Abstract
Germline mutations in BRCA1 and BRCA2 confer high risks of breast and ovarian cancer, and their identification allows genetic testing of at-risk relatives. However, estimates of these risks illustrate controversies, depending on the published series. The penetrance, the earlier onset of the disease and the effect of mutations on the risk of developing breast and ovarian cancer were evaluated in 344 females belonging to 34 families from the Basque Country in Spain, in which BRCA1 or BRCA2 mutations were transmitted. Kaplan–Meier survival curves were used to derive cumulative probability curves for breast and ovarian cancer by mutation status, birth cohort and mutation position, and significance of the differences was assessed using the log-rank test. The estimated probability for breast cancer by age 70 is about 64% in BRCA1 and 69% in BRCA2, whereas the probability of developing ovarian cancer is about 37% and 25% for BRCA1 and BRCA2, respectively. There is a marginally significant higher risk of developing ovarian cancer in BRCA1 families than in BRCA2 families. The effect of birth cohort on breast cancer cumulative incidence presents an increased risk for females born after 1966 and a decreased risk for those born before 1940. There is no association between mutation position and breast cancer; however, ovarian cancer is associated to BRCA1, presenting exon 11 as an ovarian cluster. These results are important for the breast and ovarian cancer diagnosis and prevention in at-risk families.
Keywords: Breast cancer, Ovarian cancer, Penetrance, Cumulative risk
Introduction
Since the identification of the genes BRCA1 (Miki et al. 1994) and BRCA2 (Wooster et al. 1995) more than 10 years ago, a large number of studies concerning the penetrance, the earlier onset of the disease and the effect of mutations on the risk of breast and ovarian cancer in women carrying a disease-causing mutation in one of these genes have been published. The results of these studies show that the percentages vary significantly depending on the study series and populations and that the ranges are so broad that it is almost impossible to apply them to specific families or cases. For example, the estimated cumulative lifetime risk of developing breast cancer (BC) by 70 years of age for women from families with a disease-causing mutation in BRCA1 ranges from 52% to 68%, and in BRCA2 from 45% to 75% (Antoniou et al. 2003; Antoniou et al. 2006; Milne et al. 2008; Evans et al. 2008). The ranges for ovarian cancer (OC) are even broader: 22–60% for BRCA1 and 11–49% for BRCA2 by 70 years of age (Antoniou et al. 2003; Antoniou et al. 2006; Milne et al. 2008; Evans et al. 2008).
Some authors have reported that there could be different risks depending on the type of mutation (Al-Mulla et al. 2009). Furthermore, mutation position on the gene also seems to produce different risks for patients, although the number of such studies is relatively low and controversial (Thompson et al. 2001; Thompson et al. 2002; Gayther et al. 1995). The wide range of results observed, and the limited number of publications on this topic, inspired us to evaluate the risk of developing breast or OC, the earlier onset of the development of these cancers and the effect of mutation location in families diagnosed in our hospital. For the past 8 years, our laboratory has been responsible for the genetic study of BC/OC in high-risk patients (Nice 2004). The patients studied reside in the community covered by our hospital—belonging to the Public Health Service—essentially the western half of the Basque Country in Spain. We showed in a previous report (Beristain et al. 2007) that this population contains no recurring mutations, which means that it differs from other series (Díez et al. 2003; Krajc et al. 2008), and this is why we consider this study of interest. A greater understanding of the risks is, in any case, useful for the correct handling of such families in terms of both early diagnosis of the disease and its prevention and genetic counselling.
Materials and methods
Subjects
Since 2001, our laboratory has studied BC and/or OC patients from our community, after informed consent, with high genetic risk factors (Nice 2004). The inclusion criteria adopted for genetic testing have been previously described (Beristain et al. 2007). As this work demonstrated, the majority of cases with pathological mutations were found in families with multiple affected individuals.
In this work, we have included most of the families in which a pathological mutation was formerly detected and others found later, using similar criteria. The first individual in whom a pathological mutation was identified was considered the “index” case. For each index case, the individual’s family members were also assessed and, if possible, genetic testing was undertaken. A pedigree was drawn up and relatives were asked about the occurrence of breast, ovarian and other cancers in their family and the age at which it was diagnosed.
In total, 34 of the families were included in the present study: 16 whose index case was a BRCA1 mutation carrier and 18 whose index case was a BRCA2 mutation carrier (Table 1). Twenty eight of the families had three or more individuals with BC and/or OC, four families had two individuals with BC and/or OC, and finally, there were two early-onset sporadic cases, one of them presenting various cases of associated cancers in her family. After the exclusion of all males and those girls under 18 years of age, a data set of 155 female relatives of a BRCA1 mutation carrier and 189 female relatives of a BRCA2 mutation carrier was created. Of the 155 females belonging to BRCA1 families, 40 (26%) were found to be carriers of the mutation, 22 (14%) were negative and 93 (60%) had unknown mutation status. Similarly, of the 189 BRCA2 relatives, 50 (26%) were mutation-positive, 25 (13%) were mutation-negative and 114 (60%) had unknown mutation status (Table 2).
Table 1.
Families analysed and characteristics of mutations, including the classification according to mutation position
| Gene | Family | Mutationa | Type | Nucleotide | Codon | Codon stop | A | B | C |
|---|---|---|---|---|---|---|---|---|---|
| BRCA1 | 46,61,78,85 | c.211A>G | S | 211 | 71 | 64 | 1 | 1 | 1 |
| BRCA1 | 57 | c.671-?_4675+?del | LR | 671 | 224 | 1 | 1 | 1 | |
| BRCA1 | 41 | c.798_799delTT | FS | 798 | 266 | 285 | 1 | 1 | 1 |
| BRCA1 | 81 | c.2017G>T (p.E673X) | N | 2017 | 673 | 673 | 1 | 1 | 2 |
| BRCA1 | 77 | c.3257T>G (p.L1086X) | N | 3257 | 1086 | 1086 | 1 | 2 | 2 |
| BRCA1 | 86,92 | c.3331_3334delCAAG | FS | 3331 | 1111 | 1115 | 1 | 2 | 2 |
| BRCA1 | 50 | c.4161_4162delTC | FS | 4161 | 1387 | 1389 | 1 | 2 | 2 |
| BRCA1 | 6 | c.5117G>A (p.G1706E) | M | 5117 | 1706 | 2 | 3 | 3 | |
| BRCA1 | 40,80 | c.5123C>A (p.A1708E) | M | 5132 | 1708 | 2 | 3 | 3 | |
| BRCA1 | 47 | c.5153-1G>A | S | 5153 | 1718 | 1729 | 2 | 3 | 2 |
| BRCA1 | 79 | c.5418delA | FS | 5418 | 1806 | 1833 | 2 | 3 | 2 |
| BRCA2 | 99 | c.2701delC | FS | 2701 | 901 | 903 | 2 | 2 | 1 |
| BRCA2 | 37 | c.2774_2775insT | FS | 2774 | 925 | 935 | 2 | 2 | 1 |
| BRCA2 | 7 | c.2808_2811delACAA | FS | 2808 | 936 | 958 | 2 | 2 | 1 |
| BRCA2 | 90 | c.4133_4136delCTCA | FS | 4133 | 1378 | 1386 | 1 | 1 | 2 |
| BRCA2 | 104 | c.4935_4938delGAAA | FS | 4935 | 1646 | 1668 | 1 | 1 | 2 |
| BRCA2 | 38 | c.5042_5043delTG | FS | 5042 | 1681 | 1687 | 1 | 1 | 2 |
| BRCA2 | 103 | c.5116_5119delAATA | FS | 5116 | 1706 | 1710 | 1 | 1 | 2 |
| BRCA2 | 1 | c.5141_5144delATTT | FS | 5141 | 1714 | 1723 | 1 | 1 | 2 |
| BRCA2 | 51 | c.5146_5149delTATG | FS | 5146 | 1716 | 1723 | 1 | 1 | 2 |
| BRCA2 | 58 | c.5576_5579delTTAA | FS | 5576 | 1859 | 1861 | 1 | 1 | 2 |
| BRCA2 | 43,89 | c.6275_6276delTT | FS | 6275 | 2092 | 2099 | 1 | 1 | 2 |
| BRCA2 | 64,84 | c.7007G>A (Exon12-13del) | FS | 7007 | 2336 | 2311 | 2 | 2 | 2 |
| BRCA2 | 102 | c.7601+1G>A | S | 7601 | 2 | 2 | 2 | ||
| BRCA2 | 3 | c.8167G>C (p.D2723H) | M | 8167 | 2723 | 2 | 2 | 3 | |
| BRCA2 | 26,91 | c.9026_9030del5 | FS | 9026 | 3009 | 3015 | 2 | 2 | 2 |
Table 2.
Individuals used in the study
| BRCA1 | BRCA2 | |||||
|---|---|---|---|---|---|---|
| Total N | With BC | With OC | Total N | With BC | With OC | |
| Tested − | 22 | 0 | 0 | 25 | 1 | 0 |
| Tested + | 40 | 20 | 7 | 50 | 22 | 4 |
| Probands | 16 | 14 | 2 | 16 | 14 | 3 |
| Familiars | 24 | 6 | 5 | 34 | 8 | 1 |
| Not tested | 93 | 22 | 8 | 114 | 36 | 5 |
| Total | 155 | 42 | 15 | 189 | 59 | 9 |
N number of patients, BC breast cancer, OC ovarian cancer
Screening for BRCA1 and BRCA2 mutations
The DNA extraction method and molecular analysis of the BRCA1 and BRCA2 genes have been previously described (Beristain et al. 2007). Briefly, the full coding sequences and each intron/exon boundary of the BRCA1 and BRCA2 genes were amplified by polymerase chain reaction (PCR) and conformational-sensitive gel electrophoresis (CSGE) using silver-staining as screening procedure. Sequence analysis of the genomic fragments with altered CSGE mobility pattern was carried out with an Applied Biosystems 3130xl automated DNA sequencer.
Classification of mutations by their position in the gene
For the present work, mutations were classified according to their position in the gene using three different classification techniques: two already suggested by Thompson et al. (2001, 2002) and Gayther et al. (1995), and a third one proposed by us. The first of these techniques (classification A) arranges BRCA1 mutations according to two groups or effects: effect 1, which ranges from codon 1 (exon 2) to codon 1395 (exon 13), and effect 2, which ranges from codon 1396 to the end of the gene. The second technique (classification B) sorts BRCA1 mutations into three effects (effect 1: codon 1–800; effect 2: codon 801–1397; and effect 3: codon 1398 to the end of the gene). Both classifications present the same two groups or effects for BRCA2 (effect 1: OCCR cluster, codon 1011–2210; effect 2: non-OCCR cluster). Finally, the third technique described in this paper (classification C) consists of three groups or effects for BRCA1 (effect 1: codon 1–621; effect 2: codon 622 to the end of the gene; and effect 3: missense mutations located in BRCT repeats) and three effects for BRCA2 (effect 1: codon 1–1139; effect 2: codon 1140 to the end of the gene; and effect 3: missense mutations determined as pathological by other authors) (BRCA1, GeneBank:NM_007294.2; BRCA2, GeneBank:NM_000059.3—numbering starts with the A of the ATG translation initiation codon) (Table 1).
Statistical methods
Data were analysed using survival methods to account for differences in the follow-up of each subject. Censoring age was the age at appropriate BC/OC diagnosis, age at last follow-up, age at death or age at appropriate risk-reducing surgery (oophorectomy for BC and OC; mastectomies are not performed in our Hospital).
Kaplan–Meier curves were derived to obtain the cumulative probability of BC and OC in BRCA1 and BRCA2 mutation carriers. To assess the presence of significant differences in the risk of cancer among the two genes, the log-rank statistical test was used. The same procedure (Kaplan–Meier curves together with log-rank test statistics) was used to assess differences in the cumulative probabilities between mutation status (positive, negative and unknown) separately for BRCA1 families and BRCA2 families. Moreover, the effect of birth cohort (cohort 1: 1916–1940; cohort 2: 1941–1965; cohort 3: 1966–1990) was assessed with these techniques to evaluate the presence of “anticipation” in the development of BC or OC, which, in this case, was carried out combining both BRCA1 and BRCA2 families. Finally, after excluding those females who were found not to be mutation carriers, these methods were applied to the whole sample with the aim of assessing whether the different position of a mutation might confer a higher risk of developing BC or OC. The three different classification techniques described above were applied to the mutation position to compare the utility of each one for cancer prediction. All analyses were carried out with and without probands; the former with the aim of obtaining penetrance estimates derived for the population being assessed, and the later for comparative purposes.
Results
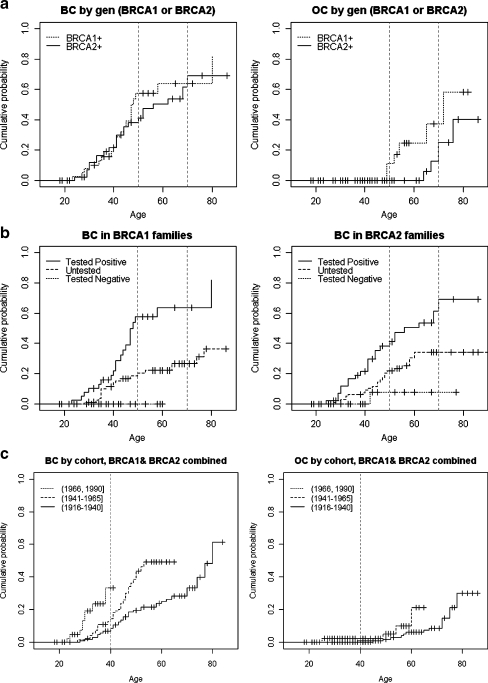
The cumulative incidence curves in BRCA1 and BRCA2 mutation carriers for BC and OC are shown in Fig. 1a, and the corresponding estimates at 50 and 70 years of age are given in Table 3 (model M1). The results indicate that the estimated probability for BC by age 70 is about 64% (39–78%) in BRCA1, a very similar value to that obtained for BRCA2 carriers (69%; 40–84%). A comparison of the results between curves according to the log-rank test (model M1 in Table 4) showed no significant differences in cancer risk by gene (p = 0.530). In contrast, the graphical representation for OC suggests differences between the curves, in particular from 50 years on, and these differences are marginally significant (p = 0.070), with a probability of developing OC of about 37% for BRCA1 and 25% for BRCA2 by the age of 70 years. Similar findings are observed without probands, even though penetrances are much lower than expected (see Tables 3 and 4).
Fig. 1.
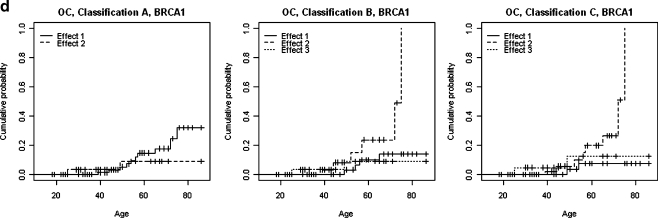
a Differences in the probability of carrier females developing BC and OC by gene mutation (BRCA1/BRCA2). b Differences in the probability of developing BC in carriers of BRCA1/BRCA2 mutations by test results. c Differences in the probability of developing BC and OC by cohort in carriers of BRCA1/BRCA2 mutations. d Association between BRCA1 and OC shown by three different classifications
Table 3.
Penetrance estimates derived from the Kaplan–Meier curves for Models 1 and 2
| Models | Breast cancer | Ovarian cancer | ||||||
|---|---|---|---|---|---|---|---|---|
| 50 years | 70 years | 50 years | 70 years | |||||
| With probands | Without | With probands | Without | With probands | Without | With probands | Without | |
| M1: by mutation (BRCA1 or BRCA2) in mutation-positive females | ||||||||
| BRCA1 | 58 (35,72) | 36 (5,57) | 64 (39,78) | 36 (05,57) | 11 (0,25) | 10 (0,27) | 37 (1,60) | 31 (0,56) |
| BRCA2 | 41 (23,55) | 26 (6,42) | 69 (40,84) | 38 (12,56) | 0 | 0 | 25 (0,48) | 0 |
| M2: between mutation-negative, mutation-positive and untested females | ||||||||
| BRCA1 | ||||||||
| − | 0 | 0 | – | – | 0 | 0 | 0 | 0 |
| Untested | 20 (10,29) | 20 (10,29) | 27 (15,37) | 27 (15,37) | 4 (0,9) | 4 (0,9) | 9 (1,16) | 9 (1,16) |
| + | 58 (35,72) | 36 (5,57) | 64 (39,78) | 36 (5,57) | 11 (0,25) | 10 (0,27) | 37 (1,60) | 31 (0,56) |
| BRCA2 | ||||||||
| − | 8 (0,21) | 8 (0,21) | 8 (0,21) | 8 (0,21) | 0 | 0 | 0 | 0 |
| Untested | 22 (12,31) | 22 (12,31) | 34 (22,45) | 34 (22,45) | 1 (0,4) | 1 (0,4) | 5 (0,11) | 5 (0,11) |
| + | 41 (23,55) | 26 (6,42) | 69 (40,84) | 38 (12,56) | 0 | 0 | 25 (0,48) | 0 |
BC breast cancer, OC ovarian cancer, M models
Table 4.
Results derived from the Cox model assessing the presence of significant differences between curves
| Models | Log rank test for BC | Log rank test for OC | ||||||
|---|---|---|---|---|---|---|---|---|
| With probands | Without | With probands | Without | |||||
| X2 | p | X2 | p | X2 | p | X2 | p | |
| M1: Differences by mutation (BRCA1 or BRCA2) in mutation-positive females | ||||||||
| 0.38 | 0.53 | 0.0 | 0.996 | 3.30 | 0.07 | 3.48 | 0.063 | |
| M2: Differences between mutation-negative, mutation-positive and untested females | ||||||||
| BRCA1 | 19.3 | <0.001a | 3.26 | 0.196 | 6.53 | 0.039a | 5.24 | 0.073 |
| BRCA2 | 13.1 | 0.001a | 2.85 | 0.240 | 3.15 | 0.217 | 0.69 | 0.710 |
| M3: Differences by cohort | ||||||||
| 23.1 | <0.001a | 6.24 | 0.044a | 5.98 | 0.050a | 7.57 | 0.023a | |
| M4: Differences by effect | ||||||||
| A, BRCA1 | 0.17 | 0.680 | 0.12 | 0.734 | 0.83 | 0.362 | 1.72 | 0.190 |
| A, BRCA2 | 0.13 | 0.717 | 0.01 | 0.934 | 0.10 | 0.747 | 0.02 | 0.901 |
| B, BRCA1 | 0.38 | 0.825 | 0.80 | 0.669 | 6.22 | 0.045a | 9.90 | 0.007a |
| B, BRCA2 | 0.13 | 0.723 | 0.01 | 0.934 | 0.10 | 0.747 | 0.02 | 0.901 |
| C, BRCA1 | 1.80 | 0.406 | 4.46 | 0.108 | 6.91 | 0.031a | 7.88 | 0.019a |
| C, BRCA2 | 1.49 | 0.474 | 0.89 | 0.642 | 0.26 | 0.880 | 0.20 | 0.904 |
BC breast cancer, OC ovarian cancer, M models, A classification A, B classification B, C classification C (see text)
aSignificantly different survival curves
A comparison of the aforementioned results for mutation carriers with the results derived for relatives with negative or unknown mutation status is given in Tables 3 and 4 (model M2). A clear trend of lower, intermediate and higher risk obtained for negative, unknown and positive carriers, respectively, is clearly observed for BC in both genes (p < 0.001 and p = 0.001 for BRCA1 and BRCA2, respectively), as graphically shown in Fig. 1b. This trend is not so clear for OC (p = 0.039 for BRCA1 and 0.217 for BRCA2) due to the low number of females with OC in the three groups. For instance, the estimated cumulative probability of BC for negative BRCA2 relatives by the age of 70 years is about 8% (0–21%), and that for untested women is 34% (22–45%), both of which are in contrast to the much higher probability in carriers (about 70%). A similar increasing trend is observed for BRCA1 relatives and for both genes in OC (see Table 3).
The effect of birth-cohort for combined BRCA1 and BRCA2 can be seen in Fig. 1c and Table 4 (model M3). The effect of the birth-cohort on BC cumulative incidence is obvious, with an increased risk observed for females born after 1966 and a decreased risk for those born before 1940. The effect of birth-cohort was found to be highly significant by the log-rank test (p < 0.001). In contrast, differences between curves as regards the cumulative incidence of OC are only marginally significant (p = 0.050).
Finally, the analyses carried out to assess the association between mutation position within the gene and the presence of BC in these families showed no evidence that any of the effects for any of the classifications is associated with an increased prevalence of BC (see Table 4, model M4). However, analysis of the cumulative incidence of OC showed that classifications B and C highlight significant differences between the curves derived for each effect for BRCA1. Thus, regarding classification B, the mutation position in group 2 is associated with a higher risk of OC (p = 0.045), and it is the same in group 2 for classification C (p = 0.031; Fig. 1d). For BRCA2, no apparent trend is observed (see Table 4, model M4).
Discussion
Since 1994/95, when the link between the BRCA1 and BRCA2 genes and the development of BC and/or OC in families with several affected members was first discovered (Miki et al. 1994; Wooster et al. 1995), numerous studies have attempted to improve the handling of these families as regards early diagnosis of the disease, its prevention and the usefulness of genetic counselling. Thus, several studies have investigated (a) the penetrance, (b) the earlier onset and (c) the effect of mutations on the risk of developing BC and/or OC for women carrying a disease-causing mutation in either of these genes; these three aspects are also covered by the work reported herein.
The penetrance, for example, varies significantly according to the study series and population, and the results ranges are so broad that it is very difficult to apply them to any particular case or family. Any further contribution is therefore useful when it comes to tipping the balance towards one side or the other. The accumulated lifetime risk of developing BC by 70 years of age calculated for the families included in this study (64% for BRCA1 and 69% for BRCA2) is similar to that calculated by other groups such as Antoniou et al. (2006) (72% for BRCA1 and 75% for BRCA2) or Evans et al. (2008) (68% for BRCA1 and 75% for BRCA2), despite the number of cases in our study being lower than in these two works. The fact that the difference between both genes is not significant, which would indicate that the likelihood of developing BC is similar for carriers of mutations in BRCA1 and BRCA2, is also similar to previously reported findings (Antoniou et al. 2006). In contrast, the accumulated risk of developing OC by 70 years of age is a little bit different for carriers of a mutation in either gene (37% for BRCA1 and 25% for BRCA2). This figure shows a clear likelihood of a woman developing OC—particularly between the ages of 50 and 70—if she carries a BRCA1 mutation, and the difference is marginally significant (p < 0.1) possibly due to the small sample size. There are some discrepancies between the various series studied as regards the development of OC. Thus, some studies have reported clear differences in the development of OC depending on the gene carrying the mutation, such as the study of Evans et al. (2008) (60% for BRCA1 and 30% for BRCA2), whereas other studies, such as that by Milne et al. (2008) in collaboration with our group, have reported very similar values (22% for BRCA1 and 18% for BRCA2). These results suggest that, generally speaking, the development of OC in Spanish women carrying a BRCA1 mutation seems to be lower than previously published, although the results in our population show a trend to a higher risk in BRCA1 carriers.
An analysis of the presence of BC and OC in tested (positive or negative) and untested individuals shows that mutation-positive women present a large number of cases of BC, whereas mutation-negative women present no cases of BC and untested women appear on a curve between these two extremes, as would be expected in light of the dominant transmission of the mutation and the resulting likelihood that around 50% of untested women might carry the disease-causing gene, and therefore, the risk of developing BC will be intermediate (Fig. 1b). It should be noted that there is a non-zero probability in the case of families with the BRCA2 mutation, even for mutation-negative women (Fig. 1b), due to the risk of developing BC in the general population.
Concerning the analysis of the earlier development of BC or OC in subsequent generations, the results obtained suggest that, for BC, the youngest generation (born after 1966) develops BC much earlier than the older generation (born before 1940; Fig. 1c). In contrast, OC provides only marginally significant results. These differences between BC and OC, which are similar to the results reported by Evans et al. (2008), could be due, in the present study, to the lower number of OC cases, which reduces the significance of these results. This earlier onset of the appearance of BC could also be due to external factors, such as better access to health care, the establishment of an early detection programme in the Basque Country in November 1995 (Asua et al. 1994), an improvement in diagnostic techniques, and environmental factors such as diet and the adoption of an increasingly stressful lifestyle over the past 10 to 20 years (Coyle 2009), rather than just genetic factors. Irrespective of the cause, however, the fact that later generations in high-risk families are likely to develop BC earlier than their parents or grandparents is important when it comes to performing a correct early diagnosis of the disease.
Finally, we also wanted to evaluate the effect of each mutation on the development of BC or OC according to its position in the BRCA1 or BRCA2 gene in order to attempt to correlate the genotype with the phenotype since other published articles are controversial. For example, Gayther et al (1995) reported that the risk of OC relative to the risk of BC was higher in families with mutations located 5´ to exon 13 of the BRCA1 gene compared with families with mutations located 3´ to exon 13. However, this finding was not confirmed in later reports (Couch et al. 1997; Frank et al. 1998). Furthermore, an ovarian cancer cluster region (OCCR) in the BRCA2 gene was first described by Gayther et al (1997), but an international collaborative study did not provide statistically significant support for the existence of that region (Neuhausen et al. 1998). Later, other authors have corroborated these results (Milne et al. 2008). This is why we wanted to study the effect of mutations on BC and OC with our patients and applying three classifications: the classification for these two genes defined by other authors (Thompson et al. 2001; Thompson et al. 2002; Gayther et al. 1995), and our own classification, which is based on both the position of the mutation in the gene and the mutation type (frame-shift or missense mutation). Our analyses show no association between BC and any subdivision of the three classifications studied for either BRCA1 or BRCA2; however, for OC, some degree of association is found for BRCA1 for those mutations located between codons 801 and 1397 (p = 0.045) in the B classification (Thompson et al. 2002) and between codon 622 and the end of the protein (p = 0.031) in our classification (C). Both classifications suggest the existence of an OC-associated region in exon 11 of BRCA1. The fact that classification A gave no significant association would suggest, as already discussed by Thompson et al. (2002), that this classification is not appropriate. However, it is clear that those deletions or insertions close to the 5′ end located in at least the first 621 amino acids (cut-off for classification C) do not increase the risk of developing OC even though the product is a very early truncated protein and, therefore, one with no BRCT domains. Some authors have proposed the “nonsense-mediated mRNA decay mechanism” as an explanation for the development of OC for both BRCA1 (Perrin-Vidoz et al. 2002) and BRCA2 (Ware et al. 2006), and this mechanism fits well with our results. According to this hypothesis, truncated proteins are pathogenic because they interfere in some way with the functioning of the normal wild-type allele or other genes, but the possibility that shortest truncated proteins completely disappear should be considered, and thus, they would not interfere. In other words, the longer the truncated protein, the greater the risk of developing OC, and the shorter the truncated protein, the lesser the likelihood of it interfering with other biological processes. Indeed, our sample clearly shows that those mutations which result in short truncated proteins (at least one third the size of the normal protein) have a better prognosis as regards the risk of developing BC and OC than other mutations, including those missense mutations located in the BRCT domain of BRCA1. For this theory to become accepted, wider ranging studies including a larger number of families carrying disease-causing mutations in BRCA1 or BRCA2 are required to achieve results which correlate the genotype with the phenotype in a significant manner. In any case, for BRCA2 no significant OC-related trend has been observed as regards the OC cluster (codons 1011–2210), results that agree with previously published results (Al-Saffar and Foulkes 2002).
Among the main limitations of this study are the sample size and a possible ascertainment bias when the purpose is to estimate population-based penetrance. This relatively small sample size produces wide confidence intervals and low power in curves comparison. For instance, the comparison between survival curves representing risk to develop BC or OC in BRCA1 families according to classification C (three effects) has a power equal to 0.75 to detect hazard ratios of 1.75, so that hazard ratios lower than 1.75 will not be depicted with the actual sample size. Regarding selection bias, it is important to remark that most of the families included in this study had multiple affected individuals, so the proportion of tested individuals with cancer may be overestimated. The exclusion of probands when the aim is to estimate population-based penetrance is usually recommended to reduce the bias, although the bias induced by using high-risk families automatically selected for high-penetrance mutations and for other genes or environmental factors that may influence expression still persists (Begg 2002). Nevertheless, our goal was to obtain penetrance estimates derived for the population being counselled, which we consider, like other authors do (Evans et al. 2008), is the penetrance of interest in clinical practice.
In conclusion, despite the limitations quoted, we believe that the results reported herein are an important contribution to previously published results for both Spanish and European/American series, in relation to the cumulative risk, the earlier onset and the genotype–phenotype correlation according to the position of the mutation in the gene. It is noticeable to mention that, although the population we addressed is a small one, it is similar to others suffering from an important immigration from other communities as it occurred in our region since the 19th century. So, as the presence of a great variety of different mutations of these types of populations makes its management difficult, our results are important for the diagnosis and prevention in at-risk families, as well as for the clinicians and practitioners who deal with these patients and who come across new mutations.
Acknowledgements
We would like to thank the Health Department of the Basque Country Government, the BIOEF foundation (BIO07/CA/006) and the KATXALIN patients’ association (BIOEF08/015), for their financial support for this work. Furthermore, we thank the families and the clinicians involved in this study for their enthusiastic help in this research.
Contributor Information
Elena Beristain, Email: eberistain001@ikasle.ehu.es.
Maria Isabel Tejada, Phone: +34-94-6006514, FAX: +34-94-6006532, Email: MARIAISABEL.TEJADAMINGUEZ@osakidetza.net.
References
- Al-Mulla F, Bland JM, Serratt D, et al. Age-dependent penetrance of different germline mutations in the BRCA1 gene. J Clin Pathol. 2009;62:350–356. doi: 10.1136/jcp.2008.062646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Saffar M, Foulkes WD. Hereditary ovarian cancer resulting from a non-ovarian cancer cluster region (OCCR) BRCA2 mutation: is the OCCR useful clinically? J Med Genet. 2002;39:e68. doi: 10.1136/jmg.39.11.e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou A, Pharoah PDP, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 and BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou A, Durocher F, Smith P, et al. INHERIT BRCAs program members, Easton DF: BRCA1 and BRCA2 mutation predictions using the BOADICEA and BRCAPRO models and penetrance estimation in high-risk French-Canadian families. Breast Cancer Res. 2006;8:R3. doi: 10.1186/bcr1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asua J, Rico R, Gutiérrez MA, Aranáez R. Detección precoz del cáncer de mama en la CAPV. Propuesta de actuación. Vitoria-Gasteiz: Departamento de Sanidad del Gobierno Vasco. Osteba; 1994. [Google Scholar]
- Begg CB. On the use of familial aggregation in population-based case probands for calculating penetrance. J Natl Cancer Inst. 2002;94:1221–1226. doi: 10.1093/jnci/94.16.1221. [DOI] [PubMed] [Google Scholar]
- Beristain E, Martínez-Bouzas C, Guerra I, et al. Differences in the frequency and distribution of BRCA1 and BRCA2 mutations in breast/ovarian cancer cases from the Basque Country with respect to the Spanish population: implications for genetic counselling. Breast Cancer Res Treat. 2007;106:255–262. doi: 10.1007/s10549-006-9489-0. [DOI] [PubMed] [Google Scholar]
- Beristain E, Martínez-Bouzas C, Mallabiabarrena G et al (2009) Is early onset breast cancer with no family history a good criterion for testing BRCA1 and BRCA2 genes?: a small population-based study. Clin Genet 75(6:)576–578 [DOI] [PubMed]
- Couch FJ, DeShano ML, Blackwood MA, et al. BRCA1 mutations in women attending clinics that evaluate the risk of breast cancer. N Engl J Med. 1997;336:1409–15. doi: 10.1056/NEJM199705153362002. [DOI] [PubMed] [Google Scholar]
- Coyle YM (2009) Lifestyle, Genes and Cancer. Methods of Molecular Biology, Cancer Epidemiology 472 [DOI] [PubMed]
- Díez O, Osorio A, Durán M, et al. Analysis of BRCA1 and BRCA2 genes in Spanish breast/ovarian cancer patients: a high proportion of mutations unique to Spain and evidence of founder effects. Hum Mutat. 2003;22:301–312. doi: 10.1002/humu.10260. [DOI] [PubMed] [Google Scholar]
- Evans DG, Shenton A, Woodward E, et al. Penetrance estimates for BRCA1 and BRCA2 based on genetic testing in a Clinical Cancer Genetics service setting: risks of breast/ovarian cancer quoted should reflect the cancer burden in the family. BMC Cancer. 2008;8:155. doi: 10.1186/1471-2407-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank TS, Manley SA, Olopade OI, et al. Sequence analysis of BRCA1 and BRCA2: correlation of mutations with family history and ovarian cancer risk. J Clin Oncol. 1998;16:2417–2425. doi: 10.1200/JCO.1998.16.7.2417. [DOI] [PubMed] [Google Scholar]
- Gayther SA, Warren W, Mazoyer S, et al. Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat Genet. 1995;11:428–433. doi: 10.1038/ng1295-428. [DOI] [PubMed] [Google Scholar]
- Gayther SA, Mangion J, Russell P, et al. Variation of risks of breast and ovarian cancer associated with different germline mutations of the BRCA2 gene. Nat Genet. 1997;15:103–105. doi: 10.1038/ng0197-103. [DOI] [PubMed] [Google Scholar]
- Krajc M, Teugels E, Zgajnar J, et al. Five recurrent BRCA1/2 mutations are responsible for cancer predisposition in the majority of Slovenian breast cancer families. BMC Med Genet. 2008;10:83. doi: 10.1186/1471-2350-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Milne RL, Osorio A, Ramón y Cajal T, et al. The average cumulative risks of breast and ovarian cancer for carriers of mutations in BRCA1 and BRCA2 attending genetic counselling units in Spain. Clin Cancer Res. 2008;14:2861–2869. doi: 10.1158/1078-0432.CCR-07-4436. [DOI] [PubMed] [Google Scholar]
- Neuhausen SL, Godwin AK, Gershoni-Baruch R, et al. Haplotype and phenotype analysis of nine recurrent BRCA2 mutations in 111 families: results of an international study. Am J Hum Genet. 1998;62:1381–1388. doi: 10.1086/301885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE Clinical Guideline 14 (May 2004) Familial Breast Cancer. Available at: [http://www.nice.org.uk/guidance/index.jsp?action=byID&o=10994]
- Perrin-Vidoz L, Sinilnikova OM, Stoppa-Lyonnet D, et al. The nonsense-mediated mRNA decay pathway triggers degradation of most BRCA1 mRNAs bearing premature termination codons. Hum Mol Genet. 2002;11:2805–2814. doi: 10.1093/hmg/11.23.2805. [DOI] [PubMed] [Google Scholar]
- Thompson D, Easton D, Breast Cancer Linkage Consortium Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet. 2001;68:410–419. doi: 10.1086/318181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D, Easton D, Breast Cancer Linkage Consortium Variation in BRCA1 cancer risks by mutation position. Cancer Epidemiol Biomark Prev. 2002;11:329–336. [PubMed] [Google Scholar]
- Ware MD, DeSilva D, Sinilnikova OM, et al. Does nonsense-mediated mRNA decay explain the ovarian cancer cluster region of the BRCA2 gene? Oncogene. 2006;25:323–328. doi: 10.1038/sj.onc.1209033. [DOI] [PubMed] [Google Scholar]
- Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]