleoplasm upon DNA damage to interfere with DNA repair processes [85]. In addition, p30 nucleolar retention signal mutants have similar functionality as wildtype p30, which raises questions about the function of nucleolar localization. A recent hypothesis suggests that p30 retention within nucleoli may serve as a reservoir for when the protein is needed in the nucleus [85]. Interestingly, it has recently been shown that hbz mRNA can regulate the production of p30, probably acting as an antisense RNA to silence its expression (Figure 3(4)) [86]. Localization of p30 within the nucleus and nucleolus suggest that this protein may mediate critical cellular processes such as cell cycle progression, DNA repair, and mRNA export [87]. Though the main cellular target of HTLV-1 is CD4+ T-cells, the virus is able to infect CD8+ T-cells, B-cells, macrophages, and dendritic cells. Intriguingly, recent studies have shown that in a macaque model, ablation of p30 within the HTLV-1 provirus severely affects infectivity and leads to reversion of the virus to the wild type genotype [35]. This observation has been confirmed in vitro by p30 knockout in HTLV-1-infected human primary and monocyte-derived dendritic cells, in which infection is not sustained over time. In T-cells, p30 is not required in vitro for efficient viral replication [35].
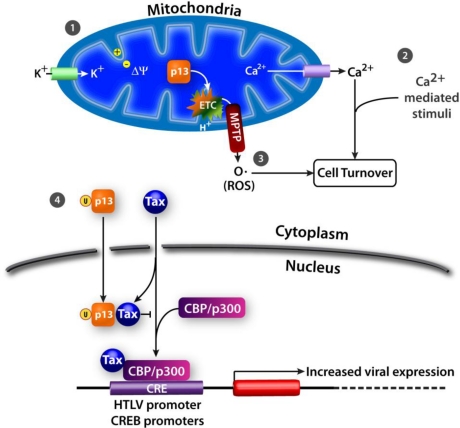
Figure 3.
Functions of p30. (1) Alternatively double spliced mRNA is translated to form the Tax and Rex regulatory proteins. (2) Tax protein localizes to the nucleus to exert its function on the LTR as a positive regulator of viral transcription. (3) Within the nucleus, Rex recognizes the Rex-responsive elements (RexRE) of viral mRNA and shuttles these transcripts to the cytoplasm while inhibiting splicing processes. However, some of the viral RNA is processed in the spliced env mRNA, the double spliced p30, (4) and the alternatively spliced tax/rex mRNA. p30 mRNA is subject to negative regulation by hbz mRNA. Once p30 protein is produced, it translocates to the nucleus and (5) interacts with p30-responsive elements (p30RE) created by the double splicing and therefore is present on tax/rex mRNA only. Moreover, p30 interacts with Rex to inhibit Rex-mediated nuclear export of double spliced viral mRNA, including tax transcripts. By preventing Tax production, p30 decreases viral transcription.
3.1. Inhibition of Nuclear Export of Tax/Rex mRNA
In contrast to HTLV-1 Tax and Rex, which enhance viral replication, p30 promotes virus latency by retaining tax/rex mRNA within the nucleus to prevent its export to the cytoplasm (Figure 3(5)) [88]. By downregulating Tax and Rex production, p30 suppresses viral replication. p30 interacts with the p30 mRNA-responsive element (p30RE) of tax/rex mRNA and with Rex at the RexBD [89]. Interestingly, p30RE spans the exon junction created after env mRNA is spliced, hence p30 binds spliced tax/rex mRNA but not the unspliced and singly spliced viral RNA [81]. The interplay between Rex and p30 is a regulatory switch between viral replication and latency. Rex binds with high affinity to the Rex-responsive element (RexRE) at the 3′ end of viral mRNA and, together with CRM1, shuttles unspliced gag/pol and singly spliced env transcripts to the cytoplasm (Figure 3(3)). Once bound to mRNA, Rex is no longer accessible to binding by p30 and is able to shuttle transcripts to the cytoplasm. However, viral mRNA-bound p30 efficiently interacts with Rex, but tax/rex transcripts are still retained in the nucleus (Figure 3(5)) [89]. Nuclear retention of viral mRNA is reversed by an excess of Rex, which displaces p30 from the p30RE. A further spliced version of tax/rex has been found in HTLV-infected cells, p21rex. During splicing of p21rex, the p30RE is removed from rex mRNA, which allows the transcript to escape p30-mediated nuclear retention. However, the function of p21rex is still unknown [88,90,91]. Thus, by retaining tax/rex mRNA, p30 decreases the translation of these two positive regulator of viral replication and promotes latency to escape host immune surveillance and to favor propagation through cell division and clonal expansion of infected cells.
3.2. Repression of the CRE Pathway
In addition to its posttranscriptional activity, p30 has been shown to function as either a transcriptional activator or repressor. The ability of p30 to induce transcriptional activation in vitro is CBP/p300-dependent [92]. CBP/p300 are known binding partners of CREB and Tax and are required for strong activation of the viral LTR [92]. Several other cellular and viral proteins bind CBP/p300, including members of the Jun-family, c-Myb, c-Fos, STAT1/2, NF-κB, p53, and TATA-binding protein (TBP) [82]. p30 disrupts CREB-Tax-p300 complex formation on the TRE (Tax-responsive element) of the viral LTR, resulting in repression of HTLV-1 transcription [82,93]. p30 has also been reported to differentially regulate transcription from the viral TRE and cellular CREB-responsive elements (CRE) in vitro and in vivo independent of Tax expression [94,95]. While p30 has been shown to repress CRE-driven gene expression in a dose-dependent manner, low concentrations of p30 enhance LTR activity [95,96]. Therefore, the expression level of p30 may play an important role in its function in the cell. Because p30 suppresses Tax production by retaining tax/rex mRNA in the nucleus, it affects CRE- and TRE-mediated transcriptional activation [88]. Interestingly, histone acetyltransferase (HAT) activity of p300 modulates p30-dependent transcriptional downregulation, whereas p30-dependent LTR repression is enhanced by deacetylation and inhibited by acetylation [82,93].
3.3. Transcriptional and Posttranscriptional Regulation
Microarray gene expression analyses of human T-cells showed that HTLV-1 p30 affects a number of cellular genes at the transcriptional level. p30 alters expression of a variety of gene families including those that have a role in transcription, translation, cell cycle progression, DNA replication and repair, cell signaling, angiogenesis, cell migration, and apoptosis [97]. Furthermore, p30 is able to retain some cellular transcripts within the nucleus, similarly to viral tax/rex mRNA. Included among these transcripts are MDM4, which is a regulator of p53, and HDAC3, which is a histone deacetylases involved in transcriptional repression [97]. Moreover, p30 has been shown to interact with the cellular transcription factor PU.1 in human macrophages. PU.1 is involved in a variety of cellular pathways including signal transduction by Toll-like receptor-4 (TLR-4) [98]. The interaction of p30 and PU.1 leads to inhibition of the DNA binding and transcriptional activity of PU.1. This, together with the p30-mediated inhibition of GSK-3β, yields decreased expression of TLR-4 at the cell surface, resulting in decreased secretion of pro-inflammatory cytokines, such as MCP-1, TNF-α and IL-8, and an increase of the anti-inflammatory cytokine IL-10 [98].
3.4. Cell Cycle and DNA Repair
Expression of p30 results in the accumulation of T-cells in the G2 phase of the cell cycle. p30 is able to enhance phosphorylation and activation of check point kinase 1 (Chk1). Chk1 is activated by ATM/ATR kinase following single strand DNA damage and results in a G2 arrest of the cell cycle [99]. p30 specifically binds to ataxia-telangiectasia mutated (ATM) and regulator of 20S proteasome activators γ (REGγ) in multiprotein high molecular weight complexes. By binding to ATM, p30 prolongs cell survival following DNA damage by inhibiting ATM autophosphorylation and subsequent activation of proteins involved in DNA repair, cell cycle check points, and apoptosis [100]. The effect of p30 interactions with REGγ remains to be determined. REGγ promotes formation of the REG-20S proteasome complex and has a critical role in a number of cellular processes, such as cell cycle progression and transcriptional regulation, and can degrade proteins in a ATP- and ubiquitin-independent manner. REGγ is localized in the nucleus where it interacts with and stabilizes p30, thereby altering p30 turnover [100]. In addition, p30 is able to bind to and prevent complex formation of cyclin E and cyclin-dependent kinase 2 (CDK2). Disruption of this complex prevents phosphorylation of retinoblastoma and subsequent E2F-mediated transcription, two key steps for G1/S transition [101]. By inhibiting cyclin E-CDK2 complex formation, p30 delays entry of cells into the S phase of the cell cycle. The inhibition of cell cycle progression is consistent with the observation that dendritic cells isolated from peripheral blood of ATL patients are unable to stimulate proliferation of CD4+ and CD8+ T-cells [102]. p30 is also able to interact with multiple proteins involved in DNA repair processes [85]. Upon DNA damage, p30 specifically delocalizes from the nucleolus to interact with and affect correct assembly of MRN complexes (Mre11-Rad50-Nbs1). MRN complexes are a key factor of DNA repair and contribute to homologous recombination (HR) during the S phase of the cell cycle. In contrast, during G1 or M phases, DNA damage is preferentially repaired by the nonconservative nonhomologous end joining (NEHJ) pathway. By interacting with these complexes, p30 activates a shift from conservative HR to the error-prone NEHJ pathway, thus favoring an accumulation of genomic alterations. Overall, these observations suggest that p30 could contribute to the accumulation of mutations that are characteristic of transformed HTLV-1-infected T-cells.
3.5. Requirement of orf-II in Viral Persistence in Animal Models
Despite the effort of multiple laboratories to define the role of HTLV-1 p30, its exact function and relevance in vivo still remains elusive. It has been previously shown in vitro that the ablation of p30 expression does not affect viral infectivity of HTLV-1 in human primary cells and that p30 is dispensable for viral replication and immortalization of primary human T-lymphocytes [31,103]. It should be noted that the in vitro infectivity of p30-ablated HTLV-1 was not sustained over time in primary dendritic cell [35]. Other observations, such as the presence of antibodies to p30 during infection may provide evidence of the importance of p30 in vivo [32,104]. It was first shown that the ablation of p30 expression in vivo result is a dramatic decrease of HTLV-1 viral load in a rabbit model [32]. However, the mutation introduced to prematurely stop the translation of p30 affected hbz as well, whose ablation alone decreases viral replication [105]. By inserting an artificial 24 base pair linker containing a premature termination codon in the p30 ORF, a significant frameshift occurred in the antisense hbz ORF [32]. The consequence of this frameshift is unknown, but it most likely affected hbz expression. Later studies have used different mutations to ablate p30 expression while preserving hbz [35]. These in vivo studies showed that p30 is not necessary for HTLV-1 infectivity in a rabbit model as viral replication was not affected by p30 ablation [35]. In contrast, the mutation of p30 severely affects virus infectivity in macaques, although a sufficient level of viral replication occurred to allow the reversion to wild type p30 over time [35].
4. HTLV-1 p13
Differential splicing of mRNA from HTLV-1 orf-II results in production of the p13 protein [20–22,106]. p13 is translated from singly spliced monocistronic mRNA to form a highly basic 87 amino acid protein that corresponds to the carboxyl terminus of p30 (Figure 1) [21]. The p13 protein has been predicted to contain a short hydrophobic leader sequence between amino acids 1–5, an amino terminus mitochondrial targeting signal (MTS) in a positively charged amphipathic alpha helix between amino acids 22–31, a transmembrane domain between amino acids 30–40, a flexible hinge region between amino acids 42–48, and a carboxyl terminus β-sheet hairpin structure between amino acids 65–75 that is homologous to an α-bungarotoxin-binding peptide [107–109]. The carboxyl terminus region contains multiple PXXP motifs that may mediate Src homology 3 (SH3) ligand binding. In addition, the carboxyl terminus region contains a cryptic nuclear localization sequence (NLS) [80]. The p13 protein is mainly localized within the inner membrane of mitochondria [107,108]. However, when expressed at high levels, p13 is able to localize within the nucleus and, when coexpressed with Tax, is directed to nuclear speckles [39]. Localization of the p13 protein within mitochondria and the nucleus suggests that this protein may modulate effects on apoptosis and transcriptional regulation (Figure 4).
Figure 4.
Functions of p13. In mitochondria, p13 mediates (1) K+ influx, inner mitochondrial membrane potential, and electron transport chain activity to affect (2) Ca2+ signaling and (3) ROS production. (4) In the presence of Tax, p13 is ubiquitinated and translocates to the nucleus. In the nucleus, p13 inhibits Tax-CBP/p300 complex formation to decrease transcription of cellular and viral genes.
4.1. K+ Influx, Inner Mitochondrial Membrane Potential, and Electron Transport Chain Activity
The p13 protein was first shown to induce changes in mitochondrial morphology and distribution [107,108,110]. Mitochondria of p13-expressing cells are clustered and have a rounded ring or crescent-like form that differs from the typical filamentous shape and interconnected mitochondrial network that occurs in normal cells. The rounded shape of mitochondria in p13-expressing cells is formed by osmotic swelling in response to energy-dependent uptake of monovalent cations, such as K+ [107,108,110]. p13 mediates this effect by altering the inner mitochondrial membrane potential (Δψ) to change K+ permeability of these organelles. The effects of p13 on K+ permeability are dose-dependent (Figure 4(1)). At low concentrations, p13 induces mitochondrial swelling without causing mitochondrial depolarization or cytochrome c release which is reversed by mitochondrial depolarization using protonophores. Cytochrome c is a component of the electron transport chain in mitochondria and is involved in initiation of apoptosis. At higher concentrations, p13 induces irreversible mitochondrial swelling, depolarization, and cytochrome c release. The change in p13-mediated mitochondrial morphology is similar to, but distinct from, the changes induced by the mitochondrial permeability transition pore (MPTP), a large nonspecific channel that regulates cytochrome c release and apoptosis.
p13-induced K+ influx and mitochondrial membrane depolarization stimulates electron transport and mitochondrial respiration, which increases O2 consumption [110]. Modulation of respiratory chain activity by p13 is accompanied by increased mitochondrial reactive oxygen species (ROS) production (discussed in Section 4.3) [110,111]. Increased ROS levels, together with mitochondrial membrane depolarization, decrease the opening threshold of the MPTP to promote pro-apoptotic signaling. Interestingly, at low concentrations of p13, increased electron transport chain activity dampens the effects of p13 on Δψ by extruding H+ from the matrix [110].
4.2. Ca2+ Homeostasis
Changes in mitochondrial Δψ also regulate intracellular Ca2+ homeostasis (Figure 4(2)) [107,110,112,113]. This effect is tightly linked to the ability of p13 to induce mitochondrial K+ influx and depolarization [113]. A p13 peptide was found to induce rapid efflux of Ca2+ from preloaded mitochondria [107]. Though p13 reduces mitochondrial Ca2+ uptake, it does not significantly affect overall change in cytosolic Ca2+ concentration, suggesting that p13-mediated mitochondrial depolarization may alter Ca2+ concentration only locally [113]. By altering Ca2+ homeostasis, p13 increases the sensitivity of cells to Ca2+-mediated stimuli [112]. Increased apoptosis is observed in p13-expressing cells upon treatment with C2 ceramide, which induces influx of Ca2+ into mitochondria and opening of the MPTP [112]. Additionally, treatment of cells with histadine results in a rise in cytosolic Ca2+ levels, leading to phosphorylation of CREB on serine 133 [112]. When p13 is expressed in cells treated with histadine, there is increased nuclear accumulation of phosphorylated CREB [112]. In cells with mitochondrial defects, such as is observed in p13-expressing cells, increased CREB phosphorylation has been shown to impair cell proliferation [114].
4.3. ROS Production
In isolated mitochondria, p13 increases ROS production and this effect is associated with K+ influx, mitochondrial membrane depolarization, and activation of the electron transport chain (Figure 4(3)) [110,111]. Unexpectedly, in transformed T-cells cultured using standard conditions, p13 does not increase ROS production [111]. However, in response to glucose deprivation, p13 increases ROS production and cell death in these cells [111]. There is a distinct gradient of ROS accumulation between primary and transformed T-cells, with very low levels observed in resting cells, higher levels in stimulated cells, and substantially higher levels in transformed cells [111]. In contrast to the effects of p13 on transformed T-cells, expression of p13 in unstimulated primary T-cells induces ROS-dependent T-cell activation and proliferation [111]. Thus, by increasing mitochondrial ROS production, p13 mediates activation of primary resting T-cells while promoting cell death in transformed T-cells. By modulating ROS levels in T-cells, it is possible that p13 has a role in lifelong persistence of HTLV-1 in the host by increasing the pool of untransformed infected cells while decreasing the number of transformed cells.
4.4. Effects on Apoptosis
Expression of p13 reduces proliferation rates of transformed cells in vitro and tumor growth in vivo. As discussed in Section 4.1, MPTP-mediated mitochondrial swelling and altered permeability has a key role in inducing apoptosis. Though p13 triggers similar effects as the MPTP, in addition to inducing cristae fragmentation, it does not directly cause apoptosis or cytochrome c release [108]. Instead, p13 increases cell sensitivity to pro-apoptotic stimuli such as Fas ligand (FasL), C2 ceramide, and glucose deprivation [111,115]. The effects of p13 on FasL-mediated apoptosis are enhanced by overexpression of Ras and antagonized by inhibiting Ras farnesylation and subsequent activation [115]. Upon FasL stimulation, farnesylated Ras traffics to mitochondria and directly binds Bcl-2 to inhibit the anti-apoptotic effects of Bcl-2 [115]. Treatment of p13-expressing cells with C2 ceramide results in increased influx of Ca2+ into mitochondria followed by opening of the MPTP, leading to cell death [111]. During glucose deprivation, p13 promotes apoptosis by increasing ROS production in transformed T-cells [111]. Consistent with the central role of mitochondria in energy production, cation flux, and apoptosis, p13 is able to affect this organelle to influence cell turnover.
4.5. Nuclear Effects
In cells expressing Tax, p13 becomes ubiquitinated and is partially localized within the nucleus (Figure 4(4)) [116]. Interestingly, Tax mediates ubiquitination of p13 though this protein contains no lysine residues. Instead, p13 is likely ubiquitinated on serine and threonine residues and this modification increases the stability of the protein. Within the nucleus, ubiquitinated p13 associates with Tax to inhibit its binding to the CBP/p300 transcriptional coactivator [116]. A decrease in Tax-CBP/p300 complex formation results in decreased Tax-mediated viral gene transcription [116]. Thus, intracellular localization of p13 may be an additional regulatory switch between viral replication and latency.
4.6. In Vivo Animal Model
CTLs and antibodies that recognize orf-II peptides can be detected in HTLV-1-infected individuals, suggesting that p13 may have an important role in vivo [104,117]. In an early study to examine the role of p13 alone in vivo, a molecular clone of HTLV-1 mutated to selectively ablate p13 failed to establish viral infection in a rabbit model [118]. However, similar to the rabbit model studies discussed in Sections 2.5 and 3.5, it is unknown whether the mutation that ablated p13 also affected expression of HBZ since the amino acid change that ablated the start codon for p13 in this study would also affect the start codon for HBZ [118]. No studies have been completed that examine the role of p13 alone in the macaque model.
5. Conclusions
HTLV-1-associated diseases have long periods of clinical latency with infected individuals having life-long persistence of viral-infected T-cell clones. Thus, HTLV-1-infected T-cells must be able to avoid immune recognition. As transcription of the provirus could lead to antigen presentation and immune recognition, it is necessary for HTLV-1 to maintain low levels of virus replication. In vivo studies demonstrate that HTLV-1 requires orf-I and orf-II for viral persistence. Since these proteins play critical roles in T-cell activation, MHC-I trafficking, cytokine expression, and virus replication, they are likely crucial for HTLV-1 maintenance of low level of virus expression in vivo. Together, p12 and p8 decrease ICAM-1, ICAM-2, and MHC-I expression at the cell surface, limiting the ability of NK cells and CTL cells to recognize infected cells. In addition, p8 inhibits proximal TCR signaling upon TCR stimulation to prevent T-cell activation. In contrast, within the ER, p12 promotes Ca2+ release and NFAT activation in resting cells. Thus, p12 decreases the IL-2 requirement for proliferation in the presence of suboptimal antigen presentation. Altogether, these data suggest that the concerted expression of p12 and p8 modulate T-cell activation and antigen presentation while promoting proliferation of resting cells, therefore providing new target cells for the virus. Orf-II protects HTLV-1-infected cells from immune system recognition by dampening the transcriptional effects of Tax. In the nucleus, p30 binds and prevents export of tax/rex mRNA to the cytosol for translation and p13, upon ubiquitination, inhibits Tax-CBP/p300 complex formation. This results in a decrease in the transcriptional activity of Tax and subsequent virus replication. In macrophages, p30 favors the production of anti-inflammatory IL-10 while decreasing the expression of pro-inflammatory cytokines by interacting with the transcription factor PU.1. Furthermore, like orf-I, p30 and p13 promote proliferation of resting T-cells. p30 inhibits DNA repair mechanisms to favor the growth and persistence of infected cells. p13 modulates the production of ROS and proliferation of resting cells while promoting apoptosis of transformed cells. The understanding of how these proteins simultaneously protect HTLV-1-infected cells from destruction and promote their expansion leads to new questions. Where, when, and at what level these proteins are expressed in vivo still must be elucidated. The answers to these questions will indicate whether these proteins may provide new targets for HTLV-1 therapeutics.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Health, National Cancer Institute, Center for Cancer Research.
References and Notes
- 1.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. U. S. A. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poiesz BJ, Ruscetti FW, Mier JW, Woods AM, Gallo RC. T-cell lines established from human T-lymphocytic neoplasias by direct response to T-cell growth factor. Proc. Natl. Acad. Sci. U. S. A. 1980;77:6815–6819. doi: 10.1073/pnas.77.11.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallo RC. The first human retrovirus. Sci. Am. 1986;255:88–98. doi: 10.1038/scientificamerican1286-88. [DOI] [PubMed] [Google Scholar]
- 4.Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, de The G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe T. HTLV-1-associated diseases. Int. J. Hematol. 1997;66:257–278. doi: 10.1016/s0925-5710(97)00077-7. [DOI] [PubMed] [Google Scholar]
- 6.Buggage RR. Ocular manifestations of human T-cell lymphotropic virus type 1 infection. Curr. Opin. Ophthalmol. 2003;14:420–425. doi: 10.1097/00055735-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat. Med. 2008;14:429–436. doi: 10.1038/nm1745. [DOI] [PubMed] [Google Scholar]
- 8.Igakura T, Stinchcombe JC, Goon PK, Taylor GP, Weber JN, Griffiths GM, Tanaka Y, Osame M, Bangham CR. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- 9.Van Prooyen N, Gold H, Andresen V, Schwartz O, Jones K, Ruscetti F, Lockett S, Gudla P, Venzon D, Franchini G. Human T-cell leukemia virus type 1 p8 protein increases cellular conduits and virus transmission. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20738–20743. doi: 10.1073/pnas.1009635107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pais-Correia AM, Sachse M, Guadagnini S, Robbiati V, Lasserre R, Gessain A, Gout O, Alcover A, Thoulouze MI. Biofilm-like extracellular viral assemblies mediate HTLV-1 cell-to-cell transmission at virological synapses. Nat. Med. 2009;16:83–89. doi: 10.1038/nm.2065. [DOI] [PubMed] [Google Scholar]
- 11.Edlich RF, Hill LG, Williams FM. Global epidemic of human T-cell lymphotrophic virus type-I (HTLV-I): an update. J. Long Term Eff. Med. Implants. 2003;13:127–140. doi: 10.1615/jlongtermeffmedimplants.v13.i2.70. [DOI] [PubMed] [Google Scholar]
- 12.Hinuma Y, Nagata K, Misoka M, Nakai T, Matsumoto T, Kiroshita K, Shirakwa S, Miyoshi I. Adult T-cell leukemia: Antigen in ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. U. S. A. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seiki M, Hattori S, Hirayama Y, Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc. Natl. Acad. Sci. U. S. A. 1983;80:3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osame M, Izumo S, Igata A, Matsumoto M, Matsumoto T, Sonoda S, Tara M, Shibata Y. Blood transfusion and HTLV-I associated myelopathy. Lancet. 1986;2:104–105. doi: 10.1016/s0140-6736(86)91636-3. [DOI] [PubMed] [Google Scholar]
- 15.Kondo T, Kono H, Nonaka H, Miyamoto N, Yoshida R, Bando F, Inoue H, Miyoshi I, Hinuma Y, Hanaoka M. Risk of adult T-cell leukaemia/lymphoma in HTLV-I carriers. Lancet. 1987;2:159. doi: 10.1016/s0140-6736(87)92359-2. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi K, Watanabe T. Human T lymphotropic virus type-I and adult T-cell leukemia in Japan. Int. J. Hematol. 2002;76:240–245. doi: 10.1007/BF03165123. [DOI] [PubMed] [Google Scholar]
- 17.Murphy EL, Hanchard B, Figueroa JP, Gibbs WN, Lofters WS, Campbell M, Goedert JJ, Blattner WA. Modelling the risk of adult T-cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type I. Int. J. Cancer. 1989;43:250–253. doi: 10.1002/ijc.2910430214. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan JE, Osame M, Kubota H, Igata A, Nishitani H, Maeda Y, Khabbaz RF, Janssen RS. The risk of development of HTLV-I-associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-I. J. Acquir. Immune Defic. Syndr. 1990;3:1096–1101. [PubMed] [Google Scholar]
- 19.Maloney EM, Cleghorn FR, Morgan OS, Rodgers-Johnson P, Cranston B, Jack N, Blattner WA, Bartholomew C, Manns A. Incidence of HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) in Jamaica and Trinidad. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1998;17:167–170. doi: 10.1097/00042560-199802010-00011. [DOI] [PubMed] [Google Scholar]
- 20.Berneman ZN, Gartenhaus RB, Reitz MS, Jr, Blattner WA, Manns A, Hanchard B, Ikehara O, Gallo RC, Klotman ME. Expression of alternatively spliced human T-lymphotropic virus type I pX mRNA in infected cell lines and in primary uncultured cells from patients with adult T-cell leukemia/lymphoma and healthy carriers. Proc. Natl. Acad. Sci. U. S. A. 1992;89:3005–3009. doi: 10.1073/pnas.89.7.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koralnik IJ, Gessain A, Klotman ME, Lo Monico A, Berneman ZN, Franchini G. Protein isoforms encoded by the pX region of human T-cell leukemia/lymphotropic virus type I. Proc. Natl. Acad. Sci. U. S. A. 1992;89:8813–8817. doi: 10.1073/pnas.89.18.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciminale V, Pavlakis GN, Derse D, Cunningham CP, Felber BK. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: Novel mRNAs and proteins produced by HTLV type I. J. Virol. 1992;66:1737–1745. doi: 10.1128/jvi.66.3.1737-1745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukumoto R, Andresen V, Bialuk I, Cecchinato V, Walser JC, Valeri VW, Nauroth JM, Gessain A, Nicot C, Franchini G. In vivo genetic mutations define predominant functions of the human T-cell leukemia/lymphoma virus p12I protein. Blood. 2009;113:3726–3734. doi: 10.1182/blood-2008-04-146928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaudray G, Gachon F, Basbous J, Biard-Piechaczyk M, Devaux C, Mesnard JM. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J. Virol. 2002;76:12813–12822. doi: 10.1128/JVI.76.24.12813-12822.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiyokawa T, Seiki M, Imagawa K, Shimizu F, Yoshida M. Identification of a protein (p40x) encoded by a unique sequence pX of human T-cell leukemia virus type I. Gann. 1984;75:747–751. [PubMed] [Google Scholar]
- 26.Kiyokawa T, Seiki M, Iwashita S, Imagawa K, Shimizu F, Yoshida M. p27x-III and p21x-III, proteins encoded by the pX sequence of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. U. S. A. 1985;82:8359–8363. doi: 10.1073/pnas.82.24.8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuoka M, Green PL. The HBZ gene, a key player in HTLV-1 pathogenesis. Retrovirology. 2009;6:71. doi: 10.1186/1742-4690-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuoka M. HTLV-1 bZIP factor gene: Its roles in HTLV-1 pathogenesis. Mol. Aspect. Med. 2010;31:359–366. doi: 10.1016/j.mam.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Younis I, Green PL. The human T-cell leukemia virus Rex protein. Front. Biosci. 2005;10:431–445. doi: 10.2741/1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chlichlia K, Khazaie K. HTLV-1 Tax: Linking transformation, DNA damage and apoptotic T-cell death. Chem. Biol. Interact. 2010;188:359–365. doi: 10.1016/j.cbi.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Derse D, Mikovits J, Ruscetti F. X-I and X-II open reading frames of HTLV-I are not required for virus replication or for immortalization of primary T-cells in vitro. Virology. 1997;237:123–128. doi: 10.1006/viro.1997.8781. [DOI] [PubMed] [Google Scholar]
- 32.Bartoe JT, Albrecht B, Collins ND, Robek MD, Ratner L, Green PL, Lairmore MD. Functional role of pX open reading frame II of human T-lymphotropic virus type 1 in maintenance of viral loads in vivo. J. Virol. 2000;74:1094–1100. doi: 10.1128/jvi.74.3.1094-1100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins ND, Newbound GC, Albrecht B, Beard JL, Ratner L, Lairmore MD. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood. 1998;91:4701–4707. [PubMed] [Google Scholar]
- 34.Silverman LR, Phipps AJ, Montgomery A, Ratner L, Lairmore MD. Human T-cell lymphotropic virus type 1 open reading frame II-encoded p30II is required for in vivo replication: Evidence of in vivo reversion. J. Virol. 2004;78:3837–3845. doi: 10.1128/JVI.78.8.3837-3845.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valeri VW, Hryniewicz A, Andresen V, Jones K, Fenizia C, Bialuk I, Chung HK, Fukumoto R, Parks RW, Ferrari MG, et al. Requirement of the human T-cell leukemia virus p12 and p30 products for infectivity of human dendritic cells and macaques but not rabbits. Blood. 2010;116:3809–3817. doi: 10.1182/blood-2010-05-284141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franchini G. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood. 1995;86:3619–3639. [PubMed] [Google Scholar]
- 37.Franchini G, Mulloy JC, Koralnik IJ, Lo Monico A, Sparkowski JJ, Andresson T, Goldstein DJ, Schlegel R. The human T-cell leukemia/lymphotropic virus type I p12I protein cooperates with the E5 oncoprotein of bovine papillomavirus in cell transformation and binds the 16-kilodalton subunit of the vacuolar H+ ATPase. J. Virol. 1993;67:7701–7704. doi: 10.1128/jvi.67.12.7701-7704.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felber BK, Derse D, Athanassopoulos A, Campbell M, Pavlakis GN. Cross-activation of the Rex proteins of HTLV-I and BLV and of the Rev protein of HIV-1 and nonreciprocal interactions with their RNA responsive elements. New Biol. 1989;1:318–328. [PubMed] [Google Scholar]
- 39.Koralnik IJ, Fullen J, Franchini G. The p12I, p13II, and p30II proteins encoded by human T-cell leukemia/lymphotropic virus type I open reading frames I and II are localized in three different cellular compartments. J. Virol. 1993;67:2360–2366. doi: 10.1128/jvi.67.4.2360-2366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson JM, Harrod R, Franchini G. Molecular biology and pathogenesis of the human T-cell leukaemia/lymphotropic virus Type-1 (HTLV-1) Int. J. Exp. Pathol. 2001;82:135–147. doi: 10.1046/j.1365-2613.2001.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding W, Albrecht B, Luo R, Zhang W, Stanley JR, Newbound GC, Lairmore MD. Endoplasmic reticulum and cis-Golgi localization of human T-lymphotropic virus type 1 p12(I): association with calreticulin and calnexin. J. Virol. 2001;75:7672–7682. doi: 10.1128/JVI.75.16.7672-7682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koralnik IJ, Lemp JF, Jr, Gallo RC, Franchini G. In vitro infection of human macrophages by human T-cell leukemia/lymphotropic virus type I (HTLV-I) AIDS Res. Hum. Retrovir. 1992;8:1845–1849. doi: 10.1089/aid.1992.8.1845. [DOI] [PubMed] [Google Scholar]
- 43.Dekaban GA, Peters AA, Mulloy JC, Johnson JM, Trovato R, Rivadeneira E, Franchini G. The HTLV-I orfI protein is recognized by serum antibodies from naturally infected humans and experimentally infected rabbits. Virology. 2000;274:86–93. doi: 10.1006/viro.2000.0406. [DOI] [PubMed] [Google Scholar]
- 44.Pique C, Dokhelar MC. In vivo production of Rof and Tof proteins of HTLV type 1: Evidence from cytotoxic T lymphocytes. AIDS Res. Hum. Retrovir. 2000;16:1783–1786. doi: 10.1089/08892220050193317. [DOI] [PubMed] [Google Scholar]
- 45.Trovato R, Mulloy JC, Johnson JM, Takemoto S, de Oliveira MP, Franchini G. A lysine-to-arginine change found in natural alleles of the human T-cell lymphotropic/leukemia virus type 1 p12(I) protein greatly influences its stability. J. Virol. 1999;73:6460–6467. doi: 10.1128/jvi.73.8.6460-6467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding W, Albrecht B, Kelley RE, Muthusamy N, Kim SJ, Altschuld RA, Lairmore MD. Human T-cell lymphotropic virus type 1 p12(I) expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. J. Virol. 2002;76:10374–10382. doi: 10.1128/JVI.76.20.10374-10382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albrecht B, D'Souza CD, Ding W, Tridandapani S, Coggeshall KM, Lairmore MD. Activation of nuclear factor of activated T cells by human T-lymphotropic virus type 1 accessory protein p12(I) J. Virol. 2002;76:3493–3501. doi: 10.1128/JVI.76.7.3493-3501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim SJ, Ding W, Albrecht B, Green PL, Lairmore MD. A conserved calcineurin-binding motif in human T lymphotropic virus type 1 p12I functions to modulate nuclear factor of activated T cell activation. J. Biol. Chem. 2003;278:15550–15557. doi: 10.1074/jbc.M210210200. [DOI] [PubMed] [Google Scholar]
- 49.Nair A, Michael B, Hiraragi H, Fernandez S, Feuer G, Boris-Lawrie K, Lairmore M. Human T lymphotropic virus type 1 accessory protein p12I modulates calcium-mediated cellular gene expression and enhances p300 expression in T lymphocytes. AIDS Res. Hum. Retrovir. 2005;21:273–284. doi: 10.1089/aid.2005.21.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nair AM, Michael B, Datta A, Fernandez S, Lairmore MD. Calcium-dependent enhancement of transcription of p300 by human T-lymphotropic type 1 p12I. Virology. 2006;353:247–257. doi: 10.1016/j.virol.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SJ, Nair AM, Fernandez S, Mathes L, Lairmore MD. Enhancement of LFA-1-mediated T cell adhesion by human T lymphotropic virus type 1 p12I1. J. Immunol. 2006;176:5463–5470. doi: 10.4049/jimmunol.176.9.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukumoto R, Dundr M, Nicot C, Adams A, Valeri VW, Samelson LE, Franchini G. Inhibition of T-cell receptor signal transduction and viral expression by the linker for activation of T cells-interacting p12(I) protein of human T-cell leukemia/lymphoma virus type 1. J. Virol. 2007;81:9088–9099. doi: 10.1128/JVI.02703-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bunn PA, Jr, Schechter GP, Jaffe E, Blayney D, Young RC, Matthews MJ, Blattner W, Broder S, Robert-Guroff M, Gallo RC. Clinical course of retrovirus-associated adult T-cell lymphoma in the United States. N. Engl. J. Med. 1983;309:257–264. doi: 10.1056/NEJM198308043090501. [DOI] [PubMed] [Google Scholar]
- 54.Clark JW, Robert-Guroff M, Ikehara O, Henzan E, Blattner WA. Human T-cell leukemia-lymphoma virus type 1 and adult T-cell leukemia-lymphoma in Okinawa. Cancer Res. 1985;45:2849–2852. [PubMed] [Google Scholar]
- 55.Migone TS, Lin JX, Cereseto A, Mulloy JC, O’Shea JJ, Franchini G, Leonard WJ. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- 56.Collins ND, D'Souza C, Albrecht B, Robek MD, Ratner L, Ding W, Green PL, Lairmore MD. Proliferation response to interleukin-2 and Jak/Stat activation of T cells immortalized by human T-cell lymphotropic virus type 1 is independent of open reading frame I expression. J. Virol. 1999;73:9642–9649. doi: 10.1128/jvi.73.11.9642-9649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mulloy JC, Crownley RW, Fullen J, Leonard WJ, Franchini G. The human T-cell leukemia/lymphotropic virus type 1 p12I proteins bind the interleukin-2 receptor beta and gammac chains and affects their expression on the cell surface. J. Virol. 1996;70:3599–3605. doi: 10.1128/jvi.70.6.3599-3605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicot C, Mulloy JC, Ferrari MG, Johnson JM, Fu K, Fukumoto R, Trovato R, Fullen J, Leonard WJ, Franchini G. HTLV-1 p12(I) protein enhances STAT5 activation and decreases the interleukin-2 requirement for proliferation of primary human peripheral blood mononuclear cells. Blood. 2001;98:823–829. doi: 10.1182/blood.v98.3.823. [DOI] [PubMed] [Google Scholar]
- 59.Jefferies WA, Burgert HG. E3/19K from adenovirus 2 is an immunosubversive protein that binds to a structural motif regulating the intracellular transport of major histocompatibility complex class I proteins. J. Exp. Med. 1990;172:1653–1664. doi: 10.1084/jem.172.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schust DJ, Tortorella D, Seebach J, Phan C, Ploegh HL. Trophoblast class I major histocompatibility complex (MHC) products are resistant to rapid degradation imposed by the human cytomegalovirus (HCMV) gene products US2 and US11. J. Exp. Med. 1998;188:497–503. doi: 10.1084/jem.188.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piguet V, Schwartz O, Le Gall S, Trono D. The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol. Rev. 1999;168:51–63. doi: 10.1111/j.1600-065x.1999.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 62.Le Gall S, Erdtmann L, Benichou S, Berlioz-Torrent C, Liu L, Benarous R, Heard JM, Schwartz O. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity. 1998;8:483–495. doi: 10.1016/s1074-7613(00)80553-1. [DOI] [PubMed] [Google Scholar]
- 63.Kerkau T, Bacik I, Bennink JR, Yewdell JW, Hunig T, Schimpl A, Schubert U. The human immunodeficiency virus type 1 (HIV-1) Vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J. Exp. Med. 1997;185:1295–1305. doi: 10.1084/jem.185.7.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Banerjee P, Feuer G, Barker E. Human T-cell leukemia virus type 1 (HTLV-1) p12I down-modulates ICAM-1 and -2 and reduces adherence of natural killer cells thereby protecting HTLV-1-infected primary CD4+ T cells from autologous natural killer cell-mediated cytotoxicity despite the reduction of major histocompatibility complex class I molecules on infected cells. J. Virol. 2007;81:9707–9717. doi: 10.1128/JVI.00887-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukudome K, Furuse M, Fukuhara N, Orita S, Imai T, Takagi S, Nagira M, Hinuma Y, Yoshie O. Strong induction of ICAM-1 in human T cells transformed by human T-cell-leukemia virus type 1 and depression of ICAM-1 or LFA-1 in adult T-cell-leukemia-derived cell lines. Int. J. Cancer. 1992;52:418–427. doi: 10.1002/ijc.2910520316. [DOI] [PubMed] [Google Scholar]
- 66.Goldstein DJ, Finbow ME, Andresson T, McLean P, Smith K, Bubb V, Schlegel R. Bovine papillomavirus E5 oncoprotein binds to the 16K component of vacuolar H(+)-ATPases. Nature. 1991;352:347–349. doi: 10.1038/352347a0. [DOI] [PubMed] [Google Scholar]
- 67.Schapiro F, Sparkowski J, Adduci A, Suprynowicz F, Schlegel R, Grinstein S. Golgi alkalinization by the papillomavirus E5 oncoprotein. J. Cell Biol. 2000;148:305–315. doi: 10.1083/jcb.148.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balotta C, Lusso P, Crowley R, Gallo RC, Franchini G. Antisense phosphorothioate oligodeoxynucleotides targeted to the vpr gene inhibit human immunodeficiency virus type 1 replication in primary human macrophages. J. Virol. 1993;67:4409–4414. doi: 10.1128/jvi.67.7.4409-4414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koralnik IJ, Mulloy JC, Andresson T, Fullen J, Franchini G. Mapping of the intermolecular association of human T cell leukaemia/lymphotropic virus type I p12I and the vacuolar H+-ATPase 16 kDa subunit protein. J. Gen. Virol. 1995;76:1909–1916. doi: 10.1099/0022-1317-76-8-1909. [DOI] [PubMed] [Google Scholar]
- 70.Finbow ME, Pitts JD, Goldstein DJ, Schlegel R, Findlay JB. The E5 oncoprotein target: A 16-kDa channel-forming protein with diverse functions. Mol. Carcinog. 1991;4:441–444. doi: 10.1002/mc.2940040605. [DOI] [PubMed] [Google Scholar]
- 71.Clague MJ, Urbe S, Aniento F, Gruenberg J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J. Biol. Chem. 1994;269:21–24. [PubMed] [Google Scholar]
- 72.Nelson N. Energizing porters by proton-motive force. J. Exp. Biol. 1994;196:7–13. doi: 10.1242/jeb.196.1.7. [DOI] [PubMed] [Google Scholar]
- 73.Prchla E, Kuechler E, Blaas D, Fuchs R. Uncoating of human rhinovirus serotype 2 from late endosomes. J. Virol. 1994;68:3713–3723. doi: 10.1128/jvi.68.6.3713-3723.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nawa M. Japanese encephalitis virus infection in Vero cells: The involvement of intracellular acidic vesicles in the early phase of viral infection was observed with the treatment of a specific vacuolar type H+-ATPase inhibitor, bafilomycin A1. Microbiol. Immunol. 1997;41:537–543. doi: 10.1111/j.1348-0421.1997.tb01889.x. [DOI] [PubMed] [Google Scholar]
- 75.Taylor JM, Brown M, Nejmeddine M, Kim KJ, Ratner L, Lairmore M, Nicot C. Novel role for interleukin-2 receptor-Jak signaling in retrovirus transmission. J. Virol. 2009;83:11467–11476. doi: 10.1128/JVI.00952-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamamoto N, Okada M, Koyanagi Y, Kannagi M, Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982;217:737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]
- 77.Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 78.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat. Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 79.Ciminale V, D'Agostino DM, Zotti L, Franchini G, Felber BK, Chieco-Bianchi L. Expression and characterization of proteins produced by mRNAs spliced into the X region of the human T-cell leukemia/lymphotropic virus type II. Virology. 1995;209:445–456. doi: 10.1006/viro.1995.1277. [DOI] [PubMed] [Google Scholar]
- 80.Ghorbel S, Sinha-Datta U, Dundr M, Brown M, Franchini G, Nicot C. Human T-cell leukemia virus type I p30 nuclear/nucleolar retention is mediated through interactions with RNA and a constituent of the 60 S ribosomal subunit. J. Biol. Chem. 2006;281:37150–37158. doi: 10.1074/jbc.M603981200. [DOI] [PubMed] [Google Scholar]
- 81.Baydoun HH, Bellon M, Nicot C. HTLV-1 Yin and Yang: Rex and p30 master regulators of viral mRNA trafficking. AIDS Rev. 2008;10:195–204. [PMC free article] [PubMed] [Google Scholar]
- 82.Michael B, Nair AM, Datta A, Hiraragi H, Ratner L, Lairmore MD. Histone acetyltransferase (HAT) activity of p300 modulates human T lymphotropic virus type 1 p30II-mediated repression of LTR transcriptional activity. Virology. 2006;354:225–239. doi: 10.1016/j.virol.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Younis I, Khair L, Dundr M, Lairmore MD, Franchini G, Green PL. Repression of human T-cell leukemia virus type 1 and type 2 replication by a viral mRNA-encoded posttranscriptional regulator. J. Virol. 2004;78:11077–11083. doi: 10.1128/JVI.78.20.11077-11083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamamoto B, Li M, Kesic M, Younis I, Lairmore MD, Green PL. Human T-cell leukemia virus type 2 post-transcriptional control protein p28 is required for viral infectivity and persistence in vivo. Retrovirology. 2008;5:38. doi: 10.1186/1742-4690-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.H HB, Pancewicz J, Nicot C. Human T-cell leukemia virus p30 inhibits homologous recombination and favors unfaithful DNA repair. Blood. 2011 doi: 10.1182/blood-2010-08-304600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choudhary G, Ratner L. The HTLV-1 hbz antisense gene indirectly promotes tax expression via down-regulation of p30(II) mRNA. Virology. 2010;410:307–315. doi: 10.1016/j.virol.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boisvert FM, van Koningsbruggen S, Navascues J, Lamond AI. The multifunctional nucleolus. Nat. Rev. Mol. Cell Biol. 2007;8:574–585. doi: 10.1038/nrm2184. [DOI] [PubMed] [Google Scholar]
- 88.Nicot C, Dundr M, Johnson JM, Fullen JR, Alonzo N, Fukumoto R, Princler GL, Derse D, Misteli T, Franchini G. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat. Med. 2004;10:197–201. doi: 10.1038/nm984. [DOI] [PubMed] [Google Scholar]
- 89.Sinha-Datta U, Datta A, Ghorbel S, Dodon MD, Nicot C. Human T-cell lymphotrophic virus type I rex and p30 interactions govern the switch between virus latency and replication. J. Biol. Chem. 2007;282:14608–14615. doi: 10.1074/jbc.M611219200. [DOI] [PubMed] [Google Scholar]
- 90.Princler GL, Julias JG, Hughes SH, Derse D. Roles of viral and cellular proteins in the expression of alternatively spliced HTLV-1 pX mRNAs. Virology. 2003;317:136–145. doi: 10.1016/j.virol.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 91.D'Agostino DM, Ciminale V, Zotti L, Chieco-Bianchi L. Influence of Rex and intronic sequences on expression of spliced mRNAs produced by human T cell leukemia virus type I. AIDS Res. Hum. Retrovir. 1999;15:1351–1363. doi: 10.1089/088922299310061. [DOI] [PubMed] [Google Scholar]
- 92.Bai XT, Baydoun HH, Nicot C. HTLV-I p30: A versatile protein modulating virus replication and pathogenesis. Mol. Aspect. Med. 2010;31:344–349. doi: 10.1016/j.mam.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang W, Nisbet JW, Bartoe JT, Ding W, Lairmore MD. Human T-lymphotropic virus type 1 p30(II) functions as a transcription factor and differentially modulates CREB-responsive promoters. J. Virol. 2000;74:11270–11277. doi: 10.1128/jvi.74.23.11270-11277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Michael B, Nair AM, Hiraragi H, Shen L, Feuer G, Boris-Lawrie K, Lairmore MD. Human T lymphotropic virus type-1 p30II alters cellular gene expression to selectively enhance signaling pathways that activate T lymphocytes. Retrovirology. 2004;1:39. doi: 10.1186/1742-4690-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang W, Nisbet JW, Albrecht B, Ding W, Kashanchi F, Bartoe JT, Lairmore MD. Human T-lymphotropic virus type 1 p30(II) regulates gene transcription by binding CREB binding protein/p300. J. Virol. 2001;75:9885–9895. doi: 10.1128/JVI.75.20.9885-9895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang JL, Sharma PL, Crumpacker CS. Enhancement of the basal-level activity of HIV-1 long terminal repeat by HIV-1 nucleocapsid protein. Virology. 2000;268:251–263. doi: 10.1006/viro.2000.0194. [DOI] [PubMed] [Google Scholar]
- 97.Taylor JM, Ghorbel S, Nicot C. Genome wide analysis of human genes transcriptionally and post-transcriptionally regulated by the HTLV-I protein p30. BMC Genomics. 2009;10:311. doi: 10.1186/1471-2164-10-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Datta A, Sinha-Datta U, Dhillon NK, Buch S, Nicot C. The HTLV-I p30 interferes with TLR4 signaling and modulates the release of pro- and anti-inflammatory cytokines from human macrophages. J. Biol. Chem. 2006;281:23414–23424. doi: 10.1074/jbc.M600684200. [DOI] [PubMed] [Google Scholar]
- 99.Datta A, Silverman L, Phipps AJ, Hiraragi H, Ratner L, Lairmore MD. Human T-lymphotropic virus type-1 p30 alters cell cycle G2 regulation of T lymphocytes to enhance cell survival. Retrovirology. 2007;4:49. doi: 10.1186/1742-4690-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Anupam R, Datta A, Kesic M, Green-Church K, Shkriabai N, Kvaratskhelia M, Lairmore MD. Human T-lymphotropic virus type 1 p30 interacts with REG{gamma} and modulates ataxia telangiectasia mutated to promote cell survival. J. Biol. Chem. 2011;286:7661–7668. doi: 10.1074/jbc.M110.176354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baydoun HH, Pancewicz J, Bai X, Nicot C. HTLV-I p30 inhibits multiple S phase entry checkpoints, decreases cyclin E-CDK2 interactions and delays cell cycle progression. Mol. Cancer. 2010;9:302. doi: 10.1186/1476-4598-9-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Makino M, Wakamatsu S, Shimokubo S, Arima N, Baba M. Production of functionally deficient dendritic cells from HTLV-I-infected monocytes: implications for the dendritic cell defect in adult T cell leukemia. Virology. 2000;274:140–148. doi: 10.1006/viro.2000.0445. [DOI] [PubMed] [Google Scholar]
- 103.Robek MD, Wong FH, Ratner L. Human T-cell leukemia virus type 1 pX-I and pX-II open reading frames are dispensable for the immortalization of primary lymphocytes. J. Virol. 1998;72:4458–4462. doi: 10.1128/jvi.72.5.4458-4462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen YM, Chen SH, Fu CY, Chen JY, Osame M. Antibody reactivities to tumor-suppressor protein p53 and HTLV-I Tof, Rex and Tax in HTLV-I-infected people with differing clinical status. Int. J. Cancer. 1997;71:196–202. doi: 10.1002/(sici)1097-0215(19970410)71:2<196::aid-ijc12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 105.Arnold J, Yamamoto B, Li M, Phipps AJ, Younis I, Lairmore MD, Green PL. Enhancement of infectivity and persistence in vivo by HBZ, a natural antisense coded protein of HTLV-1. Blood. 2006;107:3976–3982. doi: 10.1182/blood-2005-11-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Berneman ZN, Gartenhaus RB, Reitz MS, Jr, Klotman ME, Gallo RC. cDNA sequencing confirms HTLV-I expression in adult T-cell leukemia/lymphoma and different sequence variations in vivo and in vitro. Leukemia. 1992;6:S67–S71. [PubMed] [Google Scholar]
- 107.D'Agostino DM, Ranzato L, Arrigoni G, Cavallari I, Belleudi F, Torrisi MR, Silic-Benussi M, Ferro T, Petronilli V, Marin O, et al. Mitochondrial alterations induced by the p13II protein of human T-cell leukemia virus type 1. Critical role of arginine residues. J. Biol. Chem. 2002;277:34424–34433. doi: 10.1074/jbc.M203023200. [DOI] [PubMed] [Google Scholar]
- 108.Ciminale V, Zotti L, D’Agostino DM, Ferro T, Casareto L, Franchini G, Bernardi P, Chieco-Bianchi L. Mitochondrial targeting of the p13II protein coded by the x-II ORF of human T-cell leukemia/lymphotropic virus type I (HTLV-I) Oncogene. 1999;18:4505–4514. doi: 10.1038/sj.onc.1203047. [DOI] [PubMed] [Google Scholar]
- 109.Silic-Benussi M, Marin O, Biasiotto R, D'Agostino DM, Ciminale V. Effects of human T-cell leukemia virus type 1 (HTLV-1) p13 on mitochondrial K+ permeability: A new member of the viroporin family? FEBS Lett. 2010;584:2070–2075. doi: 10.1016/j.febslet.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Silic-Benussi M, Cannizzaro E, Venerando A, Cavallari I, Petronilli V, La Rocca N, Marin O, Chieco-Bianchi L, Di Lisa F, D'Agostino DM, et al. Modulation of mitochondrial K(+) permeability and reactive oxygen species production by the p13 protein of human T-cell leukemia virus type 1. Biochim. Biophys. Acta. 2009;1787:947–954. doi: 10.1016/j.bbabio.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 111.Silic-Benussi M, Cavallari I, Vajente N, Vidali S, Chieco-Bianchi L, Di Lisa F, Saggioro D, D'Agostino DM, Ciminale V. Redox regulation of T-cell turnover by the p13 protein of human T-cell leukemia virus type 1: distinct effects in primary versus transformed cells. Blood. 2010;116:54–62. doi: 10.1182/blood-2009-07-235861. [DOI] [PubMed] [Google Scholar]
- 112.Silic-Benussi M, Cavallari I, Zorzan T, Rossi E, Hiraragi H, Rosato A, Horie K, Saggioro D, Lairmore MD, Willems L, et al. Suppression of tumor growth and cell proliferation by p13II, a mitochondrial protein of human T cell leukemia virus type 1. Proc. Natl. Acad. Sci. U. S. A. 2004;101:6629–6634. doi: 10.1073/pnas.0305502101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Biasiotto R, Aguiari P, Rizzuto R, Pinton P, D'Agostino DM, Ciminale V. The p13 protein of human T cell leukemia virus type 1 (HTLV-1) modulates mitochondrial membrane potential and calcium uptake. Biochim. Biophys. Acta. 2010;1797:945–951. doi: 10.1016/j.bbabio.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 114.Arnould T, Vankoningsloo S, Renard P, Houbion A, Ninane N, Demazy C, Remacle J, Raes M. CREB activation induced by mitochondrial dysfunction is a new signaling pathway that impairs cell proliferation. EMBO J. 2002;21:53–63. doi: 10.1093/emboj/21.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hiraragi H, Michael B, Nair A, Silic-Benussi M, Ciminale V, Lairmore M. Human T-lymphotropic virus type 1 mitochondrion-localizing protein p13II sensitizes Jurkat T cells to Ras-mediated apoptosis. J. Virol. 2005;79:9449–9457. doi: 10.1128/JVI.79.15.9449-9457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Andresen V, Pise-Masison C, Sinha-Datta U, Valeri VW, Parks RW, Cecchinato V, Fukumoto R, Nicot C, Franchini G. Suppression of HTLV-1 Replication by Tax-mediated 1 Re-routing of the p13 Viral Protein to Nuclear Speckles. Blood. 2011 doi: 10.1182/blood-2010-06-293340. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pique C, Ureta-Vidal A, Gessain A, Chancerel B, Gout O, Tamouza R, Agis F, Dokhelar MC. Evidence for the chronic in vivo production of human T cell leukemia virus type I Rof and Tof proteins from cytotoxic T lymphocytes directed against viral peptides. J. Exp. Med. 2000;191:567–572. doi: 10.1084/jem.191.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hiraragi H, Kim SJ, Phipps AJ, Silic-Benussi M, Ciminale V, Ratner L, Green PL, Lairmore MD. Human T-lymphotropic virus type 1 mitochondrion-localizing protein p13(II) is required for viral infectivity in vivo. J. Virol. 2006;80:3469–3476. doi: 10.1128/JVI.80.7.3469-3476.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]