Abstract
Thalassaemia is a common and debilitating autosomal recessive disorder affecting many populations in South Asia. To date, efforts to create a regional profile of β-thalassaemia mutations have largely concentrated on the populations of India. The present study updates and expands an earlier profile of β-thalassaemia mutations in India, and incorporates comparable data from Pakistan and Sri Lanka. Despite limited data availability, clear patterns of historical and cultural population movements were observed relating to major β-thalassaemia mutations. The current regional mutation profiles of β-thalassaemia have been influenced by historical migrations into and from the Indian sub-continent, by the development and effects of Hindu, Buddhist, Muslim and Sikh religious traditions, and by the major mid-twentieth century population translocations that followed the Partition of India in 1947. Given the resultant genetic complexity revealed by the populations of India, Pakistan and Sri Lanka, to ensure optimum diagnostic efficiency and the delivery of appropriate care, it is important that screening and counselling programmes for β-thalassaemia mutations recognise the underlying patterns of population sub-division throughout the region.
Electronic supplementary material
The online version of this article (doi:10.1007/s12687-010-0026-9) contains supplementary material, which is available to authorized users.
Keywords: β-Thalassaemia, Mutation profiles, Endogamy, Consanguinity, India, Pakistan, Sri Lanka, South Asia
Introduction
It has been estimated that 1.1% of couples worldwide are at risk of bearing a child with a haemoglobin disorder, resulting in 2.7 per 1,000 conceptions being affected. If correct, this would mean that at least 5.3% of the current global population are carriers of a significant haemoglobin variant (Modell and Darlinson 2008). South Asia as a whole is a region with a significant prevalence of haemoglobin disorders. In 2010, the combined populations of India, Pakistan, Bangladesh and Sri Lanka totalled 1.56 billion, representing some 23% of the global population (PRB 2010). The combination of large population sizes and high levels of haemoglobinopathies create an issue of major public health concern, with an estimated 17 million β-thalassaemia carriers in India, ~8 million carriers in Pakistan, ~3 million in Bangladesh and ~0.5 million in Sri Lanka (Modell and Darlinson 2008).
It should, however, be noted that the calculated numbers of β-thalassaemia heterozygotes in India assumed a carrier frequency of 1.6% (Modell and Darlinson 2008), whereas frequencies of 2.78% (Mohanty et al. 2008) and 3–4% (WHO 2008) have been cited by other authorities. Furthermore, at district level in the western Indian states of Maharashtra and Gujurat, β-thalassaemia carrier frequencies ranged from 0% to 9.5% (Colah et al. 2010), indicative of the marked demographic and genetic subdivisions within Indian society (Sinha et al. 2009). The aim of the present study was to determine comparative national and regional β-thalassaemia mutation profiles for the major countries of South Asia, with the exception of Bangladesh for which only preliminary data have so far been obtained.
Subjects and methods
Data on β-thalassaemia mutations in the populations of India, Pakistan and Sri Lanka were collated from published reports. Prior to data being accepted for inclusion, two basic selection criteria were applied: (1) only studies reporting allelic frequencies for at least 10 β-globin gene mutations were included, and (2) there should be a minimum of 50 subjects per study identified by their state of origin, and/or in the case of Pakistan by specific ethnicity.
To facilitate analysis, the mutation data for each country were listed by geographical sub-division, e.g., according to state (India), or province (Pakistan). The data for Sri Lanka were not geographically subdivided since prolonged civil unrest had resulted in marked under-representation of the Tamil and Muslim minorities in the northern and eastern parts of the country. The data on β-thalassaemia alleles in the population of India were based on the recent comprehensive meta-analysis by Sinha et al. (2009) drawn from 17 studies reported between 1991 and 2009, supplemented by additional data from the East Indian state of Orissa (Nishank et al. 2009).
The collation of information on β-thalassaemia mutations in Pakistan proved more problematic, in part because of a lack of uniformity in the identifiers reported, with geographical ancestry, current residence and ethnicity used inter-changeably. For this reason, it was assumed that individuals referred to as being Punjabi correlated with ancestry and residence in Punjab, that Sindhi indicated ancestry and residence in Sindh province, Baluchi indicated ancestry and residence in Baluchistan, and that Pashtun and North West Pakistan referred to ancestry and residence in the Northwest Frontier Province (NWFP), which in 2010 was renamed as the province of Khyber Pakhtunkhwa.
After assessment of all appropriate information sources, the final data compilation for Pakistan was based on four primary sources: Varawalla et al. (1991) comprising samples from Punjab, Sindh, NWFP; Ahmed et al. (1996) with data for Punjab, Sindh, Baluchistan, NWFP; Verma et al. (1997) with data for Sindh; and Baig et al. (2006) with data for Punjab. Other potential data sources were excluded either because of deficiencies in the provincial/ethnic definitions reported (Khan and Riazuddin 1998) or incomplete data (Ghani et al. 2002).
Comparable β-thalassaemia mutation data for Sri Lanka were available from a single study (Fisher et al. 2003), conducted in eight centres in the central and western regions of the island. Of the 620 patients sampled, 93.2% were of declared Sinhalese ancestry.
As shown in Supplementary Tables S1, S2 and S3, the data were abstracted from each of the 23 selected reports and collated first at national level for India (Supplementary Table S1), Pakistan (Supplementary Table S2) and Sri Lanka (Supplementary Table S3), then by region in India (North, West, Central, East and South), and by province in Pakistan (Punjab, Sindh, Baluchistan and NWFP/Khyber Pakhtunkhwa). Pie diagrams were constructed from these data for the ten most common β-thalassaemia alleles reported at national, regional and/or provincial levels. In India, 6.6% of the disease alleles were either rare mutations or had not been identified; the equivalent figures for Pakistan and Sri Lanka were 2.2% and 5.2%, respectively.
As a number of different genomic methodologies had been employed in the different studies, including ARMS and gap polymerase chain reaction, denaturing gradient gel electrophoresis, temporal temperature gel electrophoresis, amplification refractory mutation system, reverse dot blot hybridization and direct DNA sequencing, some variability may have resulted in the mutation profiles reported by individual study groups due to technical inconsistencies.
Results
Summary of national frequencies of β-thalassaemia mutations
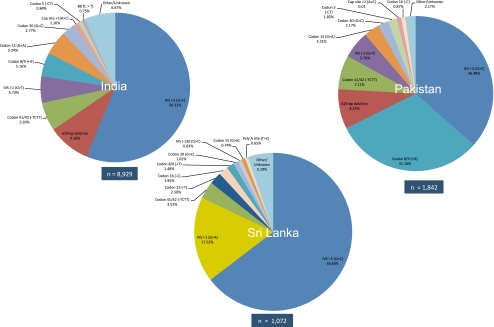
Information was collated on 8,929 β-thalassaemia alleles in India, 1,842 alleles in Pakistan and 1,021 alleles in Sri Lanka (Supplementary Table S1). Wide divergence in the alleles and allele frequencies reported for the three nations was apparent (Fig. 1.)
Fig. 1.
Pie diagrams of β-thalassaemia mutation distributions in India, Pakistan and Sri Lanka
IVS I-5 (G > C) was the most common β-thalassaemia mutation in all three countries, but its national prevalence differed markedly from 64.6% in Sri Lanka to 56.3% in India and 36.5% in Pakistan. Thereafter, the pattern of national similarities in allele type, e.g. ceased, while the second most common allele in India was a 619-bp deletion (9.2%), in Pakistan it was Codon 8/9 (+G) (31.2%), and in Sri Lanka IVS I-1 (G > A) (17.5%).
As demonstrated in Fig. 1, the high frequency of IVSI-1 (G > A) in Sri Lanka was in sharp contrast to Pakistan where the mutation had not been reported, and to India where just 29 of the 8,929 (0.32%) of β-thalassaemia alleles were IVSI-1 (G > A) (Supplementary Table S1). By comparison, in Pakistan, the frequency of Codon 8/9 (+G) (31.2%) was close to that of IVS I-5 (G > C) (36.5%), resulting in a quite different mutation profile to both India and Sri Lanka where IVS I-5 (G > C) was clearly the majority disease allele.
India
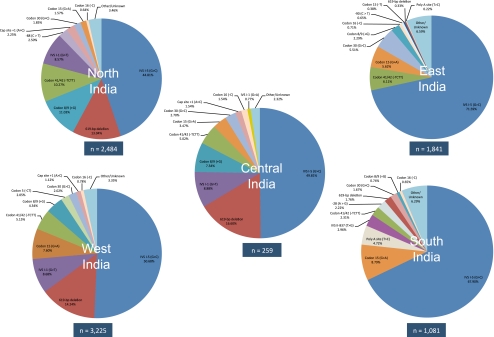
As indicated in Fig. 2, West, Central and North India showed similar mutation profiles with just two mutations, IVS I-5 (G > C) and the 619-bp deletion, accounting for 58–65% of the total in each region. In the South and East regions of India, IVS I-5 (G > C) alone accounted for 68–72% of all β-thalassaemia mutations (Fig. 2).
Fig. 2.
Pie diagrams of β-thalassaemia mutation distributions in five regions of India
A major interest in the current study was to compare the previous findings of Sinha et al. (2009) on the Sindhi contribution to the β-thalassaemia allele profile and frequency in West India with frequencies collated for the adjacent Pakistani province of Sindh. The high frequency of the 619-bp deletion in both Sindh province (28.6%) and neighbouring West India (14.2%) was an obvious similarity, and IVS I-5 (G > C), 619-bp deletion and IVS I-1 (G > T) were the three most frequent alleles in both regions, suggesting shared mutational origins. However, the proportions differed, with IVS I-5 (G > C) the majority allele in West India (50.7%) while its frequency in Sindh province (Pakistan) was 31.0% (Supplementary Tables S1 and S2).
Pakistan
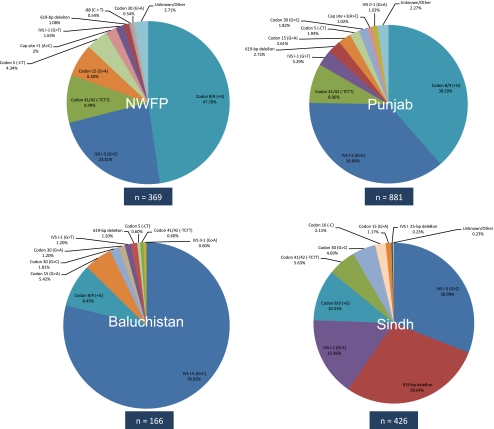
As in India, the data for Pakistan displayed a significant variability in the distribution of β-thalassaemia mutations across the four provinces (Fig. 3, Supplementary Table S2). It also was apparent that, unlike any region of India, IVS I-5(G > C) was not the most common allele in two of the four provinces, and in Sindh, it had a frequency of 31.0% by comparison with 28.6% for the 619-bp deletion. By contrast, in Baluchistan, 78.9% of alleles were IVS I-5 (G > C).
Fig. 3.
Pie diagrams of β-thalassaemia mutation distributions in four provinces of Pakistan
Codon 8/9 (+G) was the most common β-thalassaemia allele in both Punjab (38.6%) and neighbouring NWFP/Khyber Pakhtunkwha (47.7%), although it is only the fourth most common allele across India, suggesting that its origin may have been the northwest regions of Pakistan or adjoining regions of neighbouring countries, such as Afghanistan. As previously discussed, the 619-bp deletion is most common in Sindh province, suggesting a southern Pakistan origin. However, any such conclusion must be tentative given the small number of alleles analysed in a country with a population of 181 million (PRB 2010) and a calculated national carrier frequency for β-thalassaemia of 4.6% (Modell and Darlinson 2008).
Sri Lanka
The most frequent mutation was IVSI-5 G > C (64.6%), but the most obvious contrast to India and Pakistan was the high frequency of IVS I-1 (G > A) (17.5%), which is rare in the rest of South Asia and is more commonly reported in the Mediterranean region and Middle East. The Sri Lankan allele may have arisen de novo (Fisher et al. 2003). Alternatively, it could have been brought to Sri Lanka by the Indo-European founders of the modern Sinhalese population and increased in frequency via a combination of genetic bottlenecks, drift and selection. Whatever the explanation(s), within the South Asian context the high frequency of IVSI-1 (G > A) is a notable feature of this Sri Lankan population.
It also was noticeable that the Poly A mutation commonly reported in the states of South India (Sinha et al. 2009) was absent from this Sri Lankan study population (Supplementary Table S3). As individuals of Tamil ancestry accounted for just 0.5% of the study sample, the finding may in part be an artefact of sampling. Codon 8/9 (+T) was present at a frequency of 1.5%, whereas only 2/8,929 alleles of this mutation were recorded from India, both from residents of the South Indian state of Andhra Pradesh (Supplementary Table S1). Other mutations, such as Codon 15 (−T), Codon 16 (−C), Codon 41/42 (−TCTT) and Codon 30 (G > C), were present at low frequencies in Sri Lanka (Supplementary Table S3). Although Codon 15 (−T), Codon 16 (−C) and Codon 30 (G > C) also were found at low frequencies in South, East and West India, Codon 41/42 (−TCTT) (10.3%) was more common in North India (Fig. 2).
Discussion and conclusions
Despite the small numbers of alleles analysed by comparison with the large sizes of the three national populations, and the variable sources of data accessed, the meta-analysis covering samples from India, Pakistan and Sri Lanka has highlighted both the marked diversity and, in some cases, the similarities in the profiles of β-thalassaemia mutations across South Asia. The widespread distribution of the IVSI-5 (G > C) mutation in the sub-continent suggests a relatively ancient origin, especially given its reported high prevalence in indigenous tribal populations in India (Colah et al. 2009; Nishank et al. 2009).
The very high frequency of IVSI-5 (G > C) (78.9%) in the Pakistan province of Baluchistan indicates that the mutation may have originated in this region. Besides moving east with the many historical population movements into the Indian sub-continent (Thapar 1966; Spear 1970), as shown in Table 1, there also is a high incidence of IVSI-5 (G > C) (72.3%) to the west in the adjacent Iranian province of Sistan-Baluchestan (Eshghi et al. 2008).
Table 1.
Profile of the five most frequent β-thalassaemia mutations in selected South Asian and Gulf countries
| Pakistana (Baluchistan) | Iranb (Sistan-Baluchestan) | Omanc | United Arab Emiratesc | |
|---|---|---|---|---|
| IVS I-5 (G > C) | 78.9 | 72.3 | 61.6 | 59.6 |
| Codon 8 (−AA) | 2.4 | |||
| Codon 8/9 (+G) | 8.4 | 5.8 | 5.4 | |
| Codon 15 (G > A) | 5.4 | |||
| Codon 30 (G > C) | 1.2 | |||
| IVS I-1 (G > T) | 1.2 | |||
| IVS I 25-bp deletion | 5.6 | 7.8 | ||
| Codon 39 (C > T) | 1.5 | |||
| Codon 44 (−C) | 2.5 | 9.6 | ||
| IVS II-1 (G > A) | 1.5 | 3.5 | 3.6 | |
| 619-bp deletion | 4.0 | |||
| Other | 4.8 | 16.5 | 15.7 | 21.2 |
Further west again, the Baluch communities in Oman and the United Arab Emirates moved from the southern coastal district of present-day Pakistani Baluchistan to the Gulf region in the eighteenth century, both as traders and as mercenaries employed by the Sultan of Oman (Nicolini 2006, 2007). In Oman, the IVSI-5 (G > C) mutation has been reported to be responsible for 61.6–70% of β-thalassaemia cases, and it accounts for some 50–60+ percent of β-thalassaemia patients in the United Arab Emirates (White et al. 1993; Quaife et al. 1994; Daar et al. 1998; Baysal 2005; Hassan et al. 2010), but its prevalence in other Middle Eastern countries is low (Quaife et al. 1994; Zahed 2001). IVSI-5 (G > C) is strongly associated with the surname Baluchi in both the United Arab Emirates and Oman, indicative of an Indian sub-continental origin of the mutation (Quaife et al. 1994; Rajab and Patton 1997).
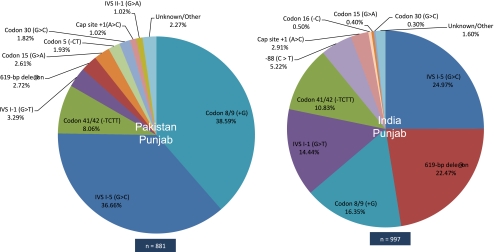
Other findings from the Indian sub-continent also are indicative of historical population movements, as in the eastward movement of the 619-bp deletion and Codon 8/9 (+G) from Sindh to West India and across Punjab state in North India. However, more recent political events also have exerted major effects, in particular the Partition of India in 1947, which resulted in the reciprocal translocation of many millions of families and individuals, with Muslims moving westward into the newly created Pakistan province of Punjab and similar numbers of Hindus and Sikhs relocating to the east into what is now the adjoining Indian state of Punjab. The resultant present-day profiles of β-thalassaemia mutations in both territories are shown in Fig. 4. In the Indian state of Punjab, IVI-5 (G > C) is the most common β-thalassaemia mutation (25.0%), followed by 619-bp deletion (22.5%), Codon 8/9 (+G) (16.4%) and IVSI-1 (G > T) (14.4%). By comparison, across the border in Pakistani Punjab, Codon 8/9 (+G) is most common (38.6%), followed by IVI-5 (G > C) (36.7%), Codon 41/42 (−TCTT) (8.1%) and IVSI-1 (G > T) (3.3%) (Supplementary Tables S1 and S2).
Fig. 4.
Pie diagrams of β-thalassaemia mutation distributions in Punjab province, Pakistan and Punjab state, India
Although the five most common mutations in both Indian and Pakistani Punjab are the same, suggesting an older shared genetic heritage, they are present at markedly different frequencies. An exception to the cross-border sharing of mutant alleles is −88 (C > T), which is present in Indian but not in Pakistani Punjab, an unsurprising finding since this mutation has previously been reported at high prevalence in the Jat Sikhs, a community which during and after Partition collectively migrated from what is now the Pakistani province of Punjab to Indian Punjab (Garewal et al. 2005).
In Sri Lanka, there was little evidence of Dravidian genetic influence from neighbouring South India on the overall profile of β-thalassaemia mutations of the predominantly Sinhalese study population. Rather, an Indo-European genetic heritage appears probable (Fisher et al. 2003; Ayub and Tyler-Smith 2009), and the high frequency of IVSI-1 (G > A) can convincingly be interpreted as stemming from a founder mutation, with subsequent expansion following long-term geographical and cultural isolation. But, as previously noted, there were very few Tamils in the study group, and more representative sampling of the entire population of Sri Lanka could result in different findings and conclusions.
The examples cited show that the flow of β-thalassaemia mutations across South Asia has largely followed well-documented invasion routes and historical population movements, with additional changes due to more recent major events, such as Partition, and in the island of Sri Lanka, the spread of founder mutations, e.g., IVSI-1 (G > A), within a population isolate. Comparable data are not yet available for Bangladesh, but in the western regions of the country the mutation profile would be expected to be similar to that of the adjacent Indian state of West Bengal, where IVS1-5 (G > C) accounted for 69.3% of mutant alleles and Codon 30 (G > C) and Codon 15 (G > A) for another 12.1% (Supplementary Table S1). Some mutation flow from Myanmar is predictable in the eastern and southeast regions of Bangladesh. As yet, data on β-thalassaemia mutations in Myanmar remain sparse, and although IVSI-1(G > T), Codon 41/42 (-TCTT) and IVSI-5(G > C) were the most common alleles reported in the capital Yangon (Ne-Win et al. 2002), the frequencies of red cell genetic disorders differ significantly between the many constituent ethnic minority populations in the country (Than et al. 2005).
From a disease screening perspective, Sinha et al. (2009) showed that in India, testing for the five most common β-thalassaemia mutations identified at national level would detect 82.5% of cases, thus offering a high level of overall screening efficiency. However, besides political and religious boundaries, caste divisions had a significant impact on the distribution and prevalence of β-thalassaemia mutations, and it seems probable that biraderis, male lineages traditionally based on occupation, exert a comparable effect in Pakistan (Hussain 2005; Bittles 2008; Bittles and Black 2010).
In South India, almost all of the β-thalassaemia mutations identified were homozygous (Bashyam et al. 2004), which would be expected given the prevailing high levels of consanguineous marriage in this region of the country (Bittles 2002). As consanguineous marriage also is common among Indian Muslims (Bittles and Hussain 2000) and in Sri Lanka (Reid 1976), and on average approximately 60% of all marital unions in Pakistan are consanguineous (Ahmed et al. 1992; www.consang.net), similar high frequencies of homozygous β-thalassaemia mutations rather than compound heterozygotes would be predicted in these populations.
To date, genetic counselling and prenatal diagnosis in India and Pakistan have largely been restricted to metropolitan and major regional centres, and although acceptable to most couples and communities (Petrou 2010), the financial costs incurred may be prohibitive, especially for couples from lower socioeconomic communities and rural backgrounds. To ensure optimum efficacy in the planning, establishment and uptake of disease screening and genetic counselling programmes, it is critical that the various distinctive population characteristics of communities are fully considered. Sustained genetic education and public awareness programmes are required for premarital screening and genetic counselling to gain optimum acceptability, incorporating mass communication methods wherever possible and delivered in the appropriate regional and local language(s).
Unfortunately, relevant and reliable health data are all too frequently unavailable in lower income countries (Byass 2008; Chan et al. 2010). This situation certainly applies with respect to β-thalassaemia in South Asia. As indicated in the “Introduction,” three estimates of the carrier rate of β-thalassaemia in India published in the same year ranged from 1.6% to 2.78% and 3–4% (Modell and Darlinson 2008; Mohanty et al. 2008; WHO 2008), a 2.5-fold overall difference. Translated into actual population numbers, and given the current total population of 1,189 million in India (PRB 2010), somewhere between 19.0 million and 47.6 million persons may be carriers of β-thalassaemia.
Clearly, such a wide range of possible values is inappropriate for health planning purposes, and in India, the situation is made even more problematic by significant regional, ethnic, religious and caste differences in the profile of β-thalassaemia disease alleles (Fig. 2; Supplementary Table S1; Sinha et al. 2009). As economically deprived communities and those living in remote rural settings have been significantly under-investigated in testing programmes (Sinha et al. 2009), the true incidence and profiles of β-thalassaemia mutations have yet to be determined for a majority of rural and urban communities, with comparable deficiencies in Pakistan and to an even greater extent in Bangladesh. Given the severe personal, social and economic costs imposed by β-thalassaemia, rapid progress in overcoming these shortcomings is overdue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
β-Thalassaemia mutation frequencies in India (XLS 32 kb)
β-Thalassaemia mutation frequencies in Pakistan (XLS 24 kb)
β-Thalassaemia mutation frequencies in Sri Lanka (XLS 36 kb)
Acknowledgements
The authors acknowledge the generous financial contribution provided by the Western Australian State Government in the establishment of the WA Centre of Excellence for Comparative Genomics and support of this project. During the course of the study, SS was in receipt of an Endeavour Executive Award from the Commonwealth Government of Australia.
References
- Ahmed T, Ali SM, Aliaga A, Arnold F, Ayub M, Bhatti MH, Bicego G, Hasan KZ, Hashmi SS, Mallick MD, Rukanuddin AR, Sathar Z, Shah NM, Sultan M (1992) Pakistan National Demographic and Health Survey 1990/91. Islamabad and Columbia MD, National Institute of Population Studies and Macro International
- Ahmed S, Petrou M, Saleem M. Molecular genetics of beta-thalassaemia in Pakistan: a basis for prenatal diagnosis. Brit J Haematol. 1996;94:476–482. [PubMed] [Google Scholar]
- Ayub Q, Tyler-Smith C. Genetic variation in South Asia: assessing the influences of geography, language and ethnicity for understanding history and disease risk. Brief Func Genom Proteom. 2009;8:395–404. doi: 10.1093/bfgp/elp015. [DOI] [PubMed] [Google Scholar]
- Baig SM, Azhar A, Hassan H, Baig JM, Aslam M, Ud Din MA, Qureshi JA, Zaman T. Prenatal diagnosis of beta-thalassemia in Southern Punjab, Pakistan. Prenat Diag. 2006;26:903–905. doi: 10.1002/pd.1523. [DOI] [PubMed] [Google Scholar]
- Bashyam MD, Bashyam L, Gorinabele R, Sangal MGV, Rama Devi AR. Molecular genetic analysis of β-thalassemia in South India reveals rare mutations in the β-globin gene. J Hum Genet. 2004;49:408–413. doi: 10.1007/s10038-004-0169-9. [DOI] [PubMed] [Google Scholar]
- Baysal E. Molecular heterogeneity of beta-thalassaemia in the United Arab Emirates. Commun Genet. 2005;8:35–39. doi: 10.1159/000083336. [DOI] [PubMed] [Google Scholar]
- Bittles AH. Endogamy, consanguinity and community genetics. J Genet. 2002;81:91–98. doi: 10.1007/BF02715905. [DOI] [PubMed] [Google Scholar]
- Bittles AH. A community genetics perspective on consanguineous marriage. Commun Genet. 2008;11:324–330. doi: 10.1159/000133304. [DOI] [PubMed] [Google Scholar]
- Bittles AH, Black ML. Consanguinity, human evolution and complex diseases. Proc Natl Acad Sci USA. 2010;107:1779–1786. doi: 10.1073/pnas.0906079106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittles AH, Hussain R. An analysis of consanguineous marriage in the Muslim population of India at regional and state levels. Ann Hum Biol. 2000;27:163–171. doi: 10.1080/030144600282271. [DOI] [PubMed] [Google Scholar]
- Byass P. The unequal world of health data. PLoS Med. 2008;6:e1000155. doi: 10.1371/journal.pmed.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M, Kazatchkine M, Lob-Levyt J, Obaid T, Schweitzer J, Sidibe M, Veneman A, Yamada T. Meeting the demand for results and accountability: a call for action on health data from eight global health agencies. PLoS Med. 2010;7:e10000223. doi: 10.1371/journal.pmed.1000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colah R, Gorakshakar A, Nadkarni A, Phanasgaonkar S, Surve R, Sawant P, Mohanty D, Ghosh K. Regional heterogeneity of β-thalassemia mutations in the multi ethnic Indian population. Blood Cells Mol Dis. 2009;42:241–246. doi: 10.1016/j.bcmd.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Colah R, Gorakshakar A, Phanasgaonkar S, D’Souza NA, Surve R, Sawant P, Master D, Patel R, Ghosh K, Mohanty D. Epidemiology of β-thalassaemia in Western India: mapping the frequencies and mutations in sub-regions of Maharashtra and Gujurat. Brit J Haematol. 2010;149:739–747. doi: 10.1111/j.1365-2141.2010.08131.x. [DOI] [PubMed] [Google Scholar]
- Daar S, Hussein HM, Merghoub T, Krishnamoorthy R. Spectrum of β-thalassemias in Oman. Ann NYAcad Sci. 1998;850:404–406. doi: 10.1111/j.1749-6632.1998.tb10504.x. [DOI] [PubMed] [Google Scholar]
- Eshghi P, Zadeh-Vakili A, Rashidi A, Miri-Moghadam E. An unusually frequent β-thalassemia mutation in an Iranian province. Hemoglobin. 2008;32:387–392. doi: 10.1080/03630260701758932. [DOI] [PubMed] [Google Scholar]
- Fisher CA, Premawardhena A, Silva S, Perera G, Rajapaksa S, Oliveri NA, Old JM, Weatherall DJ, The Sri Lanka Thalassaemia Study Group The molecular basis for the β-thalassaemias in Sri Lanka. Brit J Haematol. 2003;121:662–671. doi: 10.1046/j.1365-2141.2003.04346.x. [DOI] [PubMed] [Google Scholar]
- Garewal G, Das R, Ahluwalia J, Marwaha RK, Varma S. Nucleotide −88 (C–T) promoter mutation is a common β-thalassemia mutation in the Jat Sikhs of Punjab, India. Am J Hematol. 2005;79:252–256. doi: 10.1002/ajh.20445. [DOI] [PubMed] [Google Scholar]
- Ghani R, Manji MA, Ahmed N. Hemoglobinopathies amongst five major ethnic groups in Karachi, Pakistan. Southeast Asian J Trop Med Pub Hlth. 2002;33:855–861. [PubMed] [Google Scholar]
- Hassan SM, Hamza N, Al-Lawatiya FJ, Mohammed AJ, Harteveld CL, Rajab A, Giordano PC. Extended molecular spectrum of β- and α-thalassemia in Oman. Hemoglobin. 2010;34:127–134. doi: 10.3109/03630261003673147. [DOI] [PubMed] [Google Scholar]
- Hussain R. The effect of religious, cultural and social identity on population genetic structure among Muslims in Pakistan. Ann Hum Biol. 2005;32:145–153. doi: 10.1080/03014460500075167. [DOI] [PubMed] [Google Scholar]
- Khan SN, Riazuddin S. Molecular characterization of β-thalassemia in Pakistan. Hemoglobin. 1998;22:333–345. doi: 10.3109/03630269809071528. [DOI] [PubMed] [Google Scholar]
- Modell B, Darlinson M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull WHO. 2008;86:480–487. doi: 10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty D, Colah R, Gorakshakar A (eds) (2008) Jai Vigyan S & T mission project on community control of thalassaemia syndromes—awareness, screening, genetic counselling and prevention. A national multicentric task force study of ICMR (2000–2005), Indian Council of Medical Research, New Delhi
- Ne-Win, Harano T, Harano K, Myint T-T, Rai-Mra, Okada S, Shimono K, Aye-Aye-Myint A wider molecular spectrum of β-thalassaemia in Myanmar. Br J Haematol. 2002;117:988–992. doi: 10.1046/j.1365-2141.2002.03539.x. [DOI] [PubMed] [Google Scholar]
- Nicolini B. The Makran–Baluch–African network in Zanzibar and East Africa during the XIXth Century. Afr & Asian Stud. 2006;5:347–370. doi: 10.1163/156920906779134830. [DOI] [Google Scholar]
- Nicolini B. The Baluch role in the Persian gulf during the nineteenth and twentieth centuries. Comp Studies of S Asia, Africa & Mid East. 2007;27:84–396. [Google Scholar]
- Nishank SS, Ranjit M, Kar SK, Chhotray GP. Molecular variants and clinical importance of β-thalassemia traits found in the state of Orissa, India. Haematol. 2009;14:290–296. doi: 10.1179/102453309X439845. [DOI] [PubMed] [Google Scholar]
- Petrou M. Screening for beta thalassaemia. Ind J Hum Genet. 2010;16:1–5. doi: 10.4103/0971-6866.64934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World population data sheet. Washington: Population Reference Bureau; 2010. [Google Scholar]
- Quaife R, Al-Gazali L, Abbes S, Fitzgerald P, Fitches A, Valler D, Old JM. The spectrum of β-thalassaemia mutations in the UAE national population. J Med Genet. 1994;31:59–61. doi: 10.1136/jmg.31.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajab A, Patton MA. Major factors determining the frequencies of hemoglobinopathies in Oman. Am J Med Genet. 1997;71:240–242. doi: 10.1002/(SICI)1096-8628(19970808)71:2<240::AID-AJMG26>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Reid RM. Effects of consanguineous marriage and inbreeding on couple fertility and offspring mortality in rural Sri Lanka. Hum Biol. 1976;48:139–146. [PubMed] [Google Scholar]
- Sinha S, Black ML, Agarwal S, Colah R, Das R, Ryan K, Bellgard M, Bittles AH. Profiling β-thalassaemia mutations in India at state and regional levels: implications for genetic education, screening and counselling programmes. HUGO J. 2009;3:51–62. doi: 10.1007/s11568-010-9132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. A history of India 2. London: Penguin Books; 1970. [Google Scholar]
- Than AM, Harano T, Harano K, Myiny AA, Ogino T, Okada S. High incidence of α-thalassaemia, hemoglobin E, and glucose-6-phosphate dehydrogenase deficiency in populations of malaria-endemic southern Shan state, Myanmar. Intl J Hematol. 2005;82:119–123. doi: 10.1532/IJH97.05028. [DOI] [PubMed] [Google Scholar]
- Thapar R. A history of India 1. London: Penguin Books; 1966. [Google Scholar]
- Varawalla NY, Old JM, Sarkar R, Venkatesan R, Weatherall DJ. The spectrum of β-thalassaemia mutations on the Indian subcontinent: the basis for prenatal diagnosis. Brit J Haematol. 1991;78:242–247. doi: 10.1111/j.1365-2141.1991.tb04423.x. [DOI] [PubMed] [Google Scholar]
- Verma IC, Saxena R, Thomas E, Jain PK. Regional distribution of β-thalassemia mutations in India. Hum Genet. 1997;100:109–113. doi: 10.1007/s004390050475. [DOI] [PubMed] [Google Scholar]
- White JM, Christie BS, Nam D, Daar S, Higgs DR. Frequency and clinical significance of erythrocyte genetic abnormalities in Omanis. J Med Genet. 1993;30:396–400. doi: 10.1136/jmg.30.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2008) Joint WHO–TIF meeting on management of haemoglobin disorders (2nd: 2008: Nicosia, Cyprus) Geneva, World Health Organization. (NLM classification: WH 190)
- Zahed L. The spectrum of β-thalassemia mutations in the Arab Populations. J Biomed & Biotech. 2001;1:129–132. doi: 10.1155/S1110724301000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
β-Thalassaemia mutation frequencies in India (XLS 32 kb)
β-Thalassaemia mutation frequencies in Pakistan (XLS 24 kb)
β-Thalassaemia mutation frequencies in Sri Lanka (XLS 36 kb)