Abstract
Western health care systems are facing today increasing movement of genetic knowledge from research labs into clinical practice. This paper reports the results of a survey that addressed the confidence of primary care physicians in their ability to carry out basic medical genetic tasks. The survey was conducted in five countries (France, Germany, The Netherlands, Sweden and the UK). Stratified random samples were drawn from primary care physicians in the five countries representing a sampling frame of 139,579 physicians. Stepwise binary logistic regression procedures were performed to identify the predictor variables for self-reported confidence. Three thousand six hundred eighty-six physicians participated and filled out a self-administered questionnaire. The margin of error for accurate representation of each group of European general practitioners and specialists in the total sample is 2.9% for GP, 2.8% for obstetricians/gynaecologists (OB/GYN) and for paediatricians (PAED) 2.6% (95% confidence level). Confidence in their ability to carry out basic medical genetic tasks is low among participating primary care physicians: 44.2% are not confident, 36.5% somewhat confident, confident or very confident are 19.3%. In each country, those confident/very confident represent less than 33% of the participating physicians. Primary care physicians who report the lowest levels of confidence prove to be those least exposed to medical genetics information and training. Although there are significant differences in the way in which professional education is organised and practice is regulated across European countries, there is a need for a coordinated European effort to improve primary care physicians’ background in medical genetics.
Keywords: Genetic education, Genetic services, Primary care
Introduction
The last two decades have seen unprecedented advances in the understanding of human genetics and of genetic influence on people’s susceptibility to disease. This development strongly affects the practice of medicine. Western health care systems are facing today increasing movement of genetic knowledge from research labs into clinical practice (Nuffield Trust 2000).
However, the number of health professionals trained in medical genetics has not kept pace with the increase of genetic discovery and the demand for genetic services and genetic counselling (Harris 1997). Consequently, as genetic testing moves into the mainstream of health care, genetic tests will be increasingly administered by physicians with only limited or no training in genetics at all (Holtzman and Watson 1997). Irrespective of the speed with which genetic technologies become part of health care provision in Europe, the need for genetically informed health professionals is expected to rise.
Inadequate knowledge, skills and attitudes in medical genetics seem to be a universal problem of primary health care. In the US, the UK, The Netherlands and Switzerland, primary care physicians were found to be lacking adequate knowledge to deal appropriately with basic medical genetic problems of their patients (Suther and Goodson 2003). Studies in Canada have shown that primary care providers including general practitioners, obstetricians/gynaecologists and paediatricians have limited knowledge of genetics (Hunter et al. 1998; Bottorff et al. 2005). Primary care practitioners often do not interpret results of genetic tests ordered by them correctly and are poorly prepared to explain test results adequately (Baars et al. 2005). Many are unaware of the ethical, legal and psychosocial implications that may ensue and are not in compliance with ethical and clinical guidelines for genetic testing, especially with respect to informed consent and indications for testing (Geller 1999).
In the UK, the “Confidential Enquiry into Counselling for Genetic Disorders by Non-Geneticists (CEGEN)” (Harris and Harris 1999; Modell et al. 2000) showed that non-genetics medical specialists attach much greater importance to the clinical management of the immediate patient than to genetic counselling, screening and diagnosis designed to avoid future genetic problems in patients or their relatives.
Genetically educated and informed primary care physicians will provide better health care services and improve the quality and selectivity of referrals to geneticists (Harris 1994). One of the recommendations of the study “Concerted Action on Genetic Services in Europe (CAGSE)” on the situation of genetic counselling and genetic testing in 31 European countries was to promote education and training programmes for non-genetics health care professionals at a European level (Challen et al. 2005). European patient organisations have also identified improvement of non-genetics health professionals’ education in genetics as a priority (Smit et al. 1996).
Objectives and methods
The study “Genetic education, Improving non-genetics health professionals’ understanding of genetic testing (GenEd)”1 from which results are presented here is the first European study to address the problem of confidence of non-genetics primary health care professionals and their potential deficiencies in genetics. Five European Union member states: France, Germany, The Netherlands, Sweden and the United Kingdom participated in the GenEd study from 2002 to 2005. Together, these countries comprise more than 230 million people. The GenEd survey being part of the larger GenEd study was conducted in early 2005.
In each country, those non-genetics primary care physicians were identified who act as the main referrers to genetic services. These were obstetricians/gynaecologists, paediatricians and general practitioners. Representatives from the primary care physicians’ professional organisations in the participating countries were consulted and their cooperation in conducting the survey secured. To ensure that consumer issues in genetic testing were adequately addressed in the survey, representatives from European patient organisations (Genetic Alliance UK, United Kingdom and Vereninging Samenwerkende Ouder-en Patiëntenorganisaties, The Netherlands) were consulted.
So far, several articles describing in depth sampling, methodology and response rates, presenting results from other parts of the survey, have been published (Challen et al. 2005; Calefato et al. 2008; Julian-Reynier et al. 2008; Benjamin et al. 2009; Plass et al. 2009). One of the major objectives of the GenEd survey was to address the confidence of primary care physicians in their ability to carry out basic medical genetic tasks. The data presented here as Part 1 focus on the confidence of European primary care physicians’ as a total group. In Part 2, which will follow after this publication, differences among participating countries will be presented.
Sampling
Random samples of general practitioners, obstetricians/gynaecologists and paediatricians were drawn in each of the five participating countries, representing a total sampling frame of 139,579 physicians. Stratified sampling was used to generate—as best as possible—equal/similar numbers of physicians from each country and among general practitioners, obstetricians/gynaecologists and paediatricians. This approach minimizes the statistical influence of different country sample sizes within the total group and allows comparison of differences between the groups.
Questionnaire
The questionnaire including 28 practice and education-related questions was developed by an international multidisciplinary group of geneticists, social scientists, statisticians and representatives of the primary care providers targeted in this survey. The English questionnaire was piloted in the five participating countries among general practitioners, obstetricians/gynaecologists and paediatricians, revised and subsequently translated and back-translated.
Confidence measurement
One of the major topics addressed in the survey was the self-assessed confidence of primary care physicians in their ability to carry out basic medical genetic tasks. This study is using low confidence as an indication of an acknowledged need to improve the genetic background of the specific individual. The tasks addressed in the study represent 12 core competences in medical genetics. They were identified by consensus of the international study group and presented in the questionnaire in the following format:
“When you are caring for individuals and families with genetic conditions...”
“Please indicate how confident you feel in your ability to carry out each of the following:
Taking a family history
Identify family history of a potentially inherited condition
Identify autosomal dominant family patterns
Explain autosomal dominant family pattern to a patient
Estimate the risk for having an affected child for a couple where only one partner has a family history of an autosomal recessive disorder (e.g. cystic fibrosis or sickle cell disease)
Recognise when malformations may be genetic in origin
Counsel an individual whose father had Huntington’s disease to decide whether or not to have presymptomatic genetic testing?
Provide psychosocial counselling related to coping with a genetic test result
Identify patient support groups for rare genetic disorders
Identify relevant patient information materials for genetic disorders
Identify specialist genetic services in your area
Obtaining informed consent before taking blood for DNA (molecular) tests.”
The term “genetic conditions” was not defined in the questionnaire. The multidisciplinary group who designed the questionnaire decided that the term should be left open to the single physicians’ perception of individuals and families presenting with genetic conditions in her/his practice. The results of the pilot of the questionnaire confirmed this decision.
Data analysis
The analysis of the data was guided by four hypotheses, using null-hypothesis tests. These were:
Speciality,
Exposure to genetic teaching and training at various levels of medical education (undergraduate, postgraduate and CME/CPD),
Socio-demographic variables (age, sex and years in practice), and
Frequency of patient encounters with genetic conditions in primary care practice has a measurable impact on primary care physicians’ confidence in their ability to carry out the basic medical genetic tasks.
Respondents indicated their level of confidence in carrying out each of the 12 tasks on a four-point Likert scale (“not confident/somewhat confident/confident/very confident”).
The data were analysed using descriptive statistics as well as multivariate methods. Significance of results was tested by chi-square statistics. The contributions of the variables to improve the predictions for confident primary care physicians were calculated as odds ratios (exp(b) values); values <1 indicate negative contributions, values >1 positive ones to the fitting of the prediction. They represent the factors by which the odds for the event in question are increased or decreased. The combined effect size of each of the 12 models (tasks) was measured by “Pseudo R2”. The ratio measures the improvement of the fitted prediction compared to the unfitted. Significance for these analyses was measured by the Hosmer–Lemeshow test.
Results
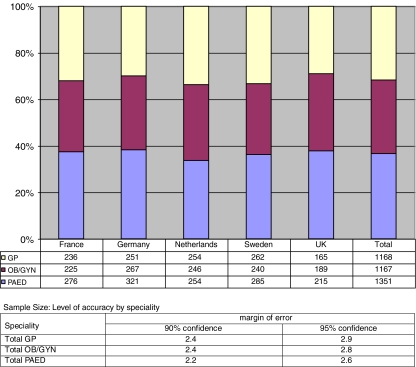
The size of the combined national samples of primary care physicians obtained from the five participating European countries is N = 3,686. The margin of error for accurate representation of each group of European general practitioners and specialists in the total sample is 2.9% for GP, 2.8% for OB/GYN and for PAED 2.6% (95% confidence level). The response rates for the individual countries were: France 50.2%, Germany 30.8%, The Netherlands 47.2%, Sweden 54.2% and the UK 29.1%. Fig. 1 gives an overview of the total sample by country and speciality and the level of accuracy of the obtained sample size for each speciality.
Fig. 1.
GenEd study: sample size by country and speciality, percentages
Demographics of the respondents
Demographics of the respondents are given in Table 1. The majority of the respondents are males (60.5%), practising for more than 10 years (85.2%) and see more than 50 patients per week (62.7%). The mean age is 50.2 years (SD 8.4).
Table 1.
Demographics of respondents
| Characteristic | Number | Valid percentage | |
|---|---|---|---|
| Sex | Male | 2,226 | 60.5 |
| Female | 1,454 | 39.5 | |
| Missing | 6 | ||
| Age | ≥50 years | 1,839 | 50.0 |
| ≤51 years | 1,837 | 50.0 | |
| Mean age | 50.2 (SEM 139) | SD 8.443 | |
| Speciality | GP | 1,168 | 31.7 |
| OB/GYN | 1,167 | 31.7 | |
| PAED | 1,351 | 36.6 | |
| Years in practice | ≤10 years | 543 | 14.8 |
| 10 ≥ 20 years | 1,272 | 34.7 | |
| ≥21 years | 1,852 | 50.5 | |
| Missing | 19 | ||
| Number of patients/week | ≥25 | 460 | 12.6 |
| 26 ≤ 50 | 903 | 24.5 | |
| 51 ≤ 100 | 1,092 | 29.6 | |
| 101 ≤ 150 | 617 | 16.7 | |
| 151 ≤ 200 | 317 | 8.6 | |
| ≥201 | 264 | 7.2 | |
| Missing | 33 | ||
Confidence levels
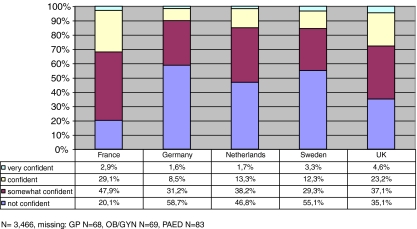
In all five countries the self-reported confidence levels of primary care physicians for carrying out the complete set of 12 basic tasks are low (Fig. 2). For all 12 tasks combined, confident/very confident physicians are a minority in all five countries: in France, this group represents 32.0%; in Germany, 10.1%; in The Netherlands, 15.0%; in Sweden, 15.6% and in the UK, 27.8% of the responding physicians.
Fig. 2.
GenEd study: confidence of primary care physicians carrying out basic medical genetic tasks by country
Specialities
Looking at the effect of the speciality variables (GP, OB/GYN, PAED), a general pattern concerning the extent of the self-reported confidence levels for all tasks is found: general practitioners are less confident (confident, very confident 9.0%) than obstetricians/gynaecologists (confident, very confident 15.4%) who, in turn, are less confident than paediatricians who are the most confident (confident, very confident 31.6%)—relatively speaking—among the primary care physicians.
Although, paediatricians were found to represent the group of specialists who are most confident in assessing their ability to carry out the specified medical genetic tasks as compared to the other primary care physicians, the confident/very confident ones never reach majority status in any of the participating countries.
Exposure to genetic information and training
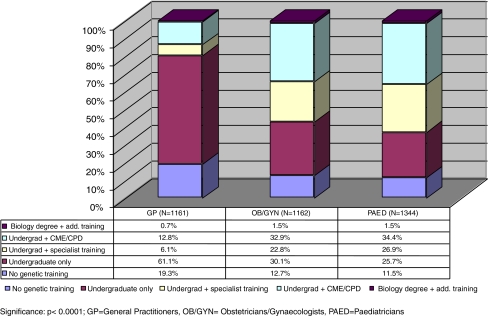
Exposure to genetic information and training at various stages of medical education was expected to have a measurable effect on the confidence level of primary care physicians. It was measured by the question: “How much education have you had in genetics?” with five answering options detailing the exposure to genetics: “None”; “Genetics content during undergraduate medical training”; “Genetics content in specialist training”; “Genetics content in courses, independent study or seminars in continuing education”; and “Degree (BSc, MSc,...) in biological sciences”. Figure 3 provides a breakdown of the obtained results by speciality:
Fig. 3.
GenEd study: medical genetics training of primary care physicians
Of general practitioners, 19.3% did not receive any genetic training, and 61.1% had only undergraduate training. The percentage of obstetricians/gynaecologists who did not receive medical genetic training was 12.7%, and 30.1% had only undergraduate training. Of the paediatricians, 11.5% report not having received any training in genetics, and 25.7% had only undergraduate training.
Obstetricians/gynaecologists and paediatricians not only get more information in medical genetics in the course of their speciality training, but a larger number of them actively seek additional information in genetics from CME/CPD courses. Of the obstetricians/gynaecologists, 32.9% and 34.4% of paediatricians attended CME/CPD courses in genetics as compared to 12.8% of the general practitioners (p < 0.000, cf. Fig. 3).
Frequency of encounters with patients with genetic conditions
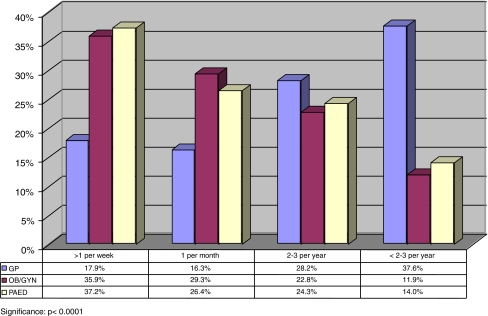
Frequency of patient contacts with medical genetic conditions broken down by speciality is shown in Fig 4.
Fig. 4.
GenEd study: frequency of contact with patients with genetic conditions by speciality
Paediatricians and obstetricians/gynaecologists report more frequent contacts (one or more per week and one or more per month) with patients with a genetic condition as compared to general practitioners (p < 0.000). Still, 34.2% of the general practitioners have at least one patient per month with a genetic condition and 17.9% report more than one patient contact due to a genetic condition per week.
Predicting primary care physicians’ confidence in their ability to perform basic medical genetic tasks
Eight predictor variables were identified: Specialities—“GP; OB/GYN; PAED”, “Exposure to genetics teaching and training in CME/CPD programmes”, “Age of respondent (<36 years)”, “Sex (F)”, “Years in practice (≤10 years)” and “Frequency of patient encounters with genetic conditions (≥1 patient with a genetic condition per week)” (Table 2).
Table 2.
Self-reported confidence of primary care physicians in their ability to carry out defined basic medical genetic tasks
| Confidence | GP | OB/GYN | PAED | Total |
|---|---|---|---|---|
| Not confident | 64.4% | 46.4% | 24.8% | 1,532 (44.2%) |
| Somewhat confident | 26.5% | 38.3% | 43.6% | 1,264 (36.5%) |
| Confident | 8.5% | 12.9% | 26.8% | 576 (16.6%) |
| Very confident | 0.5% | 2.5% | 4.8% | 94 (2.7%) |
| Total | 1,100 (100%) | 1,098 (100%) | 1,268 (100%) | 3,466 (100%) |
Missing information: 6.0%; significance: p < 0.0001
Table 3 gives an overview of the odds ratios (exp(b) values) and pseudo R2 values (Nagelkerke’s) for physicians’ confidence in their ability to carry out each one of the basic medical genetic tasks, based on the total sample of primary care physicians2 (N = 3,686). All results are significant as measured by the Hosmer–Lemeshow test. There is a split between the first six basic medical genetics tasks 1–6 and the following six ones 7–12. This split is expressed by large differences in improvements of the R2values. While the first six predictions for confident primary care physicians are improved by 12–25%, the improvements for the last six variables range only between 2% and 10%. Tasks 1–6 require different knowledge, skills and attitudes in medical genetics than tasks 7–12. The first six tasks are more closely related to clinical problems of medical genetics as compared to the remaining ones which are predominantly related to counselling and support-giving abilities.
Table 3.
Predictor variables for primary care physicians’ confidence in their ability to carry out basic medical genetic tasks, odds r atios (exp(b) values) and effect size values, CI 95% for exp(b) lower and upper limits
| Reference (….) | Speciality (other) PAED | Speciality (other) OB/GYN | Speciality (other) GP | CME/CPD (none) Genetics in CME | Age (≥36 years) <36 years | Sex (Male) Female | Years in practice (other) ≤10 years | Frequency (<1 genetic patient/w) >1 genetic patient/week | Hosmer–Lemeshow Test | Nagelkerke’s R2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Medical genetic tasks | ||||||||||
| (1) Taking a family history | 3.363 | 1.570 | 1.803 | 0.109 | 0.155 | |||||
| CI 95% for exp(b) lower–upper limit | 2.662–4.247 | 1.278–1.930 | 1.441–2.255 | |||||||
| (2) Identify family history of a potentially inherited disorder | 2.207 | 1.631 | 2.207 | 0.048 | 0.123 | |||||
| CI 95% for exp(b) lower–upper limit | 1.761–2.765 | 1.254–2.121 | 1.708–2.850 | |||||||
| (3) Identify autosomal dominant family patterns | 3.523 | 2.237 | 1.657 | 2.280 | 0.323 | 0.252 | ||||
| CI 95% for exp(b) lower–upper limit | 2.792–4.446 | 1.699–2.945 | 1.342–2.044 | 1.754–2.963 | ||||||
| (4) Explain autosomal dominant family pattern to a patient | 2.153 | 0.496 | 2.252 | 1.694 | 0.354 | 0.193 | ||||
| CI 95% for exp(b) lower–upper limit | 1.674–2.769 | 0.371–0.662 | 1.776–2.857 | 1.381–2.079 | ||||||
| (5) Estimate the risk of having an affected child to a couple where only one partner has a family history of an autosomal recessive disorder (e.g. cystic fibrosis or sickle cell disease) | 4.036 | 1.836 | 1.989 | 1.413 | 0.493 | 0.145 | ||||
| CI 95% for exp(b) lower - upper limit | 2.922–5.573 | 1.311–2.574 | 1.532–2.582 | 1.155–1.729 | ||||||
| (6) Recognise when malformation may be genetic in origin | 4.350 | 2.091 | 2.310 | 2.205 | 0.225 | 0.151 | ||||
| CI 95% for exp(b) lower–upper limit | 2.695–7.022 | 1.264–3.458 | 1.591–3.352 | 1.558–3.120 | ||||||
| (7) Counsel an individual whose father had Huntington’s disease to decide whether or not to have presymptomatic genetic testing | 0.630 | 0.650 | 0.412 | 0.022 | ||||||
| CI 95% for exp(b) lower–upper limit | 0.459–0.866 | 0.471–0.898 | ||||||||
| (8) Provide psychosocial counselling related to coping with a genetic test result | 0.716. | 0.714 | 0.656 | 1.324 | 0.848 | 0.023 | ||||
| CI 95% for exp(b) lower–upper limit | 0.563–0.911 | 0.567–0.898 | 0.437–0.985 | 1.042–1.682 | ||||||
| (9) Identify patient support groups for rare genetic disorders | 2.399 | 0.546 | 1.569 | 0.987 | 0.075 | |||||
| CI 95% for exp(b) lower–upper limit | 1.906–3.020 | 0.360–0.828 | 1.229–2.004 | |||||||
| (10) Identify relevant patient information material for genetic disorders | 2.178 | 0.678. | 0.895 | 0.042 | ||||||
| CI 95% for exp(b) lower–upper limit | 1.706–2.781 | 0.524–0.876 | ||||||||
| (11) Identify specialist genetic services in your area | 0.337. | 0.468 | 1.430 | 0.574 | 0.102 | |||||
| CI 95% for exp(b) lower–upper limit | 0.261–0.435 | 0.326–0.671 | 1.122–1.823 | |||||||
| (12) Obtain informed consent before taking blood for DNA (molecular) tests | 3.258 | 1.707 | 0.775 | 1.341 | 0.015 | 0.079 | ||||
| CI 95% for exp(b) lower–upper limit | 2.420–4.383 | 1.206–2.312 | 0.619–0.970 | 1.063–1.692 |
Primary care physicians who are confident in carrying out the first six basic medical genetic tasks are: PAED and—to a lesser extent—OB/GYN, under 36 years of age, engage in CME/CPD activities addressing genetics and have frequent encounters in their practice with patients who have genetic conditions(≥1/week). However, the variables “CME/CPD” and “Age” are mainly contributing to predicting confidence in performing clinically related medical genetics tasks 1–6.
Among all variables predicting primary care physicians’ confidence in their ability to carry out the 12 medical genetic tasks, the variable PAED is contributing most to the predictions. However, for two tasks, 8 (“Provide psychosocial counselling related to coping with a genetic test result”) and 11 (“Identify specialist genetic services in your area”), the PAED variable does not contribute to the predictions. The missing contribution of the PAED variable for task 8 indicates that paediatricians feel less confident in providing counselling as compared to providing clinical tasks. However, chances for patients for referral for counselling to genetic services may be compromised as the variable does not contribute to the prediction of task 11. Only one negative contribution of the PAED variable is found. This is made to the prediction of task 7, which relates to counselling for a late-onset disorder, a task probably not so often encountered in a paediatrician’s practice.
Obstetricians/gynaecologists are included in predictions as confident primary care physicians for two clinically oriented tasks, 5 (“Estimate the risk of an affected child…”) and 6 (“Recognise when malformation may be genetic in origin”), and one counselling task, 12 (“Obtain informed consent before taking blood for DNA tests”). General practitioners are only included in the predictions for two tasks, 4 (“Explain autosomal dominant family pattern”) and 11 (“Identify genetic services in your area”). In both cases, negative contributions are made.
The CME/CPD variable is contributing positively only to the predictions of clinically oriented tasks 2–6. It does not contribute to task 1 (“Taking a family history”). The CME/CPD variable is found contributing negatively in one of the tasks of counselling and support-giving activities.
The variable “Age (≤36 years)” makes positive contributions to the predictions of confident physicians in four (1, 3, 4 and 5) out of the six clinically oriented medical genetic tasks 1–6.
The variable “Sex: Female” contributes solely negatively to four of the predictions of the counselling and support-giving tasks.
Of the variable “Years in practice”, only recent practice (less than 10 years) contributes to the prediction. This variable contributes negatively to the predictions of tasks from the counselling and support-giving type, 8, 9 and 11.This indicates that confidence in providing counselling and support giving increases with experience in medical practice.
The variable representing frequency of patient encounters with genetic conditions (“>1 genetic patient/week”) is found to make strong positive contributions to eight out of a total of 12 tasks (1, 2, 3, 6, 8, 9, 11 and 12).
Comparing the prediction of confidence for the two groups of different tasks 1–6 and 7–12 shows that clinical tasks have clearly larger improvements. The predictions relating to counselling and support giving is shaped positively by the variable frequent encounters with patients with genetic conditions (tasks 8, 9, 11 and 12) and being a paediatrician for tasks 9, 10 and 12.
Discussion
GenEd is the first European study to address the confidence of non-genetics primary care physicians in their ability to carry out basic medical genetics tasks. The results confirm the research hypotheses. There is a measurable impact of the variables “speciality”, “exposure to genetic teaching and training in CME/CPD activities”, “age”, “sex” and “frequency of patient encounters with genetic conditions”, on primary care physicians’ reported confidence in their ability to carry out medical genetics tasks. However, the primary care physicians who expressed unrestricted confidence in their ability to carry out the complete set of the 12 medical genetics tasks were only a small group of 19.3%; moreover, those confident for all tasks are a minority in all three specialities in all participating European countries.
Confidence in the ability to carry out all 12 basic medical genetic tasks depends on the volume of genetic education and training received by the different non-genetics primary care physicians. Compared to the other two specialities represented in this study, general practitioners report the largest percentage of those who did not receive any genetic information and training. They also represent the largest group of physicians whose only base of medical genetic information is their undergraduate training. Of the general practitioners, 80.4% either had no training in medical genetics or had only undergraduate genetic information and training. It is therefore not surprising, when looking at each one of the basic genetic tasks separately, that only 16% of the general practitioners are “very confident/confident” in their ability to identify the family history of a potentially inherited disorder and 14.5% in their ability to identify autosomal dominant family patterns. Less than half of them (45.1%) report confidence in their ability to identify specialist genetic services in their area of practice. Confident general practitioners represent only 9.0% of the total sample of general practitioners. As general practitioners often are the primary care physicians of first contact for patients with genetic problems, these findings raise concern about the quality of genetic services provided by general practitioners.
Knowledge, skills and attitudes in medical genetics are acquired through structured and continuing information and training. It is, therefore, striking to see the extent to which primary care physicians did not receive any genetic information and training or rely alone on their knowledge acquired during their undergraduate training. The mean age of all primary care physicians in this survey was 50.2 years (SD 8.4). This means that the undergraduate training of a large number of primary care physicians must have taken place quite some time ago, probably too long to rely solely on its content for valid information for a fast expanding field like genetics.
The variables “CME/CPD” representing non-genetics primary care physicians actively seeking medical genetics information and the variable “Age <36 years” are found to predict primary care physicians’ confidence in their ability to perform clinically oriented medical genetics tasks (tasks 1–6). This may indicate that CME/CPD programmes seem to concentrate on the clinical aspects of medical genetics. It also documents that the situation already critically mentioned by the CEGEN Study (Harris and Harris 1999) that physicians tend to attach more importance to clinical aspects of medical genetics compared to counselling and support giving still prevails with primary care physicians. Primary care physicians under 36 years of age may have received more comprehensive information and training background in medical genetics resulting in more confidence to perform these tasks as compared to their older colleagues. These results could also be seen as indicator of a changing perception of the importance of genetics in medical education and training.
The reticence of female doctors to claim confidence in their ability to perform counselling and support-giving tasks as compared to male physicians may indicate overall gender specific differences in reporting confidence (Nomura et al. 2010).
The predictions of primary care physicians’ confidence in their ability to carry out medical genetic tasks related to “counselling and support giving” (tasks 7–12) show only minor improvements by the variables identified (“PAED”, “Frequency of patient encounters with genetic conditions”). These findings indicate that confidence in carrying out the basic tasks of medical genetics is related to frequent patient encounters with genetic conditions rather than to length of time in practice.
The results indicate that—regardless of the national setting they are practising in—the background in medical genetics of non-genetics health professionals in primary care in Europe needs revision and improvement. This requires expansion of the information and training base of primary care physicians in general and of general practitioners in particular (Emery et al. 1999; Korf 2002; Henriksson and Kristoffersson 2006; Schmidtke et al. 2006; Darzi 2008; Royal College of General Practitioners 2008; Burke et al. 2009; Manek and Allen 2009). An extended and intensified teaching approach for medical genetics in undergraduate medical education may, in the future, positively impact on the knowledge, skills, and attitudes of primary care physicians in medical genetics. However, the existing problem—identified by the GenEd study—that the majority of practising non-genetics primary health care professionals of today lack confidence in carrying out basic medical genetic tasks, require these physicians to become aware of their deficiencies now and to agree to develop an appropriate strategy to improve their background in genetics. As primary care physicians will remain the first contact for patients with genetic problems and their parents, doctors will have to actively seek to improve their competences in medical genetics. This could be achieved by CME/CPD courses in genetics, tailored to the needs of primary care practitioners (Carroll et al. 2009). The findings of the GenEd survey support the recommendations of the European Society of Human Genetics Education Committee to establish core competences for non-genetics health professionals in all European countries (EuroGentest 2010).
Limits
There are certain limits of the study results that need to be addressed. Self-assessed confidence does not automatically result in providing competent services. Although self-assessed confidence in one’s ability to perform certain tasks has long been accepted as a way to measure competence in a certain field, recent evaluations of studies using confidence as a proxy for measuring competence have shown that the results are equivocal. Most of the studies evaluated “demonstrated little, no, or an inverse relationship” between self-assessed confidence and measured competence criteria (Davis et al. 2006). On the other hand, medical services provided with little or no confidence may have a reasonably high chance to fail patients with genetic problems.
In addition, it should be kept in mind, intraprofessional and health system related administrative regulations for primary care physicians have been proliferating lately, often interfering with physicians’ activities to become more knowledgeable and skilled in medical genetics (Emery et al. 1999; Burke et al. 2009; Royal College of General Practitioners 2008; Manek and Allen 2009).
The data reported here are from 2005 and thus do not reflect whether or not improvements in the confidence to carry out basic genetic tasks have occurred among the non-genetics health professionals targeted in this survey. However, to the best of our knowledge no new empirical findings addressing this issue have been published so far for Europe.
Conclusion
The results of the study indicate that the development of confidence to carry out basic medical genetic tasks in primary care depends upon the education, training and clinical experience of the health professionals involved. The background in medical genetics of non-genetics health professionals in primary care in all European countries participating in this survey needs revision and improvement regardless of the different health care systems represented here. Although there are significant differences in the way in which professional education is organised and practice is regulated across the European countries, there is a need for a shared set of core competences in genetics that health professionals should possess. In addition, a discourse about the active role of primary care practitioners in the provision of genetic services is probably needed. To improve the quality of medical genetics, services rendered by primary care physicians and appropriate referral commitment for patients with genetic problems and a close collaboration with medical geneticists would be helpful. Although, such an approach will not substantially increase the diagnostic and treatment capacities available, it may, nevertheless, help reduce the time for patients and their families to receive valid diagnoses, counselling and preventative and treatment options, if available.
Acknowledgements
For the patient organisations: The contributions and support of Alastair Kent and Ysbrand Poortman are greatly appreciated. We also appreciate the technical support of Jean-Marc Calefato, Gaelle Santin in France; the contribution and support of Prof. Dr. E. Harms (paediatrician), Prof. Dr. W. Holzgreve (obstetrician/gynaecologist) and Prof. Dr. K. Wahle (general practitioner) in Germany; the contribution and support of Prof. Dr. Martina Cornel (community genetics), Dr. Marieke Baars (clinical geneticist), Dr. Attie Go (gynaecologist), Frans Boonekamp (general practitioner) and Dr. Michiel Weijers (paediatrician) in The Netherlands and the research and administrative support of Prof. Tony Heagerty (Professor of Medicine) and technical support of Daniel Cottam and Christine Waterman in UK.
Conflicts of interest The authors declare that they have no conflict of interest.
Footnotes
The study was funded by the EU Program FP5 Contract QLG4-CT2001-30216.
Effects of the variable “country” will be presented subsequently in Part 2.
References
- Baars MJ, Henneman L, ten Kate LP. Deficiency of knowledge of genetics and genetic tests among general practitioners, gynaecologists, and paediatricians: a global problem. Genet med. 2005;7(9):605–610. doi: 10.1097/01.gim.0000182895.28432.c7. [DOI] [PubMed] [Google Scholar]
- Benjamin CM, Anionwu EN, Kristoffersson U, ten Kate LP, Plass AM, Nippert I, Julian-Reynier C, Harris HJ, Schmidtke J, Challen K, Calefato JM, Waterman C, Powell E, Harris R, GenEd Research Group Educational priorities and current involvement in genetic practice: a survey of midwives in the Netherlands, UK and Sweden. Midwifery. 2009;25(5):483–499. doi: 10.1016/j.midw.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Bottorff JL, Blaine S, Carroll JC, Evans J, Nicolson Klimek ML, Meschino W, Ritvo P. The educational needs and professional roles of Canadian physicians and nurses regarding genetic testing and adult onset hereditary disease. Community Genet. 2005;8(2):80–87. doi: 10.1159/000084775. [DOI] [PubMed] [Google Scholar]
- Burke S, Martyn M, Stone A, Bennet C, Thomas H, Farndon P. Developing a curriculum statement based on clinical practice: genetics in primary care. Br J Gen Pract. 2009;59(559):99–103. doi: 10.3399/bjgp09X395094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calefato JM, Nippert I, Harris HJ, Kristofferson U, Schmidtke J, ten Kate LP, Anionwu E, Benjamin C, Challen K, Plass AM, Harris R, Julian-Reynier C. Assessing educational priorities in genetics for GPs and specialists in 5 countries: factor structure of genetic educational priorities (Gen-EP) scale. Genet Med. 2008;10(2):99–106. doi: 10.1097/GIM.0b013e3181614271. [DOI] [PubMed] [Google Scholar]
- Carroll J, Rideout AL, Wilson BJ, Allanson J, Blaine SM, Esplen MJ, Farrell SA, Graham GE, MacKenzie J, Meschino W, Miller F, Prakash P, Summers A, Taylor S. Genetic education for primary care providers—improving attitudes, knowledge, and confidence. Can Family Physician Le médecin de famille canadien. 2009;55:e92–e99. [PMC free article] [PubMed] [Google Scholar]
- Challen K, Harris HJ, Julian-Reynier C, Kate LP, Kristofferson U, Nippert I, Schmid-tke J, Benjamin C, Harris R. Genetic education and non-genetic health professionals: educational providers and curricula in Europe. Genet Med. 2005;7(5):302–310. doi: 10.1097/01.GIM.0000164562.18306.71. [DOI] [PubMed] [Google Scholar]
- Darzi A. Quality for all: next stage review, Final Report, London: Department of Health, 2008
- Davis DA, Mazmanian PE, Fordis M, Harrison R, Thorpe KE, Perrier L. Accuracy of physician self-assessment compared with observed measures of competence. JAMA. 2006;296(9):1094–1102. doi: 10.1001/jama.296.9.1094. [DOI] [PubMed] [Google Scholar]
- Emery J, Watson E, Rose P, Andermann A. A systematic review of the literature exploring the role of primary care in genetic services. Fam Pract. 1999;16(4):426–445. doi: 10.1093/fampra/16.4.426. [DOI] [PubMed] [Google Scholar]
- EuroGentest: Core competences in genetics for health professionals in Europe, source: http://www.eurogentest.org/web/info/public/unit6/core_competences.xhtml, July 2010
- Geller G. (1999) Americans’ attitudes toward informed consent for breast cancer susceptibility testing: questions for cross-cultural research. In: Nippert I, Neitzel H, Wolff G (eds.) The new genetics: from research into health care—social and ethical implications for users and providers. Springer, Berlin, pp 13–21
- Harris R. Genetic counselling and testing in Europe. J R Coll Physicians Lond. 1994;32(4):335–338. [PMC free article] [PubMed] [Google Scholar]
- Harris R (ed.) (1997) Genetic services in Europe: a comparative study of 31 countries. European Journal of Human Genetics 5 (Suppl 2):1–220 [PubMed]
- Harris R, Harris HJ. Clinical governance and genetic medicine. Specialist genetic centres and the confidential enquiry into counselling for genetic disorders by non-geneticists (CEGEN) J Med Genet. 1999;36:350–351. [PMC free article] [PubMed] [Google Scholar]
- Henriksson K, Kristoffersson U. Education in medical genetics for non-genetic health care providers in Sweden. Community Genet. 2006;9(4):240–245. doi: 10.1159/000094472. [DOI] [PubMed] [Google Scholar]
- Holtzman NA, Watson MS (ed.) (1997) Promoting safe and effective genetic testing in the United States. Final report of the task force on genetic testing [PubMed]
- Hunter A, Wright P, Cappelli M, Kasaboski A, Surh L. Physician knowledge and atti- tudes toward molecular genetic (DNA) testing of their patients. Clin Genet. 1998;5(6):447–455. doi: 10.1111/j.1399-0004.1998.tb02593.x. [DOI] [PubMed] [Google Scholar]
- Julian-Reynier C, Nippert I, Calefato JM, Harris HJ, Kristofferson U, Schmidtke J, Kate LP, Anionwu E, Benjamin C, Challen K, Plass AM, Harris R. Genetics in clinical practice: general practitioners’ educational priorities in European countries. Genet Med. 2008;10(2):107–113. doi: 10.1097/GIM.0b013e3181616693. [DOI] [PubMed] [Google Scholar]
- Korf BR. Integration of genetics into clinical teaching in medical school education. Genet Med. 2002;4(6, Suppl):33 S–38 S. doi: 10.1097/00125817-200211001-00007. [DOI] [PubMed] [Google Scholar]
- Manek N, Allen K. (2009) Changes to GP training, BMJ Careers, July 15, 12–14
- Modell B, Harris R, Lande B, Khan M, Darlison M, Petrou M, Old J, Layton M, Var-navides L. Informed choice in genetic screening for thalassaemia during pregnancy: audit from a national confidential inquiry. BMJ. 2000;320:337–341. doi: 10.1136/bmj.320.7231.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura K, Yano E, Fukui T. Gender differences in clinical confidence: a nationwide survey of resident physicians in Japan. Acad Med. 2010;85:647–653. doi: 10.1097/ACM.0b013e3181d2a796. [DOI] [PubMed] [Google Scholar]
- Nuffield Trust (2000) Genetics and health. Policy issues for genetic science and their implica- tions for health and health services. Nuffield Trust
- Plass AM, Baars MJH, Cornel MC, Julian-Reynier C, Nippert I, Harris HJ, Kristofferson U, Schmidtke J, Anionwu E, Benjamin C, Challen K, Harris R, Kate LP. Testing the children: do non-genetic health care providers differ in their decision to advise genetic pre-symptomatic testing of minors? A study in five countries in the EU. Genet Test Mol Biomark. 2009;13(3):367–376. doi: 10.1089/gtmb.2008.0119. [DOI] [PubMed] [Google Scholar]
- A review of GP specialty training in the UK: Interim report for the Department of Health. London: RCGP; 2008. [Google Scholar]
- Schmidtke J, Paul Y, Nippert I. Education in medical genetics for physicians: Germany. Community Genet. 2006;9(4):235–239. doi: 10.1159/000094471. [DOI] [PubMed] [Google Scholar]
- Smit C, Kent A, Poortman Y. (1996) Biomedical research and patenting: ethic, social, and legal aspects. European Platform for Patients’ Organisations, Science and Industry
- Suther S, Goodson P. Barriers to the provision of genetic services by primary care physicians: a systematic review of the literature. Am Coll Med Genetics. 2003;5(2):70–76. doi: 10.1097/01.GIM.0000055201.16487.61. [DOI] [PubMed] [Google Scholar]