A review of AMP-activated protein kinase origins and functions.
Keywords: AMP-activated protein kinase, AMPK, metabolism, autophagy, cell polarity, cell proliferation
Abstract
AMP-activated protein kinase (AMPK) is a sensor of energy status that maintains cellular energy homeostasis. It arose very early during eukaryotic evolution, and its ancestral role may have been in the response to starvation. Recent work shows that the kinase is activated by increases not only in AMP, but also in ADP. Although best known for its effects on metabolism, AMPK has many other functions, including regulation of mitochondrial biogenesis and disposal, autophagy, cell polarity, and cell growth and proliferation. Both tumor cells and viruses establish mechanisms to down-regulate AMPK, allowing them to escape its restraining influences on growth.
Living cells use ATP and ADP in a manner similar to the chemicals in a rechargeable battery. Most cellular processes require energy and are driven (directly or indirectly) by the hydrolysis of ATP to ADP and phosphate (or, less frequently, to AMP and pyrophosphate), thus “flattening the battery.” In heterotrophic organisms, the battery is recharged by catabolism; i.e., the oxidation of reduced carbon compounds of organic origin, such as glucose. In most cells (especially quiescent cells), oxidation of glucose usually proceeds completely to carbon dioxide via the process of oxidative phosphorylation. Under these conditions, most ATP synthesis occurs at the inner mitochondrial membrane, ATP being generated when protons pumped out via the respiratory chain flow back across the membrane via channels in complex V (the ATP synthase). It has been argued that the endosymbiotic acquisition of aerobic bacteria to form mitochondria was the crucial event in the development of the eukaryotes (Lane and Martin 2010). The large increase in surface area of membrane available for proton transfer (in the form of the inner mitochondrial membrane) allowed a large increase in capacity to generate ATP, which may in turn have allowed the dramatic increase in complexity displayed by eukaryotic cells and organisms. When mitochondria became the main cellular power source, one additional event required was the development of systems that sense energy status in the cytoplasm and then signal this information back to modulate mitochondrial function. Interestingly, AMP-activated protein kinase (AMPK, the subject of this review) fulfills this role and appears to be almost universal in eukaryotes. One interesting exception is Encephalitozoon cuniculi, a eukaryote with a stripped-down genome that appears to have lost not only its mitochondria, but also AMPK (Miranda-Saavedra et al. 2007). However, as it is an obligate intracellular parasite, the host cell would provide both of these missing functions.
The obvious way to achieve energy sensing would be to have proteins that monitor the cellular ratio of ATP:ADP. However, because of the very active adenylate kinases in all eukaryotic cells, which catalyze the interconversion of adenine nucleotides (2ADP ↔ ATP + AMP), the AMP:ATP ratio tends to change in concert with, and to an even greater extent than, the ADP:ATP ratio (Hardie and Hawley 2001). Thus, ratios of AMP:ATP could be monitored instead of (or in addition to) ADP:ATP, although a potential problem with this is that the concentration of AMP is usually one or two orders of magnitude lower than those of ADP and ATP (Hardie et al. 2011). There are indeed metabolic enzymes that sense cellular AMP:ATP ratios, including (1) glycogen phosphorylase and 6-phosphofructo-1-kinase in muscle, which are activated by increasing AMP:ATP, switching on two catabolic pathways (i.e., glycogenolysis and glycolysis), and (2) fructose-1,6-bisphosphatase in the liver, which is inhibited by increasing AMP:ATP, switching off an anabolic pathway, gluconeogenesis. Most other processes that are sensitive to cellular energy appear to be modulated indirectly via the AMPK system. As is discussed further below, it has recently become clear that AMPK is regulated by not only AMP and ATP, but also ADP, while it also plays a key role in maintaining the capacity of the cell for oxidative metabolism by promoting not only the production of new mitochondria, but also the disposal of dysfunctional mitochondria.
Role of AMPK orthologs in nonmammalian eukaryotes
Orthologs of AMPK are found in all eukaryotes for which genomes sequences have been completed, with the exception of the parasite E. cuniculi, as mentioned above. They appear to exist universally as heterotrimeric complexes comprising catalytic α subunits and regulatory β and γ subunits. In budding yeast (Saccharomyces cerevisiae), genes encoding the α and γ subunits (now known as SNF1 and SNF4) were isolated via mutations that caused failure to grow on carbon sources other than glucose, including alternative fermentable sugars such as sucrose and nonfermentable carbon sources such as glycerol or ethanol (Ciriacy 1977; Zimmermann et al. 1977; Carlson et al. 1981). Three alternate β subunits (Sip1, Sip2, and Gal83) were discovered later as proteins that interacted with Snf1, and knocking out all three gives the same phenotype as knocking out SNF1 or SNF4 (Schmidt and McCartney 2000).
The standard laboratory growth medium for yeast contains 2% glucose, and in this very high glucose concentration, yeast cells proliferate rapidly using fermentation (i.e., glycolysis, producing ethanol) to make ATP. This is the equivalent of the Warburg effect seen in rapidly proliferating mammalian cells (although the latter generate lactate rather than ethanol). One reason for the high glycolytic rate in rapidly proliferating cells is that the TCA cycle ceases to be a purely catabolic pathway and becomes at least partially anabolic, providing precursors for biosynthesis, especially citrate for lipid synthesis (Vander Heiden et al. 2009). As glucose in the medium runs out, however, this cannot be sustained and growth slows (a phenomenon known in yeast as the diauxic shift), and the cells switch back to the use of oxidative phosphorylation to generate ATP, which is a much more efficient process in terms of ATP generated per mole of glucose. Intriguingly, a functional SNF1 complex is required for this shift, including the switch to oxidative metabolism (Hedbacker and Carlson 2008). This suggests that an ancestral function of AMPK was to restrain growth and trigger a switch back to oxidative metabolism in response to deprivation for the preferred carbon source, glucose. When glucose runs low, snf1-null mutants behave as though they are unaware that they are starving, continuing rapid growth and fermentation and rapidly becoming nonviable. Other phenotypes of these mutants are that they do not undergo pseudohyphal growth, meiosis, and sporulation if they are diploid or invasive growth if they are haploid (Honigberg and Lee 1998; Cullen and Sprague 2000; Kuchin et al. 2002), all of which are normal responses to glucose starvation.
Consistent with an ancestral role in the response to starvation, AMPK orthologs are also required for responses to nutrient deprivation in the nematode worm Caenorhabditis elegans. Exposure of young worms to a period of starvation or other stress (heat shock or exposure to the metabolic poison azide) causes an increase in AMP:ATP ratio and an extension of subsequent life span, and the latter effect requires one of the two catalytic subunit isoforms of AMPK (AAK-2) (Apfeld et al. 2004). The germ cells are the only cells in C. elegans that do not undergo a precisely defined number of divisions; germ cell production normally arrests on dietary restriction, but this fails to occur in aak-2 mutants (Narbonne and Roy 2006). AAK-2 is also required for life span extension in response to treatment with resveratrol (an AMPK activator that is discussed further below) and to some but not all protocols of dietary restriction (Greer et al. 2007; Greer and Brunet 2009).
Also consistent with an ancestral role in the response to starvation are the phenotypes of plants lacking AMPK orthologs. In plants, darkness is equivalent to a period of fasting or starvation in an animal. In the moss Physcomitrella patens, plants lacking the two genes encoding catalytic subunit orthologs of AMPK are viable if grown under constant illumination, but fail to grow in more physiological, alternate light:dark cycles (Thelander et al. 2004). In the higher plant Arabidopsis thaliana, overexpression of the catalytic subunit causes resistance to the effects of carbohydrate starvation in cells maintained under low light levels, whereas down-regulation of the catalytic subunits results in stunted growth of plants associated with a failure to execute the normal switch in gene expression, and in the mobilization of stored starch, which occurs during a dark period (Baena-Gonzalez et al. 2007).
Mammalian AMPK—structure and regulation by adenine nucleotides
In mammals, there are seven genes encoding AMPK; i.e., two isoforms of α (α1 and α2), two of β, (β1 and β2), and three of γ (γ1, γ2, and γ3). Although there may be some preferred combinations, all 12 heterotrimeric combinations can be formed by coexpressing α, β, and γ subunits in bacteria or mammalian cells. The individual subunits are generally unstable in the absence of their binding partners; this can be a useful feature because when any one subunit is overexpressed, it acts as a dominant mutant, replacing the endogenously expressed subunit by competing for binding to the other two (Mu et al. 2001; Hawley et al. 2010).
The α subunits contain a typical serine/threonine kinase domain at the N terminus; as for many protein kinases, these only have significant activity when phosphorylated by upstream kinases (described below) at a conserved threonine residue within the activation loop (Thr 172 in human α1) (Hawley et al. 1996; Stein et al. 2000). The γ subunits contain the regulatory adenine nucleotide-binding sites and are composed of four tandem repeats of a sequence known as a CBS motif. These assemble in a pseudosymmetrical manner, such that there are four clefts where adenine nucleotides might bind (Scott et al. 2004; Xiao et al. 2007, 2011). One (site 4) appears to bind AMP very tightly, such that it does not exchange with ADP or ATP: Binding at this site may have a structural role. Two other sites within the mammalian complex (sites 1 and 3) competitively bind AMP, ADP, and ATP, and are the sites via which cellular energy status is sensed. The fourth (site 2) appears to be always unoccupied. The β subunits contain C-terminal domains that form the conserved core of the αβγ complex in both fungi and mammals, linking the C-terminal domain of the α subunit to the N-terminal region of the γ subunit (Amodeo et al. 2007; Townley and Shapiro 2007; Xiao et al. 2007). Most β subunits also contain carbohydrate-binding modules (CBMs), noncatalytic domains also found in enzymes (in both prokaryotes and eukaryotes) that metabolize starch and glycogen (Machovic and Janecek 2006). The CBMs cause mammalian AMPK to bind to glycogen in intact cells (Hudson et al. 2003; Polekhina et al. 2003, 2005; Koay et al. 2007; Bendayan et al. 2009). The physiological role of this remains uncertain, although one function may be to localize AMPK with downstream targets also bound to glycogen, such as glycogen synthase (Carling and Hardie 1989; Jorgensen et al. 2004). Intriguingly, higher plants have unique genes encoding so-called “βγ subunits,” in which a CBM appears to have become fused at the N terminus of a γ subunit. They also contain genes encoding N-terminally truncated β subunits, which have the C-terminal domain involved in the interaction with the α and γ subunits, but lack a CBM. However, all plant species also express more conventional β and γ subunits, like those in fungi and mammals (Polge and Thomas 2007).
The major upstream kinase phosphorylating Thr 172, and thus activating AMPK, in most mammalian cells is a complex between the tumor suppressor kinase LKB1 and two accessory subunits, STRAD and MO25 (Hawley et al. 2003; Woods et al. 2003; Shaw et al. 2004). Binding of AMP causes conformational changes in AMPK that promote its activation via three independent mechanisms: (1) promotion of Thr 172 phosphorylation (Hawley et al. 1995; Oakhill et al. 2010), (2) inhibition of Thr 172 dephosphorylation (Davies et al. 1995; Suter et al. 2006; Oakhill et al. 2010), and (3) allosteric activation of AMPK already phosphorylated on Thr 172 (Corton et al. 1995; Suter et al. 2006). Interestingly, it has recently been reported that mechanisms 1 and 2 can be triggered by binding of ADP as well as AMP (Oakhill et al. 2011; Xiao et al. 2011), although mechanism 3 is only observed with AMP. Sites 1 and 3 on the γ subunits have rather similar affinities for free ATP, ADP, and AMP (Xiao et al. 2011), and ATP and ADP are usually present at concentrations much higher than those of AMP, so that, under most circumstances, the kinase may be responding to fluctuations in ADP:ATP, rather than AMP:ATP as previously thought. However, under conditions of severe stress, when the levels of AMP might approach those of ADP and free ATP, the 10-fold allosteric activation caused by AMP (mechanism 3) (Suter et al. 2006) would multiply with the >200-fold activation caused by Thr 172 phosphorylation to yield >2000-fold activation overall. Thus, the mammalian kinase can respond over a wide range of cellular ADP:ATP and AMP:ATP ratios and over a very wide dynamic range.
One interesting question concerns how ADP and AMP can compete with ATP for binding to the γ subunit, when total ATP concentrations are usually much higher than those of ADP and AMP. One explanation may be that the Mg.ATP2− complex binds with a 10-fold lower affinity than free ATP4− (Xiao et al. 2011). Since the majority of ATP in the cell is present as the Mg.ATP2− complex, AMP and ADP may only have to compete with the free ATP4− form, rather than with the much more abundant Mg.ATP2− complex.
AICAR (5-aminoimidazole-4-carboxamide ribonucleoside) is an adenosine analog that is commonly used as a pharmacological activator of AMPK in experimental studies with intact cells and in vivo. AICAR is taken up into cells by adenosine transporters (Gadalla et al. 2004) and converted by adenosine kinase into the monophosphorylated form, ZMP. ZMP mimics all three effects of AMP on the AMPK system, although a much less potent activator than AMP (Corton et al. 1995).
Although it is clear that activation of the AMPK orthologs in fungi and plants requires phosphorylation of the threonine residue equivalent to Thr 172 (Estruch et al. 1992; Mackintosh et al. 1992; Wilson et al. 1996), their regulation by adenine nucleotides is less well characterized. Although AMP activation has been reported for the orthologs from Drosophila melanogaster (Pan and Hardie 2002) and C. elegans (Apfeld et al. 2004), the yeast (Wilson et al. 1996) and plant (Mackintosh et al. 1992) kinases are not allosterically activated by AMP (i.e. they lack mechanism 3, above). Activation of the yeast SNF1 complex during glucose starvation does, however, correlate with large increases in cellular AMP:ATP and ADP:ATP ratios (Wilson et al. 1996), and it seems possible that the complex might be regulated by ADP and/or AMP via mechanisms 1 and/or 2. Indeed, it has been shown that dephosphorylation and activation of a plant kinase in cell-free assays is inhibited by AMP (mechanism 2) (Sugden et al. 1999).
Although LKB1 has to be expressed in mammalian cells for agents that increase the cellular AMP:ATP and ADP:ATP ratios to cause activation of AMPK (Hawley et al. 2003), it is worth emphasizing that these effects are due to binding of adenine nucleotides to the γ subunit of AMPK and that the LKB1 complex itself appears to be constitutively active (Sakamoto et al. 2004). This might initially appear surprising, but LKB1 is also required for the activity of 12 kinases of the AMPK-related kinase family in addition to AMPK, being responsible for phosphorylation of the threonine residues equivalent to Thr 172 in all of them (Lizcano et al. 2004). The AMPK-related kinases have quite varied functions and, unlike AMPK, most do not appear to be activated by energy stress. Thus, it would make little sense for the upstream kinase to be the primary site of regulation.
In some cell types, Thr 172 can also be phosphorylated by the Ca2+/calmodulin-dependent protein kinase CaMKKβ, providing a Ca2+-activated pathway to switch on AMPK (Hawley et al. 2005; Hurley et al. 2005; Woods et al. 2005). Activation by this mechanism can occur in the absence of any change in adenine nucleotide ratios, although increases in Ca2+ can act synergistically with increases in AMP or ADP (Fogarty et al. 2010). This mechanism appears to be particularly important in neurones (Hawley et al. 2005), endothelial cells (Stahmann et al. 2006), and T lymphocytes (Tamas et al. 2006). Since increases in cytosolic Ca2+ tend to trigger energy-consuming processes, such as activation of motor proteins or secretion, this could be a mechanism to anticipate a demand for ATP before it has occurred.
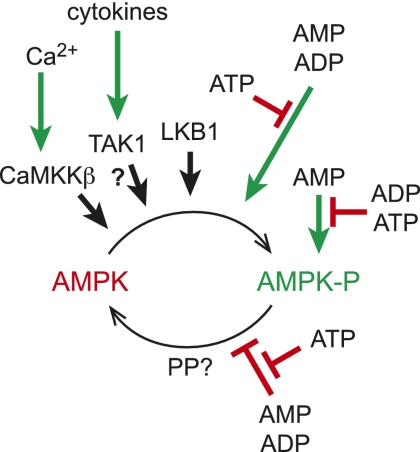
Finally, it has been reported that Thr 172 can be phosphorylated by TAK1 (also known as MAP3K7 or MEKK7) (Momcilovic et al. 2006), a protein kinase downstream from cytokine receptors that is usually thought of as acting upstream of the MAP kinase (JNK) and NF-kB signaling cascades. Although this has been implicated in activation of AMPK by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (Herrero-Martin et al. 2009), the physiological significance of this mechanism remains unclear at present. The complex mechanisms regulating AMPK are summarized in Figure 1.
Figure 1.
Regulation of AMPK by phosphorylation and adenine nucleotides. AMPK is activated >200-fold by phosphorylation at Thr 172, catalyzed by three upstream kinases: (1) LKB1, which appears to be constitutively active; (2) TAK1, which is activated by cytokines (as the physiological significance of this remains uncertain, it is shown with a question mark); and (3) CaMKKβ, which is activated by a rise in cytosol Ca2+. Phosphorylation is stimulated and dephosphorylation is inhibited by conformational changes triggered by binding of AMP or ADP to the γ subunit of AMPK, both effects being antagonized by ATP. AMP binding also causes a further 10-fold allosteric activation; this effect is antagonized by both ATP and ADP. The identity of the protein phosphatase that dephosphorylates Thr 172 (PP?) remains unclear.
Regulation of AMPK by metabolic stresses, drugs, xenobiotics, and cytokines
AMPK is activated by metabolic stresses that either interfere with catabolic generation of ATP (e.g., glucose deprivation, hypoxia, ischemia, and treatment with metabolic poisons) or accelerate ATP consumption (e.g., muscle contraction), thus increasing cellular ADP:ATP and AMP:ATP ratios (Hardie 2007). Interestingly, in cells in which AMPK activation is defective (e.g., muscle cells with a knockout of LKB1 during contraction [Sakamoto et al. 2005] or mouse hepatocytes with a double AMPK-α1/-α2 knockout treated with the metabolic poison metformin [Foretz et al. 2010]), the increases in ADP:ATP and AMP:ATP in response to metabolic stress are much greater, indicating that AMPK is acting to preserve energy homeostasis in the wild-type cells. AMPK is also activated by numerous drugs and xenobiotics. Some of these are in clinical use for the treatment of type 2 diabetes (e.g., metformin [Zhou et al. 2001] and thiazolidinediones [Fryer et al. 2002]), some are “nutraceuticals” (e.g., resveratrol from red wine [Baur et al. 2006] and epigallocatechin gallate from green tea [Hwang et al. 2007]), and some are plant products used in traditional herbal medicines in Europe (e.g., galegine, from which metformin was originally derived) (Mooney et al. 2008) or Asia (e.g., berberine [Lee et al. 2006] and hispidulin [Lin et al. 2010]). One puzzling feature was how so many xenobiotics of very varied structure could all activate AMPK; it seemed unlikely that they would all bind directly to the kinase complex. Important clues came from reports that some of these compounds inhibit mitochondrial ATP synthesis by inhibiting either complex I of the respiratory chain (e.g., metformin [El-Mir et al. 2000; Owen et al. 2000] or berberine [Turner et al. 2008]) or complex V, the ATP synthase (e.g., resveratrol) (Gledhill et al. 2007). Most of the natural products mentioned above are secondary metabolites of plants, and some appear to be produced as defense compounds to deter grazing by insects or herbivores (e.g., galegine, produced by Galega officinalis, a plant poisonous to herbivores) or ward off infection by pathogens (e.g., resveratrol, which is produced by grapes in response to fungal infection) (Romero-Perez et al. 2001). Mitochondrial inhibitors might serve well as plant defense compounds, but would also be potent activators of AMPK. To test this idea, Hawley et al. (2010) constructed cell lines expressing AMPK complexes bearing either the wild-type γ2 isoform or an R531G mutation that renders γ2 complexes insensitive to increases in ADP and AMP. This approach confirmed that most of the AMPK-activating xenobiotics, including metformin, galegine, thiazolidinediones, resveratrol, and berberine, failed to activate the R531G mutant, showing that they worked by increasing cellular AMP and/or ADP (Hawley et al. 2010).
In addition to its role in the regulation of energy balance in a cell-autonomous manner, AMPK is also modulated by numerous hormones and/or cytokines that regulate energy balance at the whole body level, including leptin, adiponectin, and ghrelin (Kahn et al. 2005); cannabinoids (Kola et al. 2006); and even thyroid hormones (Lopez et al. 2010). Many of these agents modulate AMPK in the hypothalamus. Both leptin (Minokoshi et al. 2002) and adiponectin (Yamauchi et al. 2002) have been reported to increase AMP levels in muscle cells, but the detailed molecular mechanisms by which these hormones and cytokines modulate AMPK remain unclear.
Recognition of substrates
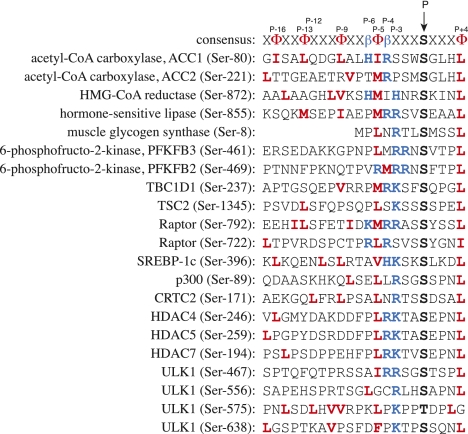
AMPK has one of the most well-defined substrate recognition motifs of any protein kinase studied to date (Fig. 2). This was elucidated using various approaches, including synthetic peptides (Weekes et al. 1993; Dale et al. 1995), site-directed mutagenesis with an recombinant substrate (Scott et al. 2002), and a peptide library approach (Gwinn et al. 2008). AMPK phosphorylates sites that have at least one basic side chain (usually R, but can be K or H) either four or three residues N-terminal (designated as P-4 and P-3) with respect to the serine or threonine residue phosphorylated. It also requires hydrophobic side chains at P-5 and P+4 (usually L or M but I, V, and F are also tolerated at P-5, and I, V, F, Q, or N at P+4). Structural modeling and site-directed mutagenesis have been used to pinpoint residues on the kinase domain (mainly in the large lobe) that are involved in binding these critical side chains on the substrate (Scott et al. 2002). Hydrophobic pockets on the kinase domain accommodate the P-5 and P+4 hydrophobic side chains on the substrate, while a patch of acidic residues (E103/D100/E143 in rat α1) interacts with the P-4 and P-3 basic side chains. The same study revealed additional positive determinants that, while not present in all substrates, improve the kinetic parameters. These include a basic residue at P-6 that interacts with another acidic patch on the kinase domain (D215/D216/D217), and an amphipathic helix from P-16 to P-5 that fits into a hydrophobic groove on the large lobe of the kinase domain. This helix has repeating hydrophobic side chains every three or four residues (including that at P-5), which line the face of the helix that binds in the groove (Scott et al. 2002). While this pattern of hydrophobic residues is difficult to discern in some substrates (and residues beyond P-7 are not even present in glycogen synthase, where the site is close to the N terminus), an amphipathic helix is present in this position in one substrate for which a crystal structure is available; i.e., HMG-CoA reductase (Istvan and Deisenhofer 2000). The peptide library approach (Gwinn et al. 2008) revealed some additional determinants, such as a bias against hydrophobic residues at P-2 and a preference for polar residues (acidic or basic) at the P+3 position. In acetyl-CoA carboxylase-1 (ACC1), the P+3 position is a histidine, and it appears to interact with Asp 56 in the small lobe of the kinase domain (Scott et al. 2002).
Figure 2.
Alignment of consensus site for recognition of substrates by AMPK (Scott et al. 2002) with the sequences (from humans) of some established physiological substrates. In the consensus sequence, hydrophobic residues are represented by Φ and basic residues are represented by β. AMPK phosphorylates serine residues (threonine also allowed) in the context of hydrophobic residues (in bold, red) at P-5 (usually M or L, but I, V, or F also allowed) and P+4 (usually L, but I, M, F, V, Q, or N allowed), and basic residues (in bold, blue; usually R, but K or H allowed) at P-4, P-3, or both. Another basic residue at P-6 is also a positive determinant, although not essential (Scott et al. 2002). Some substrates (e.g., ACC1) also have hydrophobic side chains at regular spacings of three to four residues (bold, red) running in an N-terminal direction from P-5, which form an amphipathic helix. This is clearly not essential, since the sequence of muscle glycogen synthase starts at P-7. Note that one substrate (PFKFB2) still has a βΦβ motif, but this is at P-5/P-4/P-3 rather than the more common P-6/P-5/P-4.
In one substrate (PFKFB2) (Fig. 2), a basic–hydrophobic–basic motif N-terminal to the phosphorylated serine is found at P-5, P-4, and P-3, rather than at P-6, P-5, and P-4/P-3 as usual. Thus, there may be some flexibility in the spacing between this motif and the phosphoamino acid. However, it is worth noting that another site that has a sequence motif similar to that on PFKFB2 (i.e., Ser 1177 on endothelial nitric oxide synthase [eNOS]), while apparently phosphorylated by AMPK in cell-free assays (Chen et al. 1999), does not appear to be a target in intact cells (Stahmann et al. 2006, 2010). There are also a few proteins that have been claimed to be direct substrates for AMPK that are even poorer fits to this consensus, including eukaryotic elongation factor-2 (eEF2) kinase (Browne et al. 2004), p53 (Jones et al. 2005), and p27Kip1 (Liang et al. 2007). In my opinion, it remains possible that these are indirect substrates that are phosphorylated by kinases acting downstream from AMPK (or are dephosphorylated by phosphatases inhibited by AMPK).
Regulation of metabolism
As befits its role in maintaining energy homeostasis, AMPK switches on catabolic pathways that generate ATP, while switching off anabolic pathways that consume ATP. Examples of catabolic pathways that are up-regulated include glucose uptake (via activation of both GLUT1 [Barnes et al. 2002] and GLUT4 [Holmes et al. 1999; Kurth-Kraczek et al. 1999]), glycolysis (via phosphorylation and activation of two of four isoforms of 6-phosphofructo-2-kinase, which synthesizes the glycolytic activator fructose-2,6-bisphosphate) (Marsin et al. 2000, 2002), fatty acid uptake (via translocation of the fatty acid transporter FAT/CD36) (Bonen et al. 2007), and fatty acid oxidation (via phosphorylation of the ACC2 isoform of acetyl-CoA carboxylase, thus lowering malonyl-CoA, an inhibitor of fatty acid uptake into mitochondria [Merrill et al. 1997]). GLUT4 is up-regulated at multiple levels, with a rapid effect via translocation of existing transporters to the plasma membrane (probably due in part to phosphorylation of the Rab-GAP protein TBC1D1 by AMPK) (Chen et al. 2008; Pehmoller et al. 2009) and a longer-term effect on transcription of the GLUT4 gene (probably due in part to phosphorylation of histone deacetylase-5) (McGee et al. 2008). Another way in which AMPK up-regulates catabolism is by enhancing mitochondrial biogenesis, which is discussed in a separate section below.
AMPK activation also inhibits many anabolic pathways acutely via direct phosphorylation of key metabolic enzymes. Thus, it inhibits fatty acid synthesis by phosphorylation of ACC1, isoprenoid synthesis by phosphorylation of HMG-CoA reductase, triglyceride and phospholipid synthesis by inactivation of glycerol phosphate acyl transferase (although it is not clear whether this is a direct substrate), glycogen synthesis by phosphorylation of glycogen synthase, and ribosomal RNA synthesis by phosphorylation of the RNA polymerase I transcription factor TIF-1A (RRN3) (Hardie 2007; Hoppe et al. 2009). AMPK down-regulates expression of enzymes of fatty acid synthesis at the transcriptional level by a mechanism involving phosphorylation of the transcription factor SREBP-1c, inhibiting its proteolytic processing to the active, nuclear form (Zhou et al. 2001; Li et al. 2011). It also represses transcription of mRNAs encoding enzymes involved in gluconeogenesis, such as glucose-6-phosphatase and phosphoenolpyruvate carboxykinase, apparently via multiple mechanisms. One involves phosphorylation of CRTC2 (a transcriptional coactivator of the cyclic AMP response element-binding protein CREB), causing its exclusion from the nucleus (Koo et al. 2005). A second involves phosphorylation of class IIA histone deacetylases (HDAC-4, HDAC-5, and HDAC-7), once again causing their exclusion from the nucleus. When present within the nucleus, class IIA HDACs (which are catalytically inactive) recruit the active deacetylase, HDAC3, which deacetylates and activates transcription factors of the FOXO family that are required for expression of gluconeogenic genes (Mihaylova et al. 2011). Finally, a major consumer of energy in growing cells is protein synthesis, which accounts for one-third of all ATP consumption in proliferating thymocytes (Buttgereit and Brand 1995). AMPK inhibits elongation of translation by promoting phosphorylation of eEF2 (although, as discussed above, eEF2 kinase may not be a direct target) (Horman et al. 2002; Browne et al. 2004). In addition, a particularly important target of AMPK is mTOR complex-1 (TORC1), which is switched off by direct phosphorylation of both its upstream regulator, TSC2 (Inoki et al. 2003), and the TORC1 subunit Raptor (Gwinn et al. 2008). TORC1 is stimulated by amino acids and by growth factors that activate the Akt and Raf–MEK–Erk pathways, and it promotes both the initiation and elongation of translation via phosphorylation of 4EBP1 and p70 S6 kinase (Zoncu et al. 2011).
Regulation of mitochondrial biogenesis, autophagy, and mitophagy
As discussed in the introductory section, the acquisition of mitochondria by primitive eukaryotic cells necessitated the development of mechanisms whereby a demand for energy in the cytoplasm could be converted into an increase in mitochondrial function, and AMPK appears to play an important role in this. Thus, chronic activation of AMPK in skeletal muscle for 4 wk, by repeated administration of AICAR to rats (Winder et al. 2000) or mice (Narkar et al. 2008), led to the up-regulation of nuclear-encoded mitochondrial genes and, in the mouse study, improved endurance in treadmill running tests. Interestingly, the second study led to AICAR or “any other AMPK activator” being placed on the banned list of the World Anti-Doping Agency, the body that regulates drug abuse in sports.
How does AMPK activation up-regulate mitochondrial function? The so-called “master regulator” of mitochondrial biogenesis is the transcriptional coactivator PGC-1α, which promotes transcription induced by several factors involved in expression of mitochondrial genes, including PPAR-α and PPAR-δ, which switch on genes required for fatty acid oxidation, and nuclear respiratory factors-1 and -2 (NRF-1/-2) (Lin et al. 2005). One gene switched on by NRF-1/-2 is TFAM, a mitochondrial matrix protein required for the replication of mitochondrial DNA. Thus, PGC-1α promotes biogenesis of new mitochondria as well as expression of nuclear-encoded mitochondrial genes. The first evidence that AMPK up-regulated expression of PGC-1α came from studies of mice expressing a dominant-negative mutant of AMPK, in which the induction of mitochondrial DNA and PGC-1α mRNA in response to the feeding of β-guanidinopropionic acid (a creatine analog that causes ATP depletion) was abolished (Zong et al. 2002). AMPK has been reported to directly phosphorylate PGC-1α at two sites (although neither is a good fit to the AMPK recognition motif described above), and this is proposed to activate transcription of PGC-1α from its own promoter via a positive feedback loop (Jager et al. 2007). An alternative mechanism by which AMPK may activate PGC-1α function is by deacetylation catalyzed by the NAD+-dependent deacetylase SIRT1 (Canto et al. 2010), although the exact mechanism by which AMPK activates SIRT1 remains uncertain.
Thus, AMPK activation promotes mitochondrial biogenesis and expression of nuclear-encoded mitochondrial genes by up-regulating PGC-1α. In addition, it now appears to play an important role in the disposal of dysfunctional mitochondria. Mitochondria are the major cellular site of production of reactive oxygen species and are therefore particularly susceptible to oxidative damage. Disposal of damaged mitochondria and recycling of their contents for reuse may be just as important in the preservation of overall cellular ATP-generating capacity as is the generation of new mitochondria.
Autophagy is the recycling of cytoplasmic components (including mitochondria, when the process is known as mitophagy) that are either dysfunctional or surplus to requirements by means of their engulfment by autophagic vacuoles that then fuse with lysosomes. Autophagy becomes particularly important during starvation of cells as a means to recycle amino acids for synthesis of critical proteins, and perhaps also to recycle cellular components for use as catabolic fuels. The complex cascade of events involved in autophagy was originally defined by genetic approaches in budding yeast, but the analogous pathways are now becoming well understood in mammals (Wang and Levine 2010). Autophagy is switched off by TORC1, and it was originally thought that AMPK might promote autophagy through its ability to inhibit TORC1, as discussed in a previous section. However, it is now clear that AMPK has a more direct input into autophagy that probably arose at an earlier stage during eukaryotic evolution, because the SNF1 complex appears to promote autophagy in budding yeast (Wang et al. 2001). At the top of the autophagy cascade and initiating the process are the protein kinases ULK1/ULK2 (orthologs of Atg1 in yeast). It has been found that ULK1 and ULK2 form rather stable complexes with AMPK (Behrends et al. 2010), and also that AMPK phosphorylates ULK1 at multiple sites (although the two groups identified different sites) (Egan et al. 2011; Kim et al. 2011). In isolated mouse hepatocytes deficient in either ULK1 or AMPK, there was a marked accumulation of mitochondria, indicating a defect in mitophagy. In cells in which endogenous ULK1 was replaced by either a kinase-inactive mutant or a mutant in which the four serine residues identified as AMPK sites were mutated to alanine, large numbers of mitochondria with abnormal morphology and reduced membrane potential accumulated upon starvation (Egan et al. 2011). Fewer of the cells also survived the stress of starvation, emphasizing the important role of mitophagy in cell function.
Regulation of cell growth and proliferation
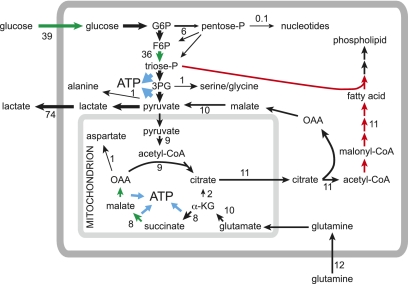
Rapid cell growth requires the active synthesis of proteins, ribosomal RNA, and lipids, all of which are switched off by AMPK activation, as discussed above (inactivation of TORC1 being a key event causing inhibition of protein synthesis). Fatty acid synthesis is a particularly active process in proliferating cells (Metallo et al. 2009), and AMPK switches this off by a dual mechanism involving phosphorylation and inactivation of ACC1 (Davies et al. 1992) and down-regulation of expression of ACC1, fatty acid synthase, and other lipogenic genes (Foretz et al. 1998; Leclerc et al. 1998). The high rate of lipid synthesis in proliferating cells helps to explain, at least in part, the well-known “Warburg effect,” in which much of the cellular ATP is generated by glycolysis, rather than oxidative phosphorylation. The large withdrawal of citrate from the TCA cycle for lipid synthesis means that the cycle can no longer be used as a purely catabolic pathway. Remarkably, in proliferating cells, the flux leaving the TCA cycle as citrate can be greater than the flux entering it from pyruvate, the additional flux to citrate coming from glutamine via reversal of part of the TCA cycle (Fig. 3; Metallo et al. 2009). This anabolic use of the TCA cycle necessitates an alternative route for ATP production; i.e., glycolysis.
Figure 3.
Metabolism in rapidly proliferating cells as revealed by metabolic flux analysis in the lung adenocarcinoma cell line A549, grown in medium containing 25 mM glucose and 4 mM glutamine (note that A549 cells do not express LKB1, so AMPK activity would be very low) (Metallo et al. 2009). Numbers refer to the estimated fluxes in nanomole per minute per milligram protein. Note that the flux out of the TCA cycle into fatty acid synthesis (presumably required for synthesis of new membrane phospholipid) is greater than the flux entering the TCA cycle from pyruvate. This deficit is made up by anaplerotic flux from glutamine, with the flow from α-ketoglutarate (αKG) to citrate operating in the reverse of the usual direction of the TCA cycle. Note also the very large flux through glucose uptake, glycolysis, and lactate production. Processes currently known to be down-regulated by AMPK activation are shown in red, and processes known to be up-regulated by AMPK are in green. In general, AMPK switches off anabolic (ATP-consuming) processes and switches on catabolic (ATP-producing) processes.
The processes of DNA replication that occurs in S phase of the cell cycle and of mitosis in M phase are both costly in terms of energy, and one might expect AMPK activation to halt progress through the cell cycle if cellular energy status was compromised. Indeed, activation of AMPK in cultured tumor cells was found to cause a G1–S-phase cell cycle arrest that involved up-regulation and/or stabilization of p53 and the cyclin-dependent kinase inhibitors p21Waf1/Cip1 and p27Kip1 (Imamura et al. 2001; Jones et al. 2005; Liang et al. 2007). These effects were proposed to be triggered by direct phosphorylation by AMPK of p53 at Ser 15 (p21Waf1/Cip1 being a transcriptional target of p53) (Jones et al. 2005) and of p27Kip1 at Thr 198 (Liang et al. 2007). However, neither of these sites is a good fit to the well-established AMPK recognition motif; the p53 site has hydrophobic residues at P-5 and P+4 but lacks the expected basic residues, while the p27 site is unusual in that Thr 198 is the last residue in the protein. Although there is no reason to doubt the findings that AMPK activation in intact cells causes phosphorylation at these sites, further work is required to confirm that that they are direct targets of AMPK.
The ability of AMPK to inhibit cell growth and proliferation is consistent with the idea that AMPK, rather than one of the other AMPK-related kinases downstream from LKB1, is responsible for the tumor suppressor effects of LKB1. Consistent with this, re-expression of LKB1 in tumor cells that have lost the upstream kinase (e.g., the G361 melanoma cell line) caused a cell cycle arrest that correlated with AMPK activation (Fogarty and Hardie 2009). This did not appear to be due to activation of an AMPK-related kinase, because the same effect was produced by activation of endogenous CaMKKβ using a Ca2+ ionophore or expression of a truncated, Ca2+-independent mutant of CaMKKβ (S Fogarty and DG Hardie, in prep.). Fogarty et al. (2010) have shown previously that while CaMKKβ phosphorylates and activates AMPK, it does not activate any of the AMPK-related kinases.
Regulation of cell polarity
Embryos that lack LKB1 or AMPK exhibit similar defects in epithelial cell polarity during development in D. melanogaster and also exhibit defects in mitosis, with many cells becoming polyploid (Martin and St Johnston 2003; Lee et al. 2007). LKB1 appears to be necessary for the establishment of epithelial cell polarity in the absence of starvation, while both LKB1 and AMPK are required for maintenance of cell polarity under starvation conditions (Mirouse et al. 2007). The defects in the lkb1-null mutants could be partially rescued by overexpression of activated AMPK mutants (containing aspartate in place of the threonine equivalent to Thr 172) (Lee et al. 2007; Mirouse et al. 2007). Although AMPK-null mouse embryos do not die at such an early stage of development as in Drosophila and do not seem to have similar defects in cell polarity, there is nevertheless evidence that the LKB1–AMPK pathway is involved in the maintenance of epithelial cell polarity in mammals. Experiments have been carried out in intestinal epithelial cancer (LS174T) cells (which lack LKB1 and exhibit no cell polarity) by re-expressing LKB1 and its regulatory subunit, STRAD, the latter via a tetracycline-inducible promoter. When STRAD expression was induced using tetracycline to activate LKB1, a rapid remodeling of the actin cytoskeleton and the formation of an apical brush border membrane was evident, even in the absence of cell:cell contacts (Baas et al. 2004). Remarkably, very similar responses were observed in LS174T cells when AMPK was activated using 2-deoxyglucose, an inhibitor of glycolysis (Lee et al. 2007). Finally, in Madin Darby canine kidney (MDCK) epithelial cells, which form polarized monolayers with tight junctions between cells, the removal of Ca2+ from the medium results in a loss of polarity and of tight junctions, while the re-addition of Ca2+ restores both, concomitant with activation of AMPK. Restoration of polarity and tight junctions is promoted by treatment with the AMPK activator AICAR, while it is blocked by expression of a dominant-negative AMPK mutant, showing that AMPK is required (Zhang et al. 2006; Zheng and Cantley 2007).
Since establishment and maintenance of cell polarity is an energy-requiring process, it might seem counterintuitive that it should be promoted by AMPK, a kinase that is activated by energy deficit. Possibly, the maintenance of epithelial barriers is so crucial to survival in multicellular organisms that it must be preserved even during periods of energetic stress, so that AMPK is helping to divert the limited amount of ATP that might remain to this crucial task. Another more speculative suggestion is that, since AMPK is a sensor of carbon nutrients in unicellular eukaryotes, primitive multicellular eukaryotes might have used the sensing of such nutrients to allow them to distinguish the outside of the organism from the inside and thus establish polarized epithelial barriers with the correct orientation.
Role of AMPK in cancer and viral infection
The discovery that LKB1 was the major upstream kinase required for activation of AMP in response to metabolic stress (Hawley et al. 2003; Woods et al. 2003) introduced for the first time a link between AMPK and cancer. LKB1 had been originally identified as the tumor suppressor responsible for an inherited susceptibility to cancer, Peutz-Jeghers syndrome (Hemminki et al. 1998; Jenne et al. 1998). Humans with Peutz-Jeghers syndrome are heterozygous for loss-of-function mutations in the LKB1 gene (STK11). Their main clinical problem is the frequent formation of benign intestinal polyps, which appear to be caused by haploinsufficiency, although they also have a greatly increased risk of malignant cancers at other sites, which are likely due to either a mutation in the second copy of STK11 or loss of heterozygosity. As expected for a tumor suppressor, the STK11 gene is also frequently mutated in spontaneous cancers, including ∼30% of non-small-cell lung cancers (Sanchez-Cespedes et al. 2002; Ji et al. 2007) and 20% of cervical cancers, including the cervical cancer in the patient Henrietta Lacks that gave rise to the HeLa cell line (Wingo et al. 2009).
Does activation of AMPK explain the tumor suppressor effects of LKB1? Although there is no formal genetic proof of this as yet, it does seem likely to be the case, because of the 14 protein kinases known to be downstream from LKB1 (the α1 and α2 isoforms of AMPK, and the 12 AMPK-related kinases), AMPK is the only one known to cause inhibition of biosynthesis and cell growth, and cell cycle arrest. When the link between LKB1 and AMPK was found, the question of whether the AMPK-activating drug metformin might protect against development of cancer was investigated. It was found that diabetics that had been treated with metformin had a significantly lower incidence of cancer than those on other treatments (Evans et al. 2005). Although this result was obtained by retrospective analysis and was not a controlled trial, the finding has been reproduced in several other diabetic populations, and prospective trials are now under way. At present, there are two ways to explain these results: (1) Metformin inhibits glucose production by the liver (possibly in part by activating AMPK). The consequent lowering of plasma glucose and insulin (and perhaps IGF1) changes the nutritional and hormonal environment of incipient tumors, thus opposing tumorigenesis indirectly. (2) Metformin activates AMPK directly in the incipient tumors, exerting a cytostatic effect. These two hypotheses, which are not necessarily mutually exclusive, cannot yet be distinguished from results with humans. However, treatment of a tumor-prone mouse model with three different activators of AMPK—i.e., metformin, phenformin (a more potent sister drug to metformin), or A-769662 (a direct activator of AMPK developed by Abbot) (Cool et al. 2006)—significantly delayed tumor development (Huang et al. 2008). The mice used in these experiments were not diabetic or insulin-resistant, so it seems unlikely that the drugs were acting via indirect effects on the liver. Moreover, since metformin/phenformin and A-769662 activate AMPK by different mechanisms (Hawley et al. 2010), it seems unlikely that the observed delays in tumorigenesis (Huang et al. 2008) were “off-target,” AMPK-independent effects.
Although there is therefore evidence that AMPK might provide protection against the development of tumors, as with any genuine tumor suppressor there is likely to be selection for loss of AMPK function in established tumors. Indeed, in a study of breast cancer, using immunohistochemistry with phospho-specific antibodies against Thr 172 on AMPK and Ser 80 on ACC1 (a well-established AMPK target), it was shown that AMPK activation appeared to be down-regulated (in the tumor compared with normal epithelium) in 90% of 350 cases. The mechanism underlying this down-regulation in breast cancer remains uncertain, but one simple explanation would be the loss of LKB1, which would prevent AMPK activation in response to metabolic stress (Hawley et al. 2003). As already mentioned, the genetic loss of LKB1 occurs in a significant proportion of lung and cervical cancers. Another mechanism for down-regulation of AMPK has been suggested in skin cancer cells (Zheng et al. 2009). A single point mutation in the proto-oncogene B-Raf (V600E, causing constitutive activation) occurs in up to half of all malignant melanomas. In melanoma cell lines carrying this mutation, LKB1 was found to be phosphorylated at two C-terminal sites by kinases acting downstream from B-Raf, and it was proposed that this interferes with the ability of LKB1 to phosphorylate and activate AMPK (Zheng et al. 2009).
Another mechanism is suggested by previous findings that phosphorylation of the α1 subunit of AMPK at Ser 485 (equivalent to Ser 491 on α2) by Akt (protein kinase B) inhibits the subsequent phosphorylation at Thr 172—and consequent activation—by LKB1 (Horman et al. 2006). Akt is activated in many tumor cells due to either activating mutations in phosphatidylinositol (PI) 3-kinase (which generates PI-3,4,5-trisphosphate [PIP3], the activating signal for Akt) or loss of the lipid phosphatase PTEN (which breaks down PIP3). Phosphorylation of Ser 485 would down-regulate AMPK in tumors containing hyperactivated Akt. Interestingly, this mechanism has been demonstrated to operate in human hepatoma cells infected with hepatitis C virus (HCV) (Mankouri et al. 2010). Tumorigenesis and viral infection bear certain analogies: In both cases, abnormal genes (activation of an oncogene or loss of a tumor suppressor in the first, insertion of the viral genome in the second) have taken over normal cellular functions and switched the cell from a quiescent state to one where there is active biosynthesis. The RNA genome of HCV encodes 10 viral proteins made by cleavage of a single polyprotein, and the virus also has a lipid envelope. Thus, replication of the virus in liver cells will require rapid protein and lipid synthesis and increase ATP turnover, which would be expected to activate AMPK (which would in turn down-regulate protein and lipid synthesis). However, when HCV-infected cells were studied, it was found that phosphorylation of AMPK at Thr 172 was reduced compared with uninfected controls. It was already known that one of the viral proteins (NS5A) binds and activates PI 3-kinase, thus switching on the Akt pathway (Street et al. 2004). Activation of Akt in virally infected cells was associated with phosphorylation of AMPK-α1 at the Akt site, Ser 485 (Mankouri et al. 2010). To confirm that this was responsible for the down-regulation of Thr 172 phosphorylation by the virus, the host cells were transiently transfected using DNAs encoding either wild-type α1, a potentially phospho-mimetic S485D mutant, or a nonphosphorylatable S485A mutant. Only in cells expressing the S485A mutant did the virus fail to replicate, suggesting that phosphorylation of Ser 485 on AMPK-α1 is necessary for viral replication. Interestingly, it was also found that this mechanism for down-regulating AMPK could be overcome by treating cells with metformin (Mankouri et al. 2010). This not only suggests that metformin could be a new treatment for chronic HCV infection, but also gives hope that metformin could be used to reverse AMPK down-regulation in tumors in which Akt is hyperactivated. Another intriguing feature of these results is that two of the complications of chronic HCV infection in humans are known to be hepatic steatosis (fatty liver) and hepatocellular carcinoma. Both might be expected to occur in cells in which AMPK was down-regulated, since AMPK switches off fatty acid and triglyceride synthesis, while, as discussed above, down-regulation of AMPK may be a prerequisite for rapid tumor growth.
Conclusions and perspectives
AMPK is a critical sensor of cellular energy in almost all eukaryotes. It appears to have arisen very early during eukaryotic evolution, where its ancestral role may have been in the response to starvation for the preferred carbon source. Although the classical allosteric activation of mammalian AMPK is only caused by AMP, it has recently been shown that ADP, as well as AMP, promotes activation by enhancing the phosphorylation of Thr 172, probably by both promoting phosphorylation and inhibiting dephosphorylation. This complex mechanism by which mammalian AMPK is regulated by increases in cellular ADP:ATP and AMP:ATP ratios means that it can respond in a dynamic, graduated manner over a very wide range of energy deficits. Although it is best known for its effects on metabolism, it is now clear that AMPK regulates almost all aspects of cellular function, including autophagy and maintenance of mitochondrial homeostasis, cell polarity, and cell growth and proliferation. Both tumor cells and viruses appear to have developed mechanisms to down-regulate AMPK and thus escape from its restraining influences on growth and biosynthesis.
Acknowledgments
Recent research in my laboratory has been funded by the Wellcome Trust and the pharmaceutical companies (AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck KGaA, and Pfizer) that support the Division of Signal Transduction in the College of Life Sciences.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.17420111.
References
- Amodeo GA, Rudolph MJ, Tong L 2007. Crystal structure of the heterotrimer core of Saccharomyces cerevisiae AMPK homologue SNF1. Nature 449: 492–495 [DOI] [PubMed] [Google Scholar]
- Apfeld J, O'Connor G, McDonagh T, Distefano PS, Curtis R 2004. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev 18: 3004–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC 2004. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116: 457–466 [DOI] [PubMed] [Google Scholar]
- Baena-Gonzalez E, Rolland F, Thevelein JM, Sheen J 2007. A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942 [DOI] [PubMed] [Google Scholar]
- Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA 2002. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK). J Cell Sci 115: 2433–2442 [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. 2006. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444: 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrends C, Sowa ME, Gygi SP, Harper JW 2010. Network organization of the human autophagy system. Nature 466: 68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendayan M, Londono I, Kemp BE, Hardie GD, Ruderman N, Prentki M 2009. Association of AMP-activated protein kinase subunits with glycogen particles as revealed in situ by immunoelectron microscopy. J Histochem Cytochem 57: 963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen A, Han XX, Habets DD, Febbraio M, Glatz JF, Luiken JJ 2007. A null mutation in skeletal muscle FAT/CD36 reveals its essential role in insulin- and AICAR-stimulated fatty acid metabolism. Am J Physiol Endocrinol Metab 292: E1740–E1749 [DOI] [PubMed] [Google Scholar]
- Browne GJ, Finn SG, Proud CG 2004. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem 279: 12220–12231 [DOI] [PubMed] [Google Scholar]
- Buttgereit F, Brand MD 1995. A hierarchy of ATP-consuming processes in mammalian cells. Biochem J 312: 163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J 2010. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab 11: 213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling D, Hardie DG 1989. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta 1012: 81–86 [DOI] [PubMed] [Google Scholar]
- Carlson M, Osmond BC, Botstein D 1981. Mutants of yeast defective in sucrose utilization. Genetics 98: 25–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE 1999. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443: 285–289 [DOI] [PubMed] [Google Scholar]
- Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, Mackintosh C 2008. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J 409: 449–459 [DOI] [PubMed] [Google Scholar]
- Ciriacy M 1977. Isolation and characterization of yeast mutants defective in intermediary carbon metabolism and in carbon catabolite repression. Mol Gen Genet 154: 213–220 [DOI] [PubMed] [Google Scholar]
- Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, et al. 2006. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab 3: 403–416 [DOI] [PubMed] [Google Scholar]
- Corton JM, Gillespie JG, Hawley SA, Hardie DG 1995. 5-Aminoimidazole-4-carboxamide ribonucleoside: a specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229: 558–565 [DOI] [PubMed] [Google Scholar]
- Cullen PJ, Sprague GF Jr 2000. Glucose depletion causes haploid invasive growth in yeast. Proc Natl Acad Sci 97: 13619–13624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale S, Wilson WA, Edelman AM, Hardie DG 1995. Similar substrate recognition motifs for mammalian AMP-activated protein kinase, higher plant HMG-CoA reductase kinase-A, yeast SNF1, and mammalian calmodulin-dependent protein kinase I. FEBS Lett 361: 191–195 [DOI] [PubMed] [Google Scholar]
- Davies SP, Carling D, Munday MR, Hardie DG 1992. Diurnal rhythm of phosphorylation of rat liver acetyl-CoA carboxylase by the AMP-activated protein kinase, demonstrated using freeze-clamping. Effects of high fat diets. Eur J Biochem 203: 615–623 [DOI] [PubMed] [Google Scholar]
- Davies SP, Helps NR, Cohen PTW, Hardie DG 1995. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2Cα and native bovine protein phosphatase-2AC. FEBS Lett 377: 421–425 [DOI] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, et al. 2011. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331: 456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X 2000. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 275: 223–228 [DOI] [PubMed] [Google Scholar]
- Estruch F, Treitel MA, Yang X, Carlson M 1992. N-terminal mutations modulate yeast SNF1 protein kinase function. Genetics 132: 639–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD 2005. Metformin and reduced risk of cancer in diabetic patients. BMJ 330: 1304–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty S, Hardie DG 2009. C-terminal phosphorylation of LKB1 is not required for regulation of AMP-activated protein kinase, BRSK1, BRSK2, or cell cycle arrest. J Biol Chem 284: 77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty S, Hawley SA, Green KA, Saner N, Mustard KJ, Hardie DG 2010. Calmodulin-dependent protein kinase kinase-β activates AMPK without forming a stable complex: synergistic effects of Ca2+ and AMP. Biochem J 426: 109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, Carling D, Guichard C, Ferré P, Foufelle F 1998. AMP-activated protein kinase Inhibits the glucose-activated expression of fatty acid synthase gene in rat hepatocytes. J Biol Chem 273: 14767–14771 [DOI] [PubMed] [Google Scholar]
- Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B 2010. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 120: 2355–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer LG, Parbu-Patel A, Carling D 2002. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct pathways. J Biol Chem 277: 25226–25232 [DOI] [PubMed] [Google Scholar]
- Gadalla AE, Pearson T, Currie AJ, Dale N, Hawley SA, Randall AD, Hardie DG, Frenguelli BG 2004. Distinct mechanisms underlie the activation of rat brain AMP-activated protein kinase and the inhibition of excitatory synaptic transmission by AICA riboside (Acadesine) in area CA1 of rat hippocampus. J Neurochem 88: 1272–1282 [DOI] [PubMed] [Google Scholar]
- Gledhill JR, Montgomery MG, Leslie AG, Walker JE 2007. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc Natl Acad Sci 104: 13632–13637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Brunet A 2009. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell 8: 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A 2007. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol 17: 1646–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ 2008. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG 2007. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8: 774–785 [DOI] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA 2001. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays 23: 1112–1119 [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D, Gamblin SJ 2011. AMP-activated protein kinase: also regulated by ADP? Trends Biochem Sci doi: 10.1016/j.tibs.2011.06.004 [DOI] [PubMed] [Google Scholar]
- Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG 1995. 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem 270: 27186–27191 [DOI] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG 1996. Characterization of the AMP-activated protein kinase kinase from rat liver, and identification of threonine-172 as the major site at which it phosphorylates and activates AMP-activated protein kinase. J Biol Chem 271: 27879–27887 [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG 2003. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG 2005. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2: 9–19 [DOI] [PubMed] [Google Scholar]
- Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, et al. 2010. Use of cells expressing gamma subunit variants to Identify diverse mechanisms of AMPK activation. Cell Metab 11: 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedbacker K, Carlson M 2008. SNF1/AMPK pathways in yeast. Front Biosci 13: 2408–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, et al. 1998. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 391: 184–187 [DOI] [PubMed] [Google Scholar]
- Herrero-Martin G, Hoyer-Hansen M, Garcia-Garcia C, Fumarola C, Farkas T, Lopez-Rivas A, Jaattela M 2009. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J 28: 677–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes BF, Kurth-Kraczek EJ, Winder WW 1999. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol 87: 1990–1995 [DOI] [PubMed] [Google Scholar]
- Honigberg SM, Lee RH 1998. Snf1 kinase connects nutritional pathways controlling meiosis in Saccharomyces cerevisiae. Mol Cell Biol 18: 4548–4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe S, Bierhoff H, Cado I, Weber A, Tiebe M, Grummt I, Voit R 2009. AMP-activated protein kinase adapts rRNA synthesis to cellular energy supply. Proc Natl Acad Sci 106: 17781–17786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M 2002. Activation of AMP-activated protein kinase leads to the phosphorylation of Elongation Factor 2 and an inhibition of protein synthesis. Curr Biol 12: 1419–1423 [DOI] [PubMed] [Google Scholar]
- Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A, Schlattner U, Wallimann T, Carling D, Hue L, et al. 2006. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase α-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem 281: 5335–5340 [DOI] [PubMed] [Google Scholar]
- Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL, McBurnie W, Fleming S, Alessi DR 2008. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J 412: 211–221 [DOI] [PubMed] [Google Scholar]
- Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG 2003. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr Biol 13: 861–866 [DOI] [PubMed] [Google Scholar]
- Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA 2005. The Ca2+/calmoldulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem 280: 29060–29066 [DOI] [PubMed] [Google Scholar]
- Hwang JT, Ha J, Park IJ, Lee SK, Baik HW, Kim YM, Park OJ 2007. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett 247: 115–121 [DOI] [PubMed] [Google Scholar]
- Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H 2001. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole- 4-carboxamide-1-β-d- ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun 287: 562–567 [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL 2003. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590 [DOI] [PubMed] [Google Scholar]
- Istvan ES, Deisenhofer J 2000. The structure of the catalytic portion of human HMG-CoA reductase. Biochim Biophys Acta 1529: 9–18 [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM 2007. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci 104: 12017–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, Muller O, Back W, Zimmer M 1998. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet 18: 38–43 [DOI] [PubMed] [Google Scholar]
- Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, et al. 2007. LKB1 modulates lung cancer differentiation and metastasis. Nature 448: 807–810 [DOI] [PubMed] [Google Scholar]
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB 2005. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 18: 283–293 [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, et al. 2004. The α2-5′AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes 53: 3074–3081 [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG 2005. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1: 15–25 [DOI] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL 2011. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13: 132–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koay A, Rimmer KA, Mertens HD, Gooley PR, Stapleton D 2007. Oligosaccharide recognition and binding to the carbohydrate binding module of AMP-activated protein kinase. FEBS Lett 581: 5055–5059 [DOI] [PubMed] [Google Scholar]
- Kola B, Boscaro M, Rutter GA, Grossman AB, Korbonits M 2006. Expanding role of AMPK in endocrinology. Trends Endocrinol Metab 17: 205–215 [DOI] [PubMed] [Google Scholar]
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, et al. 2005. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437: 1109–1114 [DOI] [PubMed] [Google Scholar]
- Kuchin S, Vyas VK, Carlson M 2002. Snf1 protein kinase and the repressors Nrg1 and Nrg2 regulate FLO11, haploid invasive growth, and diploid pseudohyphal differentiation. Mol Cell Biol 22: 3994–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW 1999. 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 48: 1667–1671 [DOI] [PubMed] [Google Scholar]
- Lane N, Martin W 2010. The energetics of genome complexity. Nature 467: 929–934 [DOI] [PubMed] [Google Scholar]
- Leclerc I, Kahn A, Doiron B 1998. The 5′-AMP-activated protein kinase inhibits the transcriptional stimulation by glucose in liver cells, acting through the glucose response complex. FEBS Lett 431: 180–184 [DOI] [PubMed] [Google Scholar]
- Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, et al. 2006. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 55: 2256–2264 [DOI] [PubMed] [Google Scholar]
- Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee SH, Shong M, Kim JM, Kim J, et al. 2007. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 447: 1017–1020 [DOI] [PubMed] [Google Scholar]
- Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, et al. 2011. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13: 376–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, et al. 2007. The energy sensing LKB1–AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol 9: 218–224 [DOI] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM 2005. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1: 361–370 [DOI] [PubMed] [Google Scholar]
- Lin YC, Hung CM, Tsai JC, Lee JC, Chen YL, Wei CW, Kao JY, Way TD 2010. Hispidulin potently inhibits human glioblastoma multiforme cells through activation of AMP-activated protein kinase (AMPK). J Agric Food Chem 58: 9511–9517 [DOI] [PubMed] [Google Scholar]
- Lizcano JM, Göransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Mäkelä TP, Hardie DG, et al. 2004. LKB1 is a master kinase that activates 13 protein kinases of the AMPK subfamily, including the MARK/PAR-1 kinases. EMBO J 23: 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M, Varela L, Vazquez MJ, Rodriguez-Cuenca S, Gonzalez CR, Velagapudi VR, Morgan DA, Schoenmakers E, Agassandian K, Lage R, et al. 2010. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med 16: 1001–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machovic M, Janecek Sh 2006. The evolution of putative starch-binding domains. FEBS Lett 580: 6349–6356 [DOI] [PubMed] [Google Scholar]
- Mackintosh RW, Davies SP, Clarke PR, Weekes J, Gillespie JG, Gibb BJ, Hardie DG 1992. Evidence for a protein kinase cascade in higher plants. 3-Hydroxy-3-methylglutaryl-CoA reductase kinase. Eur J Biochem 209: 923–931 [DOI] [PubMed] [Google Scholar]
- Mankouri J, Tedbury PR, Gretton S, Hughes ME, Griffin SD, Dallas ML, Green KA, Hardie DG, Peers C, Harris M 2010. Enhanced hepatitis C virus genome replication and lipid accumulation mediated by inhibition of AMP-activated protein kinase. Proc Natl Acad Sci 107: 11549–11554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L 2000. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol 10: 1247–1255 [DOI] [PubMed] [Google Scholar]
- Marsin AS, Bouzin C, Bertrand L, Hue L 2002. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J Biol Chem 277: 30778–30783 [DOI] [PubMed] [Google Scholar]
- Martin SG, St Johnston D 2003. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature 421: 379–384 [DOI] [PubMed] [Google Scholar]
- McGee SL, van Denderen BJ, Howlett KF, Mollica J, Schertzer JD, Kemp BE, Hargreaves M 2008. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes 57: 860–867 [DOI] [PubMed] [Google Scholar]
- Merrill GM, Kurth E, Hardie DG, Winder WW 1997. AICAR decreases malonyl-CoA and increases fatty acid oxidation in skeletal muscle of the rat. Am J Physiol 273: E1107–E1112 [DOI] [PubMed] [Google Scholar]
- Metallo CM, Walther JL, Stephanopoulos G 2009. Evaluation of 13C isotopic tracers for metabolic flux analysis in mammalian cells. J Biotechnol 144: 167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG, Downes M, Evans RM, Montminy M, Shaw RJ 2011. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell 145: 607–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB 2002. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415: 339–343 [DOI] [PubMed] [Google Scholar]
- Miranda-Saavedra D, Stark MJ, Packer JC, Vivares CP, Doerig C, Barton GJ 2007. The complement of protein kinases of the microsporidium Encephalitozoon cuniculi in relation to those of Saccharomyces cerevisiae and Schizosaccharomyces pombe. BMC Genomics 8: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouse V, Swick LL, Kazgan N, St Johnston D, Brenman JE 2007. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J Cell Biol 177: 387–392 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Momcilovic M, Hong SP, Carlson M 2006. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem 281: 25336–25343 [DOI] [PubMed] [Google Scholar]
- Mooney MH, Fogarty S, Stevenson C, Gallagher AM, Palit P, Hawley SA, Hardie DG, Coxon GD, Waigh RD, Tate RJ, et al. 2008. Mechanisms underlying the metabolic actions of galegine that contribute to weight loss in mice. Br J Pharmacol 153: 1669–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, Brozinick JT, Valladares O, Bucan M, Birnbaum MJ 2001. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7: 1085–1094 [DOI] [PubMed] [Google Scholar]
- Narbonne P, Roy R 2006. Inhibition of germline proliferation during C. elegans dauer development requires PTEN, LKB1 and AMPK signalling. Development 133: 611–619 [DOI] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, et al. 2008. AMPK and PPARδ agonists are exercise mimetics. Cell 134: 405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakhill JS, Chen ZP, Scott JW, Steel R, Castelli LA, Ling N, Macaulay SL, Kemp BE 2010. β-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK). Proc Natl Acad Sci 107: 19237–19241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, Kemp BE 2011. AMPK is a direct adenylate charge-regulated protein kinase. Science 332: 1433–1435 [DOI] [PubMed] [Google Scholar]
- Owen MR, Doran E, Halestrap AP 2000. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348: 607–614 [PMC free article] [PubMed] [Google Scholar]
- Pan DA, Hardie DG 2002. A homologue of AMP-activated protein kinase in Drosophila melanogaster is sensitive to AMP and is activated by ATP depletion. Biochem J 367: 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehmoller C, Treebak JT, Birk JB, Chen S, Mackintosh C, Hardie DG, Richter EA, Wojtaszewski JF 2009. Genetic disruption of AMPK signaling abolishes both contraction- and insulin-stimulated TBC1D1 phosphorylation and 14-3-3 binding in mouse skeletal muscle. Am J Physiol Endocrinol Metab 297: E665–E675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, et al. 2003. AMPK β-subunit targets metabolic stress-sensing to glycogen. Curr Biol 13: 867–871 [DOI] [PubMed] [Google Scholar]
- Polekhina G, Gupta A, van Denderen BJ, Feil SC, Kemp BE, Stapleton D, Parker MW 2005. Structural basis for glycogen recognition by AMP-activated protein kinase. Structure 13: 1453–1462 [DOI] [PubMed] [Google Scholar]
- Polge C, Thomas M 2007. SNF1/AMPK/SnRK1 kinases, global regulators at the heart of energy control? Trends Plant Sci 12: 20–28 [DOI] [PubMed] [Google Scholar]
- Romero-Perez AI, Lamuela-Raventos RM, Andres-Lacueva C, de La Torre-Boronat MC 2001. Method for the quantitative extraction of resveratrol and piceid isomers in grape berry skins. Effect of powdery mildew on the stilbene content. J Agric Food Chem 49: 210–215 [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Goransson O, Hardie DG, Alessi DR 2004. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab 287: E310–E317 [DOI] [PubMed] [Google Scholar]
- Sakamoto K, McCarthy A, Smith D, Green KA, Hardie DG, Ashworth A, Alessi DR 2005. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J 24: 1810–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM, Westra WH, Herman JG, Sidransky D 2002. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res 62: 3659–3662 [PubMed] [Google Scholar]
- Schmidt MC, McCartney RR 2000. β-subunits of Snf1 kinase are required for kinase function and substrate definition. EMBO J 19: 4936–4943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, Norman DG, Hawley SA, Kontogiannis L, Hardie DG 2002. Protein kinase substrate recognition studied using the recombinant catalytic domain of AMP-activated protein kinase and a model substrate. J Mol Biol 317: 309–323 [DOI] [PubMed] [Google Scholar]
- Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG 2004. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest 113: 274–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC 2004. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci 101: 3329–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahmann N, Woods A, Carling D, Heller R 2006. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase β. Mol Cell Biol 26: 5933–5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahmann N, Woods A, Spengler K, Heslegrave A, Bauer R, Krause S, Viollet B, Carling D, Heller R 2010. Activation of AMP-activated protein kinase by vascular endothelial growth factor mediates endothelial angiogenesis independently of nitric-oxide synthase. J Biol Chem 285: 10638–10652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein SC, Woods A, Jones NA, Davison MD, Carling D 2000. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J 345: 437–443 [PMC free article] [PubMed] [Google Scholar]
- Street A, Macdonald A, Crowder K, Harris M 2004. The hepatitis C virus NS5A protein activates a phosphoinositide 3-kinase-dependent survival signaling cascade. J Biol Chem 279: 12232–12241 [DOI] [PubMed] [Google Scholar]
- Sugden C, Crawford RM, Halford NG, Hardie DG 1999. Regulation of spinach SNF1-related (SnRK1) kinases by protein kinases and phosphatases is associated with phosphorylation of the T loop and is regulated by 5′-AMP. Plant J 19: 433–439 [DOI] [PubMed] [Google Scholar]
- Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D 2006. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem 281: 32207–32216 [DOI] [PubMed] [Google Scholar]
- Tamas P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, Cantrell DA 2006. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med 203: 1665–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander M, Olsson T, Ronne H 2004. Snf1-related protein kinase 1 is needed for growth in a normal day-night light cycle. EMBO J 23: 1900–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townley R, Shapiro L 2007. Crystal structures of the adenylate sensor from fission yeast AMP-activated protein kinase. Science 315: 1726–1729 [DOI] [PubMed] [Google Scholar]
- Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, Taketo MM, Cooney GJ, Kraegen EW, James DE, et al. 2008. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes 57: 1414–1418 [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RC, Levine B 2010. Autophagy in cellular growth control. FEBS Lett 584: 1417–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wilson WA, Fujino MA, Roach PJ 2001. Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol Cell Biol 21: 5742–5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weekes J, Ball KL, Caudwell FB, Hardie DG 1993. Specificity determinants for the AMP-activated protein kinase and its plant homologue analysed using synthetic peptides. FEBS Lett 334: 335–339 [DOI] [PubMed] [Google Scholar]
- Wilson WA, Hawley SA, Hardie DG 1996. The mechanism of glucose repression/derepression in yeast: SNF1 protein kinase is activated by phosphorylation under derepressing conditions, and this correlates with a high AMP:ATP ratio. Curr Biol 6: 1426–1434 [DOI] [PubMed] [Google Scholar]
- Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO 2000. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol 88: 2219–2226 [DOI] [PubMed] [Google Scholar]
- Wingo SN, Gallardo TD, Akbay EA, Liang MC, Contreras CM, Boren T, Shimamura T, Miller DS, Sharpless NE, Bardeesy N, et al. 2009. Somatic LKB1 mutations promote cervical cancer progression. PLoS ONE 4: e5137 doi: 10.1371/journal.pone.0005137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D 2003. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 13: 2004–2008 [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D 2005. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2: 21–33 [DOI] [PubMed] [Google Scholar]
- Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, Walker PA, Haire L, Eccleston JF, Davis CT, et al. 2007. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature 449: 496–500 [DOI] [PubMed] [Google Scholar]
- Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, et al. 2011. Structure of mammalian AMPK and its regulation by ADP. Nature 472: 230–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. 2002. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 6: 1288–1295 [DOI] [PubMed] [Google Scholar]
- Zhang L, Li J, Young LH, Caplan MJ 2006. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci 103: 17272–17277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Cantley LC 2007. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci 104: 819–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Jeong JH, Asara JM, Yuan YY, Granter SR, Chin L, Cantley LC 2009. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol Cell 33: 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. 2001. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann FK, Kaufmann I, Rasenberger H, Haussman P 1977. Genetics of carbon catabolite repression in Saccharomyces cerevisiae: genes involved in the derepression process. Mol Gen Genet 151: 95–103 [DOI] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM 2011. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12: 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI 2002. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci 99: 15983–15987 [DOI] [PMC free article] [PubMed] [Google Scholar]