SUMMARY
Neuroinflammation is a pathological hallmark of neurodegenerative diseases including amyotrophic lateral sclerosis (ALS), and is characterized by activated microglia at sites of neuronal injury. In ALS. neurons do not die alone; neuronal injury is noncell- autonomous and depends upon a well-orchestrated dialogue between motor neurons and microglia. Evidence from transgenic models expressing mutant superoxide dismutase 1 (SOD) suggests that the dialogue between motor neurons and microglia initially protects motor neurons. However, with increasing stress and injury within motor neurons, induced by the presence of misfolded proteins such as mSOD1, mitochondrial function and axoplasmic flow are impaired and endoplasmic reticulum stress is induced; misfolded proteins themselves or alternate signals are released from motor neurons and activate microglia. Activated microglia, in turn, switch from anti-inflammatory and neuroprotective to proinflammatory and neurotoxic. Neurotoxic signaling from motor neurons promotes microglial release of reactive oxygen species and pro-inflammatory cytokines further enhancing motor neuron stress and cell injury and initiating a self-propagating cycle of motor neuron injury and cell death. A greater understanding of how to restore the imbalance between neuroprotection and cytotoxicity will depend upon a greater understanding of the motor neuron-microglial dialogue.
KEY WORDS: Microglia, Motoneurons, ALS
Our own efforts have focused on amyotrophic lateral sclerosis (ALS) and the role of neuroinflammation in the pathogenesis of ALS. This inexorably progressive neurodegenerative disease is characterized by selective loss of lower and upper motor neurons, resulting in varying degrees of atrophy and weakness in limb musculature, spasticity, and compromised speech, swallowing, and breathing. The presence of activated microglia, astrogliosis, and infiltrating lymphocytes accompanying motor neuron injury in ALS spinal cord tissue has raised the question as to whether motor neuron cell loss is dictated solely by intracellular events – cell-autonomous – or whether other cells may be involved. This question cannot be answered directly from human studies, but has been addressed in the transgenic mouse model of ALS overexpressing a human mutation of Cu2+Zn2+ superoxide dismutase (mSOD1) (1). In both human ALS and the transgenic mSOD1 mouse, there is evidence of neuroinflammation with increased microglial activation as well as increased CD4 and CD8 T cells and dendritic cells (2, 3). Expression of the mSOD1 transgene in motor neurons alone is not sufficient to cause motor neuron injury (4). Further, expression solely in astrocytes or microglia does not lead to a motor neuron phenotype. Thus motor neurons do not die alone. To cause significant injury, mSOD1 must be expressed in motor neurons as well as surrounding cells. This non-cell autonomy suggests a potential contribution of non-motor neuron cells such as microglia to motor neuron protection as well as motor neuron injury and cell death.
Motor Neuron-Microglia Neuroprotective Signaling
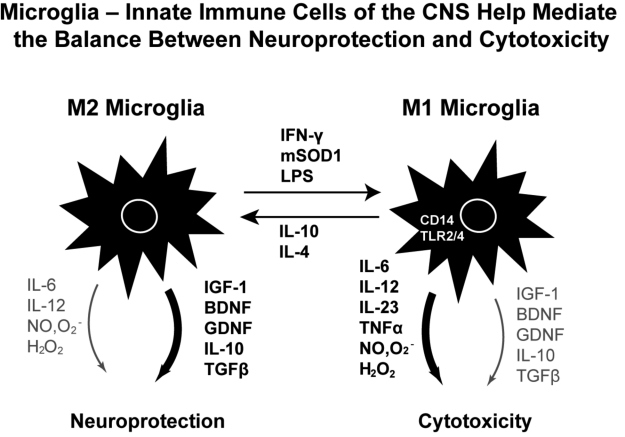
Motor neurons have been documented to promote microglia-mediated neuroprotection through at least two signals, fractalkine and CD200. The neuroprotective state of microglia characteristically releases anti-inflammatory cytokines and neurotrophic factors (Fig. 1). Microglia are the only CNS cells that express the fractalkine receptor (CX3CR1). Based on complementary expression of fractalkine (CX3CL1) on neurons and CX3CR1 on microglia, it had been proposed that neuroprotective signaling from motor neuron to microglia might be mediated through this receptor. In vivo, CX3CR1 deficiency dysregulates microglial responses, resulting in neurotoxicity. Following peripheral lipopolysaccharide injections, CX3CR1-/- mice showed cell-autonomous microglial neurotoxicity (5). Doubly transgenic mSOD/ CX3CR1-/-mice exhibited more extensive neuronal cell loss than CX3CR1+ littermate controls. Thus fractalkine release from motor neurons enhances neuroprotection, and the loss of the fractalkine receptor on microglia enhances neurotoxicity. Mice deficient for CD200, a neuronal glycoprotein whose receptor, CD200R, is expressed by all myeloid cells, show aberrant microglial physiology including morphological activation of microglia in the resting CNS and accelerated response to facial nerve transection (6). None of these attributes of altered microglial function are observed in CX3CR1-/- mice, indicating different neuroprotective pathways for CD200/CD200R and CX3CL1/CX3CR1 in regulating microglia.
Figure 1.
Microglia- Innate immune Cells of the CNS help mediate the balance between neuroprotection and cytotoxicity.
Microglia-Motor Neuron Cytotoxic Signaling
To help define the pathways for neurotoxic signaling in the microglia-motor neuron dialogue, we employed motor neurons co-cultured with microglia activated by lipopolysaccharide (LPS), which induced a proinflammatory M1 state in microglia, enhancing the production and release of NO and superoxide anion, and resulting in the formation of the extremely toxic compound peroxynitrite (8). This microglial proinflammatory state, in turn, led to motor neuron injury and cell death, mediated by reactive oxygen species and glutamate excitotoxicity. In the presence of increased NO, superoxide anion, and H2O2, extracellular glutamate interacting with the glutamate receptor on motor neurons resulted in increased entry of calcium and initiated a cell death cascade. mSOD1 microglia per se were found to be more activated than wild-type microglia, and produced and released more NO and superoxide anion than wild-type microglia, resulting in increased motor neuron cell death. Conversely, wildtype microglia were demonstrated to have increased release of neurotrophic factors IGF-1 and BDNF It was not necessary for mSOD1 to be expressed solely in microglia since the addition of extracellular mSOD1G93A protein to wild-type microglia was able to induce morphological and functional activation similar to the effects of LPS, increasing release of pro-inflammatory cytokines and free radicals (Zhao et al. 2010). Exogenous mSOD1G93A did not cause detectable direct killing of motoneurons alone. However when motoneurons were co-cultured with microglia, the addition of extracellular mSOD1G93A caused motor neuron cell death. The addition of wildtype mSOD1 protein to microglial-motor neuron cultures produced minimal motor neuron injury.
Microglial Receptors Mediating Cytotoxic Signaling
CD14 is a pattern recognition receptor for misfolded proteins and mutations in, or oxidation of, SOD1 lead to misfolded proteins (9). We were able to demonstrate that mSOD1G93A was bound to CD14. CD14 blocking antibodies attenuated the production of pro-inflammatory cytokines and free radicals and increased IGF-1 release from mSOD1G93A-treated microglia. When CD14-/- microglia were substituted for wild-type microglia, motor neuron injury and cell death were significantly attenuated. These in vitro studies are relevant to the in vivo state since expression of CD14 was significantly increased in spinal cord tissues of both ALS patients and mSOD1 mice (2, 3). Co-receptors for CD14 are the toll-like receptors TLR2 and TLR4; and previous studies suggested that CD14 and TLR contribute to the inflammatory responses initiated by microglia (10). Upregulation of CD14 and TLR2 in phagocytes are common in neurodegenerative diseases including transgenic models of Alzheimer’s disease, Parkinson’s disease, as well as ALS. Alzheimer Aß fibrils bind to CD14 and activate microglia, and anti-CD14 strategies reduce the neurotoxicity of Aß-stimulated microglia (11). Additionally, chronic stimulation of the CD14/ TLR pathway by LPS was found to exacerbate disease in ALS mice and TLR4 was necessary for LPS-sensitized hypoxic-ischemic neurodegeneration in vivo (12). In our studies microglia-mediated toxicity of motoneurons was attenuated with antibodies which blocked both TLR2 and TLR4. These data suggest that extracellular mSOD1G93A is similar to LPS, interacting with CD14, which then ligates TLR2 and TLR4, activating a proinflammatory cascade, increasing release of NO and superoxide anion, and decreasing the release of protective neurotrophic factors. Microglial release of proinflammatory factors in vitro leads to motor neuron injury and cell death. However, the addition of the cytokine IL-4 reversed LPS-induced and microglia-mediated motor neuron cytotoxicity; IL-4 suppressed nitric oxide and superoxide anion release, enhanced release of IGF-1, and promoted motor neuron survival (13). These data suggest that IL-4 may provide a significant immunomodulatory signal, protecting motor neurons from microglia-mediated neurotoxicity by suppressing the production and release of free radicals.
Motor Neuron-Microglia Cytotoxic Signaling - The role of mSOD1
A key question is whether any evidence demonstrates that microglia can be activated by the release of mSOD1 protein from motor neurons. An elegant series of papers have addressed this question directly. Chromogranins, components of neurosecretory vesicles, were documented to interact with mutant forms of superoxide dismutase but not with wild-type SOD1 (14). This interaction was confirmed by yeast two-hybrid screen and by co-immunoprecipitation assays using either lysates from Neuro2a cells coexpressing chromogranins and SOD1 mutants or lysates from spinal cord of ALS mice. Confocal and immunoelectron microscopy revealed a partial colocalization of mutant SOD1 with chromogranins in spinal cord of ALS mice. Mutant SOD1 was also found in immuno-isolated trans-Golgi network and in microsome preparations, suggesting that it could be secreted. Furthermore, chromogranins were demonstrated to act as chaperone-like proteins and promote secretion of SOD1 mutant proteins.
Motor Neuron- Microglia Cytotoxic Signaling - The role of OxidizedSOD1
Recent evidence demonstrates that oxidation of WT SOD1 results in misfolded protein that may acquire the binding and toxic properties of mSOD1, suggesting a possible shared pathway between sporadic and inherited ALS cases (15). Exposure of transfected Neuro2a cells expressing WT or amyotrophic lateral sclerosis-linked SOD1 species to H2O2 resulted in oxidized SOD1. Western blot analysis of immunoprecipitates from cell lysates revealed that, like mutant SOD1, oxidized WT SOD1 was conjugated with poly-ubiquitin, interacted with Hsp70. and was co-immunoprecipitated with Chromogranin B. Treatment of microglial cells (line BV2) with either oxidized WT SOD1 or mutant SOD1 recombinant proteins induced tumor necrosis factor-alpha and inducible nitric oxide synthase.. These results suggest that WT SOD1 may acquire binding and toxic properties of mutant forms of SOD1 through oxidative damage.
The over-expression of chromogranin in spinal cord neurons of mSOD1 transgenic mice resulted in significantly increased misfolded SOD1 species, earlier disease onset, and enhanced motor neuron degeneration (16). These findings are of relevance to human ALS since the P413L variant of chromogranin B was noted to be present in 10% of ALS patients (n = 705) as compared to 4.5% in controls (n = 751), conferring a 2.2-fold greater relative risk to develop the disease (P < 0.0001), and was associated with an earlier age of onset by almost a decade in both sporadic ALS and familial ALS cases (17).
The evidence that mutant and oxidized SOD1 can be secreted from motor neurons may also be pertinent to sporadic cases of ALS; the presence of oxidized wild-type SOD1 in sporadic ALS spinal cord motor neurons was recently described (18). Oxidized wild-type SOD1 and mutant SOD1 share a conformational epitope not present in normal wild-type SOD1, and this common epitope permitted the immunohistochemical demonstration of an aberrant wild-type misfolded SOD1 species present in motor neurons in the lumbosacral spinal cord of a subset of human sporadic ALS (SALS) cases. SOD1 immunopurified from this subset behaved similarly to familial ALS-linked mutant SOD1 and to recombinant, oxidized wild-type SOD1 in a model of axonal transport in vitro; wild-type SOD1 immunopurified from SALS tissues, oxidized wild-type SOD1, and familial ALS-linked mutant SOD1 all inhibited kinesin-based fast axonal transport whereas control wild-type SOD1 did not. Oxidative stress is one of the prominent findings in the CNS and peripheral circulation of ALS patients, and the demonstration of oxidized SOD1 in sporadic ALS motor neurons suggests an SOD1-dependent pathogenic mechanism common to FALS and SALS.
Microglia-Mediated Neuroprotection and Cytotoxicity in vivo
To evaluate the effects of microglia in vivo, we used PU.1 knockout (PU.1−/−) mice that at birth lack macrophages, neutrophils, T- and B-cells, and microglia, and require bone marrow transplation for survival (19). As a result all parenchymal microglia are derived from the bone marrow transplants, and the microglia have the genotype of the donor bone marrow cells. When we transplanted PU.1−/− mice with mSOD1G93A bone marrow, all CNS microglia were mSOD1G93A positive. However, we noted no clinical or pathological evidence of motor neuron disease. Thus mSOD1G93A microglia did not cause motor neuron disease if mSOD1G93A was not expressed in motor neurons. We then crossed PU.1−/− mice with mSOD1G93A mice to produce mSOD1G93A/PU.1−/− doubly transgenic mice, which expressed mSOD1G93A in motor neurons as well as astrocytes and other cell types, and still required a bone marrow transplant for survival. In these doubly transgenic mice the parenchymal microglia were derived from the donor transplant. mSOD1G93A/PU.1−/− mice transplanted with wild-type bone marrow had wild-type microglia throughout the parenchyma, while mSOD1G93A/PU.1−/− mice transplanted with mSOD1G93A bone marrow had mSOD1G93A microglia throughout the parenchyma. The mSOD1G93A/PU.1−/− transgenic mice with mSOD1G93A parenchymal microglia died considerably earlier and had greater motor neuron loss and shorter disease duration than the doubly transgenic mice with wild-type parenchymal microglia (19). Thus the ability of activated mSOD1 microglia to induce motor neuron injury in vitro was comparable to the mSOD1 microglia-mediated motor neuron injury in vivo, and most likely resulted from microglial-mediated release of neurotoxic substances and decreased release of neuroprotective factors. Conversely wild-type microglia mediated relative neuroprotection both in vitro and in vivo.
Summary: the Relevance of Neuroinflammation to ALS Pathogenesis
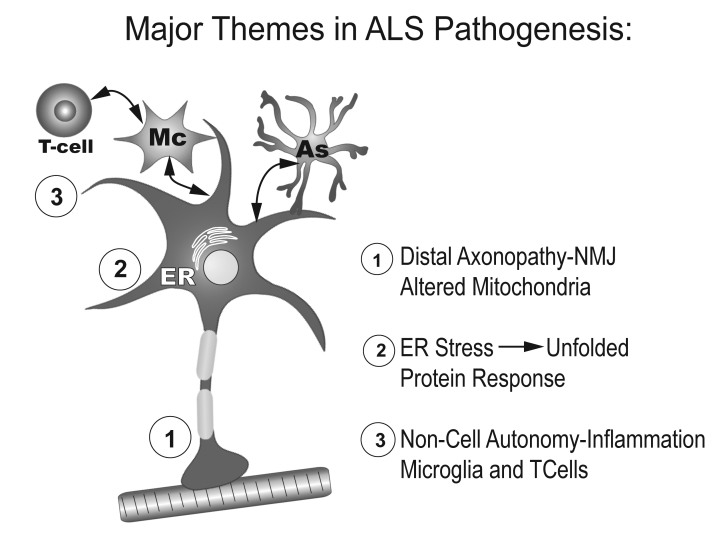
The major themes in ALS pathogenesis are depicted in Figure 2. Evidence from the mSOD1 transgenic mouse suggests that alterations in distal motor axons are among the earliest pathological changes in the pathogenesis of ALS, and the process is best explained as “dying back.” End-plate denervation is noted prior to the evidence of ventral root or cell body loss, and prior to the appearance of activated microglia surrounding affected motor neurons (20). In ALS patients, mitochondrial alterations consisting of swelling and increased calcium are present in motor axon terminals at early stages (21). Thus alterations in synaptic function and axonal connectivity may represent early and critical pathogenic events in ALS. Subsequent changes in axonal transport are also early events in ALS, impairing the transport of newly synthesized proteins and lipids and the clearance of damaged or misfolded proteins (22). A significant decrease in retrograde survival factors, including P-Trk (phospho-Trk) and P-Erk1/2, and an increase in retrograde stress factor signaling, including P-JNK (phosphorylated c-Jun N-terminal kinase), caspase-8, and p75(NTR) cleavage fragment have been documented in the mSOD1 transgenic mouse (23). Thus a shift from survival-promoting to death-promoting retrograde signaling may be a key step leading to neurodegeneration in ALS. This switch from survival-promoting to deathpromoting does not occur in mSOD1 motor neurons in vitro unless they are maintained on mSOD1 supporting cells. Thus, the evidence for non-cell autonomy necessitates the conclusion that changes within the neuron itself are insufficient to cause motor neuron death, but require motor-neuron–microglia signaling at least at the level of the cell soma. Whether the axonal death-promoting signaling is directly responsible for triggering ER stress, which further exacerbates the unfolded protein response and activates caspases, is unclear. What is clear is that motor neuron ER stress is a prominent feature of mouse and human ALS, and enhances release of neurotoxic signals, including possibly mSOD itself. These signals activate microglia to release free radicals and proinflammatory cytokines, and in turn, cause further motor neuron stress and initiate a self-propagating cytotoxic cascade. A greater understanding of the bidirectional signaling between motor neurons and microglia may lead to therapies that can restore the imbalance between neuroprotection and cytotoxicity.
Figure 2.
Major themes in ALS pathogenesis.
Acknowledgement
Supported by grants from the Muscular Dystrophy Association and the NIH.
References
- 1.Gurney ME, Pu H, Chiu AY, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 2.Henkel JS, Engelhardt JI, Siklos L, et al. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004;55:221–223. doi: 10.1002/ana.10805. [DOI] [PubMed] [Google Scholar]
- 3.Henkel JS, Beers DR, Siklós L, et al. The chemokine MCP-1 and the dendritic and myeloid cells it attracts are increased in the mSOD1 mouse model of ALS. Mol Cell Neurosci. 2006;31:427–437. doi: 10.1016/j.mcn.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Clement AM, Nguyen MD, Roberts EA, et al. Wild-type noneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 5.Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 6.Hoek RM, Ruuls SR, Murphy CA, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 7.Zhao W, Xie W, Le W, et al. Activated microglia initiate motor neuron injury by a nitric oxide and glutamate-mediated mechanism. J Neuropathol Exp Neurol. 2004;63:964–977. doi: 10.1093/jnen/63.9.964. [DOI] [PubMed] [Google Scholar]
- 8.Zhao W, Beers DR, Henkel JS, et al. Extracellular mutant SOD1 induces microglial-mediated motoneuron injury. Glia. 2010;58:231–243. doi: 10.1002/glia.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furukawa Y, Fu R, Deng HX, et al. Disulfide cross-linked protein represents a significant fraction of ALS-associated Cu, Zn-superoxide dismutase aggregates in spinal cords of model mice. Proc Natl Acad Sci USA. 2006;103:7148–7153. doi: 10.1073/pnas.0602048103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 11.Bate C, Veerhuis R, Eikelenboom P, et al. Microglia kill amyloidbeta1- 42 damaged neurons by a CD14-dependent process. Neuroreport. 2004;15:1427–1430. doi: 10.1097/01.wnr.0000132203.76836.16. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen MD, D'Aigle T, Gowing G, et al. Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2004;24:1340–1349. doi: 10.1523/JNEUROSCI.4786-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao W, Xie W, Xiao Q, et al. Protective effects of an anti-inflammatory cytokine, interleukin-4, on motoneuron toxicity induced by activated microglia. J Neurochem. 2006;99:1176–1187. doi: 10.1111/j.1471-4159.2006.04172.x. [DOI] [PubMed] [Google Scholar]
- 14.Urushitani M, Sik A, Sakurai T, et al. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci. 2006;9:108–118. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- 15.Ezzi SA, Urushitani M, Julien J. Wild-type superoxide dismutase acquires binding and toxic properties of ALS-linked mutant forms through oxidation. J Neurochem. 2007;102:170–178. doi: 10.1111/j.1471-4159.2007.04531.x. [DOI] [PubMed] [Google Scholar]
- 16.Ezzi SA, Larivière R, Urushitani M, et al. Neuronal over-expression of chromogranin A accelerates disease onset in a mouse model of ALS. J Neurochem. 2010;115:1102–1111. doi: 10.1111/j.1471-4159.2010.06979.x. [DOI] [PubMed] [Google Scholar]
- 17.Gros-Louis F, Andersen PM, Dupre N, et al. Chromogranin B P413L variant as risk factor and modifier of disease onset for amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2009;106:21777–21782. doi: 10.1073/pnas.0902174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosco DA, Morfini G, Karabacak NM, et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13:1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beers DR, Henkel JS, Xiao Q, et al. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer LR, Culver DG, Tennant P, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Siklós L, Engelhardt J, Harati Y, et al. Ultrastructural evidence for altered calcium in motor nerve terminals in amyotropic lateral sclerosis. Ann Neurol. 1996;39:203–216. doi: 10.1002/ana.410390210. [DOI] [PubMed] [Google Scholar]
- 22.Perlson E, Maday S, Fu MM, et al. Retrograde axonal transport: pathways to cell death. Trends Neurosci. 2010;3:335–344. doi: 10.1016/j.tins.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perlson E, Jeong GB, Ross JL, et al. A switch in retrograde signaling from survival to stress in rapid-onset neurodegeneration. J Neurosci. 2009;29:9903–9917. doi: 10.1523/JNEUROSCI.0813-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]