Substitution of RNA with heavy metals including selenium is of great utility for structural analysis of RNA and for studying RNA–protein interactions. This paper describes a method for synthesizing seleno-substituted nucleoside triphosphates capable of incorporation into RNA via transcription. The paper also describes methods for purifying seleno-substituted RNAs for structural and functional studies.
Keywords: modified NTPαSe, selenium-derivatization of RNA, RNA transcription, RNA crystallography, hammerhead ribozyme
Abstract
Phosphoroselenoate RNA (PSe-RNA) is nuclease resistant and has great potentials in X-ray crystal structure and function studies of noncoding RNAs and protein–RNA interactions. In order to conveniently synthesize PSe-RNA via transcription, we have developed a one-pot synthetic method for the nucleoside 5′-(α-P-seleno)-triphosphates (NTPαSe) analogs without protecting any functionality of the ribonucleosides. The NTPαSe diastereomers have been purified, fully characterized, and incorporated into RNAs by T7 RNA polymerase. The transcribed RNAs are diastereomerically pure, and the Se-derivatized ribozymes are generally active. Furthermore, we have established an affinity purification strategy by using immobilized boronate to conveniently purify NTPαSe analogs. Though the affinity-purified NTPαSe analogs are diastereomeric mixtures, they can be directly used in transcription without a significant impact on the transcription efficiency. Moreover, we found that the PSe-nucleotide is stable during polyacrylamide gel purification, indicating that the PSe-RNAs can be purified straightforwardly for crystal structural and functional studies.
INTRODUCTION
The massive discoveries of noncoding RNAs in recent years, such as small regulatory RNAs (Lee et al. 1993; Makarova and Kramerov 2007) and the riboswitch RNAs (Gelfand et al. 1999), lead to a great demand for structural insights of the functional RNAs and RNA-ligand complexes (Ferre-D'Amare et al. 1998; Kieft et al. 1999; Batey et al. 2004; Macrae et al. 2006). In addition to crystallization, however, RNA crystallography has faced the other long-standing challenge: derivatization and phasing (Ke and Doudna 2004). To address these needs, several phasing-and-crystallization strategies have been developed recently (Ferre-D'Amare et al. 1998; Keel et al. 2007; Ferre-D'Amare 2010; Koldobskaya et al. 2011). In the case of a novel protein structure, the phase problem is usually solved by the selenomethionine (Se-Met) derivatization and multiwavelength anomalous dispersion (MAD) technique (Hendrickson 1991; Deacon and Ealick 1999). Inspired by the convenient Se-derivatization of proteins, since 1998, Huang's research group has pioneered and developed the selenium derivatization of nucleic acids for X-ray crystallography (Carrasco et al. 2001; Caton-Williams and Huang 2008; Sheng and Huang 2010). We found that the selenium-derivatized RNAs and DNAs are stable and that the selenium derivatizations do not cause significant perturbation (Jiang et al. 2007; Salon et al. 2007, 2010; Sheng et al. 2007, 2010, 2011; Caton-Williams and Huang 2008; Olieric et al. 2009; Hassan et al. 2010).
There are two strategies to derivatize RNA with selenium for X-ray crystallography: indirect derivatization (noncovalent Se-derivatization via the seleno-protein binding) (Ferre-D'Amare et al. 1998; Rupert and Ferre-D'Amare 2001; Ferre-D'Amare 2010) and direct derivatization (covalent Se-derivatization via the oxygen replacement) (Caton-Williams and Huang 2008; Sheng and Huang 2010). The cocrystallization method using Se-Met-derivatized U1A protein (a RNA-binding protein) was developed by Doudna and coworkers (Ferre-D'Amare et al. 1998; Rupert and Ferre-D'Amare 2001; Ferre-D'Amare 2010). Although this U1A method has been used to solve several ribozyme structures, it is labor intensive. For direct derivatization of RNA via covalent incorporation of selenium, chemical approach (solid-phase synthesis) or enzymatic methods (RNA polymerase transcription) can be applied (Caton-Williams and Huang 2008; Sheng and Huang 2010). A general drawback of solid-phase synthesis for large-scale RNA preparation is the RNA length limit (usually <60 nt). To solve this problem, a T4 RNA ligase method using a synthetic 2′-Se-RNA was developed by Micura's group in order to prepare a longer 2′-Se-RNA. (Hobartner et al. 2005). However, due to the size limit and high cost associated with solid-phase synthesis, RNA transcription is a preferred approach for most molecular and structural biology laboratories. A large quantity of long RNA molecules can be easily prepared via transcription by T7 RNA polymerase (Ferre-D'Amare and Doudna 2001; Kieft and Batey 2004).
Phosphoroselenoate modification has been introduced into DNA through solid-phase synthesis for the structure study by Egli and coworkers (Wilds et al. 2002). This PSe-DNA modification was successfully used to facilitate the phase determination of a homo-DNA structure (Egli et al. 2006). However, the PSe-DNA prepared by solid-phase synthesis was a mixture of Rp and Sp diastereomers, which needed to be separated. Separation of the diastereomers, which are formed at the chiral center of each Se-modified phosphate linker, requires anions exchange chromatography extensively. Since the diastereomer number increases exponentially by 2n (n: the modification number), it is not practical to separate diastereomers generated by multiple phosphoroselenoates incorporated into DNA or RNA. To obtain diastereomerically pure PSe-nucleic acids, our research group has developed the enzymatic methods (Carrasco and Huang 2004; Carrasco et al. 2005; Brandt et al. 2006). In order to prepare phosphoroselenoate RNA (PSe-RNA) (Fig. 1) through transcription, we first synthesized the 5′-(α-P-seleno)-triphosphates (NTPαSe) analogs (Fig. 1; Carrasco et al. 2005; Brandt et al. 2006). Considering broad applications of the NTPαSe analogs and PSe-RNAs, it is necessary to develop a convenient strategy to synthesize the NTPαSe analogs and PSe-RNAs on a large scale. Herein, we report a novel synthetic method for the NTPαSe analogs without any nucleoside protection, which greatly reduces efforts in the synthesis and purification and increases the overall yield. We also developed a simple purification strategy for NTPαSe and the affinity-purified NTPαSe analogs can be used directly to transcribe PSe-RNAs. Moreover, stability of the phosphoroseleonate modification was confirmed under the conditions of PAGE purification.
FIGURE 1.
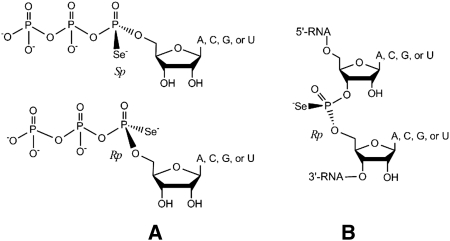
Chemical structures of the Se-derivatized nucleotides. (A) Nucleoside 5′-(α-P-seleno)triphosphates (NTPαSe, Sp and Rp). (B) Phosphoroselenoate RNA (PSe-RNA, Rp).
RESULTS
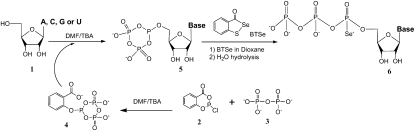
Nucleoside triphosphates, especially chemically modified ones, are normally synthesized by using protected nucleosides with free 5′-hydroxyl groups (Ludwig and Eckstein 1989; Carrasco and Huang 2004; Brandt et al. 2006). The protection is critical. If other hydroxyl groups (such as 2′-OH and 3′-OH) and amino groups on the nucleobases are not protected, they can cause formation of many by-products during the chemical synthesis of NTPs. These by-products are difficult to remove during the purification steps. In order to obtain a clean phosphorylation reaction with chemical selectivity on the 5′-hydroxyl group of the nonprotected nucleosides, we developed a selective phosphorylating reagent. This reagent (Fig. 2, 4) was generated in situ by reacting pyrophosphate (3) with 2-chloro-4H-1,3,2-benzodioxaphosphorin-4-one (2), followed by addition of the nucleosides (1) to offer the cyclic phosphite (5). After selenization and hydrolysis, NTPαSe analogs (6) were successfully synthesized without any protection on the nucleosides. The HPLC analysis of the crude products indicated these 5′-triphosphates as the dominant products.
FIGURE 2.
Facile synthesis of the NTPαSe analogs. (DMF) Dimethylformamide; (TBA) tributylamine.
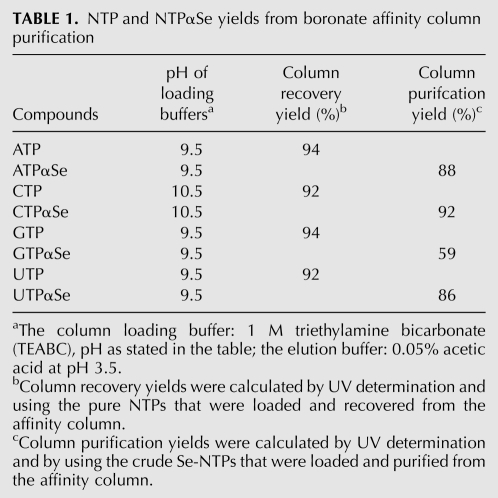
After the synthesis, NTPαSe analogs need to be purified from crude products before RNA transcription, otherwise salts and other organic impurities will inhibit the activity of RNA polymerase. In our previous study, NTPαSe analogs were purified by HPLC, which is labor-intensive and has a low capacity for larger-scale synthesis (Carrasco et al. 2005; Brandt et al. 2006). Thus, we developed the boronate affinity method for purification of NTPαSe analogs. Boronate has a high affinity to cis-diol-containing compounds (Batey et al. 1992; Liu and Scouten 2000). The cis-diols at the 2′ and 3′ positions of NTPs can interact with boronate strongly, which allows the separation of NTPαSe analogs from many other impurities. Boronate column purification of NTPαSe analogs offered satisfactory yields (Table 1). Moreover, as a comparison, we also purified NTPαSe analogs via HPLC (Supplemental Fig. S1) and performed RNA transcription (Fig. 3). Compared with HPLC, the boronate affinity column has a larger capacity (easily up to a 100 mg of NTPαSe) and requires less purification time and steps. The quality of NTPαSe analogs purified by the affinity column was examined by analytical HPLC. As a typical example, HPLC profiles of CTPαSe analogs before and after boronate column purification were shown in Supplemental Figure S2. After affinity purification, NTPαSe analogs (diastereomer mixtures) can be directly used for RNA transcription (Fig. 4). Full-length RNA products were observed with the affinity-purified NTPαSe analogs.
TABLE 1.
NTP and NTPαSe yields from boronate affinity column purification
FIGURE 3.
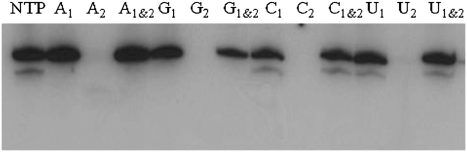
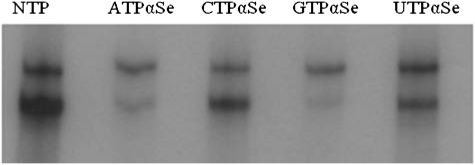
Enzymatic incorporation of NTPαSe analogs purified by HPLC to (hammerhead mutant) HHM RNA. Minor cleavage (the small faster-moving bands) was observed with the native, Se-C and Se-U ribozymes. NTP (all native nucleoside triphosphates), A1 (ATPαSe I), A2 (ATPαSe II), A1&2 (I and II; I vs II = 1:1); C1 (CTPαSe I), C2 (CTPαSe II), C1&2 (I and II; I vs II = 1:1); G1 (GTPαSe I), G2 (GTPαSe II), G1&2 (I and II; I vs II = 1:1); U1 (UTPαSe I), U2 (UTPαSe II), U1&2 (I and II; I vs II = 1:1).
FIGURE 4.
HHM RNAs transcribed with the NTPαSe analogs (diastereomer mixture) purified by boronate column. The shorter fragments are self-cleaved products.
All four NTPαSe analogs were synthesized via this facile route, and all eight diastereomers were purified and fully characterized by MS, HPLC, 1H-, 13C-, and 31P-NMR (Supplemental Tables S1–S4; Supplemental Fig. S1). This is the first full characterization of all NTPαSe analogs. Based on the literature result on the phosphorothioates (Griffiths et al. 1987), Peak 1 (NTPαSe I) and Peak 2 (NTPαSe II) in HPLC profiles (Supplemental Fig. S1) were tentatively assigned as Sp and Rp, respectively. Eight diastereomers from the four NTPαSe analogs were individually examined using T7 RNA polymerase (Fig. 3). Consistent with our previous report (Carrasco et al. 2005; Brandt et al. 2006), clearly, T7 RNA polymerase can efficiently recognize the NTPαSe I diastereomers (Sp) at the same level as the native NTPs (Fig. 3), while the NTPαSe II diastereomers (Rp) were not recognized. Our transcription data with several different DNA templates indicated that the PSe-RNA transcription efficiency and yields are similar to the native ones. Since T7 RNA polymerase only recognizes Sp-NTPαSe diastereomers and Rp-NTPαSe diastereomers are neither substrates nor inhibitors (Fig. 3), RNAs transcribed with the affinity-purified NTPαSe analogs are diasteromerically pure (Fig. 4).
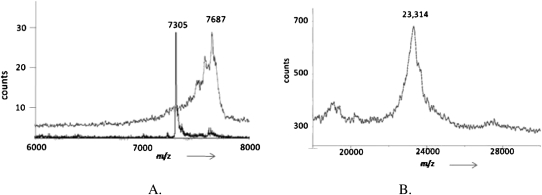
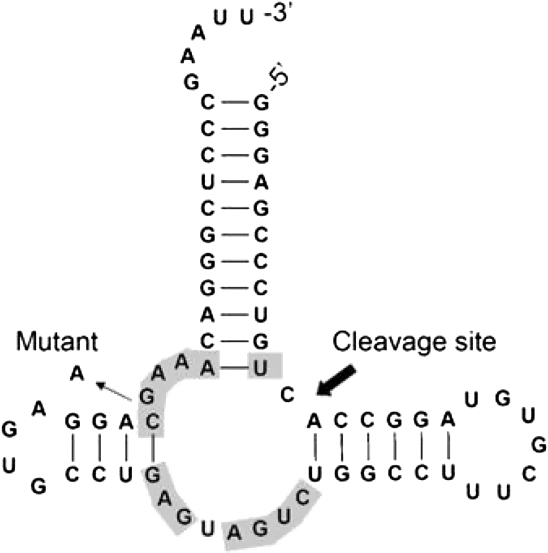
A PSe-RNA (22 nt) containing six Se atoms was first prepared by RNA transcription using UTPαSe I, and its integrity was confirmed by MS (Fig. 5A). Two hammerhead ribozymes (Fig. 6, HHN and HHM) were used later for examining the transcriptional incorporation of NTPαSe analogs. The wild-type hammerhead ribozyme (HHN) transcribed using all native NTPs could cleave itself in the transcription condition (Fig. 7). The mutant hammerhead ribozyme (HHM) is a hammerhead mutant that has a very low turnover rate. The majority of HHM transcribed could not cleave itself (Fig. 7). Low self-cleavage activities of PSe-RNA HHM (the Se-C and Se-U mutant ribozymes) were also observed (Fig. 3), while the Se-A and Se-G mutant ribozymes were not active. This is also consistent with literature that the A and G nucleotides play more critical roles in the hammerhead ribozyme, especially in the active site. Due to the presence of the A at the cleavage site and the five As in the highly conserved sequences of HHN (Fig. 6), HHN transcribed by ATPαSe showed no self-cleavage activity (Fig. 7, lane N of A). Unsurprisingly, HHN transcribed with the other Sp-NTPαSe (CTPαSe, GTPαSe, or UTPαSe) has self-cleavage activity (Fig. 7). The catalytic activity of the Se-derivatized ribozymes suggests that the selenium modifications on nonessential phosphates do not cause significant structural and functional perturbations. Furthermore, the inhibition of the ribozyme self-cleavage by ATPαSe incorporation (Fig. 7, lane N of A) indicates that the PSe-RNA is stable under the transcription conditions without deselenization, since deselenization will result in formation of the self-cleaving wild-type ribozyme. Moreover, the UTPαSe-transcribed ribozyme (HHM) containing 15 Se atoms was confirmed by MALDI-TOF (Fig. 5B).
FIGURE 5.
MALDI-TOF MS analysis of transcribed RNAs. (A) Native RNA42.1 (22 nt), molecular formula: C209H261N82O165P24; observed [M+H]+ = 7305 (calcd. [M+H]+ = 7303). PSe-U RNA42.1, molecular formula: C209H261N82O159P24Se6; observed [M+H]+ = 7687 (calcd. [M+H]+ = 7686). Their mass difference of 382 (7687 − 7305 = 382) reflects incorporation of six Se-modified U units (6Se-6O = 6 × 80 − 6 × 16 = 384). (B) PSe-U HHM, molecular formula: C657H814N264O465P68Se15 (calcd [M] = 23,144); the matrix (2′,4′,6′-trihydroxyacetophenone, THAP), molecular formula: C8H8O4, molecular weight: 168.0; observed mass of [M + THAP]: 23,314 (calcd. 23,312).
FIGURE 6.
Secondary structure of wild-type hammerhead ribozyme (HHN) derived from the satellite RNA of tobacco ringspot virus. Conserved bases are labeled in gray. The mutant hammerhead ribozyme (HHM) has a G→A mutation.
FIGURE 7.
Transcription of the hammerhead ribozymes. (M) Mutant HHM; (N) wild-type HHN; (NTP) all native NTPs; (A) ATPαSe I and other natives; (C) CTPαSe I and other natives; (G) GTPαSe I and other natives; (U) UTPαSe I and other natives. One band from each cleavage is shown here, since the other band (12 nt) ran off the gel.
To investigate stability of the phosphoroselenoate moiety during PAGE purification, pure ATPαSe I was loaded on a polyacrylamide gel for electrophoresis and recovered from gel. The original and recovered ATPαSe was analyzed by HPLC by comparing it with native ATP (Supplemental Fig. S3). HPLC analysis showed that the phosphoroselenoate moiety was quite stable under PAGE purification and recovery conditions, since only an insignificant amount of ATPαSe (<2%; profile 4 in Supplemental Fig. S3) was oxidized to native ATP after the PAGE purification. This suggests that the PSe-RNAs can be purified by ordinary polyacrylamide gel, which is the most common purification method used for preparing large-scale RNAs in X-ray crystallography. As a matter of fact, PSe-RNA is more stable in the presence of magnesium salt (or even in a solution with RNase contamination) than native RNA (Carrasco et al. 2005; Brandt et al. 2006). For example, after storing long Se-RNAs for 4–5 mo in the freezer (with 1 mM DTT), MALDI-MS analysis still confirmed their integrity.
DISCUSSION
Nucleotide triphosphates are common biological molecules in all life forms, and their analogs have wide applications for scientific and therapeutic discoveries. Our facile synthesis and purification methods for the NTPαSe analogs enable scale-up preparation of PSe-RNAs for X-ray crystallography and other potential applications. Our experimental research indicates that only NTPαSe I could be incorporated into RNAs by T7 RNA polymerase. Moreover, unless the cleavage site is modified, the Se-derivatized ribozymes are generally active, indicating that the Se-modification does not significantly affect RNA global folding and perturb their structure and function. The method described here is an efficient synthetic approach for NTPαSe without any protection of the nucleosides, which is a one-pot strategy without multiple steps of purification. This procedure allows for the synthesis of natural ribonucleotide triphosphates, α-phosphoroselenoate nucleotide triphosphates, α-phophorothioate nucleoside triphosphates, and α-borano nucleotide triphosphates by selecting the appropriate corresponding oxidizing agent. The NTPαSe analogs can be easily purified by boronate affinity column for RNA transcription. The Sp-NTPαSe analogs are efficient substrates for T7 RNA polymerase, while the Rp-NTPαSe analogs are neither inhibitors nor substrates. A full-length PSe-hammerhead ribozyme with multiple Se-modifications was transcribed and confirmed by MALDI-TOF. PSe-RNAs are stable under a PAGE gel purification condition, which allows convenient preparation of PSe-RNAs for studying ribozyme catalytic mechanism, RNA crystal structure and function, and RNA interference.
MATERIALS AND METHODS
The following procedures are normally performed under argon, and all organic solvents and chemicals used in the experiments were purchased as anhydrous compounds. Unless specifically mentioned, the reactions were performed at room temperature. All compounds are commercially available, including 2-choloro-4H-1,3,2-benzodioxaphosphorin (from Sigma, http://www.sigmaaldrich.com; SeNA Research, http://www.senaresearch.com) and 3H-1,2-benzothaselenol-3-one (BTSe) (Stawinski and Thelin 1994) (commercially available from SeNA Research).
NTPαSe synthesis
Individual nucleoside (1, 0.45 mmol, A [120 mg], C [109 mg], G [127 mg], or U [110 mg]) (Fig. 2), tributylammonium pyrophosphate (3, 426 mg, 0.9 mmol, 2 eq.), and 3H-1, 2-benzothaselenol-3-one (BTSe, 195 mg, 0.9 mmol, 2 eq.) were dried in separate flasks under high vacuum for 1 h. To the dried tributylammonium pyrophosphate (3), DMF (0.6 mL) and tributyl amine (TBA, 1.2 mL) were injected to dissolve the pyrophosphate. Anhydrous 2-chloro-4H-1,3,2-benzodioxaphosphorin-4-one (2, 182 mg, 0.9 mmol, 2 eq.) dissolved in DMF (1.2 mL) was then injected into the pyrophosphate solution (3). This reaction mixture was stirred under argon at room temperature for 30 min and then injected into the nucleoside (1, 0.45 mmol) dissolved in DMF (0.45 mL; special note: adenosine was dissolved in a mixed solvent of 0.32 mL DMF and 0.13 mL DMSO, and guanosine was dissolved in a mixed solvent of 0.22 mL DMF and 0.23 mL DMSO). After the nucleoside phosphorylating reaction was stirred under argon at room temperature for 1 h, 3H-1,2-benzothaselenol-3-one (BTSe) dissolved in dioxane (1.5 mL) was injected to the nucleoside reaction mixture. The BTSe oxidization reaction was finished at room temperature for 1 h. Then, 10 mL water (approximately twice the volume of the reaction solution) was added to the BTSe reaction mixture. After hydrolysis at room temperature for 2 h, an ethanol/NaCl precipitation (NaCl [1.7 mL, 3 M] and ethanol [50 mL] were sequentially added to the NTPαSe solution, followed by freezing at −80°C for 30 min and centrifugation at 14,000 rpm for 20 min) was performed in the presence of fresh DTT (2 mM). The pellet was redissolved in a small amount of water (200 μL), and the concentration of NTPαSe analog (6) was determined by UV-Vis spectrometry.
HPLC analysis of NTPαSe
The synthesized NTPαSe analogs (Fig. 2, 6) were analyzed by HPLC. ATPαSe, CTPαSe, and GTPαSe were eluted (1 mL/min) with a linear gradient from Buffer A (20 mM triethylammonium acetate [TEAAc] at pH 7.0) to 12.5% Buffer B (50% acetonitrile in water, 20 mM triethylammonium acetate [TEAAc] at pH 7.0) for >20 min. UTPαSe was eluted (1 mL/min) with a linear gradient from Buffer A (20 mM triethylammonium acetate [TEAAc] at pH 7.0) to 20% Buffer B (50% ethanol in water, 20 mM triethylammonium acetate [TEAAc] at pH 7.0) for >20 min.
Purification of NTPαSe analogs by boronate affinity column
Sample loading buffer (1 M triethylamine bicarbonate, TEABC) was prepared by bubbling CO2 through a solution containing 28.2 mL triethylamine in 140 mL H2O in an ice bath, until the pH dropped to 9.5. Then, the solution final volume was brought up to 200 mL by adding more deionized water. The prepared TEABC solution was stored at 4°C, and the pH was checked each time before the buffer was used. The elution buffer (1 L, 0.05% v/v acetic acid, pH 3.5) was prepared by dilution of concentrated acetic acid (0.5 mL) with 999.5 mL of deionized water. All working buffers were kept at 4°C during the affinity purification.
Boronate affigel (0.20 g, 1.2 meq/g, Bio-Rad) was hydrated in 2 mL of TE buffer (10 mM Tris Hcl at pH 8.0, 1 mM EDTA) and packed in a column (1 × 4 cm). The column was equilibrated by 5 mL of 1 M TEABC (pH 9.5) for 1 h at 4°C. After draining the excess buffer, the NTPs (0.5 μmol) or crude NTPαSe analogs (20 μmol) dissolved in deionized water (100 μL) were loaded onto the column and incubated for 1 h at 4°C. The column was then eluted with the loading buffer (1 M TEABC), and fractions (0.5 mL each) were collected. Each fraction was monitored by UV. We washed the column until the maximum absorbance dropped below 0.01 (the wavelengths monitored are the followings: ATP [259 nm], UTP [262 nm], GTP [252 nm], and CTP [271 nm]; special note: phosphoroselenoate modifications do not change UV absorbance of nucleotides). The NTPαSe on column was then eluted by the elution buffer (0.05% v/v acetic acid at pH 3.5), and fractions (0.5 mL each) were collected by tubes containing Na-PO4 buffer (100 μL, 1 M at pH 7.3). The neutralized individual fraction was analyzed by UV spectrometry. The fractions containing the eluted NTPαSe were combined and concentrated by lyophilization. The column was later washed by several column volumes of 1% acetic acid (pH 2.8) and water to regenerate it. HPLC analysis was done to confirm the quality of the affinity-purified NTPαSe analogs.
PSe-RNA transcription
Two types of hammerhead ribozymes were transcribed with either all native NTPs, or one of the NTPs was replaced with the corresponding NTPαSe. The sequence of mutant hammerhead ribozyme (HHM) was 5′-GGGAGCCCUGUCACCGGAUGUGCUUUCCGGUCUGAUGAGUCCGUGAGGACAAAACAGGGCUCCCGAAUU-3′. The sequence of wild-type hammerhead ribozyme (HHN) was 5′-GGGAGCCCUGUCACCGGAUGUGCUUUCCGGUCUGAUGAGUCCGUGAGGACGAAACAGGGCUCCCGAAUU-3′. The sequence of native RNA 42.1 was 5′-GGUCGCUGGUAAUGGGCGCCUU-3′. RNA transcribed with radioactive labeling was performed according to the protocol from the manufacturer (Epicentre T7 RNA polymerase, T7905K) for 1 h at 37°C. Each time one type of 10 mM NTPαSe was used to substitute for type of native 10 mM NTP solution in protocol (for example, in ATPαSe I transcription mixture, equal amounts of ATPαSe I, CTP, GTP, and UTP were added). RNAs were labeled by using radioactive NTPs, such as ATP[α-32P]. A typical reaction (10 μL) was performed under the final conditions of 50 ng/μL plasmid or 5 ng/μL dsDNA template prepared by PCR, 0.50 mM NTP or NTPαSe analog (diastereomerically pure), 10 mM DTT, 1X Epicentre T7 RNA transcription buffer, 1 μL of Epicentre T7 RNA polymerase (50 U), and RNase-free water to bring the volume to 10 μL. For transcription with the affinity-purified NTPαSe analogs (diastereomer mixture), the NTPαSe I concentration was kept at 0.5 mM in the final reaction solution. The transcription reaction was quenched by adding an equal volume of loading dye containing EDTA (100 mM). The transcriptions were monitored by a 15% denature PAGE gel and autoradiography.
Stability study of phosphoroselenoate nucleotides
ATPαSe I was synthesized and purified by HPLC as described above. ATP or ATPαSe (10 μL, 10 mM) was loaded on a denaturing polyacrylamide gel (12%) and recovered from the gel after gel electrophoresis. An ATPαSe band on the gel was visualized by UV shadowing and cut from the gel, then soaked in water overnight. The soaking solution was centrifuged the next day to remove gel fragments. The supernatant was collected and filtered with a 0.2-μm syringe filter. The filtered solutions were analyzed by HPLC to compare ATPαSe with the native ATP recovered in the same manner.
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
ACKNOWLEDGMENTS
This work was financially supported by USA NIH (GM095086), the Georgia Cancer Coalition (GCC) Distinguished Cancer Clinicians and Scientists, and USA NSF (CHE-0750235 and MCB-0824837).
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.2719311.
REFERENCES
- Batey RT, Inada M, Kujawinski E, Puglisi JD, Williamson JR 1992. Preparation of isotopically labeled ribonucleotides for multidimensional NMR spectroscopy of RNA. Nucleic Acids Res 20: 4515–4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batey RT, Gilbert SD, Montange RK 2004. Structure of a natural guanine-responsive riboswitch complexed with the metabolite hypoxanthine. Nature 432: 411–415 [DOI] [PubMed] [Google Scholar]
- Brandt G, Carrasco N, Huang Z 2006. Efficient substrate cleavage catalyzed by hammerhead ribozymes derivatized with selenium for X-ray crystallography. Biochemistry 45: 8972–8977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco N, Huang Z 2004. Enzymatic synthesis of phosphoroselenoate DNA using thymidine 5′-(α-P-seleno)triphosphate and DNA polymerase for X-ray crystallography via MAD. J Am Chem Soc 126: 448–449 [DOI] [PubMed] [Google Scholar]
- Carrasco N, Ginsburg D, Du Q, Huang Z 2001. Synthesis of selenium-derivatized nucleosides and oligonucleotides for X-ray crystallography. Nucleosides Nucleotides Nucleic Acids 20: 1723–1734 [DOI] [PubMed] [Google Scholar]
- Carrasco N, Caton-Williams J, Brandt G, Wang S, Huang Z 2005. Efficient enzymatic synthesis of phosphoroselenoate RNA by using adenosine 5′-(α-P-seleno)triphosphate. Angew Chem Int Ed Engl 45: 94–97 [DOI] [PubMed] [Google Scholar]
- Caton-Williams J, Huang Z 2008. Biochemistry of selenium-derivatized naturally occurring and unnatural nucleic acids. Chem Biodivers 5: 396–407 [DOI] [PubMed] [Google Scholar]
- Deacon AM, Ealick SE 1999. Selenium-based MAD phasing: setting the sites on larger structures. Structure 7: R161–R166 [DOI] [PubMed] [Google Scholar]
- Egli M, Pallan PS, Pattanayek R, Wilds CJ, Lubini P, Minasov G, Dobler M, Leumann CJ, Eschenmoser A 2006. Crystal structure of homo-DNA and nature's choice of pentose over hexose in the genetic system. J Am Chem Soc 128: 10847–10856 [DOI] [PubMed] [Google Scholar]
- Ferre-D'Amare AR 2010. Use of the spliceosomal protein U1A to facilitate crystallization and structure determination of complex RNAs. Methods 52: 159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre-D'Amare AR, Doudna JA 2001. Methods to crystallize RNA. In Current Protocols in Nucleic Acid Chemistry. Chapter 7: Unit 7 6, Wiley Online Library [DOI] [PubMed] [Google Scholar]
- Ferre-D'Amare AR, Zhou K, Doudna JA 1998. Crystal structure of a hepatitis delta virus ribozyme. Nature 395: 567–574 [DOI] [PubMed] [Google Scholar]
- Gelfand MS, Mironov AA, Jomantas J, Kozlov YI, Perumov DA 1999. A conserved RNA structure element involved in the regulation of bacterial riboflavin synthesis genes. Trends Genet 15: 439–442 [DOI] [PubMed] [Google Scholar]
- Griffiths AD, Potter BV, Eperon IC 1987. Stereospecificity of nucleases towards phosphorothioate-substituted RNA: stereochemistry of transcription by T7 RNA polymerase. Nucleic Acids Res 15: 4145–4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AE, Sheng J, Zhang W, Huang Z 2010. High fidelity of base pairing by 2-selenothymidine in DNA. J Am Chem Soc 132: 2120–2121 [DOI] [PubMed] [Google Scholar]
- Hendrickson WA 1991. Determination of macromolecular structures from anomalous diffraction of synchrotron radiation. Science 254: 51–58 [DOI] [PubMed] [Google Scholar]
- Höbartner C, Rieder R, Kreutz C, Puffer B, Lang K, Polonskaia A, Serganov A, Micura R 2005. Syntheses of RNAs with up to 100 nucleotides containing site-specific 2′-methylseleno labels for use in X-ray crystallography. J Am Chem Soc 127: 12035–12045 [DOI] [PubMed] [Google Scholar]
- Jiang J, Sheng J, Carrasco N, Huang Z 2007. Selenium derivatization of nucleic acids for crystallography. Nucleic Acids Res 35: 477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke A, Doudna JA 2004. Crystallization of RNA and RNA-protein complexes. Methods 34: 408–414 [DOI] [PubMed] [Google Scholar]
- Keel AY, Rambo RP, Batey RT, Kieft JS 2007. A general strategy to solve the phase problem in RNA crystallography. Structure 15: 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieft JS, Batey RT 2004. A general method for rapid and nondenaturing purification of RNAs. RNA 10: 988–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieft JS, Zhou K, Jubin R, Murray MG, Lau JY, Doudna JA 1999. The hepatitis C virus internal ribosome entry site adopts an ion-dependent tertiary fold. J Mol Biol 292: 513–529 [DOI] [PubMed] [Google Scholar]
- Koldobskaya Y, Duguid EM, Shechner DM, Suslov NB, Ye J, Sidhu SS, Bartel DP, Koide S, Kossiakoff AA, Piccirilli JA 2011. A portable RNA sequence whose recognition by a synthetic antibody facilitates structural determination. Nat Struct Mol Biol 18: 100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V 1993. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75: 843–854 [DOI] [PubMed] [Google Scholar]
- Liu XC, Scouten WH 2000. Boronate affinity chromatography. Methods Mol Biol 147: 119–128 [DOI] [PubMed] [Google Scholar]
- Ludwig J, Eckstein F 1989. Rapid and efficient synthesis of nucleoside 5′-0-(1-thiotriphosphates), 5′-triphosphates and 2′,3′-cyclophosphorothioates using 2-chloro-4H-1,3,2-benzodioxaphosphorin-4-one. J Org Chem 54: 631–635 [Google Scholar]
- Macrae IJ, Zhou K, Li F, Repic A, Brooks AN, Cande WZ, Adams PD, Doudna JA 2006. Structural basis for double-stranded RNA processing by Dicer. Science 311: 195–198 [DOI] [PubMed] [Google Scholar]
- Makarova JA, Kramerov DA 2007. Noncoding RNAs. Biochemistry (Mosc) 72: 1161–1178 [DOI] [PubMed] [Google Scholar]
- Olieric V, Rieder U, Lang K, Serganov A, Schulze-Briese C, Micura R, Dumas P, Ennifar E 2009. A fast selenium derivatization strategy for crystallization and phasing of RNA structures. RNA 15: 707–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupert PB, Ferre-D'Amare AR 2001. Crystal structure of a hairpin ribozyme-inhibitor complex with implications for catalysis. Nature 410: 780–786 [DOI] [PubMed] [Google Scholar]
- Salon J, Sheng J, Jiang J, Chen G, Caton-Williams J, Huang Z 2007. Oxygen replacement with selenium at the thymidine 4-position for the Se base pairing and crystal structure studies. J Am Chem Soc 129: 4862–4863 [DOI] [PubMed] [Google Scholar]
- Salon J, Sheng J, Gan J, Huang Z 2010. Synthesis and crystal structure of 2′-Se-modified guanosine containing DNA. J Org Chem 75: 637–641 [DOI] [PubMed] [Google Scholar]
- Sheng J, Huang Z 2010. Selenium derivatization of nucleic acids for X-ray crystal-structure and function studies. Chem Biodivers 7: 753–785 [DOI] [PubMed] [Google Scholar]
- Sheng J, Jiang J, Salon J, Huang Z 2007. Synthesis of a 2′-Se-thymidine phosphoramidite and its incorporation into oligonucleotides for crystal structure study. Org Lett 9: 749–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng J, Salon J, Gan J, Huang Z 2010. Synthesis and crystal structure study of 2′-Se-adenosine-derivatized DNA. Science China: Chemistry 53: 78–85 [Google Scholar]
- Sheng J, Hassan AEA, Zhang W, Zhou J, Xu B, Soares AS, Huang Z 2011. Synthesis, structure and imaging of oligodeoxyribonucleotides with tellurium-nucleobase derivatization. Nucleic Acids Res 39: 3962–3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawinski J, Thelin M 1994. Nucleoside H-phosphonates. 14. Synthesis of nucleoside phosphoroselenoates and phosphorothioselenoates via stereospecific selenization of the corresponding H-phosphonate and H-phosphonothioate diesters with the aid of new selenium-transfer reagent, 3H-1,2-benzothiaselenol-3-one. J Org Chem 59: 130–136 [Google Scholar]
- Wilds CJ, Pattanayek R, Pan C, Wawrzak Z, Egli M 2002. Selenium-assisted nucleic acid crystallography: use of phosphoroselenoates for MAD phasing of a DNA structure. J Am Chem Soc 124: 14910–14916 [DOI] [PubMed] [Google Scholar]