Abstract
Background:
Myofibroblasts in the cancer microenvironment have recently been implicated in tumour growth and metastasis of gastric cancer. However, the mechanisms responsible for the regulation of myofibroblasts in cancer-associated fibroblasts (CAFs) remain unclear. This study was performed to clarify the mechanisms for regulation of myofibroblasts in gastric cancer microenvironment.
Methods:
Two CAFs (CaF-29 and CaF-33) from the tumoural gastric wall and a normal fibroblast (NF-29) from the nontumoural gastric wall, 4 human gastric cancer cell lines from scirrhous gastric cancer (OCUM-2MD3 and OCUM-12), and non-scirrhous gastric cancer (MKN-45 and MKN-74) were used. Immunofluorescence microscopy by triple-immunofluorescence labelling (α-SMA, vimentin, and DAPI) was performed to determine the presence of α-SMA-positive myofibroblasts. Real-time RT–PCR was performed to examine α-SMA mRNA expression.
Results:
Immunofluorescence microscopy showed that the frequency of myofibroblasts in CaF-29 was greater than that in NF-29. The number of myofibroblasts in gastric fibroblasts gradually decreased with serial passages. Transforming growth factor-β (TGF-β) significantly increased the α-SMA expression level of CAFs. Conditioned medium from OCUM-2MD3 or OCUM-12 cells upregulated the α-SMA expression level of CAFs, but that from MKN-45 or MKN-74 cells did not. The α-SMA upregulation effect of conditioned medium from OCUM-2MD3 or OCUM-12 cells was significantly decreased by an anti-TGF-β antibody or Smad2 siRNA.
Conclusion:
Transforming growth factor-β from scirrhous gastric carcinoma cells upregulates the number of myofibroblasts in CAFs.
Keywords: myofibroblasts, cancer-associated fibroblasts, TGF-β, scirrhous gastric carcinoma, microenvironment, interaction
Recently, tumour progression has been recognised as the product of evolving crosstalk between cancer cells and the surrounding tissue (Kalluri and Zeisberg, 2006). The normal stroma contains few fibroblasts, but there is a dramatic increase in fibroblast-like cells within the reactive stroma surrounding inflamed or neoplastic tissue (Worthley et al, 2010). Cancer cells themselves may alter their adjacent stroma to form a permissive and supportive environment for tumour progression (Durning et al, 1984; Schor et al, 1988). Fibroblasts within the tumour stroma, known as carcinoma-associated fibroblasts (CAFs), including both fibroblasts and myofibroblasts (Semba et al, 2009), play a critical role in the regulation of tumour growth (Kalluri and Zeisberg, 2006; Guo et al, 2008; Noma et al, 2008; Shimoda et al, 2010; Yashiro and Hirakawa, 2010). Myofibroblasts, which are distinct from fibroblasts in their expression of both vimentin and α-smooth muscle actin (α-SMA), have recently been implicated in important aspects of solid tumour progression (Olumi et al, 1999; Hasebe et al, 2000; Tomasek et al, 2002; Kalluri and Zeisberg, 2006; Tsujino et al, 2007; Matsubara et al, 2009), because myofibroblasts produce a number of important factors that can directly promote growth in the adjacent epithelium (Kalluri and Zeisberg, 2006; Brenmoehl et al, 2009). Scirrhous gastric cancer cells proliferate with fibrosis when the cancer cells invade into the submucosa containing abundant stromal cells (Nakazawa et al, 2003). We have previously reported that gastric fibroblasts play an important role in the progression, growth, and spread of scirrhous gastric cancers (Yashiro and Hirakawa, 2010), and myofibroblasts in gastric fibroblasts are particularly associated with scirrhous-type gastric cancer and the poor prognosis of gastric cancer patients (Kinugasa et al, 1998; Otsuji et al, 2004).
Overexpression of transforming growth factor-β (TGF-β) is reported to be correlated with a poor prognosis for gastric tumours (Naef et al, 1997; Maehara et al, 1999; Saito et al, 2000), especially scirrhous gastric carcinoma (Kinugasa et al, 1998; Hawinkels et al, 2007), suggesting that TGF-β signalling might have an important role in the progression of scirrhous gastric cancer cells (Inoue et al, 1997; Kinugasa et al, 1998; Kawajiri et al, 2008). Transforming growth factor-β activates type II TGF-β receptors (TβR-II), which phosphorylate type I TGF-β receptors (TβR-I) (Heldin et al, 1997; Massague, 2008). Activated TβR-I kinase phosphorylates Smad2/3. Phosphorylated Smad2/3 is associated with Smad4 and translocation in the nucleus as transcriptional factors. Transforming growth factor-β remains among the key factors responsible for the development of a myofibroblastic phenotype from a variety of precursor cells, including fibroblasts (Tomasek et al, 2002; Webber et al, 2010). However, the mechanisms responsible for the upregulation of myofibroblasts remain unclear.
In this study, we investigated the effect of gastric cancer cells on normal fibroblasts and CAFs isolated from the primary tumour site to understand the mechanisms for regulation of myofibroblast expression in the cancer microenvironment.
Materials and methods
Cell culture and cell lines
We used three human gastric fibroblast cell lines and four human gastric cancer cell lines in this study. Fibroblasts cell lines were established at our department. The NF-29 and CAF-29 were established from a 68-year-old male patient with poorly differentiated gastric carcinoma who had a total gastrectomy. The NF-29 was from nontumoural gastric wall, and CaF-29 was from tumoural gastric wall. The CaF-33 was established from a 65-year-old male patient with poorly differentiated gastric carcinoma who had a distal gastrectomy. The primary culture was initiated as follows: the primary tumour was excised under aseptic conditions, and minced with forceps and scissors. The tumour pieces were cultivated in Dulbecco's modified Eagle medium (DMEM; Nikken, Kyoto, Japan) with 10% heat-inactivated fetal calf serum (FCS; Life Technologies, Inc., Grand Island, NY, USA), 100 IU ml–1 penicillin (ICN Biomedical, Costa Mesa, CA, USA), 100 μg ml–1 streptomycin (ICN Biomedical), and 0.5 mM sodium pyruvate (Cambrex, Walkersville, MD, USA), and incubated in humidified incubators at 37 °C in an atmosphere of 5% CO2 in air. The fibroblasts initially grew in a monolayer. After ∼2 weeks, fibroblasts were collected and transferred to another culture dish. Serial passages were then carried out every 4–7 days. The fibroblasts were used 3–12th passage in culture. Four human gastric cancer cell lines, including OCUM-2MD3 (poorly differentiated adenocarcinoma) (Yashiro et al, 1996), OCUM-12 (poorly differentiated adenocarcinoma) (Kato et al, 2010), MKN-45 (poorly differentiated adenocarcinoma) (Motoyama et al, 1986), and MKN-74 (well-differentiated adenocarcinoma) (Motoyama et al, 1986) were seeded in a 100-mm dish (Falcon, Lincoln Park, NJ, USA) and cultured. OCUM-2MD3 and OCUM-12 were derived from scirrhous gastric carcinoma.
Immunofluorescence microscopy
To examine incubating myofibroblast content of fibroblast, immunofluorescence microscopy was performed. Triple-immunofluorescence labelling was performed to examine the presence of α-SMA-positive myofibroblasts. Fibroblasts were washed twice with Dulbecco’s PBS and fixed with acetone for 5 min, and then blocked with 3% BSA (diluted in PBS) for 30 min at room temperature. Fibroblasts were further incubated with anti-human α-SMA antibody (R&D Systems, Minneapolis, MN, USA; 1 : 100) and vimentin (Santa Cruz, Santa Cruz, CA, USA; 1 : 50) and DAPI (Wako, Osaka, Japan; 1 : 10 000) for 60 min at room temperature. Fibroblasts were viewed under a fluorescence microscope Leica Digital Microscopy DMI 6000 (Leica Microsystems, Heidelberg, Germany) with a DAPI filter (365 nm excitation), α-SMA fluorescence with a PE filter (546 nm excitation), and vimentin with a FITC filter (450–490 nm excitation). Cells that were α-SMA positive were determined as myofibroblasts. The percentage of binding cells was calculated as follows: (number of myofibroblasts/number of total cells) × 100. The percentage of α-SMA-positive myofibroblast cells was determined in 10 random fields. At least, three independent experiments were performed.
Western blot analysis
Fibroblasts were rinsed with PBS and were lysed in a lysis buffer. Aliquots containing 30 μg of total protein were subjected to SDS–PAGE, and the protein bands were transferred to a polyvinylidene difluoride membrane (Amersham, Aylesbury, UK). The membrane was placed in the TBS-T solution containing the primary antibody, α-SMA (Dako, Glostrup, Denmark; 1 : 1000) or β-Actin (Cell Signaling, Danvers, MA, USA; 1 : 1000), and allowed to react at 4 °C overnight for western blotting. The bands were detected using an enhanced chemiluminescence system (Amersham). An immunoblot analysis was performed twice.
Preparation of conditioned medium
Conditioned medium from gastric cancer cells was prepared as follows. Gastric cancer cells (5 × 104 cells ml–1) were seeded into 100-mm plastic dishes with 10 ml of DMEM containing 2% FCS and incubated for 3 days. The number of fibroblasts and gastric cancer cells in each dish was ∼2.5 × 106 cells after 3 days of incubation. To obtain conditioned medium, fibroblasts and gastric cancer cells were washed twice with PBS and then incubated for 3 days in 3 ml of DMEM. Conditioned medium was collected from each dish and centrifuged at 1000 g for 5 min. The supernatant was stored as conditioned medium at −20 °C until use. As a control, DMEM was used instead of conditioned medium.
Quantitative real-time reverse transcriptase-polymerase chain reaction (RT–PCR)
Real-time RT–PCR was performed to examine α-SMA mRNA expression. Gastric cancer cells and fibroblasts were incubated in 3 ml DMEM containing 2% FCS with 50% each conditioned medium. After 3 days of incubation, the total cellular RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA). After removal of genomic DNA by DNAse, cDNA was prepared from 20 μg RNA using random primers (Invitrogen). To determine fold changes in each gene, real-time RT–PCR was performed on the ABI Prism 7000 (Applied Biosystems, Foster City, CA, USA), using commercially available gene expression assays for α-SMA (Hs00426835). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal standard to normalise mRNA levels. The threshold cycle (Ct) values were used to calculate the relative expression ratios between control and treated cells using the formula described by Pfaffl (Pfaffl, 2001). The α-SMA expression level was calculated relative to that of NF-29 at third passage (1.0-fold as the control). Quantitative RT–PCR reactions were performed in triplicate.
Effect of conditioned medium, TGF-β, or anti-TGF-β neutralising antibody on α-SMA expression of fibroblasts
Fibroblasts were incubated in 3 ml DMEM containing 2% FCS with 50% of each conditioned medium, 10 ng ml–1 TGF-β (R&D Systems), and 10 μM anti-TGF-β neutralising antibody. After 3 days of incubation, α-SMA expression of fibroblasts was examined by RT–PCR as described above.
The effect of Smad2 siRNA on α-SMA expression of fibroblasts
The sequences for Smad2 small interfering RNA (siRNA) are designed as: Smad2 siRNA sense, 5′-GUCCCAUGAAAAGACUUAATT-3′ antisense, 5′-UUAAGUCUUUUCAUGGGACTT-3′. Control non-targeting siRNA was purchased from Ambion (Austin, TX, USA). The transfection mixture was prepared by incubating 5 μl of siPORT Neo-Fx (Ambion) and 295 μl of Opti-MEMI. The CAF-33 cells were prepared at 50–60% confluence in six-well dishes. The transfection mixture (final siRNA concentration was 30 nM) was added to six-well dish containing 2 ml of DMEM with 10% FBS. At 24 h after transfection, CAF-33 cells were incubated in addition of conditioned medium from gastric cancer cells. After 3 days of incubation, the total cellular RNA was extracted, and RT–PCR was performed.
Statistical analysis
Data are expressed as the means±s.d. from at least three independent determinations. Significance of difference was analysed using unpaired Student’s t-tests. Values of P<0.05 were considered to indicate statistical significance.
Results
The proportion of myofibroblasts in the primary culture
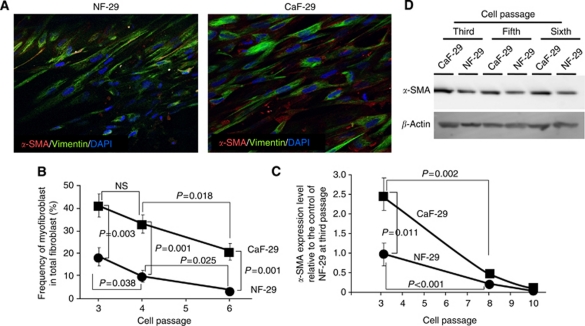
Immunofluorescence microscopy showed that NF-29 and CaF-29 fibroblasts contained α-SMA-positive (red) myofibroblast cells. A larger number of α-SMA-positive myofibroblasts were found in CaF-29 cells than in NF-29 cells. All cultured fibroblasts at the third passage were vimentin positive (green; Figure 1A). The ratios of myofibroblasts among the total fibroblasts in CaF-29 and NF-29 cultures at the third passage were 42% and 18%, respectively. The ratio of myofibroblasts in CaF-29 was significantly greater than that in NF-29 at the third (P=0.003), fourth (P=0.001), and sixth (P=0.001) passages. With each serial passage, the frequency of myofibroblasts in CaF-29 or NF-29 decreased, such that the frequency of myofibroblasts at the sixth passage was significantly decreased in CaF-29 (P=0.018) or NF-29 (P=0.025) compared with that at the fourth passage (Figure 1B). The α-SMA mRNA expression level of the cancer-associated fibroblast, CaF-29, was significantly (P=0.011) higher than that of the normal NF-29 fibroblasts at third passage. With each serial passage, the α-SMA expression level in CaF-29 or NF-29 decreased, and that in CaF-29 (P=0.002) or NF-29 (P<0.001) at the eighth passage was significantly lower in comparison with that at the third passage. There was no significant difference between the α-SMA expression levels of CaF-29 and NF-29 at the eighth and tenth passages (Figure 1C). Western blot analysis also showed that α-SMA expression level in CaF-29 was higher than that of NF-29 at each passage. The α-SMA expression level in CaF-29 at the third passage was high in comparison with that at other passages (Figure 1D).
Figure 1.
The α-smooth muscle actin (α-SMA) expression in fibroblasts. (A) Immunofluorescence of NF-29 fibroblasts and CaF-29 fibroblasts. Fibroblasts were stained with α-SMA (red), vimentin (green), and cell nuclei were stained with DAPI (blue). The percentage of myofibroblasts accompanying cancer-associated fibroblasts, CaF-29, from gastric tumour lesions was higher than that from normal fibroblasts, NF-29, derived from normal gastric tissue. (B) The proportion of myofibroblasts in the primary culture. The percentage of α-SMA-positive myofibroblast cells was determined in 10 random fields. The percentage of α-SMA-positive myofibroblasts cells of NF-29 (•) and CaF-29 (▪) at the third passage were 42% and 18%, respectively. At the fourth or sixth passage, the myofibroblast contents of both NF-29 and CaF-29 fibroblast cultures were lower than that at the third passage. (C) The expression level of α-SMA mRNA in the primary culture. The α-SMA expression level of CaF-29 (•) at the third passage was 2.5 relative to the α-SMA expression level of NF-29 (▪) as the control. The α-SMA expression level of both NF-29 and CaF-29 at the eighth or tenth passage was under 0.3 relative to the control of NF-29 at the third passage. (D) Western blot analysis. The α-SMA expression level in CaF-29 was higher than that of NF-29 at each passage. The α-SMA expression level in CaF-29 at the third passage was high in comparison with that at fifth or sixth passage.
Effect of conditioned medium from gastric cancer cells on α-SMA expression of fibroblasts
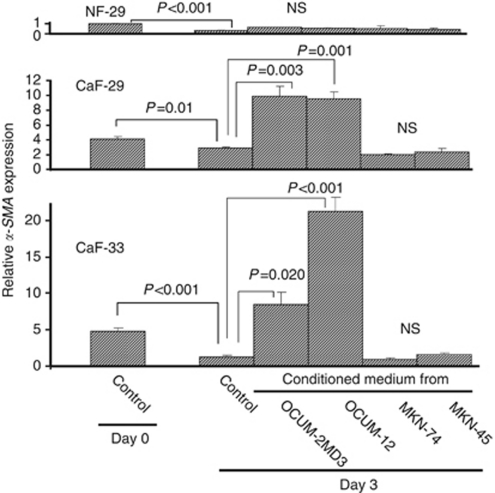
The α-SMA expression level of the controls at day 3 was significantly decreased compared with that at day 0 in NF-29 (P<0.001), CaF-29 (P=0.01), and CaF-33 (P<0.001) cells. Conditioned medium from OCUM-2MD3 and OCUM-12 cells significantly increased the α-SMA expression level of CaF-29 and CaF-33 cells, but not that from MKN-45 and MKN-74 cells. The α-SMA expression level of NF-29 cells was not increased by any conditioned medium from gastric cancer cells. The α-SMA expression level of NF-29 at day 0 was set as 1 (Figure 2).
Figure 2.
Effect of conditioned medium from gastric cancer cells on the α-SMA expression of fibroblast. The α-SMA expression levels of cancer-associated fibroblasts CaF-29 and CaF-33 were significantly increased by conditioned medium from scirrhous gastric cancer cells OCUM-2MD3 and OCUM-12, but not by conditioned medium from non-scirrhous gastric cancer cells MKN-45 and MKN-74. The α-SMA expression of normal NF-29 fibroblasts was not increased by the addition of conditioned medium from any gastric cancer cells. The graph depicts expression levels relative to control NF-29 fibroblasts at day 0.
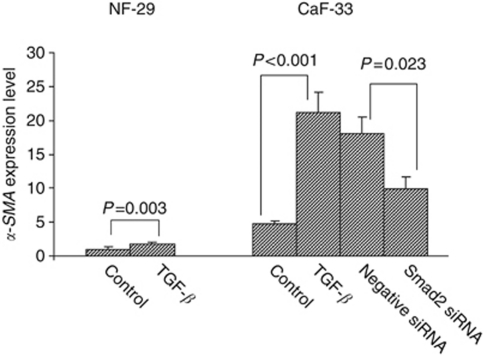
Effect of TGF-β or Smad2 siRNA on α-SMA expression of fibroblasts
Transforming growth factor-β significantly upregulated the α-SMA expressions of both NF-29 and CaF-33, whereas the α-SMA expression level of CaF-33 by TGF-β was higher than that of NF-29 by TGF-β. The TGF-β-stimulating effect of α-SMA expression in CaF-33 cells was significantly (P=0.023) decreased by Smad2 siRNA (30 nM) compared with those treated by negative control siRNA (Figure 3).
Figure 3.
Effect of TGF-β or Smad2 siRNA on α-SMA expression of fibroblasts. The TGF-β (10 ng ml–1) increased the α-SMA expression level of gastric fibroblasts. The upregulation of α-SMA expression level by TGF-β was significantly (P=0.023) decreased by Smad2 siRNA (30 nM) in CaF-33 cells. The graph depicts expression levels relative to control NF-29.
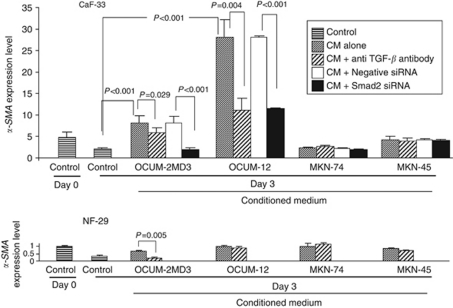
Effect of anti-TGF-β antibody or Smad2 siRNA on α-SMA-stimulating effect of conditioned medium
Anti-TGF-β antibody significantly decreased the α-SMA expression level of CaF-33 that was upregulated by conditioned medium from OCUM-2MD3 or OCUM-12 cells. The Smad2 siRNA (30 nM) significantly (P<0.001) decreased the α-SMA expression level of CAF-33 that was upregulated by conditioned medium from OCUM-2MD3 or OCUM-12 cells. In contrast, no difference of α-SMA expression level between Smad2 siRNA and negative siRNA treatment was found in CAF-33 cells with conditioned medium from MKN-45 or MKN-74. In NF-29 cells with conditioned medium from OCUM-2MD3 cells, the anti-TGF-β antibody significantly decreased the α-SMA expression level (Figure 4).
Figure 4.
Effect of anti-TGF-β antibody or Smad2 siRNA on α-SMA expression of fibroblasts. The conditioned medium (CM) from OCUM-2MD3 and OCUM-12 cells significantly increased the α-SMA expression level of cancer-associated fibroblast CaF-33 cells. The α-SMA expression level of CaF-33 with the addition of CM of OCUM-2MD3 and OCUM-12 was significantly decreased by anti-TGF-β antibody or Smad2 siRNA (30 nM). In contrast, CM from MKN-45 and MKN-74 did not affect the α-SMA expression level of CaF-33. The graph depicts expression levels relative to control NF-29 fibroblasts at day 0.
Discussion
The α-SMA expression is reported to be the most common marker for myofibroblast identification and allows the monitoring of the behaviour of this cell (Desmouliere et al, 2004), whereas there is no myofibroblast-specific immunocytochemical marker (De Wever et al, 2008). In this study, we defined myofibroblasts based on a combination of positive markers, both α-SMA and vimentin. All cultured fibroblasts at third passage were vimentin positive, which suggested that no epithelial cells were contained in the culture cells. The rate of myofibroblasts in CAFs derived from gastric tumours was greater than that in fibroblasts derived from normal gastric tissue. The number of myofibroblasts gradually decreased with serial passage in the normal tissue culture. Conditioned medium from OCUM-2MD3 or OCUM-12 cells upregulated the α-SMA expression level of CAFs. These findings might suggest that myofibroblasts are reversible to fibroblasts and that some factor(s) from scirrhous gastric cancer cells maintain the myofibroblast phenotype in CAFs. In contrast, conditioned medium from gastric cancer cells did not affect the α-SMA expression level of normal NF-29 fibroblasts, suggesting different responses of the α-SMA phenotype to conditioned medium for CAFs and normal fibroblasts. Future studies might be needed to determine which molecules of CAFs represent the myofibroblast phenotype in comparison with normal host fibroblasts.
Conditioned medium from scirrhous gastric cancer cells (OCUM-2MD3 and OCUM-12) significantly increased the number of myofibroblasts in CAFs, whereas conditioned medium from non-scirrhous gastric cancer cells (MKN-45 and MKN-74) did not; gastric cancer cells of varying differentiation had differential effects on the phenotypic features of fibroblasts. Scirrhous gastric cancer cells proliferate diffusely with extensive fibrosis, whereas most intestinal-type carcinoma cells proliferate with fewer stromal cells (Japanese Gastric Cancer, 1998). This histological difference in the volume of the stroma might be determined by the response of gastric fibroblasts to factor(s) from gastric cancer cells. Myofibroblasts represent an important prognostic factor for invasive growth that translates into a poor clinical prognosis for patients with various types of cancer (Eyden et al, 2009; Worthley et al, 2010; Yamashita et al, 2010). Myofibroblasts induced by scirrhous gastric cancer cells may create a congenial environment for the progression of scirrhous gastric carcinoma.
Transforming growth factor-β is secreted by a range of tumour cells (Mueller and Fusenig, 2004) and mediates the interaction of cancer cells with stromal fibroblasts (Kalluri and Zeisberg, 2006). Webber et al (2010) found that some cancer-derived exosomes could trigger elevated α-SMA expression and other changes consistent with the process of fibroblast differentiation into myofibroblasts. It has been reported that the cancer cell-derived TGF-β modulates myofibroblast differentiation in colon cancer (De Wever et al, 2004), breast cancer (Casey et al, 2008), and squamous cancer (Lewis et al, 2004). In this study, we found that the number of myofibroblasts of gastric fibroblasts was also upregulated by TGF-β. Moreover, our study indicated that the number of myofibroblasts was more increased by TGF-β in cancer-associated fibroblasts in comparison with normal fibroblasts. In this study, TGF-β significantly increased the α-SMA expression level of CAFs, and the α-SMA upregulation effects of conditioned medium was significantly decreased by an anti-TGF-β antibody and Smad2 siRNA. These findings suggested that TGF-β produced by tumour cells may contribute to maintaining the myofibroblastic phenotype and might play an important role in the malignant phenotype in the cancer microenvironment. Previous studies have reported that in clinical cases gastric tumours with overexpression of TGF-β imply a poor prognosis, and that TGF-β expression levels are higher in scirrhous gastric cancer cells than in non-scirrhous gastric cancer cells (Mahara et al, 1994; Kinugasa et al, 1998; Hawinkels et al, 2007). These findings might explain one of the mechanisms for the different responses of CAFs to conditioned medium from gastric cancer cells.
Transforming growth factor-β is synthesised as an inactive precursor, the large latent complex consisting of a TGF-β dimer, the latency-associated protein (LAP) and latent TGF-β-binding protein (LTBP) for localisation and binding to the ECM (Hawinkels et al, 2007). Before TGF-β can exert its biological effects, LAP and LTBP have to be dissociated. The urokinase plasminogen activator (uPA) is one factor that can activate latent TGF-β. We previously reported that scirrhous gastric cancer cells produced higher amounts of uPA (Yashiro et al, 1995). Hawinkels et al (2007) also observed a significant relation between active TGF-β levels and urokinase activity, implying plasmin, via urokinase-mediated plasminogen activation, as a principal candidate of latent TGF-β activation. The correlation between scirrhous gastric cancer and uPA suggests a role for plasmin in TGF-β activation in the tumour-specific microenvironment, resulting in transformation of resident fibroblasts to tumour-promoting myofibroblasts (Hawinkels et al, 2007). These findings might be one of the reasons for the high frequency of myofibroblasts from cancer tissue in comparison with that from normal tissue in scirrhous gastric cancer.
Myofibroblasts are a contractile cell types with actin expression (Hinz et al, 2001). Scirrhous gastric carcinomas sometimes cause a rapid contraction of the stomach wall at an advanced stage, the so-called ‘linitis plastica’. Ura et al (1991) reported that the activated form of TGF-β might contract the stomach wall. The upregulation of myofibroblasts by TGF-β released from scirrhous gastric carcinoma cells might explain the mechanisms underlying contraction of the stomach wall in cases of linitis plastica.
De Wever et al (2008) reported the multiplicity of molecules involved in the interaction between cancer cells and (myo)fibroblasts. Although TGF-β is a dominant indirect proinvasive factor for epithelial cancer cells, other factors acting in combination may also be implicated (De Wever et al, 2008). Future studies might be needed to determine which molecules of CAFs represent the myofibroblast phenotype in comparison with normal host fibroblasts.
Myofibroblasts produce a number of important factors that can directly promote growth in the adjacent epithelium (Kalluri and Zeisberg, 2006; Brenmoehl et al, 2009). The relationship between cancer cells and myofibroblasts in the tumour microenvironment might be an important target for new therapeutic approaches in controlling the growth and metastasis of cancer. The increase in myofibroblasts within the cancer microenvironment may occur through TGF-β signalling. The TGF-β receptor might therefore be a promising target molecule for cancer therapy in the gastric cancer–stroma interaction, especially in the scirrhous type of cancer.
In conclusion, TGF-β from scirrhous gastric carcinoma cells upregulates the proportion of cancer-associated myofibroblasts.
Acknowledgments
This study was supported in part by Grants-in Aid for Scientific Research (KAKENHI, Nos 20591573, 22390262, and 23390329) from the Ministry of Education, Science, Sports, Culture and Technology of Japan.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
The authors declare no conflict of interest.
References
- Brenmoehl J, Miller SN, Hofmann C, Vogl D, Falk W, Scholmerich J, Rogler G (2009) Transforming growth factor-beta 1 induces intestinal myofibroblast differentiation and modulates their migration. World J Gastroenterol 15: 1431–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey TM, Eneman J, Crocker A, White J, Tessitore J, Stanley M, Harlow S, Bunn JY, Weaver D, Muss H, Plaut K (2008) Cancer associated fibroblasts stimulated by transforming growth factor beta1 (TGF-beta 1) increase invasion rate of tumor cells: a population study. Breast Cancer Res Treat 110: 39–49 [DOI] [PubMed] [Google Scholar]
- De Wever O, Demetter P, Mareel M, Bracke M (2008) Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer 123: 2229–2238 [DOI] [PubMed] [Google Scholar]
- De Wever O, Nguyen QD, Van Hoorde L, Bracke M, Bruyneel E, Gespach C, Mareel M (2004) Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J 18: 1016–1018 [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Guyot C, Gabbiani G (2004) The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol 48: 509–517 [DOI] [PubMed] [Google Scholar]
- Durning P, Schor SL, Sellwood RA (1984) Fibroblasts from patients with breast cancer show abnormal migratory behaviour in vitro. Lancet 2: 890–892 [DOI] [PubMed] [Google Scholar]
- Eyden B, Banerjee SS, Shenjere P, Fisher C (2009) The myofibroblast and its tumours. J Clin Pathol 62: 236–249 [DOI] [PubMed] [Google Scholar]
- Guo X, Oshima H, Kitmura T, Taketo MM, Oshima M (2008) Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J Biol Chem 283: 19864–19871 [DOI] [PubMed] [Google Scholar]
- Hasebe T, Sasaki S, Imoto S, Ochiai A (2000) Proliferative activity of intratumoral fibroblasts is closely correlated with lymph node and distant organ metastases of invasive ductal carcinoma of the breast. Am J Pathol 156: 1701–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawinkels LJ, Verspaget HW, van Duijn W, van der Zon JM, Zuidwijk K, Kubben FJ, Verheijen JH, Hommes DW, Lamers CB, Sier CF (2007) Tissue level, activation and cellular localisation of TGF-beta1 and association with survival in gastric cancer patients. Br J Cancer 97: 398–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P (1997) TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 390: 465–471 [DOI] [PubMed] [Google Scholar]
- Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C (2001) Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 12: 2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Chung YS, Yashiro M, Nishimura S, Hasuma T, Otani S, Sowa M (1997) Transforming growth factor-beta and hepatocyte growth factor produced by gastric fibroblasts stimulate the invasiveness of scirrhous gastric cancer cells. Jpn J Cancer Res 88: 152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japanese Gastric Cancer Association (1998) Japanese classification of gastric carcinoma - 2nd English edition. Gastric Cancer 1: 10–24 [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6: 392–401 [DOI] [PubMed] [Google Scholar]
- Kato Y, Yashiro M, Noda S, Tendo M, Kashiwagi S, Doi Y, Nishii T, Matsuoka J, Fuyuhiro Y, Shinto O, Sawada T, Ohira M, Hirakawa K (2010) Establishment and characterization of a new hypoxia-resistant cancer cell line, OCUM-12/Hypo, derived from a scirrhous gastric carcinoma. Br J Cancer 102: 898–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawajiri H, Yashiro M, Shinto O, Nakamura K, Tendo M, Takemura S, Node M, Hamashima Y, Kajimoto T, Sawada T, Ohira M, Hirakawa K (2008) A novel transforming growth factor beta receptor kinase inhibitor, A-77, prevents the peritoneal dissemination of scirrhous gastric carcinoma. Clin Cancer Res 14: 2850–2860 [DOI] [PubMed] [Google Scholar]
- Kinugasa S, Abe S, Tachibana M, Hishikawa Y, Yoshimura H, Monden N, Dhar DK, Nagasue N, Nagaoka S (1998) Overexpression of transforming growth factor-beta1 in scirrhous carcinoma of the stomach correlates with decreased survival. Oncology 55: 582–587 [DOI] [PubMed] [Google Scholar]
- Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, Marshall JF, Thomas GJ (2004) Tumour-derived TGF-beta1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer 90: 822–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehara Y, Kakeji Y, Kabashima A, Emi Y, Watanabe A, Akazawa K, Baba H, Kohnoe S, Sugimachi K (1999) Role of transforming growth factor-beta 1 in invasion and metastasis in gastric carcinoma. J Clin Oncol 17: 607–614 [DOI] [PubMed] [Google Scholar]
- Mahara K, Kato J, Terui T, Takimoto R, Horimoto M, Murakami T, Mogi Y, Watanabe N, Kohgo Y, Niitsu Y (1994) Transforming growth factor beta 1 secreted from scirrhous gastric cancer cells is associated with excess collagen deposition in the tissue. Br J Cancer 69: 777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J (2008) TGFbeta in cancer. Cell 134: 215–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara D, Morikawa T, Goto A, Nakajima J, Fukayama M, Niki T (2009) Subepithelial myofibroblast in lung adenocarcinoma: a histological indicator of excellent prognosis. Mod Pathol 22: 776–785 [DOI] [PubMed] [Google Scholar]
- Motoyama T, Hojo H, Watanabe H (1986) Comparison of seven cell lines derived from human gastric carcinomas. Acta Pathol Jpn 36: 65–83 [DOI] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE (2004) Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 4: 839–849 [DOI] [PubMed] [Google Scholar]
- Naef M, Ishiwata T, Friess H, Buchler MW, Gold LI, Korc M (1997) Differential localization of transforming growth factor-beta isoforms in human gastric mucosa and overexpression in gastric carcinoma. Int J Cancer 71: 131–137 [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Yashiro M, Hirakawa K (2003) Keratinocyte growth factor produced by gastric fibroblasts specifically stimulates proliferation of cancer cells from scirrhous gastric carcinoma. Cancer Res 63: 8848–8852 [PubMed] [Google Scholar]
- Noma K, Smalley KS, Lioni M, Naomoto Y, Tanaka N, El-Deiry W, King AJ, Nakagawa H, Herlyn M (2008) The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis. Gastroenterology 134: 1981–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR (1999) Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59: 5002–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuji E, Kuriu Y, Okamoto K, Ochiai T, Ichikawa D, Hagiwara A, Yamagishi H (2004) Outcome of surgical treatment for patients with scirrhous carcinoma of the stomach. Am J Surg 188: 327–332 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H, Tsujitani S, Oka S, Kondo A, Ikeguchi M, Maeta M, Kaibara N (2000) An elevated serum level of transforming growth factor-beta 1 (TGF-beta 1) significantly correlated with lymph node metastasis and poor prognosis in patients with gastric carcinoma. Anticancer Res 20: 4489–4493 [PubMed] [Google Scholar]
- Schor SL, Schor AM, Grey AM, Rushton G (1988) Foetal and cancer patient fibroblasts produce an autocrine migration-stimulating factor not made by normal adult cells. J Cell Sci 90(Part 3): 391–399 [DOI] [PubMed] [Google Scholar]
- Semba S, Kodama Y, Ohnuma K, Mizuuchi E, Masuda R, Yashiro M, Hirakawa K, Yokozaki H (2009) Direct cancer-stromal interaction increases fibroblast proliferation and enhances invasive properties of scirrhous-type gastric carcinoma cells. Br J Cancer 101: 1365–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda M, Mellody KT, Orimo A (2010) Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol 21: 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3: 349–363 [DOI] [PubMed] [Google Scholar]
- Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N, Monden M (2007) Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res 13: 2082–2090 [DOI] [PubMed] [Google Scholar]
- Ura H, Obara T, Yokota K, Shibata Y, Okamura K, Namiki M (1991) Effects of transforming growth factor-beta released from gastric carcinoma cells on the contraction of collagen-matrix gels containing fibroblasts. Cancer Res 51: 3550–3554 [PubMed] [Google Scholar]
- Webber J, Steadman R, Mason MD, Tabi Z, Clayton A (2010) Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res 70: 9621–9630 [DOI] [PubMed] [Google Scholar]
- Worthley DL, Giraud AS, Wang TC (2010) Stromal fibroblasts in digestive cancer. Cancer Microenviron 3: 117–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Ogawa T, Zhang X, Hanamura N, Kashikura Y, Takamura M, Yoneda M, Shiraishi T (2010) Role of stromal myofibroblasts in invasive breast cancer: stromal expression of alpha-smooth muscle actin correlates with worse clinical outcome. Breast Cancer; e-pub ahead of print 27 October 2010 [DOI] [PubMed]
- Yashiro M, Chung YS, Nishimura S, Inoue T, Sowa M (1995) Establishment of two new scirrhous gastric cancer cell lines: analysis of factors associated with disseminated metastasis. Br J Cancer 72: 1200–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro M, Chung YS, Nishimura S, Inoue T, Sowa M (1996) Peritoneal metastatic model for human scirrhous gastric carcinoma in nude mice. Clin Exp Metastasis 14: 43–54 [DOI] [PubMed] [Google Scholar]
- Yashiro M, Hirakawa K (2010) Cancer-stromal interactions in scirrhous gastric carcinoma. Cancer Microenviron 3: 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]