Abstract
Globally, hepatocellular carcinoma (hcc) is the third most common cause of death from cancer, after lung and stomach cancer. The incidence of hcc in Canada is increasing and is expected to continue to increase over the next decade. Given the high mortality rate associated with hcc, steps are required to mitigate the impact of the disease. To address this challenging situation, a panel of 17 hcc experts, representing gastroenterologists, hepatologists, hepatobiliary surgeons, medical oncologists, pathologists, and radiologists from across Canada, convened to provide a framework that, using an evidence-based approach, will assist clinicians in optimizing the management and treatment of hcc. The recommendations, summarized here, were developed based on a rigorous methodology in a pre-specified process that was overseen by the steering committee. Specific topics were identified by the steering committee and delegated to a group of content experts within the expert panel, who then systematically reviewed the literature on that topic and drafted the related content and recommendations. The set of recommendations for each topic were reviewed and assigned a level of evidence and grade according to the levels of evidence set out by the Centre for Evidence-based Medicine, Oxford, United Kingdom. Agreement on the level of evidence for each recommendation was achieved by consensus. Consensus was defined as agreement by a two-thirds majority of the 17 members of the expert panel. Recommendations were subject to iterative review and modification by the expert panel until consensus could be achieved.
Keywords: Hepatocellular carcinoma, consensus recommendations, screening, diagnosis, staging, prognosis, surgical resection, transplantation, percutaneous ethanol injection, radiofrequency ablation, transarterial chemotherapy, systemic chemotherapy, clinical management
1. INTRODUCTION
Globally, hepatocellular carcinoma (hcc) is the third most common cause of death from cancer, after lung and stomach cancer1. At least 300,000 of the 600,000 deaths worldwide occur in China alone, with most of the other deaths occurring in sub-Saharan Africa1. In men, hcc is the fifth most common cancer, and in women, the seventh most common2. In the United States, hcc has shown an annual increase of 5.4% between 2002 and 20063. The Canadian Cancer Registry reports that the age-adjusted incidence rate in men 40–84 years of age was 5.4 per 100,000 in the years 1976–1980 and rose to 15.4 per 100,000 in the years 2006–20104. Rates for hcc in men are projected to reach 18.5 per 100,000 by 20154. The incidence rate for women in Canada is lower, but also shows an increasing trend, projected to reach 4.1 per 100,000 by 20154. In Canada, these rates imply the presentation of approximately 7672 male and 1709 female hcc patients per year by 20154. Furthermore, modelling of liver cancer prevalence and decompensated cirrhosis shows that the prevalence of hcc in the United States (and likely also in Canada) is predicted to rise beyond 2015, with a peak around 20255.
Hepatocellular carcinoma is a challenging disease to treat because it usually appears in the setting of underlying liver disease, which means that liver function impairment must be monitored before, during, and after therapy. Such monitoring is best achieved by involving multidisciplinary groups in the care of these patients. For that reason, and in light of the growing problem of hcc in Canada, a group of 17 hcc experts was convened as an expert panel to develop consensus recommendations on the management of hcc.
2. METHODS
The expert panel of 17 multidisciplinary specialists included 5 medical oncologists, 3 hepatobiliary surgeons, 3 hepatologists, 3 radiologists, 2 gastroenterologists, and 1 pathologist. Members of the panel were selected by the Steering Committee, and no members were paid an honorarium for their involvement. Funding for logistics and meeting costs was provided by Bayer Canada. Specific topics related to hcc were delegated to content experts within the expert panel who then systematically reviewed the literature and took responsibility for reviewing the draft manuscript and the recommendations for each topic.
The systematic review of the literature began with a review of reference lists found in relevant guidelines on hcc and of suggestions from the panel based on proceedings of the American Society of Clinical Oncology and the European Society for Medical Oncology. The medline database (January 2002–September 2010) was searched, with an English-language restriction, for “hepatocellular carcinoma” (mesh term) combined with the text words “ablation,” “biopsy,” “diagnosis,” “embolization,” “epidemiology,” “monitoring,” “pathology,” “prognosis,” “recurrence,” “screening,” “staging,” “surgery,” “therapy,” and “transarterial chemoembolization.” The search results were filtered to select distinct interventions that included appropriate patient outcomes and comparisons.
The expert panel was convened on September 12–13, 2010, to review chapter presentations by its members and to discuss their evidence-based recommendations. The process of achieving consensus was completed after the meeting by online anonymous voting. The recommendations were assigned a level of evidence (1–5, Table i) and a grade (A, B, C; Table ii) by the expert panel. The level of evidence defines the strength of the evidence in favour of (or against) the intervention. The method for achieving consensus was based on a modified version of the Delphi method 8. Consensus was defined as agreement by two thirds of the expert panel. The consensus methodology used a number of cycles of anonymous voting and written discussions, managed by an organizational facilitator, Core Health Services. The anonymity of the Delphi process avoids group bias and conflicts of interest, and allows for a thorough and rigorous analysis of each recommendation before consensus8.
TABLE I.
Levels of evidencea
| Level | Description |
|---|---|
| 1A | Systematic review with randomized controlled trials |
| 1B | Individual randomized controlled trial (with narrow confidence interval) |
| 1C | All-or-none case series |
| 2A | Systematic review with homogeneity of cohort studies |
| 2B | Individual cohort study |
| 2C | “Outcomes” research; ecological studies |
| 3A | Systematic review with homogeneity of case–control studies |
| 3B | Individual case–control study |
| 4 | Case series |
| 5 | Expert opinion without explicit critical appraisal, or based on physiology, bench research, or “first principles,” or descriptive epidemiology |
Adapted from the Centre for Evidence Based Medicine, Oxford Centre for Evidence-based Medicine, Levels of Evidence6.
TABLE II.
Barcelona Clinic Liver Cancer (bclc) staging system for hepatocellular carcinoma (hcc)a
| bclc stage | Tumour stage | cp class | ecog ps | Recommended therapy | |
|---|---|---|---|---|---|
| Very early (0) | 1 hcc < 2 cm | A | 0 | Resection Transplantation rfa |
|
| Early (A) | 1 hcc < 5 cm or 3 nodules < 3 cm | A or B | 0 | ||
| Intermediate (B) | Multinodular | A or B | 0 | tace | |
| Advanced (C) | Portal invasion, N1, M1 | A or B | 1–2 | Sorafenib | |
| End-stage (D) | Any | C | >2 | Symptomatic treatment |
Adapted from the 2010 American Association for the Study of Liver Diseases guidelines7. Treatment options for bclc stage 0 and A are determined by the presence or absence of increased portal pressure or bilirubin (or both) with or without associated diseases.
cp = Child–Pugh; ecog = Eastern Cooperative Oncology Group; ps = performance status; rfa = radiofrequency ablation; tace = transarterial chemoembolization; N1 = nodes; M1 = metastasis.
3. TOPICS AND RECOMMENDATIONS
3.1. HCC Risk Factors
Hepatocellular carcinoma is 4–8 times more common in men than in women, and it is associated with several important risk factors, notably hepatitis B (hbv) and C virus (hcv) infection, specific demographic factors, cirrhosis, and possibly exposure to certain toxins9,10. Based on prospective studies comparing non-infected patients with carriers of hbv, the risk of hcc is increased by a factor of 50–100 in hbv carriers11,12. Chronic hbv infection is considered the leading cause of hcc worldwide10; in North America, however, chronic hcv infection is the major underlying cause of hcc5. The effect of age is important, in that as populations infected with hcv and hbv grow older and the duration of infection increases, the risk of hcc also increases13. An increase in nonalcoholic fatty liver disease has been identified as a risk factor that will likely fuel a continued rise in the incidence of hcc in the future, even as hbv and hcv infections become less important factors13,14. The incidence of hcc in cirrhosis caused by diseases other than viral hepatitis is also high.
3.2. HCC Surveillance
Recommendation 1:
Patients at risk for developing hcc should undergo screening at 6-month intervals. (Level of evidence: 1B)
The definition of the populations at risk for hcc that might benefit from screening is important. These population groups have been well defined in the 2010 guidelines from the American Association for the Study of Liver Diseases (aasld)7.
The effectiveness of surveillance was demonstrated in a single randomized controlled trial performed in China15. That large study (18,816 subjects) used a cluster randomization protocol and demonstrated a 37% relative reduction in hcc-related mortality with screening every 6 months using ultrasonography and alpha fetoprotein (afp) testing. This improved mortality was achieved with less than 100% compliance with the screening schedule15. The study enrolled only patients with chronic hbv, and so its applicability to other liver diseases and other geographic areas is uncertain.
Several cohort studies also indicate that surveillance results in stage migration—that is, earlier diagnosis16,17. Potentially curative treatments can therefore be used much earlier in the disease course when they are more likely to achieve optimum results. However, this type of study cannot determine whether screening lowers mortality from the disease.
In cohort studies in which survival was the endpoint, the group that underwent surveillance experienced increased survival when lead-time bias was taken into account18.
Recommendation 2:
Screening for hcc should use ultrasonography alone. (Level of evidence: 2B)
Ultrasonography is more sensitive than any of the serology markers, even in patients with cirrhosis. The distinction between cirrhotic nodules and early hcc can be made using an appropriate algorithm19 (discussed later in this guideline). Obese patients may not be optimal candidates for surveillance by ultrasonography. Several studies have also indicated that survival is better after 6-monthly screening than after yearly screening.
Computed tomography (ct) imaging is untested as a surveillance method. Cost–efficacy analysis in the United States suggests that the incremental cost–effectiveness ratio for surveillance with both ultrasonography and ct imaging compared with ultrasonography alone may exceed $300,00020, making ct imaging for surveillance a very expensive option.
The best-studied serology test for hcc is afp7. The only randomized trial of screening used a combination of ultrasonography and afp15. Most studies that have investigated the use of afp were performed in patients known to have hcc21,22, and the data therefore cannot be extrapolated to surveillance studies because the performance characteristics of afp will not be the same in patients with a 4-cm cancer as in those with a 1-cm or smaller tumour. In screening for smaller cancers, the sensitivity and specificity of afp is inadequate for general use21,22. Des-gamma carboxyprothrombin is slightly more sensitive, but still inadequate as a surveillance test22, and afp-l3 (an isoform of afp) is even less sensitive than afp. Furthermore, all three markers indicate advanced disease23–25 and thus cannot be effective in uncovering early-stage disease.
In most studies, the sensitivity of afp as a screening test for hcc is only 60%21,26–33. More recently, two studies again suggested that afp is an inadequate marker for hcc surveillance. The Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis study evaluated afp concentration at hcc diagnosis and 12 months before diagnosis34. Using a 20 ng/mL cut-off at the time of diagnosis (that is, known cancer), the sensitivity of afp was 61%. At a cut-off of 200 ng/mL, sensitivity was only 22%. Another recent study from the United States assessed the utility of afp screening in 417 patients with hcc (again, established cancer) and 417 controls with cirrhosis22. Overall, afp sensitivity was only 67%.
When various population groups are analyzed, approximately 20% of patients with cirrhosis will have an afp exceeding 20 ng/mL even without hcc. In people positive for the hbv surface antigen, 13% will, over 7 years, develop an afp level greater than 20 ng/mL even without liver cancer22. Similarly, in those with hcv, 14% will, over a similar period, develop an afp level greater than 20 ng/mL in the absence of hcc33. In summary, more than 90% of the time, elevated afp found during surveillance is not related to cancer.
Surveillance for hcc requires not only screening tests, but also a well-defined institutional program or process with established quality control procedures7.
3.3. Diagnosis of HCC
Recommendation 3:
For patients with lesions in the liver that are not clearly hemangioma, the lesions should be investigated using the algorithm described in the 2010 aasld guidelines (Figure 1). (Level of evidence: 2B)
FIGURE 1.
Diagnostic algorithm for suspected hepatocellular carcinoma (hcc). Adapted from the 2010 American Association for the Study of Liver Diseases guidelines7. us = ultrasonography; mdct = multi-detector computed tomography; mri = magnetic resonance imaging; ct = computed tomography.
This recommendation applies to patients who have a high pre-test probability of hcc—that is, patients with cirrhosis, and patients with other hcc risk factors7. Other groups of patients should be evaluated using clinical judgment7.
In the confirmation of hcc, radiologic diagnostic criteria are very specific when present35–37. Arterial phase hypervascularity and venous phase or late phase washout are highly specific for hepatoma. When those features are present, the diagnosis can be made without biopsy38,39. Diagnosis of lesions smaller than 1 cm is very difficult. Most of these lesions are cirrhotic nodules, and very few turn out to be cancerous. Of the nodules that are malignant, most are slow growing, and so diagnosis is not urgent. Therefore, for these small lesions, observation over time is all that is required. Nevertheless, it is recommended that this surveillance occur more frequently than the usual 6-monthly interval: approximately every 3 months is appropriate7. Surveillance should continue until stability is assured—that is, for 18–24 months.
For lesions smaller than 1 cm that are clearly not hemangioma, the sequential use of ct and magnetic resonance (mr) imaging is highly specific40,41. However, if neither imaging method shows the typical features of hcc, then a biopsy is required.
Contrast-enhanced ultrasonography has been shown to be highly accurate in diagnosing hcc in cirrhotic livers42; however, other studies have cautioned that contrast-enhanced ultrasonography alone should not be used for the diagnosis of hcc because similar patterns of enhancement may be seen with intrahepatic cholangiocarcinoma43.
3.4. Pathology
Recommendation 4:
To increase diagnostic accuracy, all nodular lesions that are not clearly hcc should be tested using an ancillary panel of immunostains: heat shock protein 70 (Hsp70), glypican 3 (Gpc3), and glutamine synthetase (gs). (Level of evidence: 2B)
Despite advances in the histology criteria for distinguishing early-stage well-differentiated hcc from high-grade dysplastic nodules (hgdn), difficult cases remain in which ancillary techniques based on immunostaining can be helpful in making the histologic diagnosis of hcc. Immunohistochemically, Gpc3 is a marker of malignancy in hcc44–46. Its reported sensitivity and specificity are 77% and 96% respectively. Tests for Gpc3 tend to be negative in hgdn and positive in hcc. A few caveats are required with the use of Gpc3 for hcc diagnosis. In extremely well-differentiated hcc, Gcp3 sensitivity drops to about 50%47. Expression of Gpc3 can occur in benign liver tissue with active hcv and with very active necro-inflammatory injury48.
The Hsp70 protein is produced by a class of genes implicated in tumorigenesis and regulation of the cell cycle and apoptosis49,50. In early hcc, HSP70 is upregulated, and in advanced hcc compared with early hcc or in early hcc compared with precancerous lesions, it is significantly overexpressed51. In most hccs (but not in nonmalignant lesions), Hsp70 is immunoreactive. The diagnostic sensitivity of Hsp70 in resection specimens for hcc is 70%50.
Beta-catenin activates gs. Activating mutations of the beta-catenin gene and overexpression of gs are frequent pathogenetic events in hcc52,53. A stepwise increase in gs immunoreactivity is observed from hgdn to early hcc and thence to advanced hcc54. Specificity is increased when immunostaining is diffuse with strong intensity55. In a recent study, it was suggested that, using a panel of the Gpc3, Hsp70, and gs markers, the presence of at least 2 positive markers (regardless of which 2) increases the diagnostic sensitivity to 72%, with 100% specificity for hcc55.
Recommendation 5:
Biopsy for hcc should be a core biopsy and not a fine-needle aspirate. A non-targeted biopsy of adjacent non-tumorous liver for comparison purposes may be helpful. (Level of evidence: 5)
A negative biopsy of a nodule does not exclude the possibility of hcc. A negative biopsy of an anatomically visible nodule requires expert review or a second opinion, or both. Patients with liver nodules that are negative on biopsy require continued ultrasonography follow-up at short intervals. If the size or character of the lesion changes, the patient requires re-investigation with contrast-enhanced imaging or a repeat biopsy.
The recently published consensus opinion on the histologic diagnosis of early hcc from the International Consensus Group for Hepatocellular Neoplasia defined the characteristics of low- and high-grade dysplastic nodules56,57. Their criteria should be applied in all cases of uncertainty.
3.5. Staging
Recommendation 6:
The preferred staging system is the Barcelona Clinic Liver Cancer (bclc) staging system. It takes into account the anatomic extent of tumour, liver function, and the patient’s performance status. (Level of evidence: 2B)
Since 1975, at least 16 staging systems have been proposed for hcc58. Several are widely known or well disseminated. The bclc staging system is endorsed by the aasld7,59, the European Association for the Study of the Liver, and the European Society for Medical Oncology, and it has been externally validated60. Table ii illustrates the stages of the bclc system.
The bclc system has many advantages. It is the only staging system that incorporates tumour stage, liver function (as measured by the Child–Pugh score), and health status as measured by Eastern Cooperative Oncology Group performance status. It is also the only staging system that comes with treatment recommendations. The use of the bclc system has become widespread in Western countries, and the bclc system has therefore become the de facto staging system of choice for many studies of new drugs, making its use necessary when outcomes of various studies are being compared.
Unfortunately, the bclc system is not entirely comprehensive. As a treatment algorithm, it does not recognize the role of liver transplantation for patients with advanced liver failure (Child class C is considered end-stage in bclc) or the role of chemoembolization for large single tumours. It also considers failed therapy to advance the stage of the disease. Thus, a patient with failed chemoembolization (bclc stage B) becomes a candidate for treatment for bclc stage C disease.
3.6. Treatment
3.6.1. Surgical Resection
Recommendation 6:
Patients with bclc stage 0 disease—and some patients with bclc stage A disease with single lesions or with up to 3 lesions that are anatomically in close proximity (for example, satellites)—can undergo surgical resection if the future liver remnant demonstrates adequate function. (Level of evidence: 2B)
Recognizing that most patients in Western countries will develop hcc in the setting of cirrhosis, suitability for surgical resection depends in large part on the function of the liver remnant after resection. Resection is generally reserved for patients with a Child–Pugh score of 6 or lower (Child class A)61. Assessment of the functional reserve of the remnant is an inexact science. However, various groups have used the presence of portal hypertension, elevated bilirubin, or altered indocyanine green clearance as markers of poor functional reserve; the presence of those signs therefore constitutes a contraindication to resection in most patients61,62. Portal hypertension is determined by a hepatic vein pressure gradient greater than 10 mmHg, by splenomegaly and a platelet count lower than 100×109/L, or by significant esophageal varices61. None of these methods of assessing liver reserve has been properly validated, but all are in common use nonetheless.
The presence of tumour invasion into the main portal vein or into the left or the right portal vein is also a contraindication to resection, primarily because of the very high rate of recurrence after resection. Patients with branch portal vein invasion may have a lower rate of recurrence, but given the lack of an adequate assessment of recurrence in this population, no recommendation can be made for or against resection in such patients.
Surgery in patients with Child class B cirrhosis is possible63, but the survival rate is lower than with liver transplant, and transplantation is therefore a better option for these patients7.
In patients with marginal liver function, there have been attempts to improve the function of the liver remnant by embolization of the portal vein on the side of the tumour64. In theory, this procedure causes atrophy of the lobe containing the tumour and hypertrophy of the contralateral lobe. This result can be demonstrated after embolization, but it does not occur in all patients. Whether this procedure can convert a patient from a nonsurgical into a surgical candidate with an outcome equivalent to that in patients who do not need embolization is not clear64. Nor has it been demonstrated that this form of therapy has a better outcome than transplantation or transarterial chemoembolization (tace).
The use of neoadjuvant therapy before resection for hcc is not recommended. Neoadjuvant therapy may adversely affect liver function and disadvantage patient outcome. Three randomized controlled trials of neoadjuvant treatment have been performed65–67. In one study, the tace group experienced an overall survival worse than that in the control group65. In the two other trials, no survival benefit was associated with tace 66,67. This high level of evidence indicates that there is no support for the use of neoadjuvant tace for resectable hcc.
Recurrence of hcc after surgical resection remains a major problem, with approximately 70% of patients experiencing a recurrence within 5 years of their surgery61. Unfortunately, no role for adjuvant therapy after surgical resection has been established to reduce this risk of recurrence or to improve overall survival68. In three trials, transarterial chemotherapy or tace was delivered postoperatively, with one trial achieving a mild increase in overall survival and the other two trials showing no difference69.
Small randomized controlled trials in which systemic chemotherapy was used postoperatively resulted in lower survival in the treatment groups than in the control groups70.
3.6.2. Liver Transplantation
Recommendation 7:
Patients with bclc stage 0 or stage A disease are candidates for liver transplantation. (Level of evidence: 2B)
Patients with good liver function and bclc stage 0 or stage A hcc are candidates for resection and for liver transplantation (lt). Transplantation has the advantage of treating both the cancer and the underlying cirrhosis, and recurrence rates are typically less than 15% at 5 years if the Milan criteria (a single lesion 5 cm or smaller in diameter, or up to 3 lesions, none larger than 3 cm) are used for patient selection71,72. The treatment that should be offered depends on circumstances in the individual transplant centre61. In Canada, the waiting time for lt is such that resection is often a better choice, although this approach does not preclude the possibility of salvage transplantation at a later stage. In patients with poor liver function (Child–Pugh class B or C), in whom resection is not possible, transplantation is the best option. To keep patients within listing criteria while they await lt, tace or radiofrequency ablation (rfa) are often used, although strong evidence to support this practice is lacking73.
Expansion of listing beyond patients who meet the Milan criteria generally results in higher recurrence rates and therefore in survival rates that are lower than those for patients who meet the Milan criteria72,74,75. Nonetheless, based on experiences at some Ontario and Alberta centres, the survival rates for extended-criteria lt are acceptable76,77. Whether the criteria should be thus expanded for transplantation in hcc is a matter of choice for the individual transplant centre, taking into account the current wait time, drop-out while on the wait list, and the effect that failed transplantation for hcc has on the overall mortality of all patients in the program.
Downstaging is the process of taking hcc that is beyond the listing criteria by extent of disease on imaging and attempting to reduce the size of the tumour to fall within listing criteria. Although several reports have described downstaging to meet study entry criteria, the treatments applied and the outcome measurements used have been so heterogeneous as to make interpretation of the results impossible78. Downstaging is therefore not currently recommended outside of experimental protocols. Simply reducing the size of the tumour is unlikely to change its biology, and larger tumours are associated with a higher risk of post-transplant recurrence.
3.6.3. Radiofrequency Ablation
Recommendation 8:
Patients with a single hcc nodule smaller than 2.5 cm are ideal candidates for radiofrequency ablation. (Level of evidence: 2B)
Radiofrequency ablation can also be used for patients with hcc up to about 4 cm who are not candidates for resection or transplantation. There are no data indicating that rfa is superior to resection or tace. The more a lesion exceeds about 2.5 cm in size, the lower the complete ablation rate, and the higher the recurrence rate79. Similarly, more than 1 lesion can be treated, but in the presence of more than 3 lesions, the likelihood of complete ablation declines. These patients may be better managed by other forms of therapy, although there are no available data to suggest which treatment might be superior. For lesions 2 cm or smaller discovered by hcc surveillance, rfa is ideal; the procedure has a high complete response rate (97%), a low complication rate (2%), and an overall 5-year survival of 68% (in patients who would been surgical candidates)80.
In a randomized controlled trial in patients with lesions suitable for either resection or rfa, overall survival was not different between the two treatment arms81. However, a meta-analysis that included nonrandomized controlled trials found that disease recurrence rates were significantly lower with resection than with rfa82.
Radiofrequency ablation has some disadvantages. If the lesion is near a large vessel, the heat-sink effect might make the lesion difficult to ablate completely. Lesions that are on the edge of the liver might also be more difficult to ablate without causing intraperitoneal spread. Such lesions are best approached through solid liver, rather than directly. Overall, the risk of tumour seeding has been estimated to be less than 1% after rfa83.
Radiofrequency ablation can be safely delivered percutaneously as an outpatient procedure. Occasionally, a laparoscopic approach may be necessary for better access to the lesion or to protect adjacent structures. Microwave-based thermal ablation may be an alternative when heat sink is a concern.
Percutaneous ethanol injection is not as effective as rfa (Level of evidence: 1A), as attested by meta-analyses of randomized controlled trials84,85, but it can be used (for example) in patients in whom a heat-sink effect is a concern or in conjunction with rfa.
There is no good evidence to support the routine use of combined tace and rfa, but a meta-analysis of controlled trials suggests a benefit of percutaneous ethanol injection and tace in combination86.
3.6.4. Transarterial Chemoembolization
Recommendation 9:
Transarterial chemoembolization is the treatment of choice for patients who have bclc stage B disease. (Level of evidence: 1A)
Two randomized controlled trials published in 2002 were the first to demonstrated a survival advantage of tace in patients with unresectable disease87,88. Subsequently, two meta-analyses confirmed the benefits of tace over no treatment, establishing tace as the standard of care for bclc stage B patients89,90.
There is no consensus regarding the chemotherapeutic agent or the embolizing agent to be used90. There is also little agreement about the frequency or the number of treatments.
Few data are available for patients with vascular invasion. However, given that the survival of those patients is so much worse than that of patients with no vascular invasion, it cannot be assumed that the survival advantage for the latter patients will translate to those with vascular invasion. Furthermore, such patients are candidates for sorafenib (discussed later in this section).
The data supporting bland embolization is not as solid90, and therefore no recommendation can be made for or against that procedure. However, given that these patients would be candidates for tace, and that tace confers a survival advantage, tace is the preferred treatment.
Recent improvements in tace technology has involved the use of drug-eluting beads. These beads accomplish both delivery of a chemotherapeutic agent and embolization of distal vessels. A randomized control trialled found that drug-eluting beads were not superior to conventional tace, but may be associated with fewer side effects91.
3.6.5. Systemic Therapy
Recommendation 10:
Sorafenib is indicated as the standard of care for systemic treatment in bclc stage C hcc. (Level of evidence: 1B)
Sorafenib was the first targeted therapy developed for the treatment of hcc. This drug is an oral multi-targeted kinase inhibitor of vascular endothelial growth factor receptor, platelet-derived growth factor receptor-β, Raf-1, B-Raf, and C-Raf.
Randomized trials with sorafenib have demonstrated an improvement in survival of about 2–3 months in patients with advanced hcc92,93. Although this improvement seems minor, it is approximately the same as that seen in some forms of treatment for other cancers.
Level 1 evidence for sorafenib exists only in patients with Child class A cirrhosis. It appears to be safe in Child class B cirrhosis, but there is as yet no evidence of benefit in this population with a poorer prognosis (median survival of approximately 4 months)94. Many newer targeted agents are at various stages of evaluation in hcc. It is noteworthy that sunitinib, a inhibitor of vascular endothelial growth factor receptor more potent than sorafenib, was found to be inferior to sorafenib—with respect to both overall survival and tolerability—in a direct comparison in a phase iii randomized trial95.
Standard systemic chemotherapy has not been demonstrated to be effective in hcc and is not generally recommended. Doxorubicin in combination with sorafenib was shown to be superior to doxorubicin alone in a recent phase ii trial96. A phase iii trial to determine if combination therapy is superior to sorafenib alone is under way. Some chemotherapy regimens are undergoing further evaluation in trials with more homogeneous patient populations than were studied previously. Internationally, drugs such as doxorubicin, gemcitabine, or cisplatin, and oxaliplatin-based regimens are used, often in the second-line setting. In the absence of a clearly demonstrated clinical benefit, enrolment in a clinical trial is a preferred option for these patients.
Similarly, there is no benefit with tamoxifen (level of evidence: 1A)89 or octreotide (level of evidence: 1A)97 in advanced hcc.
3.6.6. Radiation
Recommendation 11:
The evidence is insufficient to support recommendations for internal or external radiation for hcc patients. (Level of evidence: 4)
The evidence is insufficient to support recommendations in favour of 131I lipiodol internal radiotherapy or conformal external-beam radiotherapy. Internal radiotherapy with transarterial radioembolization (tare) is a promising technique in which beads or resin particles labelled with beta-emitting radiation (90Y) are injected into an artery feeding the tumour. This procedure can induce significant tumour necrosis, and case series have suggested reasonable outcomes in bclc stage B and C patients98. Unfortunately, no randomized controlled trials with these agents have been conducted, and it is unknown whether they are superior to other forms of treatment for equivalent-stage disease. Randomized trials with external-beam radiation and tare are planned based on promising phase ii data.
3.7. Prognosis
The prognosis of patients with hcc depends on tumour stage, liver function, and performance status. A multivariate analysis of 72 studies found that these important predictors correlated with survival: portal vein thrombosis, tumour size, elevated afp, Child–Pugh class, bilirubin level, and clip (Italian Investigators for Cancer of the Liver Program) score99. A meta-analysis of survival rates for the untreated patients in randomized controlled trials identified performance status, Child–Pugh score, and portal vein thrombosis as important prognostic factors100.
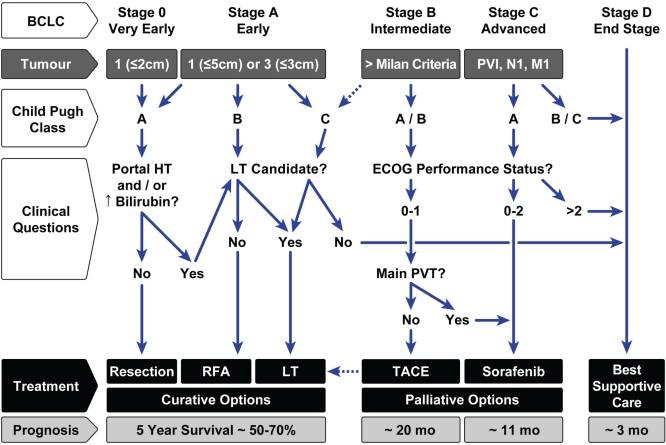
Prognosis also largely depends on the type of therapy that can be administered for hcc (Figure 2):
Carefully selected patients undergoing curative treatment options for bclc stage 0 or A may have a 5-year survival after therapy of approximately 70%. That rate includes carefully selected patients undergoing surgical resection61, those with small tumours (2 cm or smaller) undergoing rfa80, and patients who undergo liver transplantation within the Milan criteria72.
Patients with intermediate-stage hcc (bclc stage B) have a median survival of 16 months, which increases to 20 months with tace89,90.
Patients with advanced-stage hcc have a median survival of 8 months, which increases to 11 months with the use of sorafenib92.
Patients with hcc who present with advanced liver failure (Child–Pugh class C), and who are not candidates for transplantation, typically survive only 3 months on average. They should be offered palliative care only7.
FIGURE 2.
Management of hepatocellular carcinoma (hcc) in a patient with cirrhosis. Modified from the 2010 American Association for the Study of Liver Diseases guidelines 7 and the Alberta hcc Algorithm 101. bclc= Barcelona Clinic Liver Cancer; pvi= portal vein invasion; N1 = nodes positive; M1 = metastasis; ht= portal hypertension; lt= liver transplantation; ecog= Eastern Cooperative Oncology Group; pvt= portal vein thrombosis; rfa= radiofrequency ablation; tace= transarterial chemoembolization.
3.8. Treatment Algorithm
The treatment algorithm proposed here is based on the bclc staging system7 and the Alberta hcc algorithm101. It takes into account tumour stage, Child–Pugh classification, and the answers to several important clinical questions related to liver function or patient status. These factors are then linked to recommended treatment options and to prognosis, which includes the estimated 5-year survival for curative options and the estimated median survival after palliative treatments in properly selected patients (Figure 2).
Patients with bclc stage 0 or A disease are candidates for resection, rfa, or transplantation. The preferred treatment option for patients with advanced liver failure (Child–Pugh class B or C) is lt, because it treats both the cancer and the cirrhotic liver, and it has the lowest recurrence rates. Because of donor organ shortages, resection is preferred to lt for patients with preserved liver function and no significant portal hypertension. Ideally, rfa should be reserved for small tumours (2.5 cm or smaller), in patients who are not surgical candidates because of portal hypertension or liver failure (Child–Pugh class B) and not transplantation candidates because of age or comorbidities. Radiofrequency ablation is an alternative to resection in some patients, although recurrence rates are higher after rfa than after resection.
The dotted lines in the algorithm recognize that, by institutional protocol, several Canadian lt centres offer transplantation for patients who exceed the Milan criteria (single tumour 5 cm or smaller, or 3 tumours all 3 cm or smaller)76,101 and that tace has a potential role in these patients while they await lt.
For patients with bclc stage B disease and with large or multifocal hcc (that is, they exceed the Milan criteria), tace is the preferred treatment option, provided that performance status and liver function are reasonable91. Transarterial chemoembolization can be performed in selected Child–Pugh class B patients, but should be avoided in patients with poorly controlled ascites and in those with a main portal vein thrombosis90.
Sorafenib is the standard of care for advanced bclc stage C disease, and in bclc stage B patients in whom tace has failed or who are not candidates for that procedure. Level 1 evidence for the efficacy of sorafenib is available only for patients with Child–Pugh class A cirrhosis and a reasonable performance status92.
Patients may progress to bclc stage D if their performance status is poor or if they are Child–Pugh class C and not candidates for lt. These end-stage patients should be offered best supportive care only.
4. FUTURE DIRECTIONS
Currently, several open clinical studies are actively recruiting patients with advanced hcc. Because sorafenib is the standard of care in advanced disease, it should be used as the comparator arm in phase iii studies102. New agents should also be evaluated as second-line therapy after sorafenib failure.
Sorafenib is also being evaluated in combination with tace for bclc stage B patients [space (Sorafenib or Placebo in Combination with Transarterial Chemoembolization) study] and as adjuvant therapy to reduce the risk of recurrence after curative-intent surgery or rfa [storm (Sorafenib as Adjuvant Treatment in the Prevention of Recurrence of Hepatocellular Carcinoma) study].
Well-conducted randomized controlled trials are needed to determine the relative roles of rfa and resection in bclc stage 0 patients, to evaluate the role of locoregional therapy in bclc stage A patients awaiting transplantation, and to compare tare with tace in bclc stage B patients and tare with sorafenib in bclc stage C patients.
Finally, gene expression signatures that are associated with poor prognosis in hcc patients with cirrhosis have been identified103. Researchers have subclassified hcc according to these gene profiles104, which will allow for future tailoring of therapy to individual patients.
5. CONCLUSIONS
The incidence of hcc is increasing in Canada, and high-risk populations should undergo screening with abdominal ultrasonography every 6 months. Contrast-enhanced imaging with ct or mr imaging is usually sufficient to establish the diagnosis of hcc. If a biopsy is required, review by an expert pathologist and use of an ancillary panel of immunostains can help to improve the diagnostic accuracy. The bclc staging system is preferred because it takes into account tumour stage, patient status, and liver function and links those factors to specific therapies. Patients with bclc stage 0 and A disease are candidates for curative therapies of surgical resection, rfa, or transplantation. Recommended palliative therapies include tace for bclc stage B patients and sorafenib for bclc stage C patients. Patients with bclc stage D disease should receive best supportive care. Finally, a multidisciplinary approach is essential to provide optimal outcomes for patients with hcc.
Footnotes
6. CONFLICT OF INTEREST DISCLOSURES
The funding to convene a panel of experts for a 2-day meeting consisting of presentations and discussions concerning hcc was managed by Core Health Services, who received an educational grant from Bayer Healthcare Canada. Medical writing assistance was provided by Core Health Services. None of the authors received any honoraria for the development of these recommendations. Some authors (MS and KB) have, in the past, received speaking honoraria from Bayer independent of the meeting whose results are reported here. All authors declare that these past awards have had no impact on the content of the present manuscript.
7. REFERENCES
- 1.Ferenci P, Fried M, Labrecque D, et al. on behalf of the World Gastroenterology Organization Hepatocellular carcinoma (hcc): a global perspective. J Clin Gastroenterol. 2010;44:239–45. doi: 10.1097/MCG.0b013e3181d46ef2. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: globocan 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.United States, National Institutes of Health, National Cancer Institute (nci) Cancer Trends Progress Report—2009/2010 Update. Bethesda, MD: NCI; 2010. [Google Scholar]
- 4.Pocobelli G, Cook LS, Brant R, Lee SS. Hepatocellular carcinoma incidence trends in Canada: analysis by birth cohort and period of diagnosis. Liver Int. 2008;28:1272–9. doi: 10.1111/j.1478-3231.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37(suppl 2):S88–94. doi: 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 6.Howick J, Phillips B, Ball C, et al. Oxford Centre for Evidence-based Medicine—Levels of Evidence (March 2009) Oxford, U.K.: Centre for Evidence-based Medicine; 2011. [Available online at: http://www.cebm.net/index.aspx?o=1025; cited March 3, 2011] [Google Scholar]
- 7.Bruix J, Sherman M, on behalf of the American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Worth A, Nurmatov U, Sheikh A. Key components of anaphylaxis management plans: consensus findings from a national electronic Delphi study. JRSM Short Rep. 2010;1:42. doi: 10.1258/shorts.2010.010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parikh P, Malhotra H, Jelic S, on behalf of the esmo Guidelines Working Group Hepatocellular carcinoma: esmo clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19(suppl 2):ii27–8. doi: 10.1093/annonc/mdn114. [DOI] [PubMed] [Google Scholar]
- 10.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 11.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 12.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (hcv)–infected persons in the United States: a multiple cohort model of hcv prevalence and disease progression. Gastroenterology. 2010;138:513–21. 521.e1–6. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 13.Regimbeau JM, Colombat M, Mognol P, et al. Obesity and diabetes as a risk factor for hepatocellular carcinoma. Liver Transpl. 2004;10(suppl 1):S69–73. doi: 10.1002/lt.20033. [DOI] [PubMed] [Google Scholar]
- 14.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–22. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 16.Wong LL, Limm WM, Severino R, Wong LM. Improved survival with screening for hepatocellular carcinoma. Liver Transpl. 2000;6:320–5. doi: 10.1053/lv.2000.4875. [DOI] [PubMed] [Google Scholar]
- 17.Oka H, Kurioka N, Kim K, et al. Prospective study of early detection of hepatocellular carcinoma in patients with cirrhosis. Hepatology. 1990;12:680–7. doi: 10.1002/hep.1840120411. [DOI] [PubMed] [Google Scholar]
- 18.Santi V, Trevisani F, Gramenzi A, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010;53:291–7. doi: 10.1016/j.jhep.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Larcos G, Sorokopud H, Berry G, Farrell GC. Sonographic screening for hepatocellular carcinoma in patients with chronic hepatitis or cirrhosis: an evaluation. AJR Am J Roentgenol. 1998;171:433–5. doi: 10.2214/ajr.171.2.9694470. [DOI] [PubMed] [Google Scholar]
- 20.Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2008;6:1418–24. doi: 10.1016/j.cgh.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trevisani F, D’Intino PE, Morselli–Labate AM, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-hcv status. J Hepatol. 2001;34:570–5. doi: 10.1016/S0168-8278(00)00053-2. [DOI] [PubMed] [Google Scholar]
- 22.Marrero JA, Feng Z, Wang Y, et al. Alpha-fetoprotein, desgamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–18. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koike Y, Shiratori Y, Sato S, et al. Des-gamma-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: a prospective analysis of 227 patients. Cancer. 2001;91:561–9. doi: 10.1002/1097-0142(20010201)91:3<561::AID-CNCR1035>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 24.Miyaaki H, Nakashima O, Kurogi M, Eguchi K, Kojiro M. Lens culinaris agglutinin-reactive alpha-fetoprotein and protein induced by vitamin K absence ii are potential indicators of a poor prognosis: a histopathological study of surgically resected hepatocellular carcinoma. J Gastroenterol. 2007;42:962–8. doi: 10.1007/s00535-007-2117-x. [DOI] [PubMed] [Google Scholar]
- 25.Carr BI, Kanke F, Wise M, Satomura S. Clinical evaluation of lens culinaris agglutinin-reactive alpha-fetoprotein and des-gamma-carboxy prothrombin in histologically proven hepatocellular carcinoma in the United States. Dig Dis Sci. 2007;52:776–82. doi: 10.1007/s10620-006-9541-2. [DOI] [PubMed] [Google Scholar]
- 26.Pateron D, Ganne N, Trinchet JC, et al. Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. J Hepatol. 1994;20:65–71. doi: 10.1016/S0168-8278(05)80468-4. [DOI] [PubMed] [Google Scholar]
- 27.Borzio M, Bruno S, Roncalli M, et al. Liver cell dysplasia is a major risk factor for hepatocellular carcinoma in cirrhosis: a prospective study. Gastroenterology. 1995;108:812–17. doi: 10.1016/0016-5085(95)90455-7. [DOI] [PubMed] [Google Scholar]
- 28.Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432–8. [PubMed] [Google Scholar]
- 29.Solmi L, Primerano AM, Gandolfi L. Ultrasound followup of patients at risk for hepatocellular carcinoma: results of a prospective study on 360 cases. Am J Gastroenterol. 1996;91:1189–94. [PubMed] [Google Scholar]
- 30.Zoli M, Magalotti D, Bianchi G, Gueli C, Marchesini G, Pisi E. Efficacy of a surveillance program for early detection of hepatocellular carcinoma. Cancer. 1996;78:977–85. doi: 10.1002/(SICI)1097-0142(19960901)78:5<977::AID-CNCR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 31.McMahon BJ, Bulkow L, Harpster A, et al. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology. 2000;32:842–6. doi: 10.1053/jhep.2000.17914. [DOI] [PubMed] [Google Scholar]
- 32.Bolondi L, Sofia S, Siringo S, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48:251–9. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16:553–9. doi: 10.1046/j.1440-1746.2001.02470.x. [DOI] [PubMed] [Google Scholar]
- 34.Lok AS, Sterling RK, Everhart JE, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burrel M, Llovet JM, Ayuso C, et al. mri angiography is superior to helical ct for detection of hcc prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034–42. doi: 10.1053/jhep.2003.50409. [DOI] [PubMed] [Google Scholar]
- 36.Yu JS, Kim KW, Kim EK, Lee JT, Yoo HS. Contrast enhancement of small hepatocellular carcinoma: usefulness of three successive early image acquisitions during multiphase dynamic mr imaging. AJR Am J Roentgenol. 1999;173:597–604. doi: 10.2214/ajr.173.3.10470886. [DOI] [PubMed] [Google Scholar]
- 37.Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97–104. doi: 10.1002/hep.21966. [DOI] [PubMed] [Google Scholar]
- 38.Torzilli G, Minagawa M, Takayama T, et al. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology. 1999;30:889–93. doi: 10.1002/hep.510300411. [DOI] [PubMed] [Google Scholar]
- 39.Levy I, Greig PD, Gallinger S, Langer B, Sherman M. Resection of hepatocellular carcinoma without preoperative tumor biopsy. Ann Surg. 2001;234:206–9. doi: 10.1097/00000658-200108000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sangiovanni A, Manini MA, Iavarone M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010;59:638–44. doi: 10.1136/gut.2009.187286. [DOI] [PubMed] [Google Scholar]
- 41.Khalili K, Kim TK, Jang HJ, et al. Optimization of imaging diagnosis of 1–2 cm hepatocellular carcinoma: an analysis of diagnostic performance and resource utilization. J Hepatol. 2011;54:723–8. doi: 10.1016/j.jhep.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 42.Jang HJ, Kim TK, Wilson SR. Small nodules (1–2 cm) in liver cirrhosis; characterization with contrast-enhanced ultrasound. Eur J Radiol. 2009;72:418–24. doi: 10.1016/j.ejrad.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology. 2010;51:2020–9. doi: 10.1002/hep.23600. [DOI] [PubMed] [Google Scholar]
- 44.Capurro M, Wanless IR, Sherman M, et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/S0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 45.Libbrecht L, Severi T, Cassiman D, et al. Glypican-3 expression distinguishes small hepatocellular carcinomas from cirrhosis, dysplastic nodules, and focal nodular hyperplasia-like nodules. Am J Surg Pathol. 2006;30:1405–11. doi: 10.1097/01.pas.0000213323.97294.9a. [DOI] [PubMed] [Google Scholar]
- 46.Wang XY, Degos F, Dubois S, et al. Glypican-3 expression in hepatocellular tumors: diagnostic value for preneoplastic lesions and hepatocellular carcinomas. Hum Pathol. 2006;37:1435–41. doi: 10.1016/j.humpath.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 47.Shafizadeh N, Ferrell LD, Kakar S. Utility and limitations of glypican-3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Mod Pathol. 2008;21:1011–18. doi: 10.1038/modpathol.2008.85. [DOI] [PubMed] [Google Scholar]
- 48.Abdul-Al HM, Makhlouf HR, Wang G, Goodman ZD. Glypican-3 expression in benign liver tissue with active hepatitis C: implications for the diagnosis of hepatocellular carcinoma. Hum Pathol. 2008;39:209–12. doi: 10.1016/j.humpath.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun. 2001;286:433–42. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- 50.Helmbrecht K, Zeise E, Rensing L. Chaperones in cell cycle regulation and mitogenic signal transduction: a review. Cell Prolif. 2000;33:341–65. doi: 10.1046/j.1365-2184.2000.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chuma M, Sakamoto M, Yamazaki K, et al. Expression profiling in multistage hepatocarcinogenesis: identification of Hsp70 as a molecular marker of early hepatocellular carcinoma. Hepatology. 2003;37:198–207. doi: 10.1053/jhep.2003.50022. [DOI] [PubMed] [Google Scholar]
- 52.Christa L, Simon MT, Flinois JP, Gebhardt R, Brechot C, Lasserre C. Overexpression of glutamine synthetase in human primary liver cancer. Gastroenterology. 1994;106:1312–20. doi: 10.1016/0016-5085(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 53.Zucman–Rossi J, Benhamouche S, Godard C, et al. Differential effects of inactivated Axin1 and activated beta-catenin mutations in human hepatocellular carcinomas. Oncogene. 2007;26:774–80. doi: 10.1038/sj.onc.1209824. [DOI] [PubMed] [Google Scholar]
- 54.Osada T, Sakamoto M, Nagawa H, et al. Acquisition of glutamine synthetase expression in human hepatocarcinogenesis: relation to disease recurrence and possible regulation by ubiquitin-dependent proteolysis. Cancer. 1999;85:819–31. doi: 10.1002/(SICI)1097-0142(19990215)85:4<819::AID-CNCR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 55.Di Tommaso L, Franchi G, Park YN, et al. Diagnostic value of Hsp70, glypican 3, and glutamine synthetase in hepatocellular nodules in cirrhosis. Hepatology. 2007;45:725–34. doi: 10.1002/hep.21531. [DOI] [PubMed] [Google Scholar]
- 56.Terminology of nodular hepatocellular lesions. International Working Party. Hepatology. 1995;22:983–93. doi: 10.1016/0270-9139(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 57.International Consensus Group for Hepatocellular Neoplasia Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658–64. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 58.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the bclc update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 59.Bruix J, Sherman M, on behalf of the Practice Guidelines Committee, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 60.Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–16. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 61.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–40. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 62.Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 63.Ishizawa T, Hasegawa K, Aoki T, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–16. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 64.Truty MJ, Vauthey JN. Uses and limitations of portal vein embolization for improving perioperative outcomes in hepatocellular carcinoma. Semin Oncol. 2010;37:102–9. doi: 10.1053/j.seminoncol.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ, P’eng FK. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995;82:122–6. doi: 10.1002/bjs.1800820141. [DOI] [PubMed] [Google Scholar]
- 66.Yamasaki S, Hasegawa H, Kinoshita H, et al. A prospective randomized trial of the preventive effect of pre-operative transcatheter arterial embolization against recurrence of hepatocellular carcinoma. Jpn J Cancer Res. 1996;87:206–11. doi: 10.1111/j.1349-7006.1996.tb03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou WP, Lai EC, Li AJ, et al. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg. 2009;249:195–202. doi: 10.1097/SLA.0b013e3181961c16. [DOI] [PubMed] [Google Scholar]
- 68.Tan A, Aucejo F, Kim R. Is there a role for adjuvant treatment after hepatic resection for hepatocellular carcinoma? Oncology. 2010;78:161–71. doi: 10.1159/000315577. [DOI] [PubMed] [Google Scholar]
- 69.Lau WY, Lai EC, Lau SH. The current role of neoadjuvant/adjuvant/chemoprevention therapy in partial hepatectomy for hepatocellular carcinoma: a systematic review. Hepatobiliary Pancreat Dis Int. 2009;8:124–33. [PubMed] [Google Scholar]
- 70.Ono T, Nagasue N, Kohno H, et al. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: a prospective randomized study. Semin Oncol. 1997;24(suppl 6):S6-18–S6-25. [PubMed] [Google Scholar]
- 71.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 72.Mazzaferro V. Results of liver transplantation: with or without Milan criteria? Liver Transpl. 2007;13(suppl 2):S44–7. doi: 10.1002/lt.21330. [DOI] [PubMed] [Google Scholar]
- 73.Heckman JT, Devera MB, Marsh JW, et al. Bridging loco-regional therapy for hepatocellular carcinoma prior to liver transplantation. Ann Surg Oncol. 2008;15:3169–77. doi: 10.1245/s10434-008-0071-3. [DOI] [PubMed] [Google Scholar]
- 74.Yao FY. Liver transplantation for hepatocellular carcinoma: beyond the Milan criteria. Am J Transplant. 2008;8:1982–9. doi: 10.1111/j.1600-6143.2008.02351.x. [DOI] [PubMed] [Google Scholar]
- 75.Toso C, Asthana S, Bigam DL, Shapiro AM, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009;49:832–8. doi: 10.1002/hep.22693. [DOI] [PubMed] [Google Scholar]
- 76.DuBay D, Sandroussi C, Sandhu L, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumour differentiation on biopsy as an exclusion criterion. Ann Surg. 2011;253:166–72. doi: 10.1097/SLA.0b013e31820508f1. [DOI] [PubMed] [Google Scholar]
- 77.Toso C, Meeberg GA, Bigam DL, et al. De novo sirolimus-based immunosuppression after liver transplantation for hepatocellular carcinoma: long-term outcomes and side effects. Transplantation. 2007;83:1162–8. doi: 10.1097/01.tp.0000262607.95372.e0. [DOI] [PubMed] [Google Scholar]
- 78.Toso C, Mentha G, Kneteman NM, Majno P. The place of down-staging for hepatocellular carcinoma. J Hepatol. 2010;52:930–6. doi: 10.1016/j.jhep.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 79.Lam VW, Ng KK, Chok KS, et al. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J Am Coll Surg. 2008;207:20–9. doi: 10.1016/j.jamcollsurg.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 80.Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47:82–9. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 81.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–8. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10:78. doi: 10.1186/1471-230X-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of hcc. Cancer Treat Rev. 2007;33:437–47. doi: 10.1016/j.ctrv.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 84.Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453–9. doi: 10.1002/hep.22648. [DOI] [PubMed] [Google Scholar]
- 85.Orlando A, Leandro G, Olivo M, Andriulli A, Cottone M. Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009;104:514–24. doi: 10.1038/ajg.2008.80. [DOI] [PubMed] [Google Scholar]
- 86.Wang W, Shi J, Xie WF. Transarterial chemoembolization in combination with percutaneous ablation therapy in unresectable hepatocellular carcinoma: a meta-analysis. Liver Int. 2010;30:741–9. doi: 10.1111/j.1478-3231.2010.02221.x. [DOI] [PubMed] [Google Scholar]
- 87.Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–9. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 88.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–71. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 89.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 90.Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6–25. doi: 10.1007/s00270-006-0062-3. [DOI] [PubMed] [Google Scholar]
- 91.Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the precision v study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 93.Cheng AL, Kang YK, Chen Z, et al. Efficacy a nd s afety of sorafenib in patients in the Asia–Pacific region with advanced hepatocellular carcinoma: a phase iii randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 94.Kim R, Byrne MT, Tan A, Aucejo F. What is the indication for sorafenib in hepatocellular carcinoma? A clinical challenge. Oncology (Williston Park) 2011;25:283–91. 295. [PubMed] [Google Scholar]
- 95.Cheng AL, Kang Y, Lin D, et al. Phase iii trial of sunitinib (Su) versus sorafenib (So) in advanced hepatocellular carcinoma (hcc) [abstract 4000] J Clin Oncol. 2011;29 [Available online at: http://www.asco.org/ASCOv2/Meetings/abstracts?&vmview=abst_detail_view&confID=102&abstractID=78796; cited August 17, 2011] [Google Scholar]
- 96.Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154–60. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 97.Guo TK, Hao XY, Ma B, et al. Octreotide for advanced hepatocellular carcinoma: a meta-analysis of randomized controlled trials. J Cancer Res Clin Oncol. 2009;135:1685–92. doi: 10.1007/s00432-009-0615-3. [DOI] [PubMed] [Google Scholar]
- 98.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 99.Tandon P, Garcia–Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29:502–10. doi: 10.1111/j.1478-3231.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274–83. doi: 10.1002/hep.23485. [DOI] [PubMed] [Google Scholar]
- 101.Burak KW, Kneteman NM. An evidence-based multidisciplinary approach to the management of hepatocellular carcinoma (hcc): the Alberta hcc algorithm. Can J Gastroenterol. 2010;24:643–50. doi: 10.1155/2010/410574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 103.Villanueva A, Hoshida Y, Battiston C, et al. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology. 2011;140:1501–12.e2. doi: 10.1053/j.gastro.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoshida Y, Toffanin S, Lachenmayer A, Villanueva A, Minguez B, Llovet JM. Molecular classification and novel targets in hepatocellular carcinoma: recent advancements. Semin Liver Dis. 2010;30:35–51. doi: 10.1055/s-0030-1247131. [DOI] [PMC free article] [PubMed] [Google Scholar]