Abstract
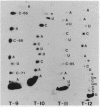
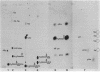
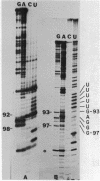
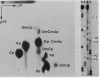
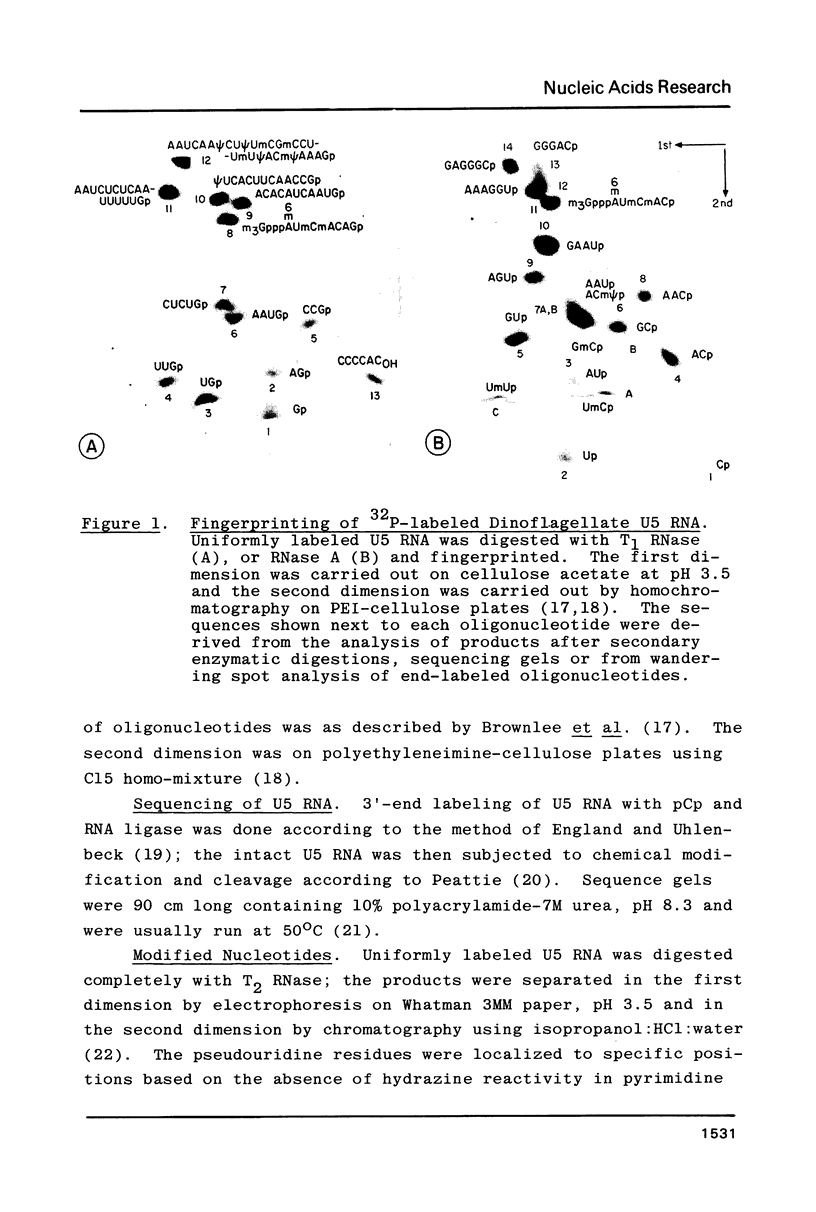
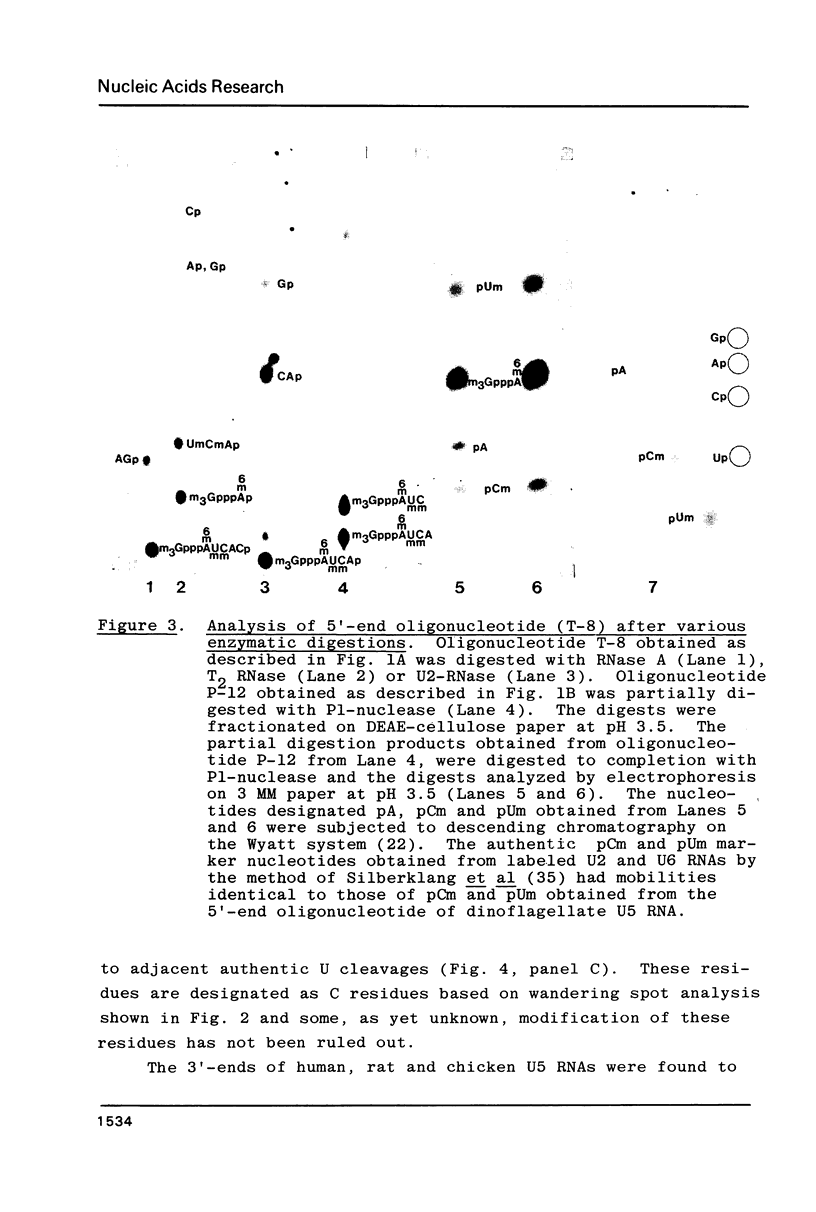
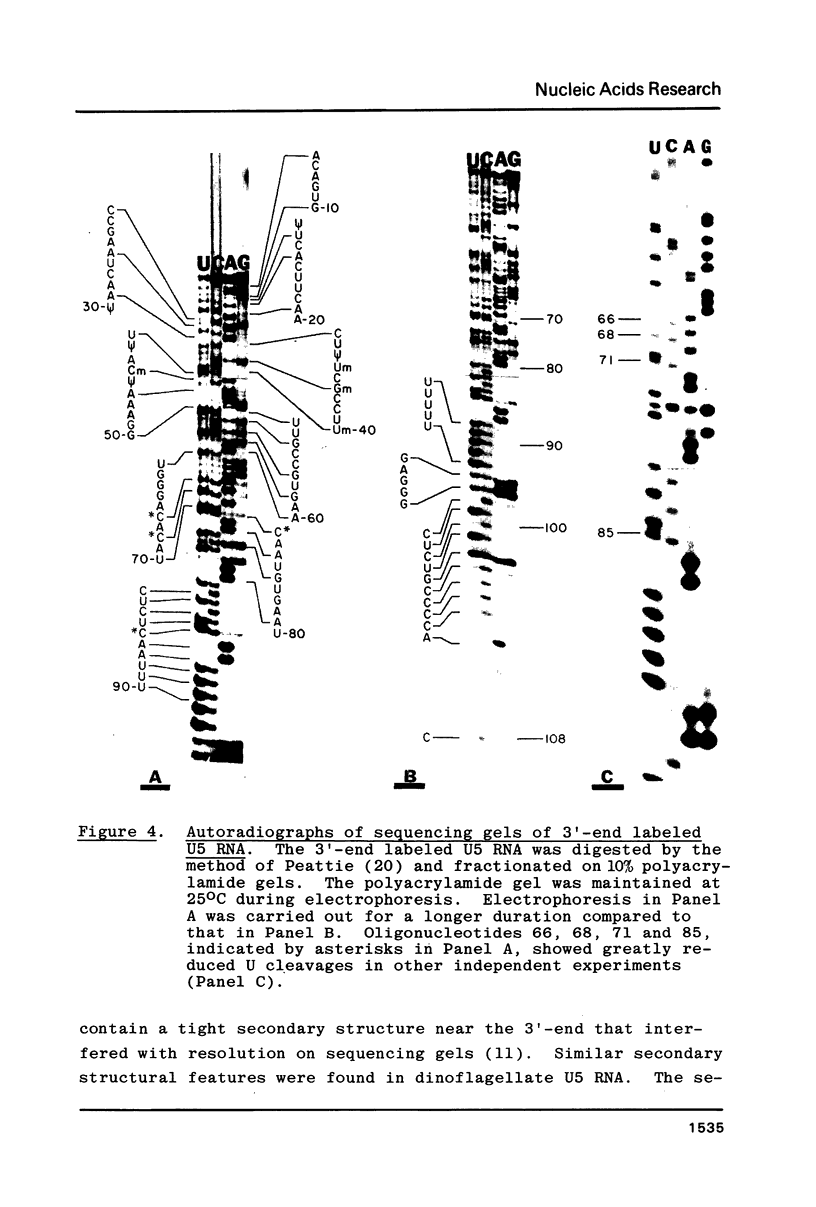
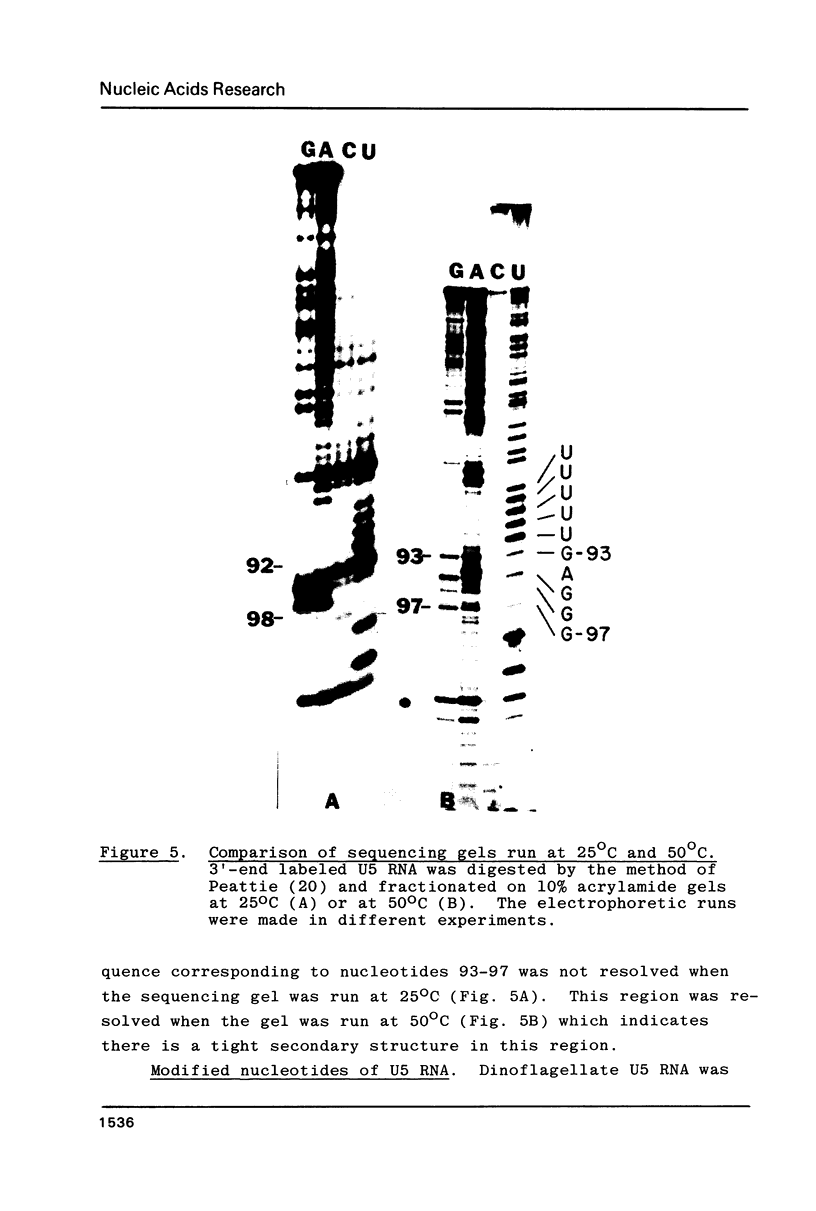
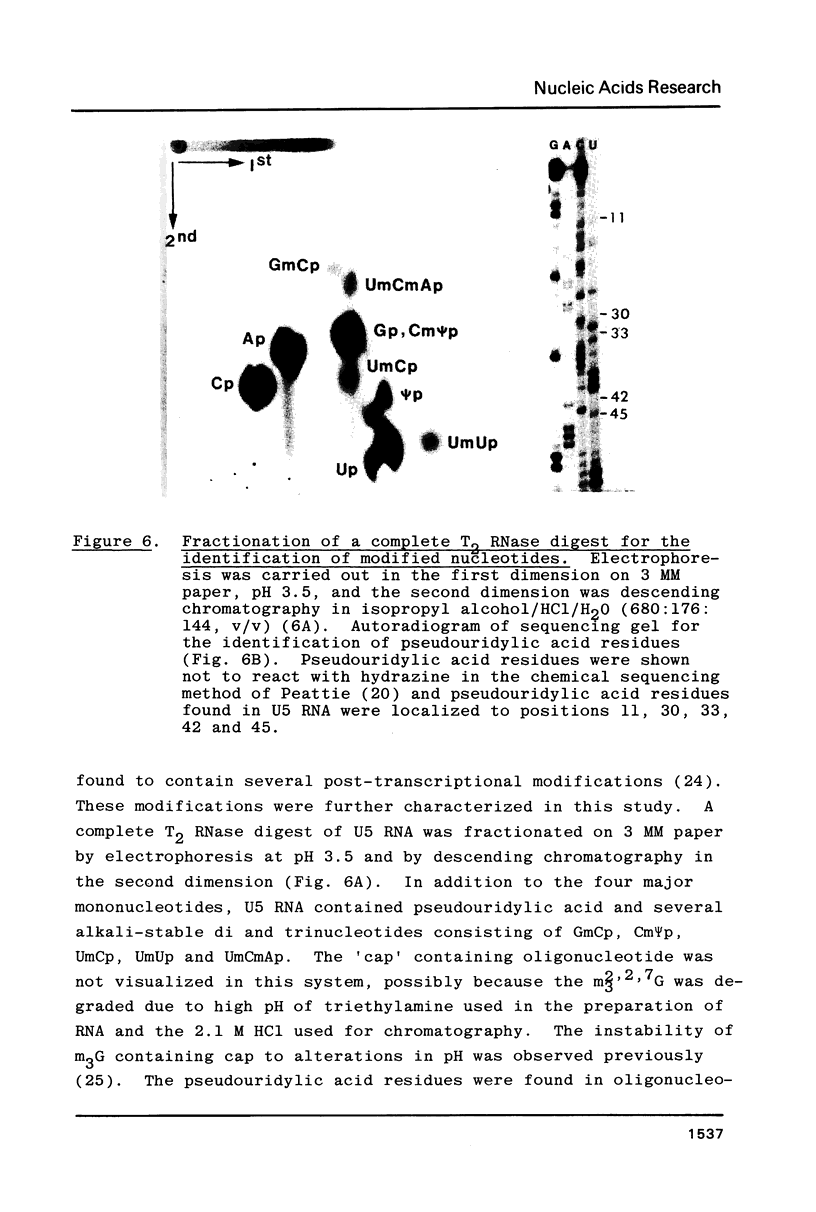
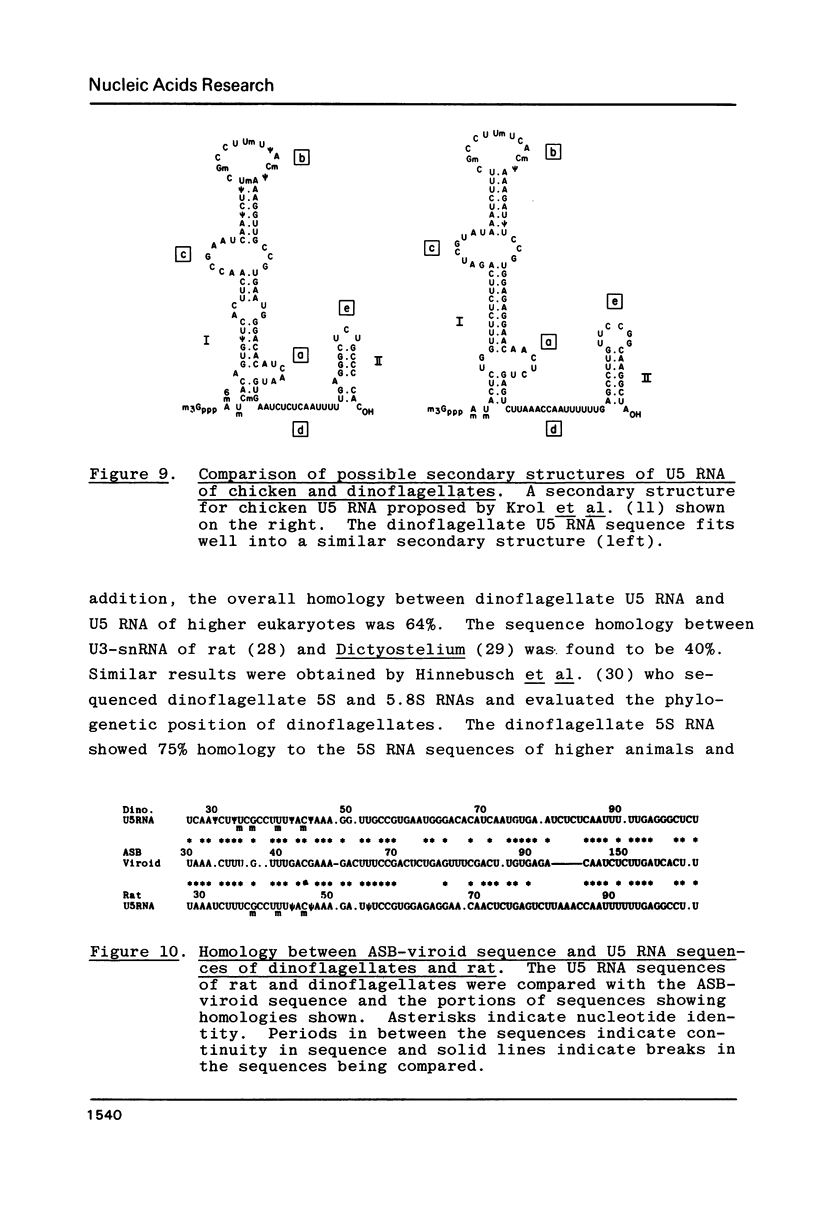
U5 RNA is one of the six capped small nuclear RNAs present in most eukaryotic cells. Like U1, U2, U4 and U6 RNAs, U5 RNA is associated with hnRNP particles and is thus probably involved in some, as yet undefined, aspects of pre-messenger RNA processing. In this study, the complete nucleotide sequence of U5 RNA of a dinoflagellate, Crypthecodinium cohnii was determined. The analysis of this dinoflagellate U5 RNA sequence showed that a) the sequence homology between human, rat and chicken U5 RNA sequences and dinoflagellate U5 RNA sequence is 64%; b) the extent and the position of post-transcriptional modifications are similar to those found in U5 RNA of higher eukaryotes; c) although the dinoflagellate U5 RNA is shorter in length (108 nucleotides long vs 117 long in human, rat and chicken cells), the RNA fits well into the same secondary structure proposed for U5 RNA of higher eukaryotes (Krol et al. (1981) Nucl. Acids Res. 9, 769); and d) the AUn nucleotide sequence protected by the Sm-antigen and the tight secondary structure found near the 3'-end of other U-RNAs was also found in dinoflagellate U5 RNA. The high order of homology observed between dinoflagellate U5 RNA and U5 RNA of higher eukaryotes indicates that dinoflagellates are more closely related to metazoans than to early eukaryotes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branlant C., Krol A., Ebel J. P., Lazar E., Gallinaro H., Jacob M., Sri-Widada J., Jeanteur P. Nucleotide sequences of nuclear U1A RNAs from chicken, rat and man. Nucleic Acids Res. 1980 Sep 25;8(18):4143–4154. doi: 10.1093/nar/8.18.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F., Barrell B. G. The sequence of 5 s ribosomal ribonucleic acid. J Mol Biol. 1968 Jun 28;34(3):379–412. doi: 10.1016/0022-2836(68)90168-x. [DOI] [PubMed] [Google Scholar]
- Busch H., Reddy R., Rothblum L., Choi Y. C. SnRNAs, SnRNPs, and RNA processing. Annu Rev Biochem. 1982;51:617–654. doi: 10.1146/annurev.bi.51.070182.003153. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Are viroids escaped introns? Proc Natl Acad Sci U S A. 1981 Aug;78(8):5014–5015. doi: 10.1073/pnas.78.8.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G., Klotz L. C., Blanken R. L., Loeblich A. R., 3rd An evaluation of the phylogenetic position of the dinoflagellate Crypthecodinium cohnii based on 5S rRNA characterization. J Mol Evol. 1981;17(6):334–337. doi: 10.1007/BF01734355. [DOI] [PubMed] [Google Scholar]
- Kato N., Harada F. Nucleotide sequence of nuclear 5S RNA of mouse cells. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1468–1476. doi: 10.1016/0006-291x(81)90784-1. [DOI] [PubMed] [Google Scholar]
- Kiss T., Solymosy F. Sequence homologies between a viroid and a small nuclear RNA (snRNA) species of mammalian origin. FEBS Lett. 1982 Aug 2;144(2):318–320. doi: 10.1016/0014-5793(82)80662-5. [DOI] [PubMed] [Google Scholar]
- Krol A., Gallinaro H., Lazar E., Jacob M., Branlant C. The nuclear 5S RNAs from chicken, rat and man. U5 RNAs are encoded by multiple genes. Nucleic Acids Res. 1981 Feb 25;9(4):769–787. doi: 10.1093/nar/9.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Liautard J. P., Sri-Widada J., Brunel C., Jeanteur P. Structural organization of ribonucleoproteins containing small nuclear RNAs from HeLa cells. Proteins interact closely with a similar structural domain of U1, U2, U4 and U5 small nuclear RNAs. J Mol Biol. 1982 Dec 15;162(3):623–643. doi: 10.1016/0022-2836(82)90392-8. [DOI] [PubMed] [Google Scholar]
- Ohshima Y., Itoh M., Okada N., Miyata T. Novel models for RNA splicing that involve a small nuclear RNA. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4471–4474. doi: 10.1073/pnas.78.7.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R., Henning D., Busch H. Nucleotide sequence of nucleolar U3B RNA. J Biol Chem. 1979 Nov 10;254(21):11097–11105. [PubMed] [Google Scholar]
- Reddy R., Li W. Y., Henning D., Choi Y. C., Nohga K., Busch H. Characterization and subcellular localization of 7-8 S RNAs of Novikoff hepatoma. J Biol Chem. 1981 Aug 25;256(16):8452–8457. [PubMed] [Google Scholar]
- Reddy R., Spector D., Henning D., Liu M. H., Busch H. Isolation and partial characterization of dinoflagellate U1-U6 small RNAs homologous to rat U small nuclear RNAs. J Biol Chem. 1983 Nov 25;258(22):13965–13969. [PubMed] [Google Scholar]
- Ro-Choi T. S., Choi Y. C., Henning D., McCloskey J., Busch H. Nucleotide sequence of U-2 ribonucleic acid. The sequence of the 5'-terminal oligonucleotide. J Biol Chem. 1975 May 25;250(10):3921–3928. [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeris C. E., Niessing J. Evidence for the existence of a structural RNA component in the nuclear ribonucleoprotein particles containing heterogeneous RNA. Biochem Biophys Res Commun. 1975 Feb 3;62(3):642–650. doi: 10.1016/0006-291x(75)90447-7. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Sri-Widada J., Liautard J. P., Brunel C., Jeanteur P. Interaction of snRNAs with rapidly sedimenting nuclear sub-structures (hnRNPs) from HeLa cells. Nucleic Acids Res. 1983 Oct 11;11(19):6631–6646. doi: 10.1093/nar/11.19.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Uhlenbeck O. C., Levine M. D. Estimation of secondary structure in ribonucleic acids. Nature. 1971 Apr 9;230(5293):362–367. doi: 10.1038/230362a0. [DOI] [PubMed] [Google Scholar]
- WYATT G. R. The purine and pyrimidine composition of deoxypentose nucleic acids. Biochem J. 1951 May;48(5):584–590. doi: 10.1042/bj0480584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G., Brownlee G. G. 3'End labelling of RNA with 32P suitable for rapid gel sequencing. Nucleic Acids Res. 1978 Sep;5(9):3129–3139. doi: 10.1093/nar/5.9.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J. A., Weiner A. M. Dictyostelium small nuclear RNA D2 is homologous to rat nucleolar RNA U3 and is encoded by a dispersed multigene family. Cell. 1980 Nov;22(1 Pt 1):109–118. doi: 10.1016/0092-8674(80)90159-2. [DOI] [PubMed] [Google Scholar]