Abstract
Original experimental studies in nonhuman primate models of focal ischemia showed flow-related changes in evoked potentials that suggested a circumferential zone of low regional cerebral blood flow with normal K+ homeostasis, around a core of permanent injury in the striatum or the cortex. This became the basis for the definition of the ischemic penumbra. Imaging techniques of the time suggested a homogeneous core of injury, while positing a surrounding ‘penumbral' region that could be salvaged. However, both molecular studies and observations of vascular integrity indicate a more complex and dynamic situation in the ischemic core that also changes with time. The microvascular, cellular, and molecular events in the acute setting are compatible with heterogeneity of the injury within the injury center, which at early time points can be described as multiple ‘mini-cores' associated with multiple ‘mini-penumbras'. These observations suggest the progression of injury from many small foci to a homogeneous defect over time after the onset of ischemia. Recent observations with updated imaging techniques and data processing support these dynamic changes within the core and the penumbra in humans following focal ischemia.
Keywords: focal ischemia, imaging, ischemic penumbra, metabolic characteristics, microvessel characteristics, molecular characteristics
Introduction
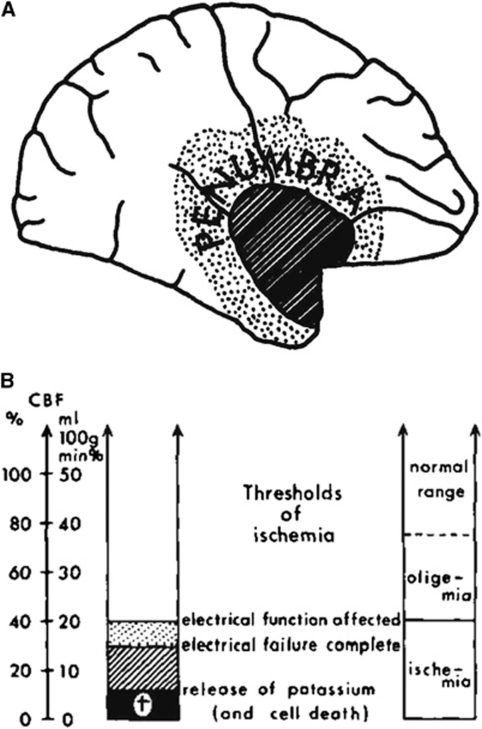
Occlusion of a brain-supplying artery for an extended period of ischemia leads to permanent injury if return of flow is inadequate. Astrup et al (1977, 1981) posited the development of a core of injury destined for tissue destruction (infarction), surrounded by a ‘penumbra' of metabolically metastable tissue that has the potential for full recovery (Figure 1A) (Branston et al, 1974, 1977). This general depiction of the ‘penumbra' has greatly influenced the concept of ischemic stroke following thrombotic and thromboembolic occlusion of brain-supplying arteries, and our attempts to enhance tissue recovery clinically. However, evolving data regarding cellular and molecular responses to ischemia, the interrelationships of vascular and neuronal activation (the ‘neurovascular unit'), experience with models of focal ischemia, and progress in acute imaging techniques in both animals and patients have suggested important refinements to the ‘penumbra' concept.
Figure 1.
The ‘penumbra'. (A) Depiction of ‘the penumbra' based on rCBF and electrophysiological studies on the nonhuman primate (from Symon (1980), with permission). (B) Depiction of the relationship among rCBF, electrical failure in the dependent cerebral tissue, and ischemia (taken from a figure in the study by Astrup et al (1977), with permission). rCBF, regional cerebral blood flow.
Although the original conceptualization of the penumbra remains solid, newer data that take into account the time course of injury development, potential interactions of boundary zones to the core, and the vascular ‘territory at risk' within the stricken hemisphere may provide even greater relevance to patient outcome than indicated by many acute intervention clinical trials. Recent work is beginning to address these issues and could point the way to further constructive developments in brain injury management.
Evolving Definitions of the Penumbra
Early approaches to modeling focal ischemia in mammals sought to develop discrete regions of neuron injury by manipulating the cerebral vascular supply. As model development moved toward the more clinically relevant occlusion of the middle cerebral artery (MCA) in the nonhuman primate, descriptions of neuron function and integrity were linked to regional changes in regional cerebral blood flow (rCBF). By necessity, the concept of the ‘penumbra' has depended in a large part on the techniques used to visualize it.
From the notion of a circumferential region of metabolic disturbance that has not reached the threshold of permanent injury, the concept has evolved to include (1) characteristic electrophysiological changes, (2) biochemical/molecular alterations, (3) responses of the microvasculature, (4) metabolic changes defined by imaging methods, and (5) observed differences between regions of abnormal tissue perfusion and diffusion regions on magnetic resonance imaging (MRI) studies.
In the original electrophysiological concept, the ‘ischemic penumbra' was an area that became electrically silent, but could recover function if the rCBF was restored in time (Heiss, 1992; Hossmann, 1994). An operational definition states that the penumbra ‘is ischemic tissue which is functionally impaired and is at risk for infarction, but has the potential to be salvaged…. If not salvaged this tissue is progressively recruited into the infarct core, which will expand with time into the maximal volume originally at risk (Baron, 1999)'. Imaging of the penumbra, using positron emission tomography (PET) has allowed measurement of rCBF, cerebral metabolic rate for oxygen (CMRO2), rCMRglc (regional cerebral metabolic rate of glucose), and oxygen extraction fraction (OEF). Infarction usually corresponds to rCBF decreased below 12 mL/100 g per min and rCMRO2 below 65 μm/100 g per min, whereas the penumbra has been defined as rCBF decreased to 12 to 22 mL/100 μg per min, the rCMRO2 above 65 μm/100 g per min, and OEF increased to 50% to 90% (Heiss, 2000). The concept of a mismatch between areas of blood perfusion and H2O diffusion in the injured territory as revealed by high field-strength magnetic MRI has provided a possible way to image the ‘penumbra' in humans.
Using animal models and methods with increased resolution, alterations in protein synthesis occur within and around the regions of injury. In regions of decreased rCBF, molecular correlates of the penumbra are: (1) decreased protein synthesis that can recover, (2) preserved ATP, (3) synthesis of heat-shock proteins (Hsps), and possibly (4) a successful unfolded protein response (Sharp et al, 2000). If these molecular criteria are met, the tissue has the capacity to be salvaged if blood flow is restored.
Once considered to be inert conduits, the microvasculature has been shown to respond rapidly and dynamically within the ‘territory at risk', containing the ischemic core and regions peripheral to the core. Microvessel adhesion receptor and basal lamina matrix expression can present as ‘mini-cores' and ‘mini-penumbras' within the early evolving core of neuron injury, after occlusion of a brain-supplying artery, in a characteristic time-dependent manner (Tagaya et al, 2001). Given that the microvasculature, which in capillaries consists of the endothelium–basal lamina matrix–astrocyte end-feet complex, serves neurons (as part of a hypothetical ‘neurovascular unit'), changes in microvessels and neurons must to be connected locally.
Each of these definitions describes the events/conditions in regions of focal ischemic injury within the ‘territory at risk'. Not considered by these definitions is whether the processes that determine the ‘penumbra' or ‘core' occur uniformly in adjacent areas over time and whether the active conversion of the penumbra to core occurs at the interface of the core. Recent data have suggested that the core of ischemic injury develops heterogeneously, and with time coalesces dynamically into a homogenous core. These data are consistent with the original concepts of the penumbra and core, but recognize the dynamic complex heterogeneous processes involved. This refinement has potential therapeutic implications.
The Ischemic Penumbra and the Ischemic Core
The ‘ischemic penumbra' was initially defined by physiologic experiments performed in large animals subjected to focal cerebral ischemia as (1) a region around a central core of low rCBF and physiologic silence, (2) a boundary region with release of intracellular K+, and (3) a region of metabolic instability that could be recovered.
Symon et al (1980) described the penumbra as a tissue of evolving injury around a homogeneous central core destined for infarction. Symon et al (1974) showed that 3 years after the rapid reduction in rCBF in the MCA territory in the nonhuman primate (Papio sp), a residual core of low rCBF remained that was congruent with the acute CBF reduction. Regions of CBF peripheral to the ‘core' had returned to normal levels. Branston et al (1974), using the same nonhuman primate model, related the loss in evoked potential (EP) amplitude to significant reductions in local CBF. Here, the rCBF threshold for 50% reduction in EP amplitude was ∼18 mL/100 g per min, when regional flow was reduced by phlebotomy and induced hypotension.
The functional threshold for neurologic dysfunction progressed from mild paresis at 22 mL/100 g per min to complete paralysis at 8 mL/100 g per min. Hence, the presence of a boundary zone in which the EP amplitude was sensitive to blood pressure/flow could be defined. Reduction in EP amplitude correlated with cellular K+ release into the surrounding tissue. When measured with H2 clearance electrodes, rCBF reduction could be observed at which the EP amplitude remained at 50% normal and K+ extravasation occurred in the ‘core', but not in the peripheral zones (Symon et al, 1974; Branston et al, 1977). Further studies have shown a differential sensitivity of K+ and Ca2+ extravasation when rCBF ranged from 8 to 15 mL/100 g per min (Harris and Symon, 1984); EP amplitude decreased when rCBF dropped below 15 mL/100 g per min even when K+ extravasation was not observed (Branston et al, 1977). On the basis of model studies, flow rates of 12 mL/100 g per min lasting for 2 to 3 hours led to large infarctions, but individual cells became necrotic after shorter intervals of time and at higher levels of residual flow. The potential for irreversible damage or recovery then seemed determined not only by the level of residual flow but also by the duration of flow disturbance for a given region.
These observations form the basis for the familiar temporal relationship among rCBF, electrical function, and brain tissue integrity characterizing regions of permanent injury and of reversible injury from ischemia onset (Figure 1B) (Jones et al, 1981). This synthesis implies that the core is homogeneous, and expands as a wave front with time into a penumbra of tissue that is at least initially not doomed to infarction. The range of perfusion between those limits—a rCBF level below which neuronal function is impaired and a lower threshold below which irreversible membrane failure and morphologic damage occur—typifies the ‘ischemic penumbra' (Astrup et al, 1981).
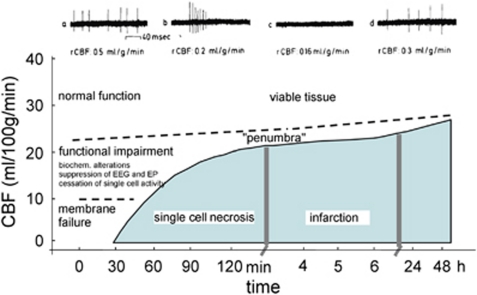
Separately, experiments using multiple electrodes in cats subjected to focal cerebral ischemia, which simultaneously assessed single-cell activity and local flow, showed pockets of low flow and cortical neuron vulnerability in closely adjacent cortical areas (Rosner et al, 1986). The altered single-cell activity with grouped or regular discharges at flow levels just above the threshold correlated with the gradual appearance of functional deficits (Figure 2). Spontaneous neuronal activity and EPs were restored when blood flow was reestablished (Heiss et al, 1976; Heiss and Rosner, 1983).
Figure 2.
Response of tissue to the duration of rCBF reduction, and development of the ‘penumbra'. Diagram of CBF thresholds required for the preservation of function and morphology of brain tissue. The activity of individual neurons is blocked when flow decreases below a certain threshold (dashed line) and returns when flow is increased again above this threshold. The upper recordings are from a single neuron before, during, and after reversible MCA occlusion. The fate of a single cell depends on the duration for which CBF is impaired below a certain level. The curved solid line demarcates conditions for structurally damaged from a functionally impaired, but morphologically intact tissue, the ‘penumbra'. The dashed line distinguishes tissue ‘not at risk' from the functionally impaired tissue. MCA, middle cerebral artery; rCBF, regional cerebral blood flow.
Metabolic Characteristics of the Penumbra and Core
Energy demands of the central nervous system are high and almost entirely provided by oxidative glucose metabolism. Increases in glucose consumption (and rCBF) evoked by functional activation are most prominent in synapse-rich regions, i.e., which contain axonal terminals, dendritic processes, and astrocytic processes that envelope the synapses and microvasculature. Energy requirements for functional activation are mostly caused by stimulation of Na+K+-ATPase activity to restore the ionic gradients across neuron cell membranes and membrane potentials that are altered by the spike activity (Sokoloff, 1999).
From its conceptual foundation in experiments in the nonhuman primate, further work on metabolic aberrations in the ‘at-risk' tissue proceeded in smaller animal models of focal cerebral ischemia. Loss of bioluminescent ATP and tissue acidosis occur in the ischemic core and penumbra (Astrup et al, 1977; Hossman and Mies, 2007) (AJ Strong, personal communication). The relationships among ischemic perfusion, functional impairment, biochemical disturbances, tissue damage, and duration of critical perfusion were shown in PET imaging studies of cats following MCA occlusion (Heiss et al, 1994, 1997). The PET imaging studies have suggested three phases of injury progression:
At flows below the threshold of energy metabolism (∼20% to 30% of preocclusion values), ‘acute' ischemic injury is usually established within minutes after the onset of ischemia (Branston et al, 1974, 1977; Astrup et al, 1977, 1981).
During the subsequent ‘subacute' phase (flows ranging from 25% to 50% of preocclusion values), the core expands into the penumbra, defined as areas of decreased CBF and O2 metabolism, but increased OEF. In most models, the core has consumed the entire penumbra within 4 to 6 hours, whereas in some animal models and in some humans (Heiss et al, 1992), the extension of the core into the penumbra can require >24 hours. This expansion is postulated to be attributed in part to peri-infarct spreading depression, and other factors related to the failure to restore CBF. Peri-infarct spreading depressions are initiated at the border of the infarct core and spread over the ipsilateral hemisphere. During spreading depression, the metabolic rate of the tissue markedly increases in response to the activated ion exchange pumps (Somjen, 2001), but is not associated with an increased rCBF in ischemic areas of the brain (review in the study by Strong (2009)). Thus, the increased metabolic workload coupled with limited O2 supply leads to transient episodes of worse hypoxia and stepwise increases in lactate with each depolarization, loss of ionic gradients, and other molecular disturbances that increase cell death (Mies et al, 1993; Hossmann, 2006).
A ‘delayed' phase of injury evolution occurs that can last for several days to weeks. Using multiparametric imaging techniques for differentiation between the core and the penumbra, by 1 hour after proximal MCA occlusion in the cat, the penumbra approximately predicts the size of the final infarct if flow is not restored (Heiss et al, 1994). However, after 3 hours, >50% of the penumbra progresses to infarction, and between 6 and 8 hours, almost all of the penumbra disappears and is converted into the irreversibly damaged infarct core (see Figure 5 below).
The flow values for irreversible damage and for functional impairment from experimental models of ischemia in cats correspond to those observed in humans. Considerable variability exists (damage below 4.8 to 17.3 mL/100 g per min, penumbra below 14.1 to 35.4 mL/100 g per min), which depends on the definition of damage/penumbra, the methods used for flow determination, the time of measurement after stroke, and whether occlusion was permanent or temporary (for review see the study by Heiss (2000)).
Molecular Characteristics of the Ischemic Penumbra and the Core
During focal ischemia, the molecular events that correspond best to the physiologic and imaging definitions of the penumbra are: (1) regions of decreased protein synthesis with preserved ATP and (2) regions of Hsp70 induction in neurons. Many molecular events occur well outside the clinically defined penumbra of ischemia-induced injury that does not lead to detectable infarction. These are associated with vascular changes during noninjurious decreases in blood flow, inflammation with loss of cells and their processes in the core, and synaptic/brain reorganization.
Kleihues and Hossmann (1971) and Hossmann (1993) first described decreased cerebral protein synthesis following focal cerebral ischemia. As rCBF decreases to ∼30% to 50% of baseline (Mies et al, 1990), blockade of translation and decreased protein synthesis are observed (Dienel et al, 1980; Kiessling et al, 1986; Bergstedt et al, 1993; Hossmann, 1994). Within the core region of ischemic injury, protein synthesis decreases early and is associated with ATP loss and irreversible translation blockade, whereas in the ‘penumbra', protein synthesis is initially depressed, ATP remains normal, and then protein synthesis recovers over time (Hossmann, 1993, 1994).
The Unfolded Protein Response
The decrease in protein synthesis in the ischemic core is mediated in part by the unfolded protein response within the cell endoplasmic reticulum. Translation blockade proceeds through initiation, maintenance, and termination (DeGracia and Hu, 2007). Initiation of the translation blockade in part involves the PKR-like endoplasmic reticulum kinase that phosphorylates eukaryotic initiation factor-2α and blocks translation in the endoplasmic reticulum. If eukaryotic initiation factor-2α is phosphorylated, the initiation complex is disrupted and translation is slowed or blocked (Hu and Wieloch, 1993). This is associated with disaggregation of polysomes, followed by the appearance of stress granules (DeGracia and Hu, 2007). The PKR-like endoplasmic reticulum kinase knockouts only show early translation blocks after ischemia, so that eukaryotic initiation factor-2α is related to the early, but not prolonged, blockade of protein synthesis following ischemia. After the initial translation blockade, other transcription factors, including eukaryotic initiation factor-4(E,F,G), likely maintain decreased protein synthesis for 4 to 24 hours and perhaps longer. This maintenance phase is followed either by (1) recovery of translation or by (2) permanent translation blockade and cell death, which constitute the termination phase (DeGracia et al, 2008). The proximate stimuli for the unfolded protein response in cerebral ischemia are still unknown, but may include increased Ca+2 in the endoplasmic reticulum, impaired amino-acid or glucose delivery, abnormal glycosylation of proteins, and other factors (Paschen, 1996, 1998; Kaufman et al, 2002, 2004; Schroder and Kaufman, 2005). It has been suggested by several groups that protein synthesis is arrested following ischemia to prevent formation of partially denatured or improperly processed proteins, which is probably protective. How protein synthesis resumes in the penumbra that will be salvaged and why protein synthesis remains suppressed in regions of penumbra that infarct are still not understood, but are under study.
Heat-Shock Protein Induction
Another major molecular responder to ischemia is the family of Hsps. The Hsps, expressed in every living cell and organism, are induced in cells during periods of ischemia and other stresses that produce intercellular denatured proteins (Lindquist and Craig, 1988; Craig et al, 1993; Lindquist and Kim, 1996; Massa et al, 1996; Yenari, 2002).
Now, Hsp70, the major inducible Hsp, is expressed at almost undetectable levels in normal brain cells. After heat stress, ischemia, and other stresses, Hsp70 is massively induced and becomes the most abundant protein in the cell because it binds denatured proteins directly and attempts to refold them (Beckmann et al, 1990, 1992; Welch, 1993; Welch and Brown, 1996). Denatured proteins induce heat-shock transcription factors that bind to heat-shock elements in the Hsp genes and induce Hsp40, Hsp70, Hsp90, and others. At this point, the injured cell appears to make a decision: Hsp70 can promote protein refolding and cell survival or allow protein degradation and progression to cell death (Hohfeld et al, 2001). Importantly, cells that synthesize Hsp70 mRNA but cannot synthesize Hsp70 protein likely die. Most cells that express both Hsp70 mRNA and Hsp70 protein appear to survive.
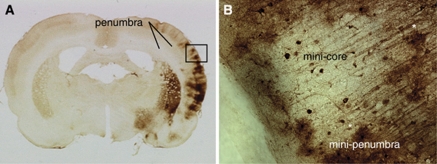
After MCA occlusion, Hsp70 mRNA is expressed throughout the MCA territory, both within the infarction and in regions adjacent to the infarction (Kinouchi et al, 1993a, 1993b, 1994). Hata et al (1998) have shown that Hsp70 transcription (RNA) and Hsp70 translation (protein) occur in regions of decreased protein synthesis, but preserved ATP. This is the area where blood flow is 18% to 20% normal and in which infarction would occur if reperfusion did not occur (Hata et al, 2000a, 2000b, 1998). Under conditions in which Hsp70 transcripts are induced in the MCA distribution, the Hsp70 protein is only synthesized in neurons peripheral to the ischemic core, whereas there is no or very little Hsp70 protein synthesized in the core (Kinouchi et al, 1993a). Thus, the ischemic core corresponds to a region of either no Hsp70 mRNA induction or induction of Hsp70 mRNA, but without translation into the Hsp70 protein. This contrasts with the molecular events within the penumbra: regions of Hsp70 mRNA induction and Hsp70 protein synthesis in the regions of denatured proteins where cells can effectively refold proteins (Figure 3). This also correlates with differential cellular vulnerability to ischemia because blood vessels that synthesize Hsp70 mRNA and Hsp70 protein may well survive in areas of infraction where neurons and glia succumb. Although the regions of Hsp70 mRNA and Hsp70 protein induction at 24 hours appear to correlate well with the clinical definition of the penumbra, it is not known whether the volume of tissue destined to infarction corresponds exactly to that represented by the Hsp70 protein, because there is no known independent molecular marker of tissue at risk for infarction.
Figure 3.
Development of ‘mini-penumbra' and ‘mini-core'. (A) Coronal section of the adult rat brain that shows Hsp70 protein (stain) 24 hours after occlusion of the right middle cerebral artery (MCA) for 10 minutes. Hsp70 immunoreactivity in the cortex, basal ganglia, and ventral thalamus and dorsal hypothalamus in the MCA distribution must be noted. Hsp70 staining delineates the entire penumbra, because this region of brain did not infarct, but would have infarcted if MCA occlusion had persisted for 2 hours. (B) The region of the cortex within the box in panel A shows a central area of little Hsp70 expression surrounded by a large number of Hsp70-stained cells that are predominantly microglia (two are denoted with green stars). For the purposes of this review, we have designated the central area that represents a possible microscopic infarct as the ‘mini-core' and the surrounding area of Hsp70-stained glia and some neurons as the ‘mini-penumbra'. This figure is adapted from the study by Zhan et al (2008). Hsp70, heat-shock protein 70.
The degree and duration of ischemia affect the regional expression of Hsp70. For example, 10 minutes of focal ischemia in the MCA distribution (using the suture model), which does not infarct rat brain, induces Hsp70 protein in neurons throughout the MCA distribution 4 to 24 hours later. Thus, the regions of Hsp70 protein induction indicate the penumbra because this is the region ‘at risk' for infarction, but which did not infarct because blood flow was restored after 10 minutes of focal ischemia (Zhan et al, 2008). In contrast, at 24 hours after 3-hour (suture-induced) MCA occlusion infarction occurs in the MCA territory, and the small area of Hsp70 induction indicates a small penumbra located between the middle and anterior, and the posterior cerebral arteries (Zhan et al, 2008). The expression of Hsp70 in neurons outside areas of infarction is presumed to protect these cells from further protein denaturation, as overexpression of the Hsp70 protein in transgenic mice markedly protects the brain against infarction (Rajdev et al, 2000).
Heterogeneity of the Molecular Responses
The ‘boundary' of the penumbra reflects the degree and duration of decreased cerebral perfusion. Outside this region, the hypoperfused ischemic brain tissue contains neurons, glia, and vascular elements together with inflammatory cells that are associated with gliosis, synaptic pruning, and other structural and molecular changes presaging recovery after stroke.
In contrast to a central zone of hypoperfused brain at risk for infarction, the pattern and evolution of the penumbra can be quite variable. For example, in some models of cerebral infarction, there are no zones that correspond to decreased protein synthesis and Hsp70 protein—and thus there is no penumbra. In other models, there are small islands of the infarcted brain (‘mini-cores') surrounded by neurons and glial cells that express Hsp70 (‘mini-penumbras') (Figure 3). The ‘most-stressed' cells at the margins of these islands are sometimes neurons and sometimes microglia. The features that determine this heterogeneity are unknown, but likely reflect a dynamic interplay between adjacent microvascular beds and complex interactions between the microvessels and the adjacent glia and neurons that differ over regions of 30 to 40 μm. How this relates to infarction without the intervening surviving tissue is still unknown. Indeed, although neurons are more vulnerable than glia to ischemia, there is eventually a discrete demarcation between the infarcted brain and the surviving brain where both viable neurons and glia exist at the margin and where their companions have perished on the infarcted side of the margin. The factors that define such a sharp margin are unknown, but may reflect interdependent survival growth factors responsible for the survival of all members of a given neurovascular unit, or regions lacking adequate microvascular supply.
The data in Figure 3 strongly support the idea of ‘mini-penumbras' and ‘mini-cores' based on the pattern of stress gene response. Histologic data obtained from the study by Hughes et al (2010) also support this concept because they found patchy neuronal loss and associated microglial activation in the salvaged cortical penumbra after brief MCA occlusion. In addition, we have shown very patchy areas of microinfarction in the striatum and occasionally in the cortex after 5- and 10-minute periods of focal ischemia produced with the suture technique in rats (Zhan et al, 2008). These ‘mini-cores' were microscopic and defined by contiguous loss of NeuN-immunostained neurons associated with contiguous loss of glial fibrillary acidic protein (GFAP)-stained astrocytes in the same region.
Vascular Characteristics of the Ischemic Penumbra and the Core
Heterogeneity in the development of cortical neuron injury has been shown in a rodent model of focal cerebral ischemia (Dawson and Hallenbeck, 1996). One provocative view has suggested that vascular and hemostatic responses vary depending on the specific organ, although little information was available about the cerebral vasculature (Rosenberg and Aird, 1999). It is now known that cellular and molecular responses of cerebral microvessels to focal ischemia are heterogeneously distributed in space and time very early in the ischemic territory.
After MCA occlusion in the nonhuman primate, neuron injury and the microvessel responses are rapid and are distributed in a characteristic topographical arrangement with regard to the development of the ischemic core. Tagaya et al (1997) have shown that in the evolving core early after injury (defined by evidence of DNA strand breaks or repair), 80.0%±6.6% of injured cells were neurons at 2 hours after MCA occlusion, but there is a much lower proportion of injured endothelial cells at later times (<2% of cells at 27 hours). This is consistent with the notion that neurons are most sensitive to ischemia, whereas vascular endothelial cells are most resistant. However, microvessels within the territories of injury are rapidly reactive, participating in inflammatory cell adhesion, and displaying changes in barrier permeability and matrix integrity. These observations indicate heterogeneity in cell vulnerability to injury within the developing core and adjacent penumbra. Indeed, in the primate corpus striatum after proximal MCA occlusion, microvessel alterations and injury within the regions of the ischemic core are distributed heterogeneously (Tagaya et al, 2001).
Microvessel Matrix Receptor Responses
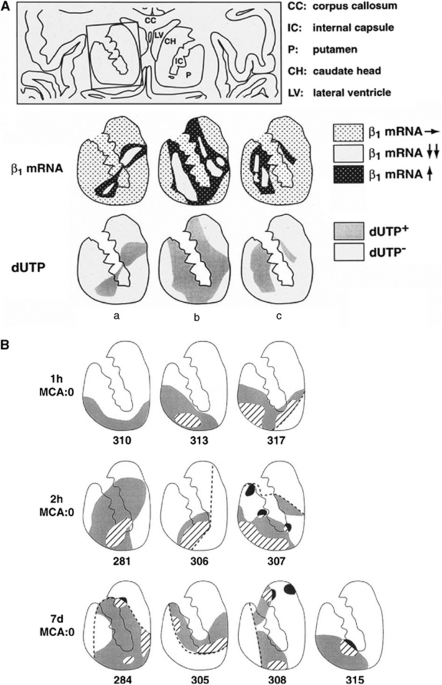
This heterogeneity in the evolution of injury is also shown by the response of integrin α1β1 expression by microvessel endothelial cells in regions of the territory at risk that become the core. Integrin α1β1 is a common receptor for endothelial cell adhesion to laminin, collagen IV, and perlecan in the microvessel basal lamina. The β1 integrins on the cerebral endothelium, and the integrin α6β4 as well as αβ-dystroglycan on astrocyte end-feet all decrease significantly within 2 hours after MCA occlusion (Wagner et al, 1997; Abumiya et al, 1999; Tagaya et al, 2001). Tagaya et al (2001) have shown that the distributions and density of microvessels suffering loss of α1 and β1 subunits were heterogeneously distributed, in a pattern that depended on the vascular supply. Microvessels in the ischemic striatum expressed increased β1 subunit transcripts in boundaries around central regions devoid of β1 integrin transcription. To help explain these observations, in vitro integrin β1 subunit mRNA expression increased when murine brain endothelial cells (grown on collagen type IV, laminin, or perlecan) were exposed to moderate oxygen–glucose deprivation (experimental ischemia) (Milner et al, 2008). Hence, the regions of endothelial cell response in the striatum of the nonhuman primate can be interpreted as multiple ‘mini-penumbras' made up of areas of β1 integrin mRNA upregulation on the edges of each core region and absent β1 integrin expression in ‘mini-cores' because of the failure of transcription and translation in the areas of infarction (Figure 4A) (Tagaya et al, 1997, 2001).
Figure 4.
Expression of microvessel-related gene products at 2 hours after MCA occlusion in the striatum. (A) Expression of the integrin β1 subunit mRNA by microvessels in the striatum of three nonhuman primate subjects (a–c) after MCA:O (Tagaya et al, 2001). Around mini-cores of absent β1 subunit mRNA, significant upregulation of the β1 subunit gene product was observed. The cores and boundary zones of β1 subunit upregulation corresponded to the regions where cells incorporated dUTP (i.e., dUTP+) (Tagaya et al, 1997). (B) Regions of VEGF expression (black), αν subunit (gray), and expression of both products (hatched) by activated microvessels in the striata of nonhuman primates that were subject to various periods of MCA occlusion (each image represents a separate animal) (Abumiya et al, 1999). The mini-cores of integrin or VEGF expression by microvessels must also be noted. MCA, middle cerebral artery; VEGF, vascular endothelial growth factor.
Angiogenesis
Another example of vascular response heterogeneity to focal ischemia is the increased expression of integrin αvβ3 and vascular endothelial growth factor (VEGF) by cerebral microvessels within the ischemic core during MCA occlusion (Abumiya et al, 1999). Vascular endothelial growth factor expression is responsible for the upregulation of integrin αvβ3, and stimulates proliferation, migration, and increased permeability of the endothelium (Senger et al, 1996). Abumiya et al have shown that during striatal ischemia, VEGF mRNA was detectable in noncapillary microvessels in relationship to the αvβ3 expression. Colocalization studies, involving microvessels reconstructed over 100 μm of their length in regions of dUTP incorporation (the ‘core'), showed a significant colocalization among proliferating cell nuclear antigen (PCNA), the integrin αvβ3, and VEGF along the length of activated microvessels (Abumiya et al, 1999). Integrin αvβ3 was upregulated mainly on 7.5- to 30.0-μm diameter microvessels within the core region. Microvessel-associated PCNA, VEGF, and integrin αvβ3 were all upregulated in this region (Figure 4B). Using a hierarchical log-linear model for the categorical data analysis, tests for pairwise interactions at different times showed that the interaction with time was not significant. Therefore, coexpression of PCNA, VEGF, and integrin αvβ3 by microvessels within the ischemic core indicated a highly significant interaction that was independent of time, and was therefore heterogeneous within the core regions.
An interesting observation in this respect suggests that in some settings, cerebral microvessels in a territory could be variously primed for response, such that their response is heterogeneous. Ruetzler et al (2001) have shown segmental heterogeneity of central nervous system vascular responses in normoxic spontaneously hypertensive stroke-prone rats exposed to lipopolysaccharide, in which circular decoration of brain tissue by manganese superoxide dismutase was seen around some microvessels and not others. This is possibly relevant to the observations made by Mabuchi et al (2005) of an ordered, but heterogeneously distributed, neuron injury within 2 hours of MCA occlusion that is related to the microvessel supply (the ‘neurovascular unit'). Of importance, these observations are not simply explained by the dependence of local injury on the fall-off of O2 diffusion as applied to cerebral capillaries (the Krogh cylinder), but suggest other mechanisms (Quistorff et al, 1977; Mabuchi et al, 2005). Whether the coordinated expression of PCNA, integrin αvβ3, and VEGF by cerebral microvessels during focal ischemia is an intrinsic characteristic of some vessels of a size class, or the heterogeneity of the injury, is not yet certain.
Heterogeneity of the Microvascular Responses
Responses of the microvasculature in the territory at risk (striatum) to focal ischemia are very rapid, nearly as rapid as those of the neurons they supply. Within the core regions, in the first minutes after ischemia onset, microvessel obstruction, endothelial cell receptor presentation, matrix degradation, and detachment of astrocyte end-feet occur initially in a heterogeneous manner in pockets (‘mini-cores'). The initial microvessel responses are neither simultaneous nor homogeneous. This suggests that in the early moments after arterial occlusion, the entire ‘core' is studded with pockets of ‘penumbra', and that these ‘mini-cores' and their ‘mini-penumbras' evolve dynamically and heterogeneously depending on local differences of microvessel perfusion.
Imaging the Ischemic Penumbra and Core
Noninvasive brain imaging modalities have evolved greatly since the first descriptions of the core–penumbra relationships in large animal models. The use of PET and MR imaging techniques in humans is now beginning to confirm and even extend observations of the core and penumbra defined in animal models.
Positron Emission Tomography Imaging
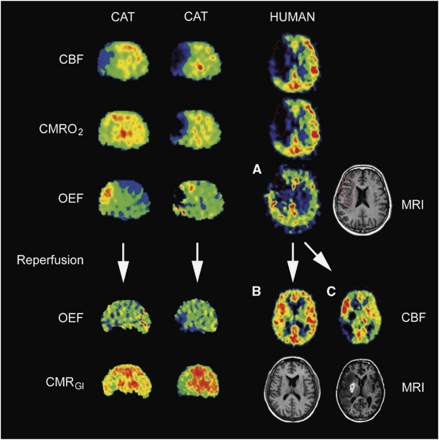
Positron emission tomography techniques have permitted classification of three regions within the disturbed vascular territory: (1) the core of ischemia which usually undergoes necrosis with a flow rate <12 mL/100 g per min, (2) a penumbra region (12 to 22 mL/100 g per min) of still viable tissue, but with uncertain chances for infarction or recovery depending on reflow, and (3) a hypoperfused area (>22 mL/100 g per min) not primarily damaged by decreased blood supply that will not proceed to infarction (see Figure 5). In humans, the brain tissue with rCBF <12 mL/100 g per min and rCMRO2 <65 μmol/100 g per min (often measured several hours after stroke) usually displays eventual evidence of infarction on late computerized tomographic scans (Ackerman et al, 1981; Baron et al, 1981a; Lenzi et al, 1982; Powers et al, 1985). Relatively preserved CMRO2 in regions of severely reduced CBF has been taken as an indicator of maintained neuronal function in regions with severely reduced CBF. This pattern, coined ‘misery perfusion', has served as a PET-derived definition of the penumbra (Baron et al, 1981b). It is characterized as the area of increased OEF (>80% from the normal value of ∼40%). Positron emission tomography investigations imply that the extent of the penumbra depends on the time of measurement relative to the onset of ischemia. The volume is large and rCBF is low if the penumbra is defined in the first hours of ischemia; the penumbra volume is small, if defined later (Heiss et al, 1992). The heterogeneity in the core of ischemia and in the penumbra over time can be seen in the occurrence of OEF especially in animal studies (Figure 5), but also seen repeatedly in human PET studies (e.g., Heiss et al, 1992).
Figure 5.
Sequential PET images of CBF, CMRO2, and OEF of permanent MCA occlusion in cats (left columns) compared with images of patients 12 hours after stroke (right columns). Two different cat subjects are shown (efficient and nonefficient perfusion) and three different patients are shown (A: early OEF defect with corresponding infarction on MRI, B: effective reperfusion without infarction, and C: ineffective/delayed reperfusion with large final partially hemorrhagic infarction). In the cat, the progressive decrease of CMRO2 and the reduction of OEF predict infarction. In the patient, the area with preserved OEF is not infarcted (outside region on late MRI, upper part of figure A). If reperfusion occurs before OEF is reduced, tissue can be salvaged (left: cat, and left: patient in the lower part of the figure B). If reperfusion is achieved after this therapeutic window, tissue cannot be salvaged (right: cat, and right: patient in lower part of the figure C). CBF, cerebral blood flow; CMRO2, cerebral metabolic rate for oxygen; MCA, middle cerebral artery; MRI, magnetic resonance imaging; OEF, oxygen extraction fraction; PET, positron emission tomography.
As these measurements require arterial blood sampling and complex logistics, a marker of neuronal integrity is preferable. The central benzodiazepine receptor ligand flumazenil (FMZ) binds to the GABA receptor that is abundant in the cerebral cortex. These receptors are sensitive to ischemic damage and can identify early neuronal loss. Flumazenil, validated in the cat MCA occlusion model, predicted the size of final infarction in patients with acute ischemic stroke, and showed the efficacy of thrombolytic treatment (Heiss, 2000). Another marker of the penumbra, 18F misonidazol is trapped in viable hypoxic tissue (Takasawa et al, 2007). In early stroke, increased 18F misonidazol uptake surrounds the core, and there is a strong association between the extent of 18F misonidazol-binding tissue that survives and functional outcome (Markus et al, 2003). 18F misonidazol uptake also assesses hypoxia in the white matter, where progression of ischemic damage is slower (Heiss et al, 2000; Spratt et al, 2007).
As PET methodology has improved, higher resolution with shorter time sequences have detected temporal and spatial heterogeneity of the core and penumbra (Kidwell et al, 2003), which were especially evident in sequential studies in animal models of focal and reversible cerebral ischemia (Heiss et al, 1994).
Magnetic Resonance Imaging
Although PET remains the imaging gold standard for identification of tissue in the penumbra in human stroke patients, MR studies using diffusion and perfusion imaging by MR have provided valuable insights into the relationships between the core and the penumbra. More than a decade ago, it was hypothesized that the early diffusion-weighted imaging (DWI) lesion estimates the ischemic core in ischemic stroke patients and adjacent critically hypoperfused tissue could be identified with perfusion-weighted imaging (PWI) (Baird et al, 1997; Barber et al, 1998). Therefore, brain regions with a PWI lesion that did not show restricted diffusion (PWI/DWI mismatch) were hypothesized to represent the penumbra. Subsequent studies have extended this notion by showing that appropriate quantification of perfusion and diffusion imaging can provide a reasonable estimate of the penumbra.
Problems with the MRI definition of the penumbra include the fact that there is no threshold within a region of oligemia (Kidwell et al, 2003; Kane et al, 2007; Toth and Albers, 2009), and that the PWI abnormality often overestimates the final infarction volume, and hence the amount of tissue at risk (Parsons et al, 2001). In addition, the initial diffusion lesion does not necessarily define the core of infarcted tissue because some diffusion lesions can be transiently or permanently reversed if blood flow is rapidly restored (Kidwell et al, 2000; Parsons et al, 2002; Chalela et al, 2004).
Kidwell et al (2003) proposed a modified model of ischemia-compromised tissue in which the penumbra includes the region of perfusion–diffusion mismatch, minus the region of benign oligemia plus a portion of the initial diffusion abnormality itself. Although several attempts have been made to identify perfusion or apparent diffusion coefficient (ADC) thresholds so as to better differentiate these regions, consensus on the best method has been hampered by the lack of methodological standardization of image postprocessing and analysis. These restrict the pooling of data and cross-comparison of results across studies (Kane et al, 2007).
Validation of MR signatures with regard to PET measurements might help in the interpretation of the respective findings and in the assessment of the accuracy of the various measures for predicting tissue outcome. Comparisons of PET and MR imaging have been performed in small groups of patients to assess perfusion, and the delineation of tissue in the penumbra (Figure 5).
To predict irreversible cortical damage, results from sequential early DWI and 11C-FMZ-PET outcomes were compared with infarct extension 24 to 48 hours later on T2-weighted MRI in 12 acute stroke patients (Heiss et al, 2004). When the volumes of tissue beyond the defined thresholds of FMZ binding and DWI intensity were compared, close correlations (1) between volumes with FMZ and DWI beyond threshold and (2) between predicted and final infarct volumes were obtained, but the volumes did not completely overlap. Whereas false-positive results were observed for 25.9% of the total volume of DWI increase, they were negligible with FMZ-PET. A time-to-peak delay of 4 seconds correlated with flow decreases below 20 mL/100 g per min as identified by H2 15O-PET (Zaro-Weber et al, 2009).
For demarcation of the volume of the penumbra, the areas of PWI/DWI mismatch were compared with those of increased OEF. Using PWI/DWI mismatch, there was considerable variability in penumbra volume, and all patients (13/13) showed areas of mismatch. However, PET detected OEF in only 8 of 13 patients. The areas of OEF elevation were always located within the areas of time-to-peak prolongation, and were significantly smaller and covered only 1% to 75% (median, 33%) of the time-to-peak area on MRI. These data show a high sensitivity, and a low specificity of the chosen thresholds to identify the penumbra as defined by PET. This suggests that the PWI/DWI volume ratios depicted by time to peak do not reliably reflect the penumbra as defined by PET.
Advances in Perfusion-Weighted Imaging Techniques
Nonetheless, the spatial correspondence between early DWI and PWI lesions, in conjunction with knowledge of whether reperfusion of the supply artery(ies) occurs, allow a reasonable (but not a highly accurate) prediction of the final infarction volume. More advanced PWI techniques, including deconvolution sequences that account for differences in arterial input to vessels with normal flow, appear to produce more accurate estimates of the regions of hypoperfusion that define the outer boundaries of the penumbra. Although PWI provides a number of parameters including mean transit time and cerebral blood volume, recently there has been focus on the maximum of the tissue residue function (Tmax) obtained by deconvolution (Zaro-Weber et al, 2010). Although the physiologic interpretation of Tmax is complex, it reflects the degree of lag between the arterial input and the tissue response, as well as dispersion and mean transit time (Calamante et al, 2010). This parameter appears to correlate with CBF values determined by Xenon computerized tomographic scans and has performed well in two prospective clinical stroke trials (Albers et al, 2006; Davis et al, 2008).
Thresholding techniques that restrict the volume of PWI lesions to brain regions where there are substantial delays in contrast arrival or transit times have also been used. This reduces the volume of benign oligemia captured by the PWI map. Prediction of the final infarction volume and salvage of the penumbra seems to be more accurate using Tmax maps with higher thresholds. In the DEFUSE (Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution) study, for instance, the correlation between infarct growth and the volume of penumbra salvaged was significantly better for PWI lesions defined by Tmax >6 seconds compared with >2 seconds (Olivot et al, 2009). In addition, in patients who did not experience early reperfusion, the >4-second threshold was a more accurate predictor of final infarction volume than Tmax >2 seconds. Similarly, in EPITHET (Echoplanar Imaging Thrombolytic Evaluation Trial), both the size and the severity of PWI lesions correlated with clinical outcomes (Parsons et al, 2010). Recent data have suggested a ∼90% sensitivity and specificity for Tmax >5.5 seconds to identify the PET-defined penumbra rCBF threshold of 20 mL/100 g per min (Zaro-Weber et al, 2010). These results suggest that quantification of the severity of PWI lesions can improve accuracy for identification of tissue that is in the penumbra.
Advances in Diffusion-Weighted Imaging Techniques
Progress has been made in quantification of DWI using ADC measurements. Animal studies and human trials are congruent, both showing correlations between CBF and the severity of ADC decline (Kohno et al, 1995; Hoehn-Berlage et al, 1995; Dijkhuizen et al, 1997; Thijs et al, 2002; Lin et al, 2003). With increasing time from symptom onset, ADC values become progressively reduced in a critically low CBF environment. Recent data have shown that only a small volume of the acute DWI lesion is reversible (Chemmanam et al, 2010). Diffusion-weighted imaging lesions are most likely to reverse if they have modest reductions in ADC and there is early reperfusion (Olivot et al, 2009). However, observations in both animal models and in human stroke patients show that reperfusion is associated with a rapid increase in ADC values, and that this increase is not necessarily reflective of tissue salvage. Therefore, an isolated ADC map can be an unreliable predictor of eventual infarct volume (Ringer et al, 2001).
The fact that early DWI lesions are not uniformly incorporated into the final infarction has an important implication: it is likely that some part of the ischemic penumbra is contained within the acute DWI lesion. Additional research is required to more accurately determine which regions of the acute DWI lesions are most likely to be salvageable, i.e., part of the penumbra.
The Core and Penumbra are Dynamic
Experimental data from vascular and molecular modeling studies, as well as recent imaging work indicate that the penumbra is dynamic. There appear to be substantial fluctuation in volumes, locations, and structures of DWI and PWI lesions during the early hours after stroke onset (Ma et al, 2009). Coregistration of acute DWI lesions with PET imaging has shown that DWI lesions are heterogeneous and include regions of variable metabolic disruption and flow-metabolism coupling (Guadagno et al, 2006). These findings are compatible with the observations discussed above regarding the relationship among rCBF, ADC, and DWI. Therefore, predicting the fate of ischemic brain tissue based on data obtained from any single imaging modality performed at only one early time point may not have high specificity and sensitivity for prediction of the core and penumbra.
Visualization of the spatial relationships between PWI and DWI lesions at different times after stroke onset can be achieved by coregistration of sequential MRI scans in stroke patients. Three-dimensional analyses have shown that a central volume of restricted diffusion (‘core') surrounded by a hypoperfused penumbra is not present in the majority of stroke patients imaged 3 to 6 hours after symptom onset (before administration of thrombolytic therapy).
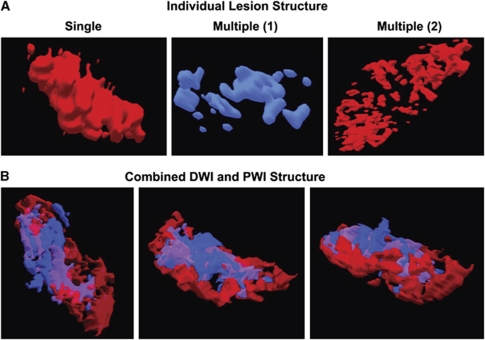
Multiple discrete regions of restricted diffusion or perfusion were documented in one-third of the patients enrolled in the DEFUSE study (Figure 6). Surprisingly, a substantial proportion of the acute DWI lesions (54%) did not have a superimposed perfusion lesion (Olivot et al, 2009). These regions of restricted diffusion without superimposed PWI lesions are assumed to represent regions where perfusion deficits have resolved or shifted to adjacent brain regions. The term RADAR (Reversible Acute Diffusion lesion Already Reperfused) has been used to describe these regions (Olivot et al, 2009). The RADAR regions appear to have an increased rate of DWI reversal. These findings emphasize the heterogeneity and complexity of the relationships between acute PWI and DWI lesions.
Figure 6.
Heterogeneity of DWI and PWI lesion structure. (A) Examples of the three-dimensional structure of DWI and PWI lesions in acute stroke patients. Left, a single PWI lesion; center, a DWI lesion with multiple individual lesion components; right, a PWI lesion with multiple components. (B) Three-dimensional imaging of DWI and PWI lesions in acute stroke patients reveals intertwined regions of mismatch, DWI/PWI overlap, and early reperfusion. Regions that contain DWI lesions without superimposed PWI lesions (early reperfusion) are shown in blue; areas where DWI lesions have superimposed PWI lesions are shown in purple. The red areas are PWI lesions without superimposed DWI lesions (regions of mismatch). Reprinted from Stroke with permission from Olivot et al (2009). DWI, diffusion-weighted imaging; PWI, perfusion-weighted imaging.
The Penumbra Revisited
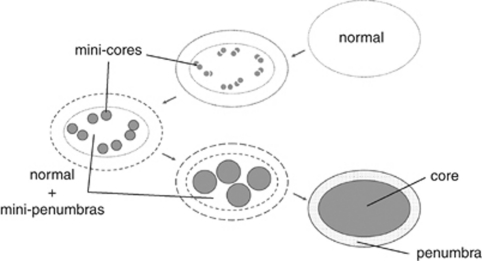
The original notion that occlusion of a brain-supplying artery produces a central core of tissue injury destined for infarction that is surrounded by a penumbra of metabolically metastable tissue with the potential for full recovery still has great merit. This model is strongly supported by the pathologic findings of stroke that show confluent regions of tissue infarction in animal models and patients. Recent experimental and imaging work offer the refinement that in the early minutes and hours after ischemia onset, the core contains pockets of injury which we characterize as ‘mini-cores', surrounded by ‘mini-penumbras'. We hypothesize that unless salvaged, such embedded ‘mini-penumbras' are consumed by the expanding ‘mini-cores' of metabolic and functional failure to generate a lesion that is ultimately homogenous and can grow into the surrounding injured tissue (Figure 7). Furthermore, it is hypothesized that the ‘mini-cores' contain tissues in which neurovascular units have been irreversibly damaged because of flow cessation and its consequences, whereas the ‘mini-penumbras' have a proportion of neurovascular units that are viable. The separate networks of microvessels, neurons, and glia associate the ‘mini-cores' with the ‘mini-penumbras'. In this way, if unimpeded, these mini-cores can grow into their respective mini-penumbras to encompass a larger region of injury.
Figure 7.
A new hypothetical construct of the penumbra. The architecture and reversibility depend on time and location of rCBF reduction in the territory at risk after occlusion of a brain-supplying artery. The outside boundary represents the territory at risk of cerebral tissue that functions normally until rCBF is reduced for an extended period. Mini-cores coalesce with the duration of rCBF reduction devouring micro-penumbras. The period of evolution from normal function to the final state of the territory at risk may depend on a number of factors, including tissue location, depth of reduction of rCBF, inflammatory state at baseline and/or degree of inflammatory response, and other factors. The location of the mini-cores and mini-penumbras appear heterogeneously distributed. However, they in fact reflect the microvascular supply of tissue and cell vulnerabilities. rCBF, regional cerebral blood flow.
This refined view has several implications:
Multiple ‘mini-penumbras' are apparent at the molecular, cellular, and microvessel levels, such that higher imaging resolution will be necessary to follow their fate in patients,
these cell and microvessel events are related to local microvascular flow,
serial imaging studies are required to show the evolution of this dynamic core and penumbra,
inflammatory cell–endothelial interactions, peri-infarct depolarization, flow changes, and other processes contribute to the engulfment of ‘mini-penumbras' into the evolving ischemic core,
these observations underscore the need for extremely early interventions, and
depending on the vascular territory involved and the timing and severity of the ischemic event, injury evolution may occur at different speeds within different ‘mini-cores'/‘mini-penumbras', such that certain interventions might have benefit later.
Future studies will need to determine whether the presence or absence of ‘mini-cores' and ‘mini-penumbras' might affect the efficacy of different types of treatment, the timing of treatment, or impact ultimate prognosis.
Acknowledgments
The authors thank G Berg for manuscript preparation.
The authors declare no conflict of interest.
Footnotes
The work reported here has been supported in part by NIH grants NS 053716, NS 038710, and NS 036945 to Dr del Zoppo, NIH grants NS066845 and NS054652 to Dr Sharp, and NIH grants NS39325 and NS044848 to Dr Albers, and funding by the WDH Foundation to Dr Heiss.
References
- Abumiya T, Lucero J, Heo JH, Tagaya M, Koziol JA, Copeland BR, del Zoppo GJ. Activated microvessels express vascular endothelial growth factor and integrin alpha(v)beta3 during focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:1038–1050. doi: 10.1097/00004647-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Ackerman RH, Correia JA, Alpert NM, Baron JC, Gouliamos A, Grotta JC, Brownell GL, Taveras JM. Positron imaging in ischemic stroke disease using compounds labeled with oxygen 15. Initial results of clinicophysiologic correlations. Arch Neurol. 1981;38:537–543. doi: 10.1001/archneur.1981.00510090031002. [DOI] [PubMed] [Google Scholar]
- Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- Astrup J, Symon L, Branston NM, Lassen NA. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke. 1977;8:51–57. doi: 10.1161/01.str.8.1.51. [DOI] [PubMed] [Google Scholar]
- Baird AE, Benfield A, Schlaug G, Siewert B, Lovblad KO, Edelman RR, Warach S. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol. 1997;41:581–589. doi: 10.1002/ana.410410506. [DOI] [PubMed] [Google Scholar]
- Barber PA, Darby DG, Desmond PM, Yang Q, Gerraty RP, Jolley D, Donnan GA, Tress BM, Davis SM. Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology. 1998;51:418–426. doi: 10.1212/wnl.51.2.418. [DOI] [PubMed] [Google Scholar]
- Baron JC. Mapping the ischaemic penumbra with PET: implications for acute stroke treatment. Cerebrovasc Dis. 1999;9:193–201. doi: 10.1159/000015955. [DOI] [PubMed] [Google Scholar]
- Baron JC, Bousser MG, Comar D, Soussaline F, Castaigne P. Noninvasive tomographic study of cerebral blood flow and oxygen metabolism in vivo. Potentials, limitations, and clinical applications in cerebral ischemic disorders. Eur Neurol. 1981a;20:273–284. doi: 10.1159/000115247. [DOI] [PubMed] [Google Scholar]
- Baron JC, Bousser MG, Rey A, Guillard A, Comar D, Castaigne P. Reversal of focal ‘misery-perfusion syndrome' by extra-intracranial arterial bypass in hemodynamic cerebral ischemia. A case study with 15O positron emission tomography. Stroke. 1981b;12:454–459. doi: 10.1161/01.str.12.4.454. [DOI] [PubMed] [Google Scholar]
- Beckmann RP, Lovett M, Welch WJ. Examining the function and regulation of hsp 70 in cells subjected to metabolic stress. J Cell Biol. 1992;117:1137–1150. doi: 10.1083/jcb.117.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Bergstedt K, Hu BR, Wieloch T. Postischaemic changes in protein synthesis in the rat brain: effects of hypothermia. Exp Brain Res. 1993;95:91–99. doi: 10.1007/BF00229658. [DOI] [PubMed] [Google Scholar]
- Branston NM, Strong AJ, Symon L. Extracellular potassium activity, evoked potential and tissue blood flow. Relationships during progressive ischaemia in baboon cerebral cortex. J Neurol Sci. 1977;32:305–321. doi: 10.1016/0022-510x(77)90014-4. [DOI] [PubMed] [Google Scholar]
- Branston NM, Symon L, Crockard HA, Pasztor E. Relationship between the cortical evoked potential and local cortical blood flow following acute middle cerebral artery occlusion in the baboon. Exp Neurol. 1974;45:195–208. doi: 10.1016/0014-4886(74)90112-5. [DOI] [PubMed] [Google Scholar]
- Calamante F, Christensen S, Desmond PM, Ostergaard L, Davis SM, Connelly A. The physiological significance of the time-to-maximum (Tmax) parameter in perfusion MRI. Stroke. 2010;41:1169–1174. doi: 10.1161/STROKEAHA.110.580670. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Kang DW, Luby M, Ezzeddine M, Latour LL, Todd JW, Dunn B, Warach S. Early magnetic resonance imaging findings in patients receiving tissue plasminogen activator predict outcome: insights into the pathophysiology of acute stroke in the thrombolysis era. Ann Neurol. 2004;55:105–112. doi: 10.1002/ana.10781. [DOI] [PubMed] [Google Scholar]
- Chemmanam T, Campbell BC, Christensen S, Nagakane Y, Desmond PM, Bladin CF, Parsons MW, Levi CR, Barber PA, Donnan GA, Davis SM. Ischemic diffusion lesion reversal is uncommon and rarely alters perfusion-diffusion mismatch. Neurology. 2010;75:1040–1047. doi: 10.1212/WNL.0b013e3181f39ab6. [DOI] [PubMed] [Google Scholar]
- Craig EA, Gambill BD, Nelson RJ. Heat shock proteins: molecular chaperones of protein biogenesis. Microbiol Rev. 1993;57:402–414. doi: 10.1128/mr.57.2.402-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, Barber PA, Bladin C, De Silva DA, Byrnes G, Chalk JB, Fink JN, Kimber TE, Schultz D, Hand PJ, Frayne J, Hankey G, Muir K, Gerraty R, Tress BM, Desmond PM. Effects of alteplase beyond 3 hours after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Hallenbeck JM. Acute focal ischemia-induced alterations in MAP2 immunostaining: description of temporal changes and utilization as a marker for volumetric assessment of acute brain injury. J Cereb Blood Flow Metab. 1996;16:170–174. doi: 10.1097/00004647-199601000-00020. [DOI] [PubMed] [Google Scholar]
- DeGracia DJ, Hu BR. Irreversible translation arrest in the reperfused brain. J Cereb Blood Flow Metab. 2007;27:875–893. doi: 10.1038/sj.jcbfm.9600388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGracia DJ, Jamison JT, Szymanski JJ, Lewis MK. Translation arrest and ribonomics in post-ischemic brain: layers and layers of players. J Neurochem. 2008;106:2288–2301. doi: 10.1111/j.1471-4159.2008.05561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Pulsinelli WA, Duffy TE. Regional protein synthesis in rat brain following acute hemispheric ischemia. J Neurochem. 1980;35:1216–1226. doi: 10.1111/j.1471-4159.1980.tb07878.x. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Berkelbach van der Sprenkel JW, Tulleken KA, Nicolay K. Regional assessment of tissue oxygenation and the temporal evolution of hemodynamic parameters and water diffusion during acute focal ischemia in rat brain. Brain Res. 1997;750:161–170. doi: 10.1016/s0006-8993(96)01343-1. [DOI] [PubMed] [Google Scholar]
- Guadagno JV, Warburton EA, Jones PS, Day DJ, Aigbirhio FI, Fryer TD, Harding S, Price CJ, Green HA, Barret O, Gillard JH, Baron JC. How affected is oxygen metabolism in DWI lesions? A combined acute stroke PET-MR study. Neurology. 2006;67:824–829. doi: 10.1212/01.wnl.0000233984.66907.db. [DOI] [PubMed] [Google Scholar]
- Harris RJ, Symon L. Extracellular pH, potassium, and calcium activities in progressive ischaemia of rat cortex. J Cereb Blood Flow Metab. 1984;4:178–186. doi: 10.1038/jcbfm.1984.26. [DOI] [PubMed] [Google Scholar]
- Hata R, Maeda K, Hermann D, Mies G, Hossmann KA. Dynamics of regional brain metabolism and gene expression after middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000a;20:306–315. doi: 10.1097/00004647-200002000-00012. [DOI] [PubMed] [Google Scholar]
- Hata R, Maeda K, Hermann D, Mies G, Hossmann KA. Evolution of brain infarction after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2000b;20:937–946. doi: 10.1097/00004647-200006000-00006. [DOI] [PubMed] [Google Scholar]
- Hata R, Mies G, Wiessner C, Hossmann KA. Differential expression of c-fos and hsp72 mRNA in focal cerebral ischemia of mice. Neuroreport. 1998;9:27–32. doi: 10.1097/00001756-199801050-00006. [DOI] [PubMed] [Google Scholar]
- Heiss WD. Experimental evidence of ischemic thresholds and functional recovery. Stroke. 1992;23:1668–1672. doi: 10.1161/01.str.23.11.1668. [DOI] [PubMed] [Google Scholar]
- Heiss WD. Ischemic penumbra: evidence from functional imaging in man. J Cereb Blood Flow Metab. 2000;20:1276–1293. doi: 10.1097/00004647-200009000-00002. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Graf R, Lottgen J, Ohta K, Fujita T, Wagner R, Grond M, Weinhard K. Repeat positron emission tomographic studies in transient middle cerebral artery occlusion in cats: residual perfusion and efficacy of postischemic reperfusion. J Cereb Blood Flow Metab. 1997;17:388–400. doi: 10.1097/00004647-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Graf R, Wienhard K, Lottgen J, Saito R, Fujita T, Rosner G, Wagner R. Dynamic penumbra demonstrated by sequential multitracer PET after middle cerebral artery occlusion in cats. J Cereb Blood Flow Metab. 1994;14:892–902. doi: 10.1038/jcbfm.1994.120. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Hayakawa T, Waltz AG. Cortical neuronal function during ischemia. Effects of occlusion of one middle cerebral artery on single-unit activity in cats. Arch Neurol. 1976;33:813–820. doi: 10.1001/archneur.1976.00500120017003. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Huber M, Fink GR, Herholz K, Pietryzyk V, Wagner R, Weinhard K. Progressive derangement of peri-infarct viable tissue in ischemic stroke. J Cereb Blood Flow Metab. 1992;12:193–203. doi: 10.1038/jcbfm.1992.29. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Kracht L, Grond M, Rudolf J, Bauer B, Wienhard K, Pawlik G. Early [(11)C]Flumazenil/H(2)O positron emission tomography predicts irreversible ischemic cortical damage in stroke patients receiving acute thrombolytic therapy. Stroke. 2000;31:366–369. doi: 10.1161/01.str.31.2.366. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Rosner G. Functional recovery of cortical neurons as related to degree and duration of ischemia. Ann Neurol. 1983;14:294–301. doi: 10.1002/ana.410140307. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Sobesky J, Smekal U, Kracht LW, Lehnhardt FG, Thiel A, Jacobs AH, Lackner K. Probability of cortical infarction predicted by flumazenil binding and diffusion-weighted imaging signal intensity: a comparative positron emission tomography/magnetic resonance imaging study in early ischemic stroke. Stroke. 2004;35:1892–1898. doi: 10.1161/01.STR.0000134746.93535.9b. [DOI] [PubMed] [Google Scholar]
- Hoehn-Berlage M, Norris DG, Kohno K, Mies G, Leibfritz D, Hossmann KA. Evolution of regional changes in apparent diffusion coefficient during focal ischemia of rat brain: the relationship of quantitative diffusion NMR imaging to reduction in cerebral blood flow and metabolic disturbances. J Cereb Blood Flow Metab. 1995;15:1002–1011. doi: 10.1038/jcbfm.1995.126. [DOI] [PubMed] [Google Scholar]
- Hohfeld J, Cyr DM, Patterson C. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep. 2001;2:885–890. doi: 10.1093/embo-reports/kve206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossman K-A, Mies G.2007Multimodal mapping of the ischemic penumbra in animal models The Ischemic Penumbra: Pathophysiology, Imaging and Therapy(Donnan GA, Baron J-C, Davis SM, Sharp FR, eds),New York: Informa Healthcare; 77–92. [Google Scholar]
- Hossmann KA. Disturbances of cerebral protein synthesis and ischemic cell death. Prog Brain Res. 1993;96:161–177. doi: 10.1016/s0079-6123(08)63265-3. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Pathophysiology and therapy of experimental stroke. Cell Mol Neurobiol. 2006;26:1057–1083. doi: 10.1007/s10571-006-9008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BR, Wieloch T. Stress-induced inhibition of protein synthesis initiation: modulation of initiation factor 2 and guanine nucleotide exchange factor activities following transient cerebral ischemia in the rat. J Neurosci. 1993;13:1830–1838. doi: 10.1523/JNEUROSCI.13-05-01830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JL, Beech JS, Jones PS, Wang D, Menon DK, Baron JC. Mapping selective neuronal loss and microglial activation in the salvaged neocortical penumbra in the rat. Neuroimage. 2010;49:19–31. doi: 10.1016/j.neuroimage.2009.08.047. [DOI] [PubMed] [Google Scholar]
- Jones TH, Morawetz RB, Crowell RM, Marcoux FW, Fitzgibbon SJ, DeGirolami U, Ojemann RG. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg. 1981;54:773–782. doi: 10.3171/jns.1981.54.6.0773. [DOI] [PubMed] [Google Scholar]
- Kane I, Carpenter T, Chappell F, Rivers C, Armitage P, Sandercock P, Wardlaw J. Comparison of 10 different magnetic resonance perfusion imaging processing methods in acute ischemic stroke: effect on lesion size, proportion of patients with diffusion/perfusion mismatch, clinical scores, and radiologic outcomes. Stroke. 2007;38:3158–3164. doi: 10.1161/STROKEAHA.107.483842. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Regulation of mRNA translation by protein folding in the endoplasmic reticulum. Trends Biochem Sci. 2004;29:152–158. doi: 10.1016/j.tibs.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ, Scheuner D, Schroder M, Shen X, Lee K, Liu CY, Arnold SM. The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol. 2002;3:411–421. doi: 10.1038/nrm829. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729–2735. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Saver JL, Mattiello J, Starkman S, Vinuela F, Duckwiler G, Gobin YP, Jahan R, Vespa P, Kalafut M, Alger JR. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol. 2000;47:462–469. [PubMed] [Google Scholar]
- Kiessling M, Dienel GA, Jacewicz M, Pulsinelli WA. Protein synthesis in postischemic rat brain: a two-dimensional electrophoretic analysis. J Cereb Blood Flow Metab. 1986;6:642–649. doi: 10.1038/jcbfm.1986.119. [DOI] [PubMed] [Google Scholar]
- Kinouchi H, Sharp FR, Chan PH, Koistinaho J, Sagar SM, Yoshimoto T. Induction of c-fos, junB, c-jun, and hsp 70 mRNA in cortex, thalamus, basal ganglia and hippocampus following middle cerebral artery occulsion. J Cereb Blood Flow Metab. 1994;14:808–817. doi: 10.1038/jcbfm.1994.101. [DOI] [PubMed] [Google Scholar]
- Kinouchi H, Sharp FR, Hill MP, Koistinaho J, Sagar SM, Chan PH. Induction of 70-kDa heat shock protein and hsp70 mRNA following transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1993a;13:105–115. doi: 10.1038/jcbfm.1993.13. [DOI] [PubMed] [Google Scholar]
- Kinouchi H, Sharp FR, Koistinaho J, Hicks K, Kamii H, Chan PH. Induction of heat shock hsp70 mRNA and HSP70 kDa protein in neurons in the ‘penumbra' following focal cerebral ischemia in the rat. Brain Res. 1993b;619:334–338. doi: 10.1016/0006-8993(93)91630-b. [DOI] [PubMed] [Google Scholar]
- Kleihues P, Hossmann KA. Protein synthesis in the cat brain after prolonged cerebral ischemia. Brain Res. 1971;35:409–418. doi: 10.1016/0006-8993(71)90484-7. [DOI] [PubMed] [Google Scholar]
- Kohno K, Back T, Hoehn-Berlage M, Hossmann K. Relationship between diffusion-weighted magnetic resonance images, cerebral blood flow and energy state in experimental brain infarction. Magn Reson Imaging. 1995;13:65–71. doi: 10.1016/0730-725x(94)00080-m. [DOI] [PubMed] [Google Scholar]
- Lenzi GL, Frackowiak RSJ, Jones T. Cerebral oxygen metabolism and blood flow in human cerebral ischemic infarction. J Cereb Blood Flow Metab. 1982;2:321–335. doi: 10.1038/jcbfm.1982.33. [DOI] [PubMed] [Google Scholar]
- Lin W, Lee JM, Lee YZ, Vo KD, Pilgram T, Hsu CY. Temporal relationship between apparent diffusion coefficient and absolute measurements of cerebral blood flow in acute stroke patients. Stroke. 2003;34:64–70. doi: 10.1161/01.str.0000048151.28173.0d. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Kim G. Heat-shock protein 104 expression is sufficient for thermotolerance in yeast. Proc Natl Acad Sci USA. 1996;93:5301–5306. doi: 10.1073/pnas.93.11.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Zavala JA, Teoh H, Churilov L, Gunawan M, Ly J, Wright P, Phan T, Arakawa S, Davis SM, Donnan GA. Fragmentation of the classical magnetic resonance mismatch ‘penumbral' pattern with time. Stroke. 2009;40:3752–3757. doi: 10.1161/STROKEAHA.109.555011. [DOI] [PubMed] [Google Scholar]
- Mabuchi T, Lucero J, Feng A, Koziol JA, del Zoppo GJ. Focal cerebral ischemia preferentially affects neurons distant from their neighboring microvessels. J Cereb Blood Flow Metab. 2005;25:257–266. doi: 10.1038/sj.jcbfm.9600027. [DOI] [PubMed] [Google Scholar]
- Markus R, Reutens DC, Kazui S, Read S, Wright P, Chambers BR, Sachinidis JI, Tochon-Danguy HJ, Donnan GA. Topography and temporal evolution of hypoxic viable tissue identified by 18F-fluoromisonidazole positron emission tomography in humans after ischemic stroke. Stroke. 2003;34:2646–2652. doi: 10.1161/01.STR.0000094422.74023.FF. [DOI] [PubMed] [Google Scholar]
- Massa SM, Swanson RA, Sharp FR. The stress gene response in brain. Cerebrovasc Brain Metab Rev. 1996;8:95–158. [PubMed] [Google Scholar]
- Mies G, Iijima T, Hossmann KA. Correlation between peri-infarct DC shifts and ischaemic neuronal damage in rat. Neuroreport. 1993;4:709–711. doi: 10.1097/00001756-199306000-00027. [DOI] [PubMed] [Google Scholar]
- Mies G, Paschen W, Ebhardt G, Hossmann KA. Relationship between of blood flow, glucose metabolism, protein synthesis, glucose and ATP content in experimentally-induced glioma (RG1 2.2) of rat brain. J Neurooncol. 1990;9:17–28. doi: 10.1007/BF00167064. [DOI] [PubMed] [Google Scholar]
- Milner R, Hung S, Wang X, Berg GI, Spatz M, del Zoppo GJ. Responses of endothelial cell and astrocyte matrix-integrin receptors to ischemia mimic those observed in the neurovascular unit. Stroke. 2008;39:191–197. doi: 10.1161/STROKEAHA.107.486134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivot JM, Mlynash M, Thijs VN, Purushotham A, Kemp S, Lansberg MG, Wechsler L, Bammer R, Marks MP, Albers GW. Relationships between cerebral perfusion and reversibility of acute diffusion lesions in DEFUSE: insights from RADAR. Stroke. 2009;40:1692–1697. doi: 10.1161/STROKEAHA.108.538082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MW, Barber PA, Chalk J, Darby DG, Rose S, Desmond PM, Gerraty RP, Tress BM, Wright PM, Donnan GA, Davis SM. Diffusion- and perfusion-weighted MRI response to thrombolysis in stroke. Ann Neurol. 2002;51:28–37. doi: 10.1002/ana.10067. [DOI] [PubMed] [Google Scholar]
- Parsons MW, Christensen S, McElduff P, Levi CR, Butcher KS, De Silva DA, Ebinger M, Barber PA, Bladin C, Donnan GA, Davis SM, Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) investigators Pretreatment diffusion- and perfusion-MR lesion volumes have a crucial influence on clinical response to stroke thrombolysis. J Cereb Blood Flow Metab. 2010;30:1214–1225. doi: 10.1038/jcbfm.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MW, Yang Q, Barber PA, Darby DG, Desmond PM, Gerraty RP, Tress BM, Davis SM. Perfusion magnetic resonance imaging maps in hyperacute stroke: relative cerebral blood flow most accurately identifies tissue destined to infarct. Stroke. 2001;32:1581–1587. doi: 10.1161/01.str.32.7.1581. [DOI] [PubMed] [Google Scholar]
- Paschen W. Disturbances of calcium homeostasis within the endoplasmic reticulum may contribute to the development of ischemic-cell damage. Med Hypotheses. 1996;47:283–288. doi: 10.1016/s0306-9877(96)90068-7. [DOI] [PubMed] [Google Scholar]
- Paschen W, Gissel C, Linden T, Althausen S, Doutheil J. Activation of gadd153 expression through transient cerebral ischemia: evidence that ischemia causes endoplasmic reticulum dysfunction. Brain Res Mol Brain Res. 1998;60:115–122. doi: 10.1016/s0169-328x(98)00180-6. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Grubb RL, Jr, Darriet D, Raichle ME. Cerebral blood flow and cerebral metabolic rate of oxygen requirements for cerebral function and viability in humans. J Cereb Blood Flow Metab. 1985;5:600–608. doi: 10.1038/jcbfm.1985.89. [DOI] [PubMed] [Google Scholar]
- Quistorff B, Chance B, Hunding A. An experimental model of the Krogh tissue cylinder: two dimensional quantitation of the oxygen gradient. Adv Exp Med Biol. 1977;94:127–136. doi: 10.1007/978-1-4684-8890-6_18. [DOI] [PubMed] [Google Scholar]
- Rajdev S, Hara K, Kokubo Y, Mestril R, Dillmann W, Weinstein PR, Sharp FR. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47:782–791. [PubMed] [Google Scholar]
- Ringer TM, Neumann-Haefelin T, Sobel RA, Moseley ME, Yenari MA. Reversal of early diffusion-weighted magnetic resonance imaging abnormalities does not necessarily reflect tissue salvage in experimental cerebral ischemia. Stroke. 2001;32:2362–2369. doi: 10.1161/hs1001.096058. [DOI] [PubMed] [Google Scholar]
- Rosenberg RD, Aird WC. Vascular-bed–specific hemostasis and hypercoagulable states. N Engl J Med. 1999;340:1555–1564. doi: 10.1056/NEJM199905203402007. [DOI] [PubMed] [Google Scholar]
- Rosner G, Graf R, Kataoka K, Heiss WD. Selective functional vulnerability of cortical neurons following transient MCA-occlusion in the cat. Stroke. 1986;17:76–82. doi: 10.1161/01.str.17.1.76. [DOI] [PubMed] [Google Scholar]
- Ruetzler CA, Furuya K, Takeda H, Hallenbeck JM. Brain vessels normally undergo cyclic activation and inactivation: evidence from tumor necrosis factor-alpha, heme oxygenase-1, and manganese superoxide dismutase immunostaining of vessels and perivascular brain cells. J Cereb Blood Flow Metab. 2001;21:244–252. doi: 10.1097/00004647-200103000-00008. [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Senger DR, Ledbetter SR, Claffey KP, Papdopoulos-Sergiou A, Peruzzi CA, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the αVβ3 integrin, osteopontin, and thrombin. Am J Pathol. 1996;149:293–305. [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Lu A, Tang Y, Millhorn DE. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:1011–1032. doi: 10.1097/00004647-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. Energetics of functional activation in neural tissues. Neurochem Res. 1999;24:321–329. doi: 10.1023/a:1022534709672. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001;81:1065–1096. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- Spratt NJ, Howells DW, Donnan GA.2007Imaging the penumbra: positron emission tomography fluoromisonidazole The Ischemic Penumbra: Pathophysiology, Imaging and Therapy(Donnan GA, Baron J-C, Davis SM, Sharp FR, eds),New York: Informa Healthcare; 149–164. [Google Scholar]
- Strong AJ. Spreading depolarisations: Tsunamis in the injured brain. Adv Clin Neurosci Rehab. 2009;9:32–35. [Google Scholar]
- Symon L. The relationship between CBF, evoked potentials and the clinical features in cerebral ischaemia. Acta Neurol Scand Suppl. 1980;78:175–190. [PubMed] [Google Scholar]
- Symon L, Pasztor E, Branston NJM. The distribution and density of reduced cerebral blood flow following acute middle cerebral artery occlusion: an experimental study by the technique of hydrogen clearance in baboons. Stroke. 1974;5:355–364. doi: 10.1161/01.str.5.3.355. [DOI] [PubMed] [Google Scholar]
- Tagaya M, Haring H-P, Stuiver I, Wagner S, Abumiya T, Lucero J, Lee P, Copeland BR, Seiffert D, del Zoppo GJ. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab. 2001;21:835–846. doi: 10.1097/00004647-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Tagaya M, Liu K-F, Copeland B, Seiffert D, Engler R, Garcia JH, del Zoppo GJ. DNA scission after focal brain ischemia. Temporal differences in two species. Stroke. 1997;28:1245–1254. doi: 10.1161/01.str.28.6.1245. [DOI] [PubMed] [Google Scholar]
- Takasawa M, Beech JS, Fryer TD, Hong YT, Hughes JL, Igase K, Jones PS, Smith R, Aigbirhio FI, Menon DK, Clark JC, Baron JC. Imaging of brain hypoxia in permanent and temporary middle cerebral artery occlusion in the rat using 18F-fluoromisonidazole and positron emission tomography: a pilot study. J Cereb Blood Flow Metab. 2007;27:679–689. doi: 10.1038/sj.jcbfm.9600405. [DOI] [PubMed] [Google Scholar]
- Thijs VN, Adami A, Neumann-Haefelin T, Moseley ME, Albers GW. Clinical and radiological correlates of reduced cerebral blood flow measured using magnetic resonance imaging. Arch Neurol. 2002;59:233–238. doi: 10.1001/archneur.59.2.233. [DOI] [PubMed] [Google Scholar]
- Toth G, Albers GW. Use of MRI to estimate the therapeutic window in acute stroke: is perfusion-weighted imaging/diffusion-weighted imaging mismatch an EPITHET for salvageable ischemic brain tissue. Stroke. 2009;40:333–335. doi: 10.1161/STROKEAHA.108.525683. [DOI] [PubMed] [Google Scholar]
- Wagner S, Tagaya M, Koziol JA, Quaranta V, del Zoppo GJ. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin alpha6beta4 during focal cerebral ischemia/reperfusion. Stroke. 1997;28:858–865. doi: 10.1161/01.str.28.4.858. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Heat shock proteins functioning as molecular chaperones: their roles in normal and stressed cells. Philos Trans R Soc Lond B Biol Sci. 1993;339:327–333. doi: 10.1098/rstb.1993.0031. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996;1:109–115. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari MA. Heat shock proteins and neuroprotection. Adv Exp Med Biol. 2002;513:281–299. doi: 10.1007/978-1-4615-0123-7_10. [DOI] [PubMed] [Google Scholar]
- Zaro-Weber O, Moeller-Hartmann W, Heiss WD, Sobesky J. The performance of MRI-based cerebral blood flow measurements in acute and subacute stroke compared with 15O-water positron emission tomography: identification of penumbral flow. Stroke. 2009;40:2413–2421. doi: 10.1161/STROKEAHA.108.540914. [DOI] [PubMed] [Google Scholar]
- Zaro-Weber O, Moeller-Hartmann W, Heiss WD, Sobesky J. Maps of time to maximum and time to peak for mismatch definition in clinical stroke studies validated with positron emission tomography. Stroke. 2010;41:2817–2821. doi: 10.1161/STROKEAHA.110.594432. [DOI] [PubMed] [Google Scholar]
- Zhan X, Kim C, Sharp FR. Very brief focal ischemia simulating transient ischemic attacks (TIAs) can injure brain and induce Hsp70 protein. Brain Res. 2008;1234:183–197. doi: 10.1016/j.brainres.2008.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]