Abstract
Genetic testing is a term used in different settings, often with very different meanings. There are only very few studies published about the various possible definitions of “genetic testing”, and evidence is lacking from its use in professional practise. The need for precise definitions is particularly felt when producing legislation, policy recommendations or professional guidelines. EuroGentest Unit 3 (Clinical, Community and Public Health Genetics) had, as one of its objectives, to analyse definitions of “genetic testing” and propose consensus working definitions, if possible. To assess what was meant when using this term, in each individual professional context, a questionnaire was developed to evaluate if a consensus definition was desirable and achievable and what items or information should be included in the scope of such a definition. The questionnaire was sent to all EuroGentest partners and other registered users of its website; 135 answers were received, a response rate of 22%. The need for a consensus definition was acknowledged by the vast majority, although there was much less concordance about the possibility of attaining one. Clinical geneticists were the most supportive for context-dependent definitions. Conflicting perspectives arose, however, when discussing the inclusion of some type of tests, material or technology used. At issue seemed to be the distinction between the concepts of genetic material-based testing and genetic information.
Keywords: DNA-based genetic testing, Genetic information, Molecular genetic testing, Cytogenetics, Biochemical genetics, Family history
Introduction
Genetic testing is a term used in many different settings (from medical care and epidemiology research to the insurance business and regulatory agencies), often with very different meanings. There are very few studies published about the possible definitions of ‘genetic testing’ (Holtzman 1994; Harper 1997; Zimmern 1999), and evidence is still lacking from its use in daily practise.
The need for a specific definition of ‘genetic testing’ is particularly felt when producing legislation, policy recommendations or professional guidelines. For example, the document from a European Commission (EC) expert group (McNally et al. 2004) acknowledges the existence of numerous definitions, and stresses, as its very first recommendation, the need that ‘any official statement or position should refer precisely to an explicit definition of the terms used’. Furthermore, the document suggests that ‘a consensus definition of genetic testing should be developed globally by all respective public and private bodies involved (including the World Health Organisation, the Organisation for Economic Co-operation and Development, the European Commission, the International Federation of Genetic Societies, and the International Conference on Harmonisation)’. Additionally, the suggestion was made that the EC should take the initiative in that matter.
For the purpose of that document, a broad definition of genetic testing was used: ‘any test that yields genetic data’, including germinal and somatic genetic data. Nevertheless, its focus was on genetic information transmissible at the germinal level, pertaining to heritable diseases or traits, recommending a specific working group to be set up to discuss the issues relevant to genetic testing for acquired genetic properties.
Genetic information is often viewed as a different kind of health/medical information, either because of a deterministic understanding of its significance or the perennial status that is frequently associated to it. But, genetic information is part of the entire spectrum of all health information and, for some, it should not represent a separate category. High standards of quality and confidentiality should be met, at all times, concerning all medical data, including genetic information.
EuroGentest (EUGT) is a Network of Excellence of the EC-funded Sixth Framework Programme with five units looking at all aspects of genetic testing (www.eurogentest.org). Through a series of initiatives, EUGT encourages the harmonisation of standards and practise in these areas throughout the EU and beyond. Unit 3 (Clinical, Community and Public Health Genetics) of EUGT had, as one of its objectives, to analyse the various definitions of ‘genetic testing’ adopted by different organisations (professional, governmental, international) and propose consensus definitions, if possible (Sequeiros and Guimarães 2007; Varga and Sequeiros 2009).
The purpose of this work was to obtain evidence needed to address directly the recommendation of the EC expert group, and aimed at contributing to the discussion of such consensus definitions and its applicability, by assessing specific parameters and components in the definitions of ‘genetic testing’, among persons associated with EUGT.
Materials and methods
To assess what was meant when using the expression ‘genetic testing’ in each professional context, a questionnaire was developed and sent to all EUGT partners (unit leaders, project participants and researchers, invited experts and members of the external advisory board) and to all other registered users of the website (other professionals with different backgrounds working in fields related to genetic testing).
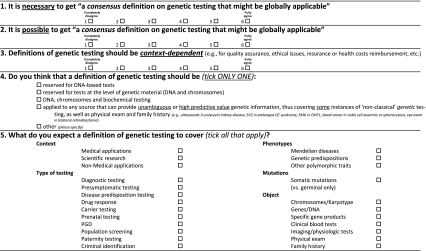
The questionnaire consisted of two pages (five questions and a space for free remarks) and was preceded by a brief explanation of its aims. Time consumed should have been under 10–15 min. Questions are shown as Table 1. Answers to questions 1 to 3 were classified as ‘completely or partially disagree’ (when graded 1–3) and as ‘completely or partially agree’ (if graded 4–6).
Table 1.
Questions in the survey
A link to the questionnaire was sent by email, by the EUGT informatics team, to all users of the website (614 registered, at the time). Two reminders were then sent, 2 weeks and 2 months after the first email.
All answers were compared between (1) EUGT partners or external registered users of its website, (2) medical doctors (MDs) vs. non-MDs, (3) geneticists vs. non-geneticists, (4) clinical geneticists vs. other MDs and (5) clinical vs. laboratory geneticists.
The statistical analysis was performed using the SPSS software package; proportions were compared with the Pearson chi-square test (questions 4 and 5), and trends with the linear-by-linear association (questions 1 to 3).
Results
We received 135 filled questionnaires (126 validated), which represents a 22% response rate. Of these, 58 were from EUGT partners and 68 were other users of the EUGT website. Respondents were from 32 countries (Table 2). Their professional background corresponded to 44 MDs (24 clinical geneticists and 20 other MDs) and 82 non-MDs (58 laboratory geneticists and 24 other professionals—genetic counsellors, lawyers, ethicists, sociologists, economists, regulators, etc.).
Table 2.
Distribution of respondents by country
| Country | Respondents | Country | Respondents |
|---|---|---|---|
| Argentina | 1 | Italy | 13 |
| Belgium | 13 | Japan | 1 |
| Bulgaria | 1 | Malta | 1 |
| Canada | 1 | Netherlands | 6 |
| Croatia | 3 | Norway | 2 |
| Czech Republic | 4 | Poland | 3 |
| Denmark | 3 | Portugal | 2 |
| Ecuador | 2 | Rep. Macedonia | 1 |
| Egypt | 1 | Romania | 1 |
| Finland | 4 | Serbia | 2 |
| France | 4 | Spain | 16 |
| Germany | 7 | Sweden | 1 |
| Greece | 1 | Switzerland | 6 |
| Hungary | 3 | Turkey | 6 |
| Iceland | 1 | UK | 13 |
| Ireland | 1 | USA | 2 |
Are consensus definitions on genetic testing necessary and possible? Should they be context-dependent?
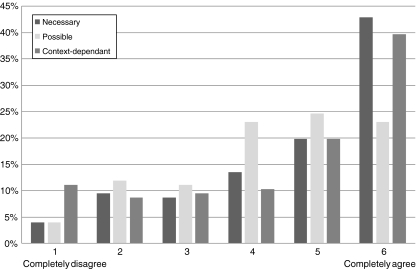
When asked if a consensus definition of genetic test was needed (question 1), more agreed (76.2%) than not (p < 0.0001, Fig. 1), and 42.9% completely agreed. In particular, external users of the website agreed more than EUGT partners (p = 0.002).
Fig. 1.
Distribution of answers to questions 1–3: (1) ‘Is it necessary to get a consensus definition on genetic testing?’ (n = 124); (2) ‘Is it possible to get a consensus definition on genetic testing?’ (n = 123) and (3) ‘Should such definition be context-dependant?’ (n = 125)
Regarding question 2, a majority agreed (70.6%), but only 23% completely agreed it was possible to achieve a consensus definition (p < 0.0001, Fig. 1); significantly fewer felt that it was possible, than needed—question 1 (p < 0.0001). EUGT partners disagreed more (p = 0.001); other professionals (social scientists, economists, etc.) were less agreeable than laboratory geneticists (p = 0.003).
The feeling that such a definition should be context dependent (question 3) prevailed, answers approaching those to question 1 (p < 0.0001; Fig. 1). Physicians appeared to be more in favour than non-physicians and clinical geneticists more so than laboratory geneticists (although this did not reach statistical significance).
Scope of genetic testing and sources of genetic information
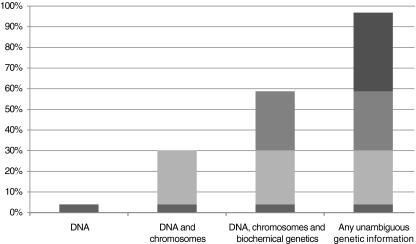
In question 4, only five respondents (4%) thought that ‘genetic testing’ should be reserved to DNA-based testing. About one third of the respondents chose DNA or DNA and chromosomal analysis; about two thirds included those as well as biochemical genetics tests; 38%, however, included also in the definition any unambiguous genetic information, independently of its origin. A majority of respondents were thus in favour of a broader definition (p < 0.0001, Fig. 2); though not statistically significant, EUGT partners, physicians and, in particular, clinical geneticists seemed to be more in favour of a broader definition.
Fig. 2.
Distribution of answers to question 4 (n = 126), concerning ‘What tests/information should be covered by the definition of genetic test’. The representation is cumulative, each category comprising the sum of its answers with the previous ones; 3.2% proposed an alternative framework for the definition of genetic testing
The ‘scope of genetic testing’ was also ascertained in question 5, when inquiring professionals what they expected such a definition to cover. Five major areas were evaluated.
Context
One fourth of all respondents would leave out scientific research (p < 0.0001, if comparing to medical applications only); 43% would include also non-medical applications (p < 0.0001); there were no differences according to the respondents background.
Type of tests
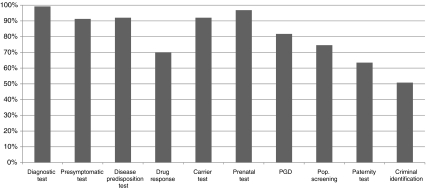
Concordance with diagnostic tests (99.2%) was used as comparison: presymptomatic (PST), carrier, prenatal (PND) and susceptibility testing were also chosen by over 90% of respondents (Fig. 3); on the other hand, preimplantation genetic diagnosis (PGD) was picked only by 82% (p < 0.0001). Also, pharmacogenetic testing—approximately 70% (p < 0.0001), population screening—75% (p < 0.0001), paternity testing—64% (p < 0.0001) and criminal identification—51% (p < 0.0001), were less often answered. PST (p = 0.006), susceptibility (p = 0.023) and carrier testing (p = 0.023) were also less perceived as ‘genetic testing’ (mostly due to non-geneticists), whereas there was no difference for PND relatively to a diagnostic test. PGD was less perceived as ‘genetic testing’ by non-geneticists MDs (p = 0.047). Population screening was less considered as ‘genetic testing’ by non-EUGT partners (p = 0.025) and non-geneticists (p = 0.045; due to non-geneticists MDs—p = 0.036). Other MDs (n.s.) and non-EUGT partners (p = 0.016) tended also to see pharmacogenetics less as ‘genetic testing’. For most clinical geneticists, all these were ‘genetic testing’.
Fig. 3.
Distribution of answers to question 5b—‘Type of tests’ that should be included in the definition of genetic testing (n = 126; PST presymptomatic, PND prenatal, PGD preimplantation)
Phenotypes
Testing for Mendelian diseases was included in the scope by virtually all, but testing for genetic predispositions was less often so (88.1%, p < 0.0001) and testing for other polymorphic traits was even less (61.1%, p < 0.0001). Clinical geneticists were more in favour of including also genetic predispositions (n.s.) and other polymorphic traits (p = 0.042).
Mutations (germinal vs. somatic)
Somatic mutations were included by 73.0%, overall, but only by 69% EUGT partners (n.s.), 61.4% MDs (p = 0.031) and 62.5% clinical geneticists (n.s.).
Object of the test
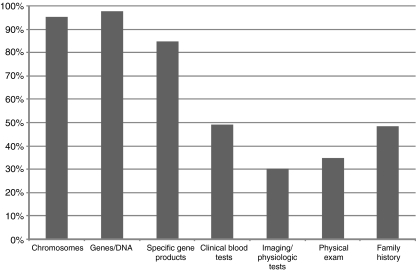
Genes and DNA (97.6%) were more perceived as the material of ‘genetic testing’ than chromosomes (95.2%, n.s.), gene products (84.9%, p < 0.0001), other blood tests (49.2%, p < 0.0001), family history (48.4%, p < 0.0001), physical exam (34.9%, p < 0.0001) or physiological tests/imaging (30.2%, p < 0.0001) (Fig. 4). EUGT partners, physicians and, mainly, clinical geneticists tended to include these other sources of genetic information, as well.
Fig. 4.
Distribution of answers to question 5e—‘Object’ (material/method used to derive genetic information) that should be included in the definition (n = 126). Two modal sets are clear, corresponding to concepts of ‘genetic-material based testing’ (first three items) and of ‘genetic information’ (the previous plus the last four items)
Discussion
In the health-care context, the term ‘genetic testing’ is often used to refer to the detection of the presence or absence of, or alteration in, a particular gene, chromosome or gene product (ACGT 1998). A different definition had been proposed before (Harper 1997): the analysis of a specific gene, its product or function, or other DNA and chromosome analysis, to detect or exclude an alteration likely to be associated with a genetic disorder. Zimmern (1999) discusses two different usages of ‘genetic information’, and distinguishes ‘gene tests’ (DNA or chromosomes) from ‘genetic testing’, this including also any clinical, haematological, radiological or biochemical test, from which information about the gene or the inheritability of a disorder to be inferred. In fact, in some instances, even a detailed physical examination or a family history can yield genetic information with high predictive value; the understanding of what a genetic test is, however, varies considerably, even among genetics professionals.
Tests on nucleic acids are performed in many medical areas, other than clinical genetics, and may share technical approaches, in particular molecular biology techniques. Not all medical tests based on genetic material (as those testing non-human genetic material and non-genetic disorders, e.g., viral and bacterial infections) are medical genetics tests. The most sensitive issues when defining ‘genetic testing’ concern mainly heritable changes and genetic disorders, as its psycho-social impact is potentially greater, particularly in asymptomatic persons.
To the best of our knowledge, this is the first report presenting any evidence about the usage of the term ‘genetic testing’ among the various professionals who are more involved with it. The practise of medical genetics, in general, and of genetic testing, in particular, involves many different professionals of various backgrounds, including clinical and laboratory geneticists, genetic counsellors and nurses, clinical psychologists, bioethicists, social scientists and lawyers.
Therefore, the question was if the working setting and background would yield differences in what should be covered by the definition of ‘genetic testing’, and if the need for a consensus (all-purpose) definition was felt, and if this was possible to attain.
Although this sample may not be representative of the genetics professionals working in this field, and potential biases exist, the answers received are still illustrative of the diversity of opinions existing and of the specific issues that may or may not gather agreement.
The need for a consensus definition was acknowledged by all professional groups, although these were also somewhat sceptical about the possibility of achieving one (Fig. 1). Two reasons that may explain this are the very diverse settings in which such a definition would have to apply and stand, and a concern that it would be too vague and, therefore, useless. This may also explain why all groups and, in particular, clinical geneticists would be more in favour of context-dependent definitions.
Most professionals tended to consider also other means of genetic information (Fig. 2), though laboratory geneticists were less supportive of including sources other than DNA and cytogenetics tests. The general consensus was that non-medical applications and genetic tests in a research setting should not be covered (most respondents were professionally involved mainly with the application of medical genetics tests and, thus, biased in that regard). Many genetic tests, particular for rare diseases, are developed in research laboratories; though not recommended, (OECD 2007), on occasion, results obtained in a research setting are used for clinical (diagnostic and counselling) purposes.
There was also no consensus regarding the type of tests to be covered in this definition. Diagnostic, PST, PND, carrier and susceptibility testing gathered a consensus (more than 90%; Fig. 3). PGD, however, was less seen as a genetic test than prenatal diagnosis; pharmacogenetics and population genetic screening were also less consensual (all well under 90%). If the first does not address directly (a diagnosis or carrier status for) a genetic disorder, the second does, though outside the context of a family-centred genetic diagnosis and counselling. Forensic tests were considered by more than half of the respondents, but well below other tests.
Both monogenic disorders and susceptibility testing met a vast consensus; however, non-disease phenotypes would be excluded by 50%. Somatic mutations belonged within the definition only for 3/4; but, laboratory geneticists were much more in favour of it than clinical geneticists and other MDs.
DNA, chromosomes and gene products were almost unanimously seen as within the scope of ‘genetic testing’. On the other hand, other possible sources of genetic information were perceived as covered by almost half of the clinical geneticists, but significantly less so by the other groups.
Context-dependent definitions could be useful and convenient, as they would be more suited for practical purposes and everyday decision making in a specific setting, as for legal or regulatory purposes (Sequeiros 2010). Some clinical and family information may suggest a genotype or disclose persons at risk; forgetting it in a definition in legal documents that may lead to continued discrimination, e.g., in employment or insurance.
Attaining a consensus definition of ‘genetic testing’ is a difficult endeavour. But, if this is to be taken, it should undoubtedly cover (1) medical applications and, namely, (2) diagnostic, PST, carrier, susceptibility testing, PND and PGD; (3) Mendelian diseases and genetic predispositions; and (4) cytogenetics, molecular and biochemical genetics. More questionable is if (5) research-based tests, (6) pharmacogenetics, (7) population screening, (8) somatic mutations and (9) other tests/exams not based on genetic material, though also of high predictive value, should fall within its scope, as well. The issue may be more on the distinction between the concept of ‘genetic-material based testing’ and that of ‘genetic information’.
Acknowledgements
EuroGentest—Harmonizing Genetic Testing across Europe is a European Commission-funded Network of Excellence (http://www.eurogentest.org/). The authors wish to thank all respondents, and Ségoléne Aymée for her review of the survey form. We thank also Nick Nagels, web manager for EuroGentest for his availability and proficient work.
References
- Report on genetic testing for late-onset disorders. London: Health Departments of the UK; 1998. [Google Scholar]
- Harper PS. What do we mean by genetic testing? J Med Genet. 1997;34:749–752. doi: 10.1136/jmg.34.9.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman N. Benefits and risks of emerging genetic technologies: the need for regulation. Clin Chem. 1994;40:1652–1657. [PubMed] [Google Scholar]
- McNally E, Cambon-Thomsen A, Brazell C, Cassiman JJ, Kent A, Lindpaintner K, Lobato de Faria P, Niese D, Abbing HR, Solbakk JH, Tack H, Tambuyzer E, Weihrauch TR, Wendel E. 25 Recommendations on the ethical, legal and social implications of genetic testing, Brussels, Directorate-General for research. Luxembourg: Office for Official Publications of the European Communities; 2004. [Google Scholar]
- OECD (2007) OECD guidelines for quality assurance in molecular genetic testing, organisation for economic co-operation and development. Directorate for Science, Technology and Industry, 2007; at: http://www.oecd.org/dataoecd/43/6/38839788.pdf
- Sequeiros J (2010) Regulating genetic testing: the relevance of appropriate definitions. Chapter 3. In: Kristoffersson U, Schmidtke J, Cassiman J-J (eds) Quality issues in clinical genetic services, Springer (ISBN: 978-90-481-3918-7)
- Sequeiros J, Guimarães B (2007) Definitions of Genetic Testing. EuroGentest Unit 3 (WP3.4); available at: http://www.eurogentest.org/web/info/public/unit3/DefinitionsGeneticTesting-3rdDraf18Jan07.xhtml
- Varga O, Sequeiros J (2009) Definitions of genetic testing in European and other legal documents. EuroGentest Unit 3 (WP3.4), 2009; available at: http://www.eurogentest.org/web/files/public/unit6/core_competences/BackgroundDocDefinitionsLegislationV10-FinalDraft.pdf [DOI] [PMC free article] [PubMed]
- Zimmern RL. Genetic testing: a conceptual exploration. J Med Ethics. 1999;25:151–156. doi: 10.1136/jme.25.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]