Abstract
Background:
The relationships between body mass index (BMI) during early and middle-late adulthood and incidence of prostate cancer (PCa) by subtype of the disease (localised, advanced) and fatal PCa is unclear.
Methods:
A population-based cohort of 36 959 Swedish men aged 45–79 years was followed up from January 1998 through December 2008 for incidence of PCa (1530 localised and 554 advanced cases were diagnosed) and through December 2007 for PCa mortality (225 fatal cases).
Results:
From a competing-risks analysis, incidence of localised PCa was observed to be inversely associated with BMI at baseline (middle-late adulthood; rate ratio (RR) for 35 kg m–2 when compared with 22 kg m–2 was 0.69 (95% CI 0.52–0.92)), but not at age 30. For fatal PCa, BMI at baseline was associated with a nonstatistically significant increased risk (RR for every five-unit increase: 1.12 (0.88–1.43)) and BMI at age 30 with a decreased risk (RR for every five-unit increase: 0.72 (0.51–1.01)).
Conclusion:
Our results indicate an inverse association between obesity during middle-late, but not early adulthood, and localised PCa. They also suggest a dual association between BMI and fatal PCa – a decreased risk among men who were obese during early adulthood and an increased risk among those who were obese during middle-late adulthood.
Keywords: prostate cancer, body mass index, obesity, body size, prospective cohort study
The aetiology of prostate cancer (PCa), the most common cancer malignancy in men in the developed countries and the second most common one worldwide (Jemal et al, 2011), is still largely unknown (Gronberg, 2003). It seems nonetheless to differ depending on the subtype of the disease at the time of diagnosis, namely localised and advanced PCa cases (Hsing et al, 2007).
Body adiposity is related to both sex hormones and insulin-like growth factor I (IGF-I) (Zumoff, 1988; Pasquali et al, 1991; Yamamoto and Kato, 1993; Kaaks et al, 2000; Lima et al, 2000; Lukanova et al, 2002). Owing to possible relationships between sex hormones and PCa (Platz et al, 2005; Severi et al, 2006) and between IGF-I and PCa (Roddam et al, 2008), body mass index (BMI) has itself been studied as a potential risk factor (Freedland and Platz, 2007). The results, however, are inconsistent.
For incidence of localised PCa, four studies observed a statistically significant inverse relationship with BMI (Gong et al, 2006; Littman et al, 2007; Rodriguez et al, 2007; Wright et al, 2007), seven studies observed nonstatistically significant association (Schuurman et al, 2000; MacInnis et al, 2003; Kurahashi et al, 2006; Giovannucci et al, 2007; Pischon et al, 2008; Wallstrom et al, 2009; Stocks et al, 2010) and two studies with a limited number of cases observed a suggestion of direct association, although nonstatistically significant (Cerhan et al, 1997; Putnam et al, 2000).
For incidence of advanced PCa, three studies observed a statistically significant direct association with BMI (Gong et al, 2006; Giovannucci et al, 2007; Rodriguez et al, 2007), six observed no association (Schuurman et al, 2000; Littman et al, 2007; Wright et al, 2007; Pischon et al, 2008; Wallstrom et al, 2009; Stocks et al, 2010), and among four other studies with a small number of cases, two observed a statistically significant direct association (Putnam et al, 2000; MacInnis et al, 2003), but not others (Cerhan et al, 1997; Kurahashi et al, 2006).
To the best of our knowledge, only one study adjusted the multivariable models for BMI during early adulthood when examining the possible relationships between BMI during middle-late adulthood and risk of localised and advanced PCa (Giovannucci et al, 2007).
BMI during middle-late adulthood as well as in earlier stages of life could be critical for the development of PCa (Hsing, 1996; Giovannucci et al, 1997; Gapstur et al, 2002). However, only a limited number of prospective studies examined the relationship between BMI during early adulthood and incidence of PCa by subtype of the disease. Among the studies of localised PCa, inconsistent results were observed with statistically significant inverse (Wright et al, 2007), direct (Schuurman et al, 2000) and null associations (Littman et al, 2007). Four prospective studies examined the association between BMI in early adulthood and incidence of advanced PCa, observing null (Schuurman et al, 2000; Littman et al, 2007; Wright et al, 2007) and inverse associations (Giovannucci et al, 1997).
As the available evidence is limited and the results are inconsistent, the aim of our population-based cohort study was to examine the relationships between BMI during early adulthood (age 30 years) and during middle-late adulthood (age 45–79 years) regarding incidence of localised, advanced and fatal PCa.
Materials and methods
The population-based cohort of Swedish men was established during 1997 to 1998, when all eligible men (n=100 303) aged 45–79 years residing in Västmanland and Örebro counties in central Sweden received an invitation to participate in the study along with a self-administered questionnaire. The questionnaire included questions about current weight, weight at age 30 years, height, educational level, smoking habit, family history of PCa, physical activity and diet. A total of 48 645 men returned the questionnaire.
We excluded participants who returned an incomplete questionnaire (n=92), died before 1 January 1998 (n=55), had a previous cancer diagnosis (n=2592) or had BMI at baseline age or at age 30 <15, >40 kg m–2 (n=196) or missing (n=8751), thus leaving 36 959 subjects available for the analyses. This population-based cohort is representative of Swedish males aged 45–79 years in terms of age distribution, educational level and prevalence of overweight (Norman et al, 2002). Incidence rates in 1998 per 100 000 men are also comparable: for example, the incidence rate among men aged 65–69 years is 603 in our cohort and 595 in the entire Sweden (NBHW, 2000; Orsini et al, 2009).
The BMI is a surrogate measurement for body adiposity as it is highly correlated with the percentage of body fat calculated hydrostatically by body submersion (Revicki and Israel, 1986) and is calculated as weight in kilograms divided by height in metres squared (kg m–2). Validity of BMI estimates based on self-reported weight and height as measured by a slope of linear regression was 0.89 in the Swedish adult population (Kuskowska-Wolk et al, 1989).
Information about usual physical activity levels during the previous year was collected using five questions relating to occupation, housework, walking or cycling, leisure-time exercise and inactive leisure time. To calculate the activity score of the specific activity type, the intensity of these activities (expressed as MET-h) was multiplied by the reported time in hours. Based on questionnaire data we estimated a total activity score (expressed as MET-h per day) by adding the single activity scores (Norman et al, 2001).
Incident cases of PCa were ascertained by computerised record linkage with the Swedish National Cancer Register and the Regional Cancer Register covering the study area, both of which are estimated to be almost 100% complete (Mattsson and Wallgren, 1984). Information on tumour–node–metastasis (TNM) stage, Gleason grade and value of prostate-specific antigen (PSA) at PCa diagnosis were available from the Swedish Prostate Cancer Quality Registry. Incident cases were classified by subtype as localised (T1–2 and NX-0 and (MX-0 or PSA <20 ng ml–1 or Gleason grade ⩽7)) or advanced (T3–4 and NX-1 and (MX-1 or PSA >100 ng ml–1 or Gleason grade >7)).
Information on fatal PCa cases was ascertained through linkage to the Swedish Register of Death Causes at the National Board of Health and Welfare. Classification of deaths was based on International Classification of Diseases (ICD-10, code 61 for PCa).
From 1 January 1998 to 31 December 2008, during 371 792 person-years, we documented 2336 incident cases of PCa. Of these, 1530 were classified as localised and 554 as advanced cases. From 1 January 1998 to 31 of December 2007, during 333 702 person-years, we documented 225 cases of fatal PCa.
Statistical analysis
The Cox proportional hazards model was used to estimate PCa incidence rate ratios (RRs) and 95% Wald confidence intervals associated with BMI at baseline age and at age 30 years. Each subject accrued follow-up time from 1 January 1998 until the date of PCa diagnosis, death from any cause or study end (31 December 2008), whichever came first. For fatal PCa analysis, each participant accrued follow-up time from 1 January 1998 until the date of PCa death, death from any cause or study end (31 December 2007), whichever came first.
We categorised BMI at baseline age in six predefined groups (<21, 21–22.9, 23–24.9, 25–27.4, 27.5–29.9 or ⩾30 kg m–2) in order to investigate the study participants’ characteristics at baseline.
We calculated the RRs from the Cox proportional hazards models for the BMI at baseline age and BMI at age 30 years in correspondence with the midpoints of the aforementioned categories: 18, 22 (reference), 24, 26.25, 28.75 and 35 kg m–2 to present results in a tabular form. This reference was chosen because in a pooled analysis of 1.46 million white adults, the lowest risk of cancer mortality was observed in the BMI category between 20.0 and 22.5 kg m–2 (Berrington de Gonzalez et al, 2010).
BMI values at baseline age and at age 30 years were mutually adjusted in both the age-adjusted and multivariable models and were modelled as continuous variables using fractional polynomials (Royston et al, 1999) whenever this provided a better overall fit of the model calculated using the Akaike information criterion (AIC) (Akaike, 1974). All the multivariable analyses were adjusted for baseline age (years), total energy intake (kcal), total physical activity (<37.9, 38–40.9, 41–44.9, ⩾45 MET-h per day or missing), years of education (1–9, 9–12 or >12 years), smoking status (current, former or never smoker), family history of PCa (yes, no or don’t know) and personal history of diabetes (yes or no).
We checked whether the proportional hazard assumption was reasonable by means of scaled Schoenfeld's residuals, which were regressed against the natural logarithm of the survival time. There was no evidence of departure from this assumption.
It is well known that BMI is associated with overall mortality (Berrington de Gonzalez et al, 2010). Therefore, we performed a sensitivity analysis using the competing-risks regression, where all the deaths from other causes than PCa were considered as competing events. This analysis allowed us to evaluate a potential effect of competing risks on the observed results (Fine and Gray, 1999).
All reported P-values are two sided. All statistical analyses were performed with Stata release 11 (StataCorp, College Station, TX, USA).
Results
Age-standardised baseline characteristics by category of BMI at baseline age of the 36 959 study participants are shown in Table 1. Mean age and mean total energy intake did not change significantly across the six levels of BMI at baseline age, as well as prevalence of subjects with family history of PCa. Higher levels of BMI at baseline age were associated with higher values of BMI at age 30 years (Pearson's correlation coefficient=0.6). Compared with men in the lowest group of BMI at baseline age, those in the higher groups were more likely to have a personal history of diabetes and less likely to be physically active, well-educated or current smokers.
Table 1. Age-standardised baseline characteristics by level of BMI at baseline age in the cohort of 36 959 Swedish men aged 45–79 years.
|
BMI at baseline age, kg m–2 |
||||||
|---|---|---|---|---|---|---|
| Characteristics a | <21 | 21–22.9 | 23–24.9 | 25–27.4 | 27.5–29.9 | ⩾30 |
| No. of subjects | 1863 | 5123 | 9551 | 11 244 | 5597 | 3581 |
| Age at baseline (mean, years) | 60 | 59 | 59 | 59 | 59 | 59 |
| BMI at age 30 years (mean, kg m–2) | 20 | 21 | 22 | 23 | 24 | 26 |
| History of diabetes (yes, %) | 4 | 5 | 7 | 12 | 18 | 31 |
| Family history of prostate cancer (yes, %) | 7 | 7 | 6 | 6 | 7 | 6 |
| Smoking status (%) | ||||||
| Current smoker | 33 | 25 | 25 | 24 | 23 | 24 |
| Former smoker | 26 | 33 | 37 | 41 | 45 | 46 |
| Never smoker | 41 | 41 | 38 | 35 | 33 | 29 |
| Physical activity (⩾45 MET-h per day, %) | 20 | 21 | 21 | 20 | 18 | 16 |
| Total energy intake (mean, kcal) | 2813 | 2817 | 2773 | 2733 | 2720 | 2682 |
| Years of education (>12 years, %) | 24 | 23 | 20 | 17 | 14 | 12 |
Abbreviations: BMI=body mass index; MET=metabolic equivalent of task.
All factors, except age and BMI at age 30 years, were directly standardised to the age distribution of the study participants. Percentages may not sum to 100 because of rounding.
Mean age at PCa diagnosis for localised and advanced cases was 69 and 74 years, respectively. Mean age at death from PCa was 75 years.
Age-adjusted and multivariable RRs for PCa incidence of localised, advanced and fatal PCa in the study population according to BMI levels at baseline age and at age 30 years are presented in Tables 2 and 3, respectively.
Table 2. Rate ratios for incidence of total prostate cancer and its subtypes by levels of BMI at baseline age in the cohort of 36 959 Swedish men aged 45–79 years.
|
BMI at baseline age (reference point), kg m–2 |
|||||||
|---|---|---|---|---|---|---|---|
| <21 (18) | 21–22.9 (22) | 23–24.9 (24) | 25–27.4 (26.25) | 27.5–29.9 (28.75) | ⩾30 (35) | For every 5 kg m–2 BMI increase | |
| Localised prostate cancer | |||||||
| Age-adjusted model | |||||||
| No. of cases/person-years | 63/18 017 | 247/51 462 | 408/96 263 | 475/113 744 | 212/56 659 | 125/35 647 | |
| RR (95% CI)a | 0.77 (0.54–1.11) | 1 | 0.99 (0.93–1.05) | 0.93 (0.84–1.03) | 0.85 (0.74–0.97) | 0.65 (0.50–0.85) | —b |
| Multivariable modelc | |||||||
| No. of cases/person-years | 62/17 487 | 245/50 419 | 401/94 253 | 467/111 322 | 204/55 507 | 124/34 885 | |
| RR (95% CI)a | 0.78 (0.54–1.13) | 1 | 1.00 (0.94–1.06) | 0.95 (0.86–1.05) | 0.88 (0.76–1.02) | 0.71 (0.53–0.94) | —b |
| RR (95% CI)a,d | 0.70 (0.49–1.01) | 1 | 1.01 (0.95–1.07) | 0.97 (0.87–1.07) | 0.89 (0.77–1.03) | 0.69 (0.52–0.92) | —b |
| Advanced prostate cancer | |||||||
| Age-adjusted model | |||||||
| No. of cases/person-years | 28/18 017 | 76/51 462 | 165/96 263 | 157/113 744 | 80/56 659 | 48/35 647 | |
| RR (95% CI)a | 1.01 (0.89–1.14) | 1 | 1.00 (0.94–1.06) | 0.99 (0.87–1.13) | 0.99 (0.81–1.21) | 0.98 (0.66–1.45) | 0.99 (0.85–1.15) |
| Multivariable modelc | |||||||
| No. of cases/person-years | 27/17 487 | 72/50 419 | 163/94 253 | 150/111 322 | 79/55 507 | 47/34 885 | |
| RR (95% CI)a | 0.97 (0.85–1.10) | 1 | 1.02 (0.95–1.08) | 1.03 (0.90–1.18) | 1.05 (0.85–1.31) | 1.11 (0.73–1.68) | 1.04 (0.88–1.22) |
| RR (95% CI)a,d | 0.96 (0.84–1.09) | 1 | 1.02 (0.96–1.09) | 1.05 (0.91–1.20) | 1.07 (0.86–1.33) | 1.15 (0.75–1.74) | 1.05 (0.90–1.24) |
| Fatal prostate cancer | |||||||
| Age-adjusted model | |||||||
| No. of cases/person-years | 11/16 931 | 35/48 500 | 62/90 692 | 61/106 984 | 31/53 086 | 25/33 396 | |
| RR (95% CI)a | 0.89 (0.74–1.07) | 1 | 1.06 (0.97–1.16) | 1.13 (0.93–1.38) | 1.22 (0.89–1.67) | 1.47 (0.81–2.69) | 1.16 (0.92–1.46) |
| Multivariable modelb | |||||||
| No. of cases/person-years | 11/16 426 | 35/47 524 | 62/88 804 | 59/104 705 | 29/51 989 | 23/32 679 | |
| RR (95% CI)a | 0.91 (0.75–1.11) | 1 | 1.05 (0.95–1.16) | 1.11 (0.89–1.36) | 1.16 (0.83–1.63) | 1.34 (0.70–2.55) | 1.12 (0.87–1.43) |
| RR (95% CI)a,d | 0.91 (0.75–1.10) | 1 | 1.05 (0.95–1.15) | 1.10 (0.90–1.36) | 1.17 (0.85–1.62) | 1.36 (0.73–2.53) | 1.12 (0.88–1.43) |
Abbreviations: CI=confidence interval; RR=rate ratio; BMI=body mass index.
The RRs and 95% CIs were calculated in correspondence with the reference points.
No RR for every 5 kg m–2 BMI at baseline age increase was calculated, as the relationship was modelled in a nonlinear fashion using second-degree fractional polynomials.
Multivariable RRs were adjusted for BMI at age 30 years (kg m–2), age at baseline (years), total energy intake (kcal), total physical activity (<37.9, 38–40.9, 41–44.9, ⩾45 MET-h per day or missing), years of education (1–9, 9–12 or >12 years), smoking status (current, former or never smoker), family history of prostate cancer (yes, no or don’t know) and personal history of diabetes (yes or no).
The RRs and 95% CIs calculated using competing-risks analysis. All the deaths from other causes than PCa were considered as competing events.
Table 3. Rate ratios for incidence of total prostate cancer and its subtypes by levels of BMI at age 30 years in the cohort of 36 959 Swedish men aged 45–79 years.
|
BMI at age 30 years (reference point), kg m–2 |
|||||||
|---|---|---|---|---|---|---|---|
| <21 (18) | 21–22.9 (22) | 23–24.9 (24) | 25–27.4 (26.25) | 27.5–29.9 (28.75) | ⩾30 (35) | For every 5 kg m–2 BMI increase | |
| Localised prostate cancer | |||||||
| Age-adjusted model | |||||||
| No. of cases/person-years | 290/67 977 | 550/120 481 | 472/116 166 | 161/49 565 | 42/11 629 | 15/5975 | |
| RR (95% CI)a | 1.01 (0.92–1.12) | 1 | 0.99 (0.94–1.04) | 0.99 (0.89–1.10) | 0.98 (0.83–1.16) | 0.96 (0.69–1.32) | 0.98 (0.87–1.11) |
| Multivariable modelb | |||||||
| No. of cases/person-years | 287/66 730 | 539/117 845 | 467/113 617 | 154/48 573 | 41/11 308 | 15/5800 | |
| RR (95% CI)a | 1.01 (0.91–1.12) | 1 | 0.99 (0.94–1.05) | 0.99 (0.89–1.10) | 0.98 (0.82–1.16) | 0.96 (0.69–1.34) | 0.98 (0.87–1.12) |
| RR (95% CI)a,c | 1.03 (0.93–1.14) | 1 | 0.99 (0.94–1.04) | 0.97 (0.87–1.08) | 0.96 (0.81–1.14) | 0.92 (0.66–1.28) | 0.97 (0.85–1.10) |
| Advanced prostate cancer | |||||||
| Age-adjusted model | |||||||
| No. of cases/person-years | 112/67 977 | 192/120 481 | 166/116 166 | 70/49 565 | 9/11 629 | 5/5975 | |
| RR (95% CI)a | 1.09 (0.93–1.28) | 1 | 0.96 (0.88–1.04) | 0.91 (0.77–1.08) | 0.87 (0.66–1.14) | 0.76 (0.45–1.28) | 0.90 (0.73–1.10) |
| Multivariable modelb | |||||||
| No. of cases/person-years | 108/66 730 | 185/117 845 | 164/113 617 | 69/48 573 | 8/11 308 | 4/5800 | |
| RR (95% CI)a | 1.09 (0.92–1.29) | 1 | 0.96 (0.88–1.04) | 0.91 (0.77–1.09) | 0.87 (0.65–1.15) | 0.76 (0.44–1.30) | 0.90 (0.73–1.11) |
| RR (95% CI)a,c | 1.11 (0.94–1.31) | 1 | 0.95 (0.87–1.03) | 0.90 (0.75–1.07) | 0.84 (0.63–1.11) | 0.71 (0.41–1.23) | 0.88 (0.71–1.08) |
| Fatal prostate cancer | |||||||
| Age-adjusted model | |||||||
| No. of cases/person-years | 50/64 052 | 78/113 654 | 69/109 099 | 22/46 355 | 4/10 879 | 2/5553 | |
| RR (95% CI)a | 1.31 (1.02–1.701) | 1 | 0.87 (0.77–0.99) | 0.75 (0.57–0.98) | 0.63 (0.41–0.97) | 0.41 (0.18–0.94) | 0.71 (0.52–0.98) |
| Multivariable modelb | |||||||
| No. of cases/person-years | 49/62 868 | 75/111 148 | 68/106 729 | 22/45 419 | 3/10 579 | 2/5384 | |
| RR (95% CI)a | 1.28 (0.99–1.67) | 1 | 0.88 (0.77–1.01) | 0.77 (0.58–1.02) | 0.66 (0.42–1.03) | 0.45 (0.19–1.05) | 0.73 (0.53–1.02) |
| RR (95% CI)a,c | 1.30 (1.00–1.71) | 1 | 0.88 (0.77–1.00) | 0.75 (0.57–1.01) | 0.64 (0.41–1.01) | 0.42 (0.18–1.02) | 0.72 (0.51–1.01) |
Abbreviations: CI=confidence interval; RR=rate ratio; BMI=body mass index.
The RRs and 95% CIs were calculated in correspondence with the reference points.
Multivariable RRs were adjusted for BMI at age 30 years (kg m–2), age at baseline (years), total energy intake (kcal), total physical activity (<37.9, 38–40.9, 41–44.9, ⩾45MET-h per day or missing), years of education (1–9, 9–12 or >12 years), smoking status (current, former or never smoker), family history of prostate cancer (yes, no or don’t know) and personal history of diabetes (yes or no).
RRs and 95% CIs calculated using competing-risks analysis. All the deaths from other causes than PCa were considered as competing events.
For localised PCa we observed in the age-adjusted model a left-skewed ‘inverse U’-shaped relationship with BMI at baseline age. Further adjustment for potential confounders did not substantially change the shape of the relationship. In correspondence with BMI level at baseline age of 35 kg m–2, the multivariable model showed a decreased incidence of 29% (6–47%) compared with that at the reference value (22 kg m–2). No statistically significant association was observed between BMI at age 30 years and incidence of localised PCa, with a decreased risk of 2% (−13 to 12%) for every five-unit increment. By modelling BMI at baseline age as a fractional polynomial and BMI at age 30 years linearly, the global fit of the model slightly improved with respect to the model, with both variables modelled in a linear fashion (AIC=36 616 vs AIC=36 614).
For advanced PCa a direct but statistically nonsignificant association was observed with BMI at baseline age as well as BMI at age 30; in the multivariable model, the RR for BMI at baseline age increased linearly by 4% (−12 to 22%), whereas the RR for BMI at age 30 years decreased linearly by 10% (−27 to 11%), both for every five-unit increment.
For fatal PCa we observed in the multivariable analysis a nonstatistically significant direct association with BMI at baseline age and an inverse linear association with BMI at age 30 years (P-value=0.06). For every five-unit increment, the RR for BMI at baseline age increased linearly by 12% (−13 to 43%), whereas the RR for BMI at age 30 years decreased linearly by 27% (−47 to 2%). These results may also suggest a dual effect of BMI on death from PCa, but none of them reached statistical significance. Of the 225 documented cases of fatal PCa, 50 (22%) were classified at diagnosis as localised and 141 (63%) as advanced cases, whereas the remaining 34 cases (15%) were unclassified.
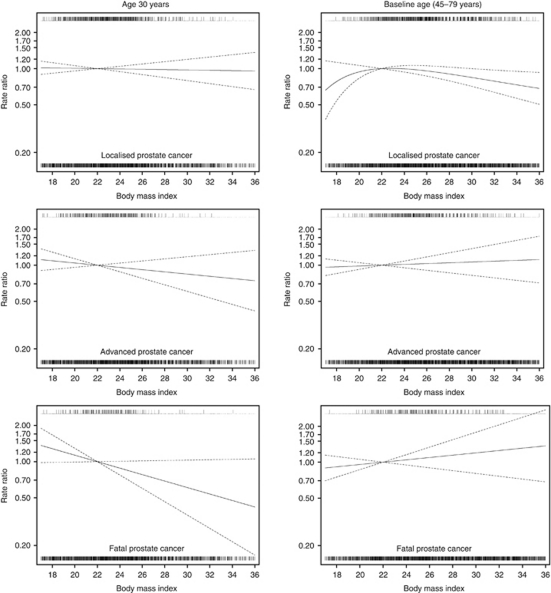
The relationships between BMI at baseline age and at age 30 years modelled as continuous variables and the incidence of localised, advanced and fatal PCa are displayed graphically in Figure 1.
Figure 1.
Multivariable rate ratios for BMI at age 30 years and at baseline age as predictors of incidence of localised, advanced and fatal prostate cancer. Data were fitted using a Cox proportional hazards model. BMI values at baseline age (kg m–2) and at age 30 years (kg m–2) were mutually adjusted and entered as continuous variables into the model; reference value was set at 22 kg m–2. Nonlinear relationships were modelled using second-degree fractional polynomials. Data were adjusted for age at baseline (years), total energy intake (kcal), total physical activity (<37.9, 38–40.9, 41–44.9, ⩾45 MET-h per day or missing), years of education (1–9, 9–12 or >12 years), smoking status (current, former or never smoker), family history of prostate cancer (yes, no or don’t know) and personal history of diabetes (yes or no). Dashed lines represent 95% confidence limits. Vertical lines above the curve represent cases of prostate cancer, whereas vertical lines below the curve represent non-cases of prostate cancer.
In order to examine whether preclinical symptoms of cancer might have affected the BMI at baseline age, leading to biased results, we excluded the first 2 years of follow-up from all the analyses. The multivariable results after this exclusion did not appreciably change (data not shown).
Compared with the results obtained using the Cox proportional hazards model, those obtained with the competing-risks regression did not significantly change, but became slightly stronger (Tables 2 and 3).
Discussion
BMI during middle-late adulthood and incidence of localised, advanced and fatal PCa
In this population-based prospective cohort study, we observed that high levels of BMI during middle-late adulthood are inversely associated with the incidence of localised PCa. This result is in agreement with some previous prospective studies (Gong et al, 2006; Littman et al, 2007; Rodriguez et al, 2007; Wright et al, 2007), but not all (Cerhan et al, 1997; Putnam et al, 2000; Schuurman et al, 2000; MacInnis et al, 2003; Kurahashi et al, 2006; Giovannucci et al, 2007; Pischon et al, 2008; Wallstrom et al, 2009; Stocks et al, 2010). Among those studies where a statistically significant association was not observed, two suggested an inverse association (Schuurman et al, 2000; Pischon et al, 2008), whereas two studies with a small number of cases suggested a direct association (Cerhan et al, 1997; Putnam et al, 2000).
In contrast to localised PCa, we observed that high BMI levels during middle-late adulthood were associated with a nonsignificant increased risk of advanced PCa. A statistically significant direct association between BMI during middle-late adulthood and incidence of advanced PCa was observed in some previous prospective studies (Putnam et al, 2000; MacInnis et al, 2003; Gong et al, 2006; Giovannucci et al, 2007; Rodriguez et al, 2007), but not all (Cerhan et al, 1997; Schuurman et al, 2000; Kurahashi et al, 2006; Littman et al, 2007; Wright et al, 2007; Pischon et al, 2008; Wallstrom et al, 2009; Stocks et al, 2010).
Our results suggesting an increased risk of fatal PCa are in line with the majority of the previous prospective studies showing a statistically significant positive association between increased BMI during middle-late adulthood and risk of death from PCa (Calle et al, 2003; Giovannucci et al, 2007; Wright et al, 2007; Stocks et al, 2010), but not all studies (Rodriguez et al, 2001). Similar to the present analysis, in only one previous study the authors adjusted the multivariable analyses also for BMI during early adulthood when examining the relationships between BMI during middle-late adulthood and risk of localised, advanced and fatal PCa (Giovannucci et al, 2007).
BMI during early adulthood and incidence of localised, advanced and fatal PCa
In our study we observed a nonstatistically significant association between BMI during early adulthood (age 30 years) and risk of localised and advanced PCa. Only three prospective studies examined the relationship between BMI during early adulthood and incidence of localised PCa, but they observed inconsistent results: statistically significant direct (Schuurman et al, 2000), inverse (Wright et al, 2007) and no associations (Littman et al, 2007). Four prospective studies examined the association between BMI during early adulthood and incidence of advanced PCa, observing null (Schuurman et al, 2000; Littman et al, 2007; Wright et al, 2007) and inverse associations (Giovannucci et al, 1997).
In our study, a weak evidence of an inverse association between BMI at age 30 years and fatal PCa was observed. Our study is the largest one among the previous prospective studies in terms of the number of cases. The existing evidence from prospective studies about a possible association between early-adult BMI and risk of death from PCa is limited, as only three studies with a small number of cases are available. Of these, two studies observed a null relationship with fatal PCa (Wright et al, 2007; Burton et al, 2010), whereas one observed a direct association (Okasha et al, 2002), although nonstatistically significant. A dual effect of obesity is suggested by comparing the observed associations between BMI at age 30 years, BMI at baseline age and incidence of fatal PCa: a decreased risk of fatal PCa among men who were obese during early adulthood and an increased risk among those who were obese during middle-late adulthood.
The inconsistent results in studies regarding BMI during late-adulthood and risk of PCa might be because of complex relationships between obesity and hormones, like testosterone and IGF-I. In particular, it is known that obesity is associated with lower serum testosterone concentrations in men (Zumoff, 1988; Pasquali et al, 1991; Lima et al, 2000). Type II diabetes, which is related to obesity, was also observed to be associated with lower levels of testosterone (Giovannucci et al, 1998). Lower testosterone concentrations were in turn observed to be associated with an increased risk of aggressive tumours in two prospective cohort studies (Platz et al, 2005; Severi et al, 2006). Moreover, decreased levels of serum testosterone at PCa diagnosis were also observed to be associated with more aggressive tumours (Hoffman et al, 2000; Schatzl et al, 2001; D’Amico, et al, 2002; Massengill et al, 2003). It has been therefore hypothesised that lower serum testosterone levels may be associated with an increased risk of aggressive tumours and a decreased risk of the nonaggressive ones (Freedland and Platz, 2007; Hsing et al, 2007). Our results are in line with this hypothesis: a higher risk of advanced and fatal PCa and a lower risk of localised PCa among obese men. However, not all studies observed this relationship between aggressiveness of the tumour and serum testosterone levels (Fodstad et al, 2002).
The highest levels of IGF-I were observed in men with a BMI between ∼24 and 26 kg m–2 (Yamamoto and Kato, 1993; Kaaks and Lukanova, 2001; Lukanova et al, 2002). High IGF-I concentrations were observed to be directly associated with PCa incidence (Roddam et al, 2008). This would, at least partly, explain the highest incidence of localised PCa among men within the normal BMI range that we observed in our study and that was also observed among low-grade tumours in the Health Professionals Follow-up Study (Giovannucci et al, 2007). However, other studies observed a linear association between obesity and IGF-I concentrations (Nam et al, 1997; Kaaks et al, 2000). Furthermore, the association between IGF-I concentrations and type II diabetes is complex and it seems to be related to time since diagnosis – IGF-I may increase following insulin resistance and then decrease due to hypoinsulinaemia, as a result of damaged pancreatic β-cells (Bell and Polonsky, 2001).
It was suggested that physiologic changes during the years before age 30 may play an important role in the development of PCa (Hsing, 1996; Giovannucci et al, 1997). Obesity during adolescence, which was observed to persist in early adulthood (The et al, 2010), was also observed to be associated with delayed pubertal development (Wang, 2002). As puberty is associated with a steep increase in IGF-I (Keenan et al, 1993; Juul et al, 1994), a delay in this increase could mean a lower cumulative exposure to IGF-I and/or less exposure at crucial ages, and thus a possible reduced risk of PCa among men obese during early adulthood. This is in line with what we observed for advanced and fatal PCa.
The principal limitation of this study is the self-reported, questionnaire-based collection of current weight and weight at age 30 years and height, which are less accurate than anthropometric measures obtained directly by trained professionals. Nonetheless, self-reported current weight and height are shown to be highly correlated with measured weight and height in the Swedish adult population (Kuskowska-Wolk et al, 1989). However, some degree of nondifferential misclassification could have affected the recalled weight at age 30. Our study was observational and therefore we cannot completely exclude the possibility of residual confounding. Nevertheless, age-adjusted and multivariable-adjusted analyses provided overall similar estimates, suggesting that residual confounding is unlikely to explain totally our observed findings. As obesity has been observed to be associated with lower PSA values, and as in obese men the detection of PCa through digital rectal examination may be more complicated (Price et al, 2008), it is possible that some cases of incident PCa might have gone undetected among obese subjects, leading to detection bias. However, there is no national recommendation in Sweden for PSA-based PCa screening and the annual proportion of men aged 55–69 years who underwent a PSA test in the two study counties between 1997 and 2007 is estimated to be between 0% and 7% (Jonsson et al, 2011); thus, any bias introduced by PSA testing should be of limited relevance in our data.
The major strengths of this study include the relatively large size of the cohort, its population-based and prospective design, the relatively large number of incident PCa cases and the completeness of case ascertainment through the Regional and National Cancer Register. These study features substantially reduced the potential risk of selection bias and increased the generalisability of the study findings. As information on exposure was collected prospectively, any nondifferential misclassification would probably weaken rather than exaggerate the true relationship between body size and PCa incidence. Death from other causes than PCa could have impeded the study subjects to develop PCa, especially among obese and underweight men, thus leading to biased estimates. However, we observed only small differences when comparing results from the Cox proportional hazards with the competing-risks models, suggesting that our results were not affected by competing events.
In conclusion, we found some evidence that obesity in middle-aged and elderly men may decrease the risk of localised PCa. On the other hand, our results indicate that there might be a dual effect of obesity on advanced and fatal PCa: an inverse relationship for BMI at age 30 years and a direct relationship for BMI during middle-late adulthood. From a public health perspective, encouraging obesity is not a realistic way to reduce PCa morbidity. The biologic mechanisms behind the relationship between obesity and PCa incidence remain unclear, and thus replication of epidemiological studies and further work in understanding the underlying biologic mechanisms is necessary.
Acknowledgments
The work was supported by research grants from the Swedish Cancer Foundation and the Swedish Research Council/Committee for Infrastructure.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Akaike H (1974) New look at statistical model identification. IEEE Trans Automat Contr AC 19(6): 716–723 [Google Scholar]
- Bell GI, Polonsky KS (2001) Diabetes mellitus and genetically programmed defects in beta-cell function. Nature 414(6865): 788–791 [DOI] [PubMed] [Google Scholar]
- Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ (2010) Body-mass index and mortality among 1.46 million white adults. N Engl J Med 363(23): 2211–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton A, Martin R, Galobardes B, Davey Smith G, Jeffreys M (2010) Young adulthood body mass index and risk of cancer in later adulthood: historical cohort study. Cancer Causes Control 21(12): 2069–2077 [DOI] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348(17): 1625–1638 [DOI] [PubMed] [Google Scholar]
- Cerhan JR, Torner JC, Lynch CF, Rubenstein LM, Lemke JH, Cohen MB, Lubaroff DM, Wallace RB (1997) Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States). Cancer Causes Control 8(2): 229–238 [DOI] [PubMed] [Google Scholar]
- D′Amico AV, Chen MH, Malkowicz SB, Whittington R, Renshaw AA, Tomaszewski JE, Samofalov Y, Wein A, Richie JP (2002) Lower prostate specific antigen outcome than expected following radical prostatectomy in patients with high grade prostate and a prostatic specific antigen level of 4 ng/ml. Or less. J Urol 167(5): 2025–2030; discussion 2030–2031 [DOI] [PubMed] [Google Scholar]
- Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94(446): 496–509 [Google Scholar]
- Fodstad P, Bjoro T, Torlakovic G, Fossa SD (2002) No association of serum gonadal or pituitary hormones with prognostic parameters in stages T1 to T3 pN0M0 prostate cancer. J Urol 168(3): 1188–1192 [DOI] [PubMed] [Google Scholar]
- Freedland SJ, Platz EA (2007) Obesity and prostate cancer: making sense out of apparently conflicting data. Epidemiol Rev 29: 88–97 [DOI] [PubMed] [Google Scholar]
- Gapstur SM, Gann PH, Kopp P, Colangelo L, Longcope C, Liu K (2002) Serum androgen concentrations in young men: a longitudinal analysis of associations with age, obesity, and race. The CARDIA male hormone study. Cancer Epidemiol Biomarkers Prev 11(10 Part 1): 1041–1047 [PubMed] [Google Scholar]
- Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC (2007) Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer 121(7): 1571–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC (1997) Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 6(8): 557–563 [PubMed] [Google Scholar]
- Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC (1998) Diabetes mellitus and risk of prostate cancer (United States). Cancer Causes Control 9(1): 3–9 [DOI] [PubMed] [Google Scholar]
- Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW, Lippman SM, Platz EA, Pollak MN, Thompson IM, Kristal AR (2006) Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev 15(10): 1977–1983 [DOI] [PubMed] [Google Scholar]
- Gronberg H (2003) Prostate cancer epidemiology. Lancet 361(9360): 859–864 [DOI] [PubMed] [Google Scholar]
- Hoffman MA, DeWolf WC, Morgentaler A (2000) Is low serum free testosterone a marker for high grade prostate cancer? J Urol 163(3): 824–827 [PubMed] [Google Scholar]
- Hsing AW (1996) Hormones and prostate cancer: where do we go from here? J Natl Cancer Inst 88(16): 1093–1095 [DOI] [PubMed] [Google Scholar]
- Hsing AW, Sakoda LC, Chua Jr S (2007) Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr 86(3): s843–s857 [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2): 69–90 [DOI] [PubMed] [Google Scholar]
- Jonsson H, Holmstrom B, Duffy SW, Stattin P (2011) Uptake of prostate-specific antigen testing for early prostate cancer detection in Sweden. Int J Cancer; doi:10.1002/ijc.25846 (in press) [DOI] [PubMed]
- Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jorgensen K, Muller J, Hall K, Skakkebaek NE (1994) Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab 78(3): 744–752 [DOI] [PubMed] [Google Scholar]
- Kaaks R, Lukanova A (2001) Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc 60(1): 91–106 [DOI] [PubMed] [Google Scholar]
- Kaaks R, Lukanova A, Sommersberg B (2000) Plasma androgens, IGF-1, body size, and prostate cancer risk: a synthetic review. Prostate Cancer Prostatic Dis 3(3): 157–172 [DOI] [PubMed] [Google Scholar]
- Keenan BS, Richards GE, Ponder SW, Dallas JS, Nagamani M, Smith ER (1993) Androgen-stimulated pubertal growth: the effects of testosterone and dihydrotestosterone on growth-hormone and insulin-like growth factor-I in the treatment of short stature and delayed puberty. J Clin Endocr Metab 76(4): 996–1001 [DOI] [PubMed] [Google Scholar]
- Kurahashi N, Iwasaki M, Sasazuki S, Otani T, Inoue M, Tsugane S (2006) Association of body mass index and height with risk of prostate cancer among middle-aged Japanese men. Br J Cancer 94(5): 740–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuskowska-Wolk A, Karlsson P, Stolt M, Rossner S (1989) The predictive validity of body mass index based on self-reported weight and height. Int J Obes 13(4): 441–453 [PubMed] [Google Scholar]
- Lima N, Cavaliere H, Knobel M, Halpern A, Medeiros-Neto G (2000) Decreased androgen levels in massively obese men may be associated with impaired function of the gonadostat. Int J Obes Relat Metab Disord 24(11): 1433–1437 [DOI] [PubMed] [Google Scholar]
- Littman AJ, White E, Kristal AR (2007) Anthropometrics and prostate cancer risk. Am J Epidemiol 165(11): 1271–1279 [DOI] [PubMed] [Google Scholar]
- Lukanova A, Soderberg S, Stattin P, Palmqvist R, Lundin E, Biessy C, Rinaldi S, Riboli E, Hallmans G, Kaaks R (2002) Nonlinear relationship of insulin-like growth factor (IGF)-I and IGF-I/IGF-binding protein-3 ratio with indices of adiposity and plasma insulin concentrations (Sweden). Cancer Causes Control 13(6): 509–516 [DOI] [PubMed] [Google Scholar]
- MacInnis RJ, English DR, Gertig DM, Hopper JL, Giles GG (2003) Body size and composition and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 12(12): 1417–1421 [PubMed] [Google Scholar]
- Massengill JC, Sun L, Moul JW, Wu H, McLeod DG, Amling C, Lance R, Foley J, Sexton W, Kusuda L, Chung A, Soderdahl D, Donahue T (2003) Pretreatment total testosterone level predicts pathological stage in patients with localized prostate cancer treated with radical prostatectomy. J Urol 169(5): 1670–1675 [DOI] [PubMed] [Google Scholar]
- Mattsson B, Wallgren A (1984) Completeness of the Swedish Cancer Register. Non-notified cancer cases recorded on death certificates in 1978. Acta Radiol Oncol 23(5): 305–313 [DOI] [PubMed] [Google Scholar]
- Nam SY, Lee EJ, Kim KR, Cha BS, Song YD, Lim SK, Lee HC, Huh KB (1997) Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord 21(5): 355–359 [DOI] [PubMed] [Google Scholar]
- NBHW (2000) Cancer Incidence in Sweden 1998: The National Board of Health and Welfare, Centre of Epidemiology: Stockholm. Report no. 91-7201-450-4
- Norman A, Bellocco R, Bergstrom A, Wolk A (2001) Validity and reproducibility of self-reported total physical activity–differences by relative weight. Int J Obes Relat Metab Disord 25(5): 682–688 [DOI] [PubMed] [Google Scholar]
- Norman A, Bellocco R, Vaida F, Wolk A (2002) Total physical activity in relation to age, body mass, health and other factors in a cohort of Swedish men. Int J Obes Relat Metab Disord 26(5): 670–675 [DOI] [PubMed] [Google Scholar]
- Okasha M, McCarron P, McEwen J, Smith GD (2002) Body mass index in young adulthood and cancer mortality: a retrospective cohort study. J Epidemiol Community Health 56(10): 780–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini N, Bellocco R, Bottai M, Pagano M, Andersson SO, Johansson JE, Giovannucci E, Wolk A (2009) A prospective study of lifetime physical activity and prostate cancer incidence and mortality. Br J Cancer 101(11): 1932–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R, Casimirri F, Cantobelli S, Melchionda N, Morselli Labate AM, Fabbri R, Capelli M, Bortoluzzi L (1991) Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism 40(1): 101–104 [DOI] [PubMed] [Google Scholar]
- Pischon T, Boeing H, Weikert S, Allen N, Key T, Johnsen NF, Tjonneland A, Severinsen MT, Overvad K, Rohrmann S, Kaaks R, Trichopoulou A, Zoi G, Trichopoulos D, Pala V, Palli D, Tumino R, Sacerdote C, Bueno-de-Mesquita HB, May A, Manjer J, Wallstrom P, Stattin P, Hallmans G, Buckland G, Larranaga N, Chirlaque MD, Martinez C, Redondo Cornejo ML, Ardanaz E, Bingham S, Khaw KT, Rinaldi S, Slimani N, Jenab M, Riboli E (2008) Body size and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 17(11): 3252–3261 [DOI] [PubMed] [Google Scholar]
- Platz EA, Leitzmann MF, Rifai N, Kantoff PW, Chen YC, Stampfer MJ, Willett WC, Giovannucci E (2005) Sex steroid hormones and the androgen receptor gene CAG repeat and subsequent risk of prostate cancer in the prostate-specific antigen era. Cancer Epidemiol Biomarkers Prev 14(5): 1262–1269 [DOI] [PubMed] [Google Scholar]
- Price MM, Hamilton RJ, Robertson CN, Butts MC, Freedland SJ (2008) Body mass index, prostate-specific antigen, and digital rectal examination findings among participants in a prostate cancer screening clinic. Urology 71(5): 787–791 [DOI] [PubMed] [Google Scholar]
- Putnam SD, Cerhan JR, Parker AS, Bianchi GD, Wallace RB, Cantor KP, Lynch CF (2000) Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Ann Epidemiol 10(6): 361–369 [DOI] [PubMed] [Google Scholar]
- Revicki DA, Israel RG (1986) Relationship between body mass indices and measures of body adiposity. Am J Public Health 76(8): 992–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddam AW, Allen NE, Appleby P, Key TJ, Ferrucci L, Carter HB, Metter EJ, Chen C, Weiss NS, Fitzpatrick A, Hsing AW, Lacey Jr JV, Helzlsouer K, Rinaldi S, Riboli E, Kaaks R, Janssen JA, Wildhagen MF, Schroder FH, Platz EA, Pollak M, Giovannucci E, Schaefer C, Quesenberry Jr CP, Vogelman JH, Severi G, English DR, Giles GG, Stattin P, Hallmans G, Johansson M, Chan JM, Gann P, Oliver SE, Holly JM, Donovan J, Meyer F, Bairati I, Galan P (2008) Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Ann Intern Med 149(7): 461–471, W83-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez C, Freedland SJ, Deka A, Jacobs EJ, McCullough ML, Patel AV, Thun MJ, Calle EE (2007) Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 16(1): 63–69 [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ (2001) Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev 10(4): 345–353 [PubMed] [Google Scholar]
- Royston P, Ambler G, Sauerbrei W (1999) The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 28(5): 964–974 [DOI] [PubMed] [Google Scholar]
- Schatzl G, Madersbacher S, Thurridl T, Waldmuller J, Kramer G, Haitel A, Marberger M (2001) High-grade prostate cancer is associated with low serum testosterone levels. Prostate 47(1): 52–58 [DOI] [PubMed] [Google Scholar]
- Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA (2000) Anthropometry in relation to prostate cancer risk in the Netherlands Cohort Study. Am J Epidemiol 151(6): 541–549 [DOI] [PubMed] [Google Scholar]
- Severi G, Morris HA, MacInnis RJ, English DR, Tilley W, Hopper JL, Boyle P, Giles GG (2006) Circulating steroid hormones and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 15(1): 86–91 [DOI] [PubMed] [Google Scholar]
- Stocks T, Hergens MP, Englund A, Ye W, Stattin P (2010) Blood pressure, body size and prostate cancer risk in the Swedish Construction Workers cohort. Int J Cancer 127(7): 1660–1668 [DOI] [PubMed] [Google Scholar]
- The NS, Suchindran C, North KE, Popkin BM, Gordon-Larsen P (2010) Association of adolescent obesity with risk of severe obesity in adulthood. JAMA 304(18): 2042–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallstrom P, Bjartell A, Gullberg B, Olsson H, Wirfalt E (2009) A prospective Swedish study on body size, body composition, diabetes, and prostate cancer risk. Br J Cancer 100(11): 1799–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y (2002) Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics 110(5): 903–910 [DOI] [PubMed] [Google Scholar]
- Wright ME, Chang SC, Schatzkin A, Albanes D, Kipnis V, Mouw T, Hurwitz P, Hollenbeck A, Leitzmann MF (2007) Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer 109(4): 675–684 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kato Y (1993) Relationship between plasma insulin-like growth factor I (IGF-I) levels and body mass index (BMI) in adults. Endocr J 40(1): 41–45 [DOI] [PubMed] [Google Scholar]
- Zumoff B (1988) Hormonal abnormalities in obesity. Acta Med Scand Suppl 723: 153–160 [DOI] [PubMed] [Google Scholar]