Abstract
Following receptor-mediated uptake into endocytic vesicles and escape from the endosome, adenovirus is transported by cytoplasmic dynein along microtubules to the perinuclear region of the cell. How motor proteins are recruited to viruses for their own use has begun to be investigated only recently. We review here the evidence for a role for dynein and other motor proteins in adenovirus infectivity. We also discuss the implications of recent studies on the mechanism of dynein recruitment to adenovirus for understanding the relationship between pathogenic and physiological cargo recruitment and for the evolutionary origins of dynein-mediated adenovirus transport.
Keywords: adenovirus, molecular motors, cytoplasmic dynein, intracellular motility
1. Introduction—Role of Microtubule Motors in Adenovirus Transport
Adenoviruses consist of nonenveloped, double-stranded DNA-containing capsids that commonly cause mild and self-limited infections in healthy individuals, but can be fatal for immunocompromised patients. At least 55 serotypes, which are divided into seven subgroups (A–G), have been identified since adenoviruses were first isolated from adenoid tissue in 1953 [1]. The individual serotypes share a similar structure, which has been solved at near atomic resolution for the prototypical subgroup C adenovirus 5 (Ad5) by cryo-electron microscopy and for an Ad5/Ad35 chimera by x-ray crystallography [2,3]. The protein shell of the virus has a diameter of 90–100 nm and consists of three major and four minor capsid proteins [4]. The trimeric fiber protein protrudes from the capsid and can interact with the Coxsackie and Adenovirus Receptor (CAR) (Figure 1). Fiber is attached to the capsid through the pentameric penton base, which is situated at each of the vertices of the icosahedral capsid. The other major capsid protein, hexon, is the most abundant subunit with 240 hexon trimers in each virus, 12 of which comprise each facet of the icosahedral virion.
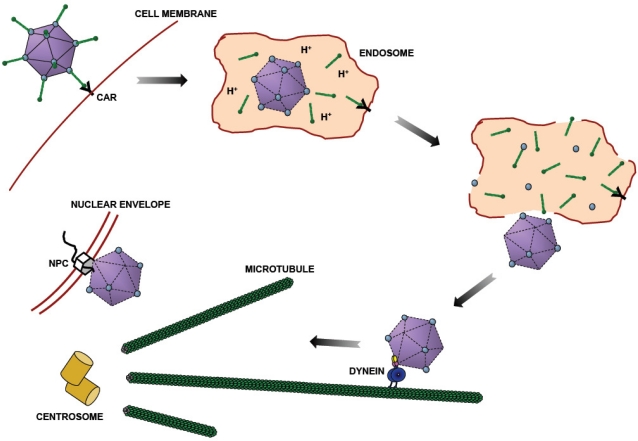
Figure 1.
Adenovirus Entry Pathway. After binding to the plasma membrane through the Coxsackie and Adenovirus (CAR) receptor, adenovirus is taken up by endocytosis [8,9]. Some capsid proteins are lost in the acidic endosomal lumen [10–12]. Following endosomalysis, adenovirus moves bidirectionally along microtubules (MTs) [13], using dynein for transport towards MT minus ends, which are typically focused at the centrosome and the vicinity of the nucleus. Finally, adenovirus binds to the nuclear pore complex (NPC) [14] through which it injects its genome for viral reproduction.
The extreme resistance of adenovirus to pH, temperature, and ionic strength [5] is likely due to the tight quarternary structure of hexon [6] and the contributions of additional minor capsid proteins, such as protein IIIa, VI, VIII and IX (reviewed in Vellinga et al. [7]) that act as “cement proteins”, bridging gaps between the major components. Inside the capsid are the 36 kb virus genome, the viral protease, and the DNA-associated proteins V, VII, μ, and the terminal protein.
For most adenovirus serotypes, uptake into the cell is initiated by binding to CAR via the knob domain at the tip of the fiber protein (Figure 1) and then strengthened by further interaction between the penton base RGD motif with cell surface-associated α5 integrins. These binding events lead to endocytosis of the capsid into clathrin-coated pits [8,9] and activation of PKA and p38/MAPK [15]. Inside the endosome, acidification to pH 4.6–6.0 [16] has been found to induce a number of structural changes in the virion, consistent with low pH-dependent structural effects in vitro [17]. Fiber proteins are shed from the capsid [10], and protein VI leaves the inside of the capsid and is presumably released into the endosomal lumen [11]. The amino-terminal end of protein VI has an amphipathic helix that then partially ruptures the endosomal membrane by inducing positive curvature in the inner leaflet of the lipid bilayer [18], thus allowing the capsid to escape into the cytoplasm. pH-dependent changes in the conformation of the remaining capsid proteins, including hexon, have also been described [17].
By about 15 minutes post-infection (p.i.), adenovirus subgroups A, C, D, E, and F have escaped the early endosome [19], whereas subgroup B adenoviruses remain in the endosomal/lysosomal pathway for up to eight hours [20,21]. Within 30–45 min after endosomal escape, subgroup C adenovirus reaches the nuclear envelope or the vicinity of the centrosome, depending on cell type. That this redistribution might involve microtubules (MTs) and MT-based transport was initially suggested by EM images of adenovirus-infected HeLa cells, which revealed partial coincidence of capsids with MTs [22,23]. More direct evidence came from live cell analysis of cytoplasmic viruses, which revealed capsid transport along linear trajectories consistent with the organization of MTs [13,24,25]. Furthermore, the use of nocodazole to depolymerize MTs in infected cells strongly inhibited adenovirus redistribution and directed transport [13,24]. Infectivity was also found to be markedly reduced, indicating an essential role for microtubules in the adenovirus infectious cycle [26].
Live cell analysis of virions after endosomal escape has indicated capsid transport along MTs in both the MT minus- and plus-end directions, presumably driven by cytoplasmic dynein and kinesin motors, respectively. While little is known about the function of kinesins in adenovirus transport (discussed below), interference with cytoplasmic dynein by RNAi, microinjection of function blocking antibodies, and expression of dynein inhibitors (dynamitin, and truncation mutants of the dynactin subunit p150Glued and the dynein heavy chain) strongly inhibited adenovirus redistribution to the cell center based on fixed and live cell analysis [13,24,25]. Adenovirus interacts with the nuclear pore protein CAN/Nup214 [14], but can accumulate in the cell center even in enucleated lung endothelial cells [27], illustrating the importance of MT minus end transport alone in adenovirus redistribution.
Because endosomes are subject to microtubule-mediated transport, adenovirus motility could to some extent reflect the behavior of the enclosing vesicular structures. Several lines of evidence, however, indicate an association of capsids with dynein after endosomal escape. Adenovirus tagged with a pH-sensitive dye revealed the particles to be at neutral pH by 30–40 min following infection [24], and almost no particles colocalized with early endosomal markers at this time [25], but most viruses showed positive immunoreactivity using antibodies to several dynein subunits and regulatory factors. These data, therefore, suggest that endosomal escape occur before adenovirus particles are typically imaged for directed transport.
2. Identification of Viral Capsid Proteins Involved in Cytoplasmic Dynein-Mediated Motility
The latter evidence indicates that following endosomal escape, adenovirus binds dynein as a naked capsid. The number of proteins potentially exposed on the capsid surface is limited (hexon, penton base, fiber, proteins IIIa, VI, VIII, IX), and candidates for a dynein interaction are further reduced by the stepwise dismantling of the virus and shedding of capsid components that occurs during early entry steps. Fiber is lost close to the cell surface. Penton base molecules are substantially removed during passage through the endosome [10,12], though this subunit can still be detected in association with nuclear envelope-associated virus particles [25]. The smaller capsid proteins IIIa, VI, and VIII are internal [2,3] and are mostly lost from the virus core within the first 20 min after infection [11,12,28]. Nevertheless, a mutation in the protein VI ubiquitinylation sequence has been found to reduce redistribution of incoming virus to the nucleus [28]. However, whether protein VI participates directly in dynein-mediated virus transport remains uncertain, because the protein is thought to remain associated with only a minority of cytoplasmic virus particles [11,12,28]. Protein IX is the only minor capsid protein accessible on the outer surface of the virion, and it also remains with the virus genome until its delivery into the nucleus [29]. An adenovirus mutant lacking protein IX, however, shows the same intracellular motility characteristics as the wild-type virus [30], arguing against a role in motor recruitment. Hexon is the most abundant capsid protein, and remains associated with the viral DNA until virus attachment to the nuclear pore complex [12,14,31,32].
To address more directly the mechanisms responsible for mediating the dynein-adenovirus interaction, we conducted a series of biochemical tests using whole purified brain cytoplasmic dynein, virus capsid, and individual capsid proteins [25]. We found that, among the reasonable candidate proteins, only hexon showed evidence of an interaction with cytoplasmic dynein. Penton base, proteins V, VII, and X were all negative in these tests. The behavior of hexon, itself, proved to be highly dependent on solvent conditions. However, once this variable was identified, robust interaction with dynein was observed, as discussed below. In addition, infection of HeLa cells heterologously expressing hexon displaced dynein from incoming adenovirus in vivo [25].
3. pH-Dependent Biochemical Characteristics of the Adenovirus Capsid and Hexon
Adenovirus purified by conventional CsCl banding has been reported to show some affinity for microtubules, which could be diminished in the presence of ATP or in the absence of MAPs, consistent with involvement of a motor protein [33]. However, we observed no detectable interaction of purified virus with cytoplasmic dynein at neutral pH [25]. To test whether the virus might be primed for cytoplasmic transport by its passage through the endosomal pathway, we exposed purified Ad5 to acidic pH conditions, and then returned the virus to neutrality. We observed a clear enhancement in dynein binding after exposure to pH levels in the 4.4–5.4 range. These values are based on conditions reported for intracellular adenovirus-containing acidic vesicles [16] and are somewhat lower than the range reported for early endosomes of uninfected cells (pH5.0 to pH6.2) [34–36].
We also tested for dynein binding by hexon alone, typically immunoisolated from lysates of Ad5-infected cells, but also purified chromatographically [37]. Hexon mimicked the behavior of the intact virus, showing a clear ability to pull down purified dynein, but only after low pH priming (Figure 2). These results strongly support a mechanism for dynein recruitment to adenovirus involving pH-dependent priming of hexon in the endosome. The correlation in the behavior of hexon with that of intact capsids is an additional piece of evidence supporting a predominant role for this subunit. The effects of reduced pH on dynein recruitment are in addition to other consequences of low pH exposure on infectivity such as rearrangement of capsid composition and possibly endosomal membrane disruption [11,17,38]. Thus, our data are consistent with physiological evidence for the importance of adenovirus exposure to endosomal pH for efficient infection (though see Smith et al. [39]). We also point out that exposure of hexon to low pH may be a useful mechanism to ensure differential transport of incoming virus capsids vs. newly expressed hexon trimers later in infection. At this stage hexon trimers redistribute from the cytoplasm to the nucleus using the nuclear localization signal of protein VI for nuclear targeting. No role for direct MT-based hexon transport in this process has been reported [40].
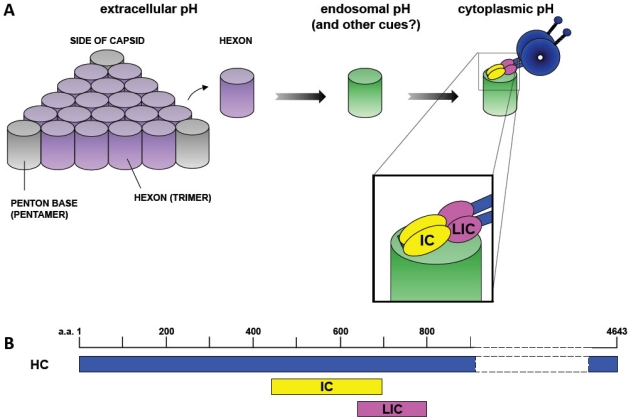
Figure 2.
Mechanism of Dynein Recruitment to Adenovirus. (A) At extracellular pH values (indicated by purple shading), adenovirus interacts weakly if at all with cytoplasmic dynein. Short-term transient exposure of virus or purified hexon trimer to the low pH values (green shading) characteristic of the endosomal lumen results in stronger dynein binding upon return to neutrality. Hexon binds directly and specifically to the dynein intermediate (IC) and light intermediate (LIC) chains [25]. (B) Representation of the binding sites of the dynein intermediate and light intermediate chains on the dynein heavy chain (HC) [41]. ICs and LICs associate with contiguous sites within the tail domain of the dynein complex and could, potentially, provide a continuous binding interface for hexon. Numbers indicate amino acid residues.
The nature of the low pH effect on hexon structure or conformation is uncertain. Hexon has been reported to exhibit an increase in hydrophobicity at pH values below pH 5.5 as judged by solvent partitioning experiments using Triton X-114 [17] and an increase in susceptibility to proteolysis [42]. These reports support a pH-induced conformational change, but the molecular details underlying this mechanism remain to be explored more fully.
4. Identification of Hexon-Interacting Cytoplasmic Dynein Subunit
Cytoplasmic dynein is a complex of two copies of a heavy chain (HC); intermediate chains (IC1 and IC2); light intermediate chains (LIC1 and LIC2); and three classes of light chains (LC8, TcTex, and LC7/Roadbock) [43,44]. The HC consists of a C-terminal motor domain (∼380 kDa) and an N-terminal tail domain, which is responsible for self-association and cargo binding, which, in turn, involves the ICs, LICs, and LCs. The ICs form a dimeric subcomplex, which is able to sequester the LCs and appears generally to block their interaction with most if not all other known binding partners [45,46]. This arrangement argues against a role for the LCs in directly linking cytoplasmic dynein to other proteins, but, instead, a role in modulating the stability or other conformational properties of the ICs. The extent to which the ICs serve directly in dynein cargo binding remains incompletely explored [47]. However, the ICs and LCs interact with two important dynein regulatory complexes, dynactin [48,49] and LIS1-NudE/NudEL [50], each of which, in turn, are involved in physiological cargo binding as well as dynein motor regulation.
The LICs are the least well studied of the cytoplasmic dynein subunits. They have been implicated in specific aspects of dynein cargo binding, interacting with pericentrin, Par3, and Rab11-FIB3 [51–53]. However, they have general roles in lysosome/late endosome motility and mitosis [54,55], though the mechanisms underlying these functions are incompletely understood.
To test which of these dynein components are involved in recruitment to adenovirus we used lysates from cultured mammalian cells overexpressing individual dynein polypeptides [25]. These studies reveal that the products of both dynein IC1 and IC2 genes and LIC1 are able to bind to acid-primed immunopurified hexon (Figure 2). LIC2 and the dynein light chains TcTex-1, RP3 and LC8 were negative in these assays [25].
5. Involvement of Cytoplasmic Dynein Regulators in Adenovirus Transport
Cytoplasmic dynein is regulated by a diversity of factors. The two best known and most extensively studied are the dynactin complex and a complex of NudE or NudEL with the product of the smooth brain gene, LIS1. Dynactin, NudE and NudEL have each been implicated in dynein recruitment to physiological forms of cargo, such as Golgi elements, components of the endosomal pathway, the G2 nuclear envelope, and mitotic kinetochores [50,56–59]. In addition, these factors regulate dynein motor behavior. Dynactin has been found to stimulate dynein processivity in vitro [60,61]. NudE and LIS1 together modify dynein to participate in high force functions, such as nuclear migration [62].
Immunocytochemical analysis revealed that both dynactin and NudE/NudEL colocalize along with cytoplasmic dynein on a high percentage of post-endosomal adenovirus particles (∼80%) in infected cells, though LIS1 colocalization was quite low. To test whether the NudE, NudEL, and dynactin interactions with virus were direct, adenovirus was used in pull downs from infected cell lysates [25]. Cytoplasmic dynein clearly associated with the virus particles in these assays, but dynactin and NudE/NudEL were undetectable. Furthermore, neither purified dynactin [63] nor recombinant NudE [62] showed an interaction with acid-primed hexon [64]. Together with our evidence for an interaction between purified dynein and virus, these results suggest that dynactin and NudE or NudEL are not involved in dynein recruitment to virus, but may, nonetheless, play regulatory roles in virus transport.
Interference with dynactin function has repeatedly been found to inhibit virus redistribution toward the cell center [13,25]. Whether this is due to a decrease in virus run-length along MTs or to a loss of dyneins from the capsid surface has been uncertain, though expression of the CC1 dynactin fragment has been found to reduce virus travel distance toward MT plus ends [65]. However, inhibition of NudE/NudEL and LIS1 by injection of function-blocking antibodies or expression of dominant negative fragments showed no obvious effect on the overall redistribution of virus to the cell center [25]. These results support an important role for dynactin in virus transport, but reveal no apparent role for NudE, NudEL, and LIS1. Why NudE and NudEL are associated with virus particles in situ remains to be resolved, but it is possible that they represent passive passengers during virus transport. The presence of NudE alone is inhibitory to dynein motility in in vitro single molecule and biochemical assays [62], and an association with adenovirus could imply a minimal or even inhibitory role in virus transport. Hence, blocking NudE/NudEL function might, potentially, result in faster capsid transport towards the nucleus. Recruitment of LIS1 to the virus particle might be as needed, e.g., during transport through cell constrictions or crowded cytoplasmic regions.
ZW10, an additional dynein recruitment factor, which interacts with dynein directly [66] or through dynactin [67,68], showed minimal colocalization with incoming adenovirus. Furthermore, ZW10 RNAi had no effect on virus progression towards the nucleus [25].
6. Evolutionary Origin of Dynein Recruitment by Viruses
Several features of the adenovirus-dynein interaction suggest an evolutionary origin independent of existing interactions between the motor protein and physiological forms of cargo (Figure 3). The latter is proving to be very complicated, and the complete mechanisms have not been worked out for any individual physiological form of cargo. It is known, however, that dynein is commonly attached to cargo through recruitments factors, such as dynactin, NudE, NudEL, and ZW10, which may, in turn, be recruited by additional factors (Figure 3B). In contrast, at least some reported LIC interactions (Par3, pericentrin, Rab4, and the Rab11 effector FIP3 [51–53]), or IC interactions (beta-catenin [47]) may be a manifestation of direct cargo-recruitment functions.
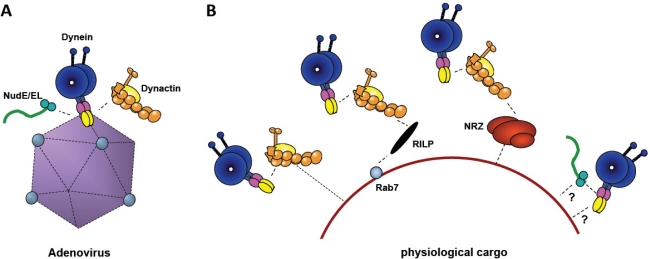
Figure 3.
Comparison of Adenovirus-mediated Dynein Recruitment with Physiological Mechanisms. (A) Cytoplasmic dynein is shown binding to adenovirus directly, and dynactin (yellow) and NudE/NudEL (green), indirectly. (B) Physiological cargoes (e.g., lysosomes, as shown) feature a variety of different dynein recruitment mechanisms, several involving dynactin. Both dynactin and NudE/NudEL are required for motility, and may, potentially, contribute either to dynein recruitment or to mechanochemical regulation, or both.
Our own recent evidence indicated that adenovirus interacts with dynein directly and through IC and the LIC1 subunits (Figure 3A). Both the directness of the interaction and the involvement of two different dynein subunits in dynein recruitment to a single form of cargo is atypical. It is of interest that the ICs and LICs are arrayed in tandem within the tail portion of the dynein HC [41] (Figure 2). This arrangement raises the possibility that the two dynein subunits might provide a contiguous binding surface for adenovirus. African swine fever and herpesviruses also seem to interact directly with cytoplasmic dynein [69,70]. In the case of African swine fever virus, it has been reported that the interaction is mediated through the dynein light chain LC8 and the viral p54 protein and can be selectively inhibited by a small LC8-derived peptide [71]. However, p54 and the dynein ICs appear to bind to a common groove within the LC8 dimer [45,71]. Herpes virus proteins of the inner tegument are the most likely candidates to recruit cytoplasmic dynein. Interestingly, the same pool of proteins has also been shown to bind directly and independently to dynactin and kinesins [70]. The functional consequences of earlier reports indicating an interaction between the herpesvirus protein VP26 (UL35) and the dynein light chains RP3 and TcTex-1 or UL34 with the dynein intermediate chain remain to be fully resolved [72–74].
Preliminary evidence suggests that the binding site for adenovirus hexon within the dynein IC is non-overlapping with that for dynactin and NudE/NudEL [75]. This arrangement should allow dynein binding to adenovirus while preserving dynein interactions with its regulators. Thus, despite the ability of adenovirus to recruit dynein directly, dynactin is still important for transport. A role for dynactin has also been reported for Herpes simplex virus and African swine fever virus [69,70,76], suggesting that dynactin may be generally required for virus transport. As noted above, there is at present no known role for NudE and NudEL in adenovirus transport, despite their clear colocalization with capsids after endosomal escape.
We also note that, although expression of hexon in cultured mammalian cells interferes with dynein recruitment to adenovirus capsids and their transport to the nucleus, hexon had no effect on Golgi morphology [25]. These results support distinct, noncompeting interactions of the dynein LIC1 and IC subunits with hexon vs. physiological forms of cargo, and argue that development of reagents to block virus transport specifically is feasible.
7. Bidirectional Intracellular Adenovirus Motility
Post-endosomal adenovirus exhibits bidirectional linear movements along microtubules [13,15,24,25]. These observations suggest that capsids are transported not only by cytoplasmic dynein but also by at least one form of kinesin. However, microinjection of established function-blocking anti-kinesin-1 monoclonal antibodies had no effect on virus redistribution [24]. Thus, the molecular mechanism responsible for plus end directed adenovirus transport remains unresolved. However, alpha- and gammaherpes viruses have been found to interact with kinesin-1 and -2 [70,77,78]. Vaccinia virus has been reported to recruit kinesin-1 heavy chain directly via an interaction with the F12 subunit, which structurally mimics the kinesin light chain [79]. Furthermore, parvovirus, influenza virus, human foamy virus, and human immunodeficiency virus also show directed translocation along MTs [80–83].
The physiological significance of bidirectional virus transport is not fully understood. Plus end movement might represent part of the host response to prevent virus from reaching the nucleus [84]. This model seems reasonable, as the virus would be expected to reach the nucleus much more efficiently using dynein alone. It has been suggested, however, that virus reaching the centrosomal region of the infected cell may still require kinesin for microtubule plus end directed transport to the nuclear envelope [85,86]. Analysis of adenovirus motility using high-resolution particle tracking revealed a near-zero net flux in the direction of virus movement from the periphery to the center of infected cells [13,25,30]. This result raises the possibility that viruses may reach the cell center via an assisted random walk mechanism. In this view motor proteins may allow virus particles to rapidly and more fully explore the cytoplasm until they encounter a nuclear pore complex, to which they bind with high affinity.
8. Conclusions
Adenoviruses rely on minus end directed transport along MTs by cytoplasmic dynein for efficient infection of their host cells, as seems to be true for at least some and likely many other pathogens. Adenovirus seems not to have modified existing physiological dynein recruitment mechanisms for its own use. Rather, it developed an unusual, if not unique, mechanism for hijacking dynein and using it to hitchhike to the nucleus. This insight may allow for development of specific inhibitors of viral infections with minimal effect on normal cellular behavior. A molecular understanding of motor recruitment also provides an inventory of factors, which must be preserved for successful use of adenovirus as a vector in gene therapy and vaccination trials. Adenovirus vectors are increasingly being used as therapeutic agents because of their large capacity for extra-genomic DNA as well as their ability to infect dividing and non-dividing cells. Preservation and improvements in the efficiency of adenovirus may be of great value in these efforts.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References and Notes
- 1.Rowe WP, Huebner RJ, Gilmore LK, Parrott RH, Ward TG. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med. 1953;84:570–573. doi: 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- 2.Reddy VS, Natchiar SK, Stewart PL, Nemerow GR. Crystal structure of human adenovirus at 3.5 A resolution. Science. 2010;329:1071–1075. doi: 10.1126/science.1187292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu H, Jin L, Koh SB, Atanasov I, Schein S, Wu L, Zhou ZH. Atomic structure of human adenovirus by cryo-EM reveals interactions among protein networks. Science. 2010;329:1038–1043. doi: 10.1126/science.1187433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart PL, Fuller SD, Burnett RM. Difference imaging of adenovirus: Bridging the resolution gap between X-ray crystallography and electron microscopy. EMBO J. 1993;12:2589–2599. doi: 10.1002/j.1460-2075.1993.tb05919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rexroad J, Wiethoff CM, Green AP, Kierstead TD, Scott MO, Middaugh CR. Structural stability of adenovirus type 5. J Pharm Sci. 2003;92:665–678. doi: 10.1002/jps.10340. [DOI] [PubMed] [Google Scholar]
- 6.Rux JJ, Kuser PR, Burnett RM. Structural and phylogenetic analysis of adenovirus hexons by use of high-resolution X-ray crystallographic, molecular modeling, and sequence-based methods. J Virol. 2003;77:9553–9566. doi: 10.1128/JVI.77.17.9553-9566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vellinga J, Van der Heijdt S, Hoeben RC. The adenovirus capsid: Major progress in minor proteins. J Gen Virol. 2005;86:1581–1588. doi: 10.1099/vir.0.80877-0. [DOI] [PubMed] [Google Scholar]
- 8.Patterson S, Russell WC. Ultrastructural and immunofluorescence studies of early events in adenovirus-HeLa cell interactions. J Gen Virol. 1983;64:1091–1099. doi: 10.1099/0022-1317-64-5-1091. [DOI] [PubMed] [Google Scholar]
- 9.Chardonnet Y, Dales S. Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome. Virology. 1970;40:462–477. doi: 10.1016/0042-6822(70)90189-3. [DOI] [PubMed] [Google Scholar]
- 10.Nakano MY, Boucke K, Suomalainen M, Stidwill RP, Greber UF. The first step of adenovirus type 2 disassembly occurs at the cell surface, independently of endocytosis and escape to the cytosol. J Virol. 2000;74:7085–7095. doi: 10.1128/jvi.74.15.7085-7095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiethoff CM, Wodrich H, Gerace L, Nemerow GR. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J Virol. 2005;79:1992–2000. doi: 10.1128/JVI.79.4.1992-2000.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greber UF, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 13.Suomalainen M, Nakano MY, Keller S, Boucke K, Stidwill RP, Greber UF. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J Cell Biol. 1999;144:657–672. doi: 10.1083/jcb.144.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trotman LC, Mosberger N, Fornerod M, Stidwill RP, Greber UF. Import of adenovirus DNA involves the nuclear pore complex receptor CAN/Nup214 and histone H1. Nat Cell Biol. 2001;3:1092–1100. doi: 10.1038/ncb1201-1092. [DOI] [PubMed] [Google Scholar]
- 15.Suomalainen M, Nakano MY, Boucke K, Keller S, Greber UF. Adenovirus-activated PKA and p38/MAPK pathways boost microtubule-mediated nuclear targeting of virus. EMBO J. 2001;20:1310–1319. doi: 10.1093/emboj/20.6.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin-Fernandez M, Longshaw SV, Kirby I, Santis G, Tobin MJ, Clarke DT, Jones GR. Adenovirus type-5 entry and disassembly followed in living cells by FRET, fluorescence anisotropy, and FLIM. Biophys J. 2004;87:1316–1327. doi: 10.1529/biophysj.103.035444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seth P, Willingham MC, Pastan I. Binding of adenovirus and its external proteins to Triton X-114. Dependence on pH. J Biol Chem. 1985;260:14431–14434. [PubMed] [Google Scholar]
- 18.Maier O, Galan DL, Wodrich H, Wiethoff CM. An N-terminal domain of adenovirus protein VI fragments membranes by inducing positive membrane curvature. Virology. 2010;402:11–19. doi: 10.1016/j.virol.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gastaldelli M, Imelli N, Boucke K, Amstutz B, Meier O, Greber UF. Infectious adenovirus type 2 transport through early but not late endosomes. Traffic. 2008;9:2265–2278. doi: 10.1111/j.1600-0854.2008.00835.x. [DOI] [PubMed] [Google Scholar]
- 20.Miyazawa N, Crystal RG, Leopold PL. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. J Virol. 2001;75:1387–1400. doi: 10.1128/JVI.75.3.1387-1400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazawa N, Leopold PL, Hackett NR, Ferris B, Worgall S, Falck-Pedersen E, Crystal RG. Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors. J Virol. 1999;73:6056–6065. doi: 10.1128/jvi.73.7.6056-6065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miles BD, Luftig RB, Weatherbee JA, Weihing RR, Weber J. Quantitation of the interaction between adenovirus types 2 and 5 and microtubules inside infected cells. Virology. 1980;105:265–269. doi: 10.1016/0042-6822(80)90177-4. [DOI] [PubMed] [Google Scholar]
- 23.Dales S, Chardonnet Y. Early events in the interaction of adenoviruses with HeLa cells. IV. Association with microtubules and the nuclear pore complex during vectorial movement of the inoculum. Virology. 1973;56:465–483. doi: 10.1016/0042-6822(73)90050-0. [DOI] [PubMed] [Google Scholar]
- 24.Leopold PL, Kreitzer G, Miyazawa N, Rempel S, Pfister KK, Rodriguez-Boulan E, Crystal RG. Dynein- and microtubule-mediated translocation of adenovirus serotype 5 occurs after endosomal lysis. Hum Gene Ther. 2000;11:151–165. doi: 10.1089/10430340050016238. [DOI] [PubMed] [Google Scholar]
- 25.Bremner KH, Scherer J, Yi JL, Vershinin M, Gross SP, Vallee RB. Adenovirus transport via direct interaction of cytoplasmic dynein with the viral capsid hexon subunit. Cell Host Microbe. 2009;6:523–535. doi: 10.1016/j.chom.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mabit H, Nakano MY, Prank U, Saam B, Dohner K, Sodeik B, Greber UF. Intact microtubules support adenovirus and herpes simplex virus infections. J Virol. 2002;76:9962–9971. doi: 10.1128/JVI.76.19.9962-9971.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey CJ, Crystal RG, Leopold PL. Association of adenovirus with the microtubule organizing center. J Virol. 2003;77:13275–13287. doi: 10.1128/JVI.77.24.13275-13287.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wodrich H, Henaff D, Jammart B, Segura-Morales C, Seelmeir S, Coux O, Ruzsics Z, Wiethoff CM, Kremer EJ. A capsid-encoded PPxY-motif facilitates adenovirus entry. PLoS Pathog. 2010;6:e1000808. doi: 10.1371/journal.ppat.1000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meulenbroek RA, Sargent KL, Lunde J, Jasmin BJ, Parks RJ. Use of adenovirus protein IX (pIX) to display large polypeptides on the virion—Generation of fluorescent virus through the incorporation of pIX-GFP. Mol Ther. 2004;9:617–624. doi: 10.1016/j.ymthe.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Gazzola M, Burckhardt CJ, Bayati B, Engelke M, Greber BS, Koumoutsakos P. A stochastic model for microtubule motors describes the in vivo cytoplasmic transport of human adenovirus. PLoS Comput Biol. 2009;5:e1000623. doi: 10.1371/journal.pcbi.1000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saphire AC, Guan T, Schirmer EC, Nemerow GR, Gerace L. Nuclear import of adenovirus DNA in vitro involves the nuclear protein import pathway and hsc70. J Biol Chem. 2000;275:4298–4304. doi: 10.1074/jbc.275.6.4298. [DOI] [PubMed] [Google Scholar]
- 32.Greber UF, Suomalainen M, Stidwill RP, Boucke K, Ebersold MW, Helenius A. The role of the nuclear pore complex in adenovirus DNA entry. EMBO J. 1997;16:5998–6007. doi: 10.1093/emboj/16.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelkar SA, Pfister KK, Crystal RG, Leopold PL. Cytoplasmic dynein mediates adenovirus binding to microtubules. J Virol. 2004;78:10122–10132. doi: 10.1128/JVI.78.18.10122-10132.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tycko B, Maxfield FR. Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin. Cell. 1982;28:643–651. doi: 10.1016/0092-8674(82)90219-7. [DOI] [PubMed] [Google Scholar]
- 35.Cain CC, Sipe DM, Murphy RF. Regulation of endocytic pH by the Na+,K+-ATPase in living cells. Proc Natl Acad Sci U S A. 1989;86:544–548. doi: 10.1073/pnas.86.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat Rev Mol Cell Biol. 2009;10:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 37.Waris M, Halonen P. Purification of adenovirus hexon protein by high-performance liquid chromatography. J Chromatogr. 1987;397:321–325. doi: 10.1016/s0021-9673(01)85015-9. [DOI] [PubMed] [Google Scholar]
- 38.Seth P, Pastan I, Willingham MC. Adenovirus-dependent increase in cell membrane permeability. J Biol Chem. 1985;260:9598–9602. [PubMed] [Google Scholar]
- 39.Smith JG, Wiethoff CM, Stewart PL, Nemerow GR. Adenovirus. Curr Top Microbiol Immunol. 2010;343:195–224. doi: 10.1007/82_2010_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wodrich H, Guan T, Cingolani G, Von Seggern D, Nemerow G, Gerace L. Switch from capsid protein import to adenovirus assembly by cleavage of nuclear transport signals. EMBO J. 2003;22:6245–6255. doi: 10.1093/emboj/cdg614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tynan SH, Gee MA, Vallee RB. Distinct but overlapping sites within the cytoplasmic dynein heavy chain for dimerization and for intermediate chain and light intermediate chain binding. J Biol Chem. 2000;275:32769–32774. doi: 10.1074/jbc.M001537200. [DOI] [PubMed] [Google Scholar]
- 42.Everitt E, Persson MJ, Wohlfart C. Ph-Dependent Exposure of endoproteolytic cleavage sites of the Adenovirus-2 hexon protein. Fems Microbiol Lett. 1988;49:229–233. [Google Scholar]
- 43.Vallee RB, Wall JS, Paschal BM, Shpetner HS. Microtubule-associated protein 1C from brain is a two-headed cytosolic dynein. Nature. 1988;332:561–563. doi: 10.1038/332561a0. [DOI] [PubMed] [Google Scholar]
- 44.Pfister KK, Fisher EM, Gibbons IR, Hays TS, Holzbaur EL, McIntosh JR, Porter ME, Schroer TA, Vaughan KT, Witman GB, et al. Cytoplasmic dynein nomenclature. J Cell Biol. 2005;171:411–413. doi: 10.1083/jcb.200508078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams JC, Roulhac PL, Roy AG, Vallee RB, Fitzgerald MC, Hendrickson WA. Structural and thermodynamic characterization of a cytoplasmic dynein light chain-intermediate chain complex. Proc Natl Acad Sci U S A. 2007;104:10028–10033. doi: 10.1073/pnas.0703614104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barbar E. Dynein light chain LC8 is a dimerization hub essential in diverse protein networks. Biochemistry. 2008;47:503–508. doi: 10.1021/bi701995m. [DOI] [PubMed] [Google Scholar]
- 47.Ligon LA, Karki S, Tokito M, Holzbaur EL. Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nat Cell Biol. 2001;3:913–917. doi: 10.1038/ncb1001-913. [DOI] [PubMed] [Google Scholar]
- 48.Vaughan KT, Vallee RB. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karki S, Holzbaur EL. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- 50.Stehman SA, Chen Y, McKenney RJ, Vallee RB. NudE and NudEL are required for mitotic progression and are involved in dynein recruitment to kinetochores. J Cell Biol. 2007;178:583–594. doi: 10.1083/jcb.200610112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmoranzer J, Fawcett JP, Segura M, Tan S, Vallee RB, Pawson T, Gundersen GG. Par3 and dynein associate to regulate local microtubule dynamics and centrosome orientation during migration. Curr Biol. 2009;19:1065–1074. doi: 10.1016/j.cub.2009.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tynan SH, Purohit A, Doxsey SJ, Vallee RB. Light intermediate chain 1 defines a functional subfraction of cytoplasmic dynein which binds to pericentrin. J Biol Chem. 2000;275:32763–32768. doi: 10.1074/jbc.M001536200. [DOI] [PubMed] [Google Scholar]
- 53.Horgan CP, Hanscom SR, Jolly RS, Futter CE, McCaffrey MW. Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J Cell Sci. 2010;123:181–191. doi: 10.1242/jcs.052670. [DOI] [PubMed] [Google Scholar]
- 54.Tan SC, Scherer J, Vallee RB. Recruitment of dynein to late endosomes and lysosomes through light intermediate chains. Mol Biol Cell. 2011;22:467–477. doi: 10.1091/mbc.E10-02-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palmer KJ, Hughes H, Stephens DJ. Specificity of cytoplasmic dynein subunits in discrete membrane-trafficking steps. Mol Biol Cell. 2009;20:2885–2899. doi: 10.1091/mbc.E08-12-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roghi C, Allan VJ. Dynamic association of cytoplasmic dynein heavy chain 1a with the Golgi apparatus and intermediate compartment. J Cell Sci. 1999;112:4673–4685. doi: 10.1242/jcs.112.24.4673. [DOI] [PubMed] [Google Scholar]
- 58.Vergnolle MA, Taylor SS. Cenp-F links kinetochores to Ndel1/Nde1/Lis1/dynein microtubule motor complexes. Curr Biol. 2007;17:1173–1179. doi: 10.1016/j.cub.2007.05.077. [DOI] [PubMed] [Google Scholar]
- 59.Liang Y, Yu W, Li Y, Yu L, Zhang Q, Wang F, Yang Z, Du J, Huang Q, Yao X, et al. Nudel modulates kinetochore association and function of cytoplasmic dynein in M phase. Mol Biol Cell. 2007;18:2656–2666. doi: 10.1091/mbc.E06-04-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- 61.Kardon JR, Reck-Peterson SL, Vale RD. Regulation of the processivity and intracellular localization of Saccharomyces cerevisiae dynein by dynactin. Proc Natl Acad Sci U S A. 2009;106:5669–5674. doi: 10.1073/pnas.0900976106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McKenney RJ, Vershinin M, Kunwar A, Vallee RB, Gross SP. LIS1 and NudE induce a persistent dynein force-producing state. Cell. 2010;141:304–314. doi: 10.1016/j.cell.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bingham JB, King SJ, Schroer TA. Purification of dynactin and dynein from brain tissue. Methods Enzymol. 1998;298:171–184. doi: 10.1016/s0076-6879(98)98017-x. [DOI] [PubMed] [Google Scholar]
- 64.Scherer J. Neither purified dynactin nor NudE interact with acidified hexon Department of Pathology and Cell Biology. Columbia University; New York, NY, USA: 2011. Unpublished work. [Google Scholar]
- 65.Engelke MF, Burckhardt CJ, Morf MK, Greber UF. The dynactin complex enhances the speed of microtubule-dependent motions of adenovirus both towards and away from the nucleus. Viruses. 2011;3:233–253. doi: 10.3390/v3030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whyte J, Bader JR, Tauhata SB, Raycroft M, Hornick J, Pfister KK, Lane WS, Chan GK, Hinchcliffe EH, Vaughan PS, et al. Phosphorylation regulates targeting of cytoplasmic dynein to kinetochores during mitosis. J Cell Biol. 2008;183:819–834. doi: 10.1083/jcb.200804114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Varma D, Dujardin DL, Stehman SA, Vallee RB. Role of the kinetochore/cell cycle checkpoint protein ZW10 in interphase cytoplasmic dynein function. J Cell Biol. 2006;172:655–662. doi: 10.1083/jcb.200510120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Starr DA, Williams BC, Hays TS, Goldberg ML. ZW10 helps recruit dynactin and dynein to the kinetochore. J Cell Biol. 1998;142:763–774. doi: 10.1083/jcb.142.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alonso C, Miskin J, Hernaez B, Fernandez-Zapatero P, Soto L, Canto C, Rodriguez-Crespo I, Dixon L, Escribano JM. African swine fever virus protein p54 interacts with the microtubular motor complex through direct binding to light-chain dynein. J Virol. 2001;75:9819–9827. doi: 10.1128/JVI.75.20.9819-9827.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Radtke K, Kieneke D, Wolfstein A, Michael K, Steffen W, Scholz T, Karger A, Sodeik B. Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures. PLoS Pathog. 2010;6:e1000991. doi: 10.1371/journal.ppat.1000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hernaez B, Tarrago T, Giralt E, Escribano JM, Alonso C. Small peptide inhibitors disrupt a high-affinity interaction between cytoplasmic dynein and a viral cargo protein. J Virol. 2010;84:10792–10801. doi: 10.1128/JVI.01168-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Douglas MW, Diefenbach RJ, Homa FL, Miranda-Saksena M, Rixon FJ, Vittone V, Byth K, Cunningham AL. Herpes simplex virus type 1 capsid protein VP26 interacts with dynein light chains RP3 and Tctex1 and plays a role in retrograde cellular transport. J Biol Chem. 2004;279:28522–28530. doi: 10.1074/jbc.M311671200. [DOI] [PubMed] [Google Scholar]
- 73.Ye GJ, Vaughan KT, Vallee RB, Roizman B. The herpes simplex virus 1 U(L)34 protein interacts with a cytoplasmic dynein intermediate chain and targets nuclear membrane. J Virol. 2000;74:1355–1363. doi: 10.1128/jvi.74.3.1355-1363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Döhner K, Radtke K, Schmidt S, Sodeik B. Eclipse phase of herpes simplex virus type 1 infection: Efficient dynein-mediated capsid transport without the small capsid protein VP26. J Virol. 2006;80:8211–8224. doi: 10.1128/JVI.02528-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scherer J, Vallee R. Mechanism of cytoplasmic dynein recruitment by adenovirus. Mol Biol Cell. 2010;21 Abstract No. 1683. [Google Scholar]
- 76.Döhner K, Wolfstein A, Prank U, Echeverri C, Dujardin D, Vallee R, Sodeik B. Function of dynein and dynactin in herpes simplex virus capsid transport. Mol Biol Cell. 2002;13:2795–2809. doi: 10.1091/mbc.01-07-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sathish N, Zhu FX, Yuan Y. Kaposi's sarcoma-associated herpesvirus ORF45 interacts with kinesin-2 transporting viral capsid-tegument complexes along microtubules. PLoS Pathog. 2009;5:e1000332. doi: 10.1371/journal.ppat.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Diefenbach RJ, Miranda-Saksena M, Diefenbach E, Holland DJ, Boadle RA, Armati PJ, Cunningham AL. Herpes simplex virus tegument protein US11 interacts with conventional kinesin heavy chain. J Virol. 2002;76:3282–3291. doi: 10.1128/JVI.76.7.3282-3291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morgan GW, Hollinshead M, Ferguson BJ, Murphy BJ, Carpentier DCJ, Smith GL. Vaccinia protein F12 has structural similarity to kinesin light chain and contains a motor binding motif required for virion export. PLoS Pathog. 2010;6:e1000785. doi: 10.1371/journal.ppat.1000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suikkanen S, Aaltonen T, Nevalainen M, Valilehto O, Lindholm L, Vuento M, Vihinen-Ranta M. Exploitation of microtubule cytoskeleton and dynein during parvoviral traffic toward the nucleus. J Virol. 2003;77:10270–10279. doi: 10.1128/JVI.77.19.10270-10279.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lakadamyali M, Rust MJ, Babcock HP, Zhuang X. Visualizing infection of individual influenza viruses. Proc Natl Acad Sci U S A. 2003;100:9280–9285. doi: 10.1073/pnas.0832269100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Petit C, Giron ML, Tobaly-Tapiero J, Bittoun P, Real E, Jacob Y, Tordo N, De The H, Saib A. Targeting of incoming retroviral Gag to the centrosome involves a direct interaction with the dynein light chain 8. J Cell Sci. 2003;116:3433–3442. doi: 10.1242/jcs.00613. [DOI] [PubMed] [Google Scholar]
- 83.McDonald D, Vodicka MA, Lucero G, Svitkina TM, Borisy GG, Emerman M, Hope TJ. Visualization of the intracellular behavior of HIV in living cells. J Cell Biol. 2002;159:441–452. doi: 10.1083/jcb.200203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Döhner K, Nagel CH, Sodeik B. Viral stop-and-go along microtubules: Taking a ride with dynein and kinesins. Trends Microbiol. 2005;13:320–327. doi: 10.1016/j.tim.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 85.Greber UF, Way M. A superhighway to virus infection. Cell. 2006;124:741–754. doi: 10.1016/j.cell.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 86.Radtke K, Dohner K, Sodeik B. Viral interactions with the cytoskeleton: A hitchhiker's guide to the cell. Cell Microbiol. 2006;8:387–400. doi: 10.1111/j.1462-5822.2005.00679.x. [DOI] [PubMed] [Google Scholar]